Abstract
The protein TRB3 (tribbles 3), also called NIPK (neuronal cell death-inducible protein kinase), was recently identified as a protein–protein interaction partner and an inhibitor of PKB (protein kinase B). To explore the hypothesis that TRB3/NIPK might act as a negative regulator of insulin signalling in the liver, this protein was overexpressed by adenoviral transduction of primary cultures of rat hepatocytes, and various aspects of insulin action were investigated. The insulin-induced phosphorylation of Ser-473 and Thr-308 of PKB was found to be undiminished in transduced hepatocytes with a molar excess of TRB3/NIPK over PKB of more than 25-fold. Consistent with unimpaired insulin activation of PKB, the stimulation of Ser-21 and Ser-9 phosphorylation of glycogen synthase kinase 3-α and -β, and the apparent phosphorylation level of 4E-BP1 (eukaryotic initiation factor 4-binding protein 1), were similar in transduced and control hepatocytes. The induction by insulin of the mRNAs encoding glucokinase and SREBF1 (sterol-regulatory-element-binding factor 1) were also normal in TRB3/NIPK hepatocytes. In contrast, the insulin-dependent induction of these two genes, as well as the activation of PKB, were shown to be suppressed in hepatocytes treated with the lipid ether compound PIA6 (phosphatidylinositol ether lipid analogue 6), a recently discovered specific inhibitor of PKB. Since TRB3/NIPK was reported to be increased in the liver of fasting mice, the effects of glucagon, glucocorticoids and insulin on the level of endogenous TRB3/NIPK mRNA in primary hepatocytes were investigated. No significant change in mRNA level occurred under any of the hormonal treatments. The present study does not support the hypothesis that the physiological role of TRB3/NIPK might be to put a brake on insulin signalling in hepatocytes.
Keywords: glucokinase, insulin, neuronal death-induced protein kinase, protein kinase B (Akt), sterol-regulatory-element-binding protein, tribbles 3
Abbreviations: CMV, cytomegalovirus; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; GCK, glucokinase; GSK3, glycogen synthase kinase 3; HA, haemagglutinin; HEK-293 cells, human embryonic kidney 293 cells; IGF1, insulin-like growth factor 1; MOI, multiplicity of infection; PCK1, phosphoenolpyruvate carboxykinase 1; PIA6, phosphatidylinositol ether lipid analogue 6; PKB, protein kinase B; SREBF1, sterol-regulatory-element-binding factor 1; TRB3/NIPK, tribbles 3/neuronal cell death-inducible protein kinase
INTRODUCTION
PKB (protein kinase B), also known as AKT, is thought to play a key role in insulin signal transduction [1]. The three isoforms of PKB (PKBα, PKBβ and PKBγ), which are variously expressed in different tissues [2], are subjected to activation by insulin in a phosphoinositide 3-kinase-dependent manner [3]. The activation of PKB is effected by phosphorylation of Thr-308 and Ser-473 (amino acid numbers refer to the PKBα sequence), catalysed respectively by phosphoinositide-dependent protein kinase 1 [4,5] and a second protein kinase suggested to be DNA-dependent protein kinase [6], although other kinases have also been incriminated [7]. Once activated, PKB can phosphorylate several protein substrates of metabolic significance. The first ‘metabolic’ substrate to be identified was GSK3 (glycogen synthase kinase 3), which is inactivated by PKB-dependent phosphorylation [8]. Inactivation of GSK3 is viewed as a key mechanism resulting in the dephosphorylation and activation of glycogen synthase and the stimulation of glycogen synthesis in insulin-stimulated cells. Other metabolic substrates of PKB include the heart isoform of the regulatory enzyme of glycolysis 6-phosphofructokinase-2 [9] and the rate-controlling enzyme of fatty acid synthesis ATP citrate-lyase [10]. Site-specific phosphorylation of these enzymes by PKB may contribute to their activation, accounting partly for the insulin stimulation of glycolysis in cardiac myocytes and lipogenesis in adipocytes. Additionally, PKB has been shown to phosphorylate and functionally de-activate the transcription factor FOXO1 (forkhead box class O factor 1) [11], leading to the repression of the gluconeogenic enzyme glucose 6-phosphatase [12]. These examples illustrate the critical role of PKB in coupling upstream signalling events with enzymes or other effector proteins, which shape up the metabolic response of target cells to insulin.
A number of proteins other than its substrates were reported to interact with PKB, with either positive or negative effects on PKB activity [7]. Protein partners of PKB that stimulate its activity might conceivably enhance insulin action, and proteins that inhibit PKB activity might cause insulin resistance. In the latter category, much interest has recently focused on a mammalian homologue of the Drosophila protein TRB3 (tribbles 3), also known as NIPK (neuronal cell death-inducible putative protein kinase), which was identified as a PKB-interacting protein in a yeast two-hybrid screen of a mouse preadipocyte cDNA library [13]. When overexpressed with PKB in HEK-293 cells (human embryonic kidney 293 cells), TRB3/NIPK inhibited the activation of PKB by IGF1 (insulin-like growth factor 1). Under pathophysiological conditions, TRB3/NIPK mRNA was reported to be increased in the livers of fasted or diabetic mice, and the overexpression of TRB3/NIPK in mouse liver after adenoviral transduction was found to compromise the accumulation of hepatic glycogen during refeeding and to cause modest glucose intolerance [13]. These findings suggested that TRB3/NIPK might be a master regulator of metabolism during fasting, and if dysregulated, a cause of insulin resistance in diabetes [14,15].
A major effect of insulin in liver is the transcriptional induction of GCK (glucokinase) [16]. This effect has been extensively analysed in this laboratory using primary cultures of rat hepatocytes, and several lines of evidence suggesting a critical role of PKB activation in the insulin induction of hepatic GCK have been obtained [17,18]. It was therefore of interest to ask whether PKB-mediated insulin signalling and GCK induction in primary hepatocytes would be impaired during overexpression of TRB3/NIPK. A second important issue was to investigate the multihormonal regulation of endogenous TRB3/NIPK mRNA in primary hepatocytes, with a focus on agents such as the glucocorticoids and cAMP, which might contribute to the reported increase in hepatic TRB3/NIPK during fasting. The results of this study do not support a role of TRB3/NIPK as a key regulator of insulin signalling in rat liver cells.
EXPERIMENTAL
Materials
The PIA6 (phosphatidylinositol ether lipid analogue 6) inhibitor of PKB was purchased under the name AKT inhibitor III from Calbiochem (Juro Supply GmbH, Lucerne, Switzerland). The suppliers of the antibodies to various antigens were the following: HA (haemagglutinin) epitope (12CA5), Roche Molecular Biochemicals (Rotkreuz, Switzerland); pleckstrin homology domain of PKB-α, Upstate Biotechnology (Lake Placid, NY, U.S.A.); total PKB (α, β and γ), PKB phosphorylated at Ser-473, PKB phosphorylated at Thr-308, GSK3-α and -β phosphorylated at Ser-21 and Ser-9 respectively, Cell Signaling Technology (Beverly, MA, U.S.A.); GSK3, Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.); and 4E-BP1 (eukaryotic initiation factor 4E-binding protein-1, also termed PHAS-1), Zymed Laboratories (San Francisco, CA, U.S.A.). Secondary antibodies were affinity-purified goat antibodies against rabbit or mouse IgG conjugated with peroxidase (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Reagents for enhanced chemiluminescence (ECL®; SuperSignal West Pico) were from Pierce. Electrocompetent BJ5183-AD-1 bacterial cells were bought from Stratagene (La Jolla, CA, U.S.A.).
Adenovirus vectors
A DNA fragment with the coding sequence for rat TRB3/NIPK was produced by PCR. The template was plasmid pSport1-NIPK, kindly provided by Dr S. Kojima (Shionogi and Co., Developmental Research Laboratories, Osaka, Japan) [19]. The sequence of the upstream primer (5′-CGCAagcttgggtggtcccATGCGAGCCACATCTCTGGC-3′) contained a cleavage site for HindIII followed by 15 nt (lower case) encoding a SLGGP linker peptide and the first 20 nt (boldface) of the coding sequence for rat TRB3/NIPK. The downstream primer (5′-ACGATATCAAGCTTCTCGAGTCTAGCCATACAGCCCCACC-3′) contained cleavage sites for multiple restriction enzymes including HindIII, followed by the last 19 nt (boldface) of the rat TRB3/NIPK coding sequence. The PCR product was digested with HindIII and inserted into the pShuttle-CMV-HA (where CMV stands for cytomegalovirus) vector to create plasmid pShuttle-CMV. This plasmid encodes a protein with an N-terminal HA epitope attached through the SLGGP linker to the complete coding sequence for rat TRB3/NIPK, which was verified by sequencing. The pShuttle-CMV-HA vector was derived by excision of the PKB-AAA sequence from the previously described plasmid pShuttle-CMV HA-PKB-AAA [18].
The pShuttle-CMV HA-TRB3/NIPK plasmid was linearized by digestion with PmeI and electrotransformed into recombination-proficient BJ5183-AD-1 bacterial cells. The resulting cloned recombinant plasmid AdEasy-1 HA-TRB3/NIPK was transfected into HEK-293 cells to produce recombinant adenovirus. The titre of virus stocks was estimated by a test for cytopathic effect in HEK-293 cells.
Culture and transduction of hepatocytes
Unless specified otherwise, hepatocytes were isolated from adult male Wistar rats fasted for 48 h. The methods for hepatocyte isolation and primary culture were as described previously [17]. Details of the method for viral transduction with adenovirus vectors have also been published [18].
Protein and RNA analysis
Protein extracts were prepared from hepatocyte monolayers as described previously [20]. Procedures for immunoblotting with the various antibodies were the same as in earlier work [17,18]. The methods for isolation of total RNA and Northern-blot analysis have been published [21]. The cDNA probes for hybridization are described in the following publications: GCK cDNA [22], SREBF1 (sterol-regulatory-element-binding factor 1) cDNA [23], PCK1 (phosphoenolpyruvate carboxykinase 1) cDNA [21] and S26 small ribosomal protein cDNA [21]. The TRB3/NIPK probe was a cDNA fragment isolated from plasmid pSport1-NIPK [19].
RESULTS
Stoichiometry of HA–TRB3/NIPK overexpression in hepatocytes
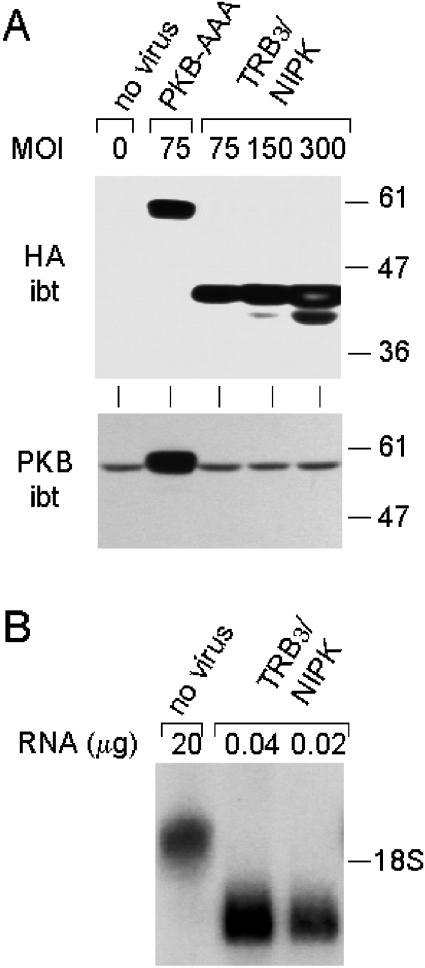
After transduction with an adenoviral vector encoding HA-tagged TRB3/NIPK, hepatocytes expressed an immunoreactive protein with electrophoretic mobility corresponding to apparent Mr of 43000, compatible with 41000 calculated from the HA–TRB3/NIPK amino acid sequence. The amount of this protein increased as a function of the MOI (multiplicity of infection) (Figure 1A, upper panel). This protein was absent in non-transduced hepatocytes or in control transduced hepatocytes, which had been infected with a virus encoding HA-tagged PKB-AAA. The latter cells, however, expressed an HA-immunoreactive protein with apparent Mr of 59000 as expected for HA–PKB-AAA.
Figure 1. Quantitative analysis of TRB3/NIPK overexpression in primary hepatocytes.
Rat hepatocytes, 5 h after being placed in primary culture, were transduced for 75 min with the AdEasy-1 HA–TRB3/NIPK adenovirus at the indicated MOI. Control hepatocytes were left uninfected or transduced with the AdEasy-1 HA–PKB-AAA vector, as indicated at the top of the Figure (HA omitted). Hepatocytes were cultured for 19 h after transduction before harvesting cells for the isolation of total protein or RNA. (A) Upper panel, immunoblot (ibt) analysis of hepatocytes using antibody to the HA epitope protein; lower panel: immunoblot analysis with antibody to the pleckstrin homology domain of PKB. PAGE with SDS was performed with 30 μg of protein per lane. The positions of Mr markers are shown to the right. (B) Northern-blot analysis using 32P-labelled TRB3/NIPK cDNA probe. Agarose gel electrophoresis using indicated amounts of total cell RNA. Position of 18 S rRNA shown on the right.
Immunoblot analysis with antibodies to PKB revealed that the PKB protein was overexpressed approx. 6-fold in the control hepatocytes transduced with HA–PKB-AAA, compared with non-transduced hepatocytes or hepatocytes transduced with the HA–TRB3/NIPK virus (Figure 1A, lower panel). Since the HA signal was equivalent in the HA–PKB-AAA hepatocytes and in hepatocytes transduced with HA–TRB3/NIPK at a MOI of 75, it follows that the molar excess of HA–TRB3/NIPK over endogenous PKB was approx. 6-fold in these hepatocytes (and multiple of this factor at higher MOI), assuming equal reactivity of the HA tag of the two proteins in the immunoblot assay.
The level of TRB3/NIPK mRNA in uninfected and transduced hepatocytes was measured by Northern-blot assay. In non-transduced hepatocytes, a single mRNA species approx. 2 kb in length was detected by hybridization to radiolabelled TRB3/NIPK cDNA (Figure 1B). An mRNA 1.3 kb in length hybridizing to the same probe was visualized in transduced hepatocytes, as expected from the length of the HA–TRB3/NIPK coding sequence inserted between the CMV promoter and the poly(A) addition signal in the adenoviral vector. As can be seen in Figure 1(B), the intensity of the 1.3 kb mRNA of vector origin was approximately equal to that of the 2 kb endogenous TRB3/NIPK mRNA band when inputs between 20 and 40 ng of RNA from hepatocytes transduced at an MOI of 300 were compared with 20 μg of RNA from non-transduced hepatocytes. Thus virally encoded HA–TRB3 transcripts in cells transduced under these conditions were approx. 700 times more abundant than endogenous TRB3/NIPK transcripts in native hepatocytes.
It is important to note that the level of expression of HA–TRB3/NIPK in transduced hepatocytes was quantified in all subsequent experiments and found to be highly reproducible. Thus, the molar excess of HA–TRB3/NIPK protein over that of endogenous PKB, ascertained by the indirect approach illustrated in Figure 1, comprised between 16- and 25-fold in the experiments illustrated in Figures 2 and 3. Similarly, at the TRB3/NIPK mRNA level, the virally encoded HA–TRB3/NIPK transcript was consistently 700–1000-fold more abundant than endogenous TRB3/NIPK mRNA in transduced hepatocytes (information available from P. B. I. on request).
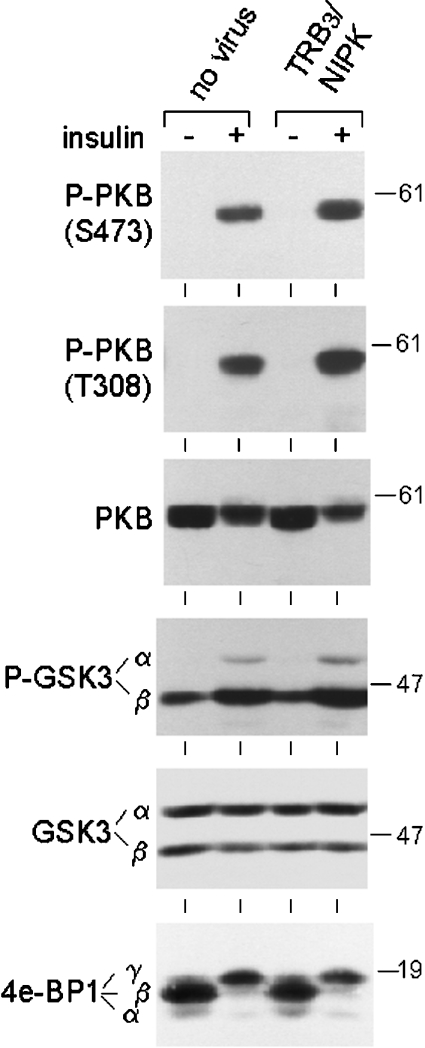
Figure 2. Unimpaired insulin signalling through PKB in hepatocytes overexpressing TRB3/NIPK.
Hepatocytes were placed in culture and transduced after 5 h with the AdEasy-1 HA–TRB3/NIPK adenovirus at an MOI of 300 or left uninfected. Cell culture was continued for 18 h after transduction. Insulin was then added (or not added for control cells) to culture medium at a concentration of 3×10−8 M for 60 min before cell harvest for the extraction of protein, SDS/PAGE and immunoblot analysis of phosphorylated and total PKB, phosphorylated and total GSK3 and total 4E-BP1. Protein load per lane was 40 μg for P-PKB Ser-473, 60 μg for P-PKB Thr-308 and total PKB, and 70 μg for P-GSK3, total GSK3 and 4E-BP1. The experiment was repeated twice with identical results. Positions of Mr markers are indicated on the right.
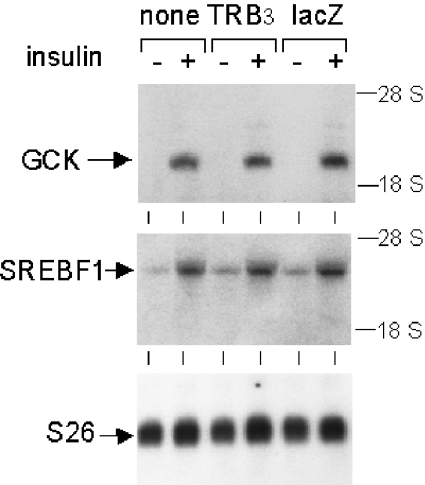
Figure 3. Unimpaired insulin induction of GCK and SREBF1 mRNAs in hepatocytes overexpressing TRB3/NIPK.
The method for hepatocyte culture and transduction was as described in Figure 2, except that cells were harvested for isolation of total RNA after 7 h of insulin stimulation. Both uninfected hepatocytes and hepatocytes transduced with an AdEasy-1 lacZ vector were used as controls. Agarose gel electrophoresis was performed with 16 μg of RNA per lane. Northern blotting was done on a nylon membrane hybridized sequentially to 32P-labelled cDNA probes for GCK, SREBF1 and ribosomal protein S26. Positions of 28 and 18 S rRNAs are shown on the right.
Effect of TRB3/NIPK overexpression on PKB-mediated insulin signalling
The effect of insulin on PKB activation was compared in control hepatocytes and hepatocytes overexpressing TRB3/NIPK by immunoblot analysis of cellular proteins using phosphospecific antibodies. In both control and TRB3/NIPK-overexpressing hepatocytes, insulin strongly stimulated the phosphorylation of PKB at Ser-473, an effect previously shown to correlate with the extent of PKB activation in hepatocytes as well as other cell types [18]. The magnitude of this effect was similar in the two types of cells (Figure 2, first panel). Site-specific phosphorylation of PKB at Thr-308 was also markedly increased by insulin treatment, again with the same amplitude in control and TRB3-overexpressing hepatocytes (Figure 2, second panel). Immunoblotting with antibodies to total PKB allowed me to verify that the amount of PKB did not vary between the various cell samples (Figure 2, third panel).
To monitor insulin signalling events downstream of PKB, the phosphorylation status of GSK3-α and -β at Ser-21 or Ser-9 respectively, which are target sites for phosphorylation by PKB, was analysed with phosphospecific antibodies. Consistent with the data on PKB activation, both GSK isoforms were hyperphosphorylated in insulin-stimulated cells, and the insulin effect was completely unaffected by TRB3/NIPK overexpression (Figure 2, fourth panel). The amount of GSK3 was virtually constant in all cell samples (Figure 2, fifth panel). Finally, the insulin-dependent mobility shift of the translation regulatory protein 4E-BP1 (also called PHAS1), reflecting phosphorylation by mammalian target of rapamycin, which is itself activated directly or indirectly by PKB [24–26], was present in TRB3/NIPK-overexpressing cells to the same extent as in the control hepatocytes (Figure 2, sixth panel).
Insulin action in the liver is characterized by the induction or repression of several genes, for which an involvement of the phosphoinositide 3-kinase/PKB signalling pathway has been proposed [27,28]. In line with intact signalling along this pathway, insulin caused a strong induction of GCK and SREBF1 mRNAs in hepatocytes overexpressing TRB3/NIPK (Figure 3). Induced mRNA levels were similar in the TRB3/NIPK cells and in nontransduced hepatocytes, as well as in additional control hepatocytes transduced with a lacZ adenovirus vector.
Inhibition of PKB-mediated insulin action in hepatocytes by ether lipid analogue PIA6
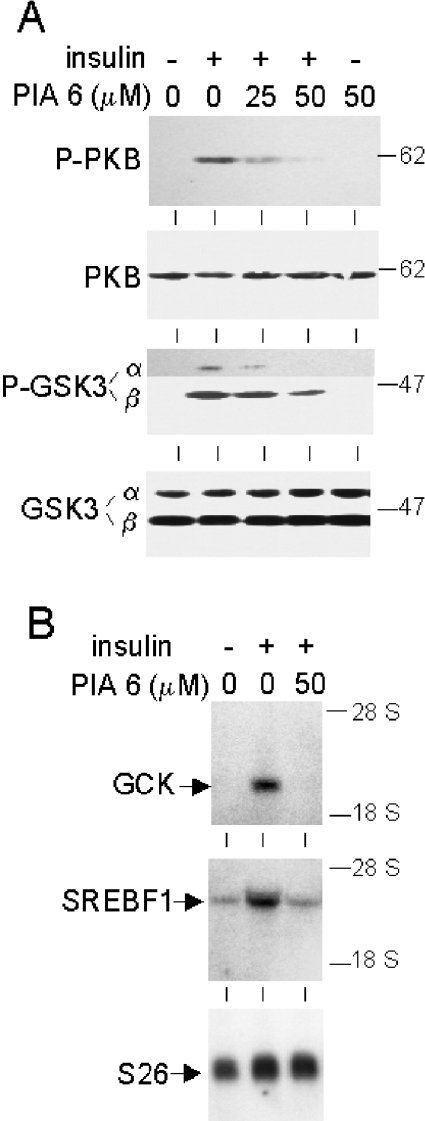
Ether lipid analogues of phosphatidylinositol 3,4,5-trisphosphate were recently shown to inhibit PKB specifically in cancer cells [29,30]. As shown in Figure 4(A), cultured hepatocytes pretreated with one such analogue, termed PIA6, exhibited dose-dependent inhibition of insulin-stimulated phosphorylation of PKB at Ser-473. This was accompanied by partial inhibition of the site-specific phosphorylation of GSK3-α and -β. Furthermore, the insulin-dependent increases in GCK and SREBF1 mRNAs were completely suppressed by PIA6 (Figure 4B). These effects of PIA6 are in sharp contrast with the inability of overexpressed TRB3/NIPK to antagonize insulin signalling and action.
Figure 4. Inhibition of PKB-mediated insulin signalling and gene induction in hepatocytes treated with PIA6 ether lipid.
Hepatocytes were cultured under standard conditions and stimulated with 3×10−8 M insulin after 23 h in culture. Ether lipid PIA6 at the indicated concentrations or DMSO as control was added to cells 1 h before insulin. Hepatocytes for extraction of protein were harvested 1 h after the addition of insulin. Hepatocytes for isolation of RNA were harvested 7 h after the addition of insulin. (A) Immunoblots using antibodies to PKB phosphorylated at Ser-473, total PKB, GSK3-α and -β phosphorylated at Ser-21 and Ser-9 respectively, and total GSK3. Protein load per lane was as in Figure 2. In the upper part of the P-GSK3 immunoblot, the image of a longer film exposure is shown to visualize P-GSK3-α. (B) Northern blot of total RNA (16 μg per lane) hybridized with 32P-labelled cDNA probes for GCK, SREBF1 and ribosomal protein S26. The experiment was repeated twice with identical results.
Lack of evidence for hormonal regulation of TRB3/NIPK expression in hepatocytes
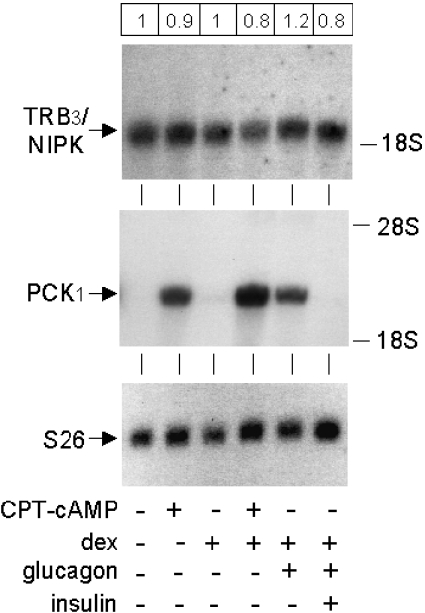
The previously reported increase in TRB3/NIPK mRNA in mouse liver during fasting, and in hepatoma cells in the presence of dexamethasone and forskolin [13], suggested that TRB3/NIPK might exhibit multihormonal regulation similar to that of the cytosolic gluconeogenic enzyme PCK1. In the present experiments, the levels of TRB3/NIPK and PCK1 mRNAs were measured by Northern-blot assay in rat hepatocytes cultured in the presence of dexamethasone, glucagon or its second messenger cAMP, and insulin. The relative amount of TRB3/NIPK mRNA remained virtually constant under all conditions tested, with no change exceeding 20% of the level seen in untreated control hepatocytes (Figure 5, top panel). An additional experiment in which RNA was extracted from hepatocytes 16 h after the addition of hormonal effectors also failed to reveal significant changes in TRB3/NIPK mRNA under any of the tested conditions (information available from P. B. I. on request). In contrast, glucagon or cAMP induced massive increases in PCK1 mRNA. Dexamethasone potentiated the effect of cAMP or glucagon on PCK1 mRNA and insulin counteracted the glucagon effect (Figure 5, middle panel). In these experiments, Northern-blot analysis of the mRNA of small ribosomal subunit protein 26, which is generally considered invariant [31], was performed as a control (Figure 5, bottom panel).
Figure 5. Constitutive expression of TRB3/NIPK mRNA in primary hepatocytes.
Hepatocytes were prepared and cultured under standard conditions, except that they were isolated from male Wistar rats fed ad libitum. Hormonal agents were added to the cells after 23 h of culture. Final concentrations were: CPT-cAMP, 10−4 M; dexamethasone, 10−8 M; glucagon, 10−9 M; insulin, 3×10−8 M. Cells were harvested for isolation of total RNA 6.5 h after hormone addition. Agarose gel electrophoresis was performed using 21 μg of RNA per lane. The membrane was hybridized to a 32P-labelled cDNA probe for TRB3/NIPK, dehybridized and hybridized to probes for PCK1 and S26. Numbers in the frame at the top of the Figure are the mean values for TRB3/NIPK mRNA levels quantified by phosphorimaging in two separate cell experiments, normalized for the level of S26 mRNA and expressed relative to the value in control untreated hepatocytes. CPT, 8-(4-chlorophenylthio).
DISCUSSION
The present study provided no evidence for the intracellular protein TRB3/NIPK acting as a negative regulator of insulin signalling in primary rat hepatocytes. In hepatocytes synthesizing massive amounts of TRB3/NIPK protein, following adenovirus-mediated gene transfer, the effect of insulin to stimulate the phosphorylation of the two critical amino acids of PKB necessary for activation, Ser-473 and Thr-308, was fully preserved. Consistent with this finding, the site-specific phosphorylation of the key PKB protein substrates GSK3-α and -β occurred normally after insulin stimulation. Similarly, the mobility shift in SDS/PAGE of 4E-BP1, reflecting mammalian target of rapamycin activation downstream of PKB, was present in TRB3/NIPK overexpressing hepatocytes to the same extent as in control hepatocytes. Finally, as end-marker of insulin action in the hepatocytes, insulin induction of the GCK and SREBF1 mRNAs was normal.
The ether lipid PIA6, a structural analogue of phosphatidylinositol 3,4,5-trisphosphate, was recently shown to reduce the steady-state and growth-factor-stimulated levels of PKB activity in tumour cells [30]. In contrast with overexpressed TRB3/NIPK, PIA6 effectively inhibited the insulin-dependent activation of PKB and downstream signalling events in primary hepatocytes. The insulin induction of GCK and SREBF1 was completely suppressed in PIA6-treated hepatocytes, providing further support for a key role of PKB in these insulin effects.
The inability of overexpressed TRB3/NIPK to inhibit PKB phosphorylation in the present experiments differs from the findings of Du et al. [13]. These authors showed a partial inhibition of IGF1-induced Ser-473 and Thr-308 phosphorylation of PKB in HEK-293 cells transiently co-transfected with PKB and TRB3/NIPK plasmid vectors. The reason for the discrepancy between Du's results and the present study is not clear. An important difference between the two studies relates to the cell systems used, primary hepatocytes in the present study and tumoral HEK-293 cells by Du et al. [13]. Furthermore, exogenously expressed PKB was analysed in the HEK-293 cells, as opposed to endogenous PKB in the hepatocytes. Conceivably, distinct subcellular localization or interaction of PKB with dissimilar scaffold proteins in the two cell-types might affect the susceptibility to TRB3/NIPK inhibition.
Another unexpected finding in this work was the lack of regulation of TRB3/NIPK gene expression by the glucocorticoids, glucagon or insulin in rat hepatocytes. In FAO rat hepatoma cells, both dexamethasone and forskolin, an activator of adenylate cyclase, were reported to induce a 2.5-fold increase in TRB3/NIPK mRNA [13]. Differences in hormone responses between hepatoma cells and primary cultures of hepatocytes are not without precedent. In the present study, the invariant expression of TRB3/NIPK was not due to inadequate hormone responsiveness of the culture system, as shown by the robust regulation of PCK1 in the same cells. The induction of PCK1 mRNA by glucagon and its second messenger cAMP, synergized by the glucocorticoids, and the de-induction by insulin recapitulated the multihormonal regulation supposed to be important for metabolic adaptation in the whole animal [32]. The present results establish a clear distinction between the gluconeogenic enzyme PCK1 and TRB3/NIPK with respect to the regulation by the hormones at play during feeding, fasting or diabetes. It should be added that the induction during food deprivation of a protein serving to attenuate insulin responsiveness, as has been proposed for TRB3/NIPK, is somewhat counterintuitive. Instead, one might expect the tissues of a fasted animal to remain highly insulin-sensitive, in anticipation for efficient uptake and storage of nutrient when food becomes available. A recently reported example for this type of adaptation might be the increase in the level of IRS-2 (insulin receptor substrate 2) in the livers of fasted mice [33].
Acknowledgments
The help of Dr P. Ribaux in producing the AdEasy-1 HA–TRB3/NIPK adenovirus is acknowledged. Thanks are expressed to A. Gjinovci for his assistance with the isolation of primary hepatocytes. This research was supported by grant no. 3200-063423.00 from the Swiss National Science Foundation.
References
- 1.Whiteman E. L., Cho H., Birnbaum M. J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z. Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E., Hemmings B. A. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 3.Walker K. S., Deak M., Paterson A., Hudson K., Cohen P., Alessi D. R. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem. J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessi D. R., Deak M., Casamayor A., Caudwell F. B., Morrice N., Norman D. G., Gaffney P., Reese C. B., MacDougall C. N., Harbison D., et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 5.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Feng J., Park J., Cron P., Hess D., Hemmings B. A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 7.Hanada M., Feng J., Hemmings B. A. Structure, regulation and function of PKB/AKT – a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 9.Rider M. H., Bertrand L., Vertommen D., Michels P. A., Rousseau G. G., Hue L. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem. J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berwick D. C., Hers I., Heesom K. J., Moule S. K., Tavare J. M. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 11.Rena G., Guo S., Cichy S. C., Unterman T. G., Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 12.Vander Kooi B. T., Streeper R. S., Svitek C. A., Oeser J. K., Powell D. R., O'Brien R. M. The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J. Biol. Chem. 2003;278:11782–11793. doi: 10.1074/jbc.M212570200. [DOI] [PubMed] [Google Scholar]
- 13.Du K., Herzig S., Kulkarni R. N., Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 14.Saltiel A. R. Putting the brakes on insulin signaling. N. Engl. J. Med. 2003;349:2560–2562. doi: 10.1056/NEJMcibr031668. [DOI] [PubMed] [Google Scholar]
- 15.Koo S. H., Satoh H., Herzig S., Lee C. H., Hedrick S., Kulkarni R., Evans R. M., Olefsky J., Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat. Med. (N.Y.) 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 16.Iynedjian P. B., Jotterand D., Nouspikel T., Asfari M., Pilot P. R. Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon-cAMP system. J. Biol. Chem. 1989;264:21824–21829. [PubMed] [Google Scholar]
- 17.Iynedjian P. B., Roth R. A., Fleischmann M., Gjinovci A. Activation of protein kinase B/cAkt in hepatocytes is sufficient for the induction of expression of the gene encoding glucokinase. Biochem. J. 2000;351:621–627. [PMC free article] [PubMed] [Google Scholar]
- 18.Ribaux P. G., Iynedjian P. B. Analysis of the role of protein kinase B (cAKT) in insulin-dependent induction of glucokinase and sterol regulatory element-binding protein 1 (SREBP1) mRNAs in hepatocytes. Biochem. J. 2003;376:697–705. doi: 10.1042/BJ20031287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayumi-Matsuda K., Kojima S., Suzuki H., Sakata T. Identification of a novel kinase-like gene induced during neuronal cell death. Biochem. Biophys. Res. Commun. 1999;258:260–264. doi: 10.1006/bbrc.1999.0576. [DOI] [PubMed] [Google Scholar]
- 20.Ribaux P., Gjinovci A., Sadowski H. B., Iynedjian P. B. Discrimination between signaling pathways in regulation of specific gene expression by insulin and growth hormone in hepatocytes. Endocrinology. 2002;143:3766–3772. doi: 10.1210/en.2002-220304. [DOI] [PubMed] [Google Scholar]
- 21.Iynedjian P. B., Marie S., Gjinovci A., Genin B., Deng S. P., Buhler L., Morel P., Mentha G. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes. J. Clin. Invest. 1995;95:1966–1973. doi: 10.1172/JCI117880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayzer D. J., Iynedjian P. B. Alternative splicing of glucokinase mRNA in rat liver. Biochem. J. 1990;270:261–263. doi: 10.1042/bj2700261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tontonoz P., Kim J. B., Graves R. A., Spiegelman B. M. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott P. H., Brunn G. J., Kohn A. D., Roth R. A., Lawrence J. C., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nave B. T., Ouwens M., Withers D. J., Alessi D. R., Shepherd P. R. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 26.Inoki K., Li Y., Zhu T., Wu J., Guan K. L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann M., Iynedjian P. B. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem. J. 2000;349:13–17. doi: 10.1042/0264-6021:3490013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao J., Barthel A., Nakatani K., Roth R. A. Activation of protein kinase B/Akt is sufficient to repress the glucocorticoid and cAMP induction of phosphoenolpyruvate carboxykinase gene. J. Biol. Chem. 1998;273:27320–27324. doi: 10.1074/jbc.273.42.27320. [DOI] [PubMed] [Google Scholar]
- 29.Kozikowski A. P., Sun H., Brognard J., Dennis P. A. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J. Am. Chem. Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- 30.Castillo S. S., Brognard J., Petukhov P. A., Zhang C., Tsurutani J., Granville C. A., Li M., Jung M., West K. A., Gills J. G., et al. Preferential inhibition of Akt and killing of Akt-dependent cancer cells by rationally designed phosphatidylinositol ether lipid analogues. Cancer Res. 2004;64:2782–2792. doi: 10.1158/0008-5472.can-03-1530. [DOI] [PubMed] [Google Scholar]
- 31.Vincent S., Marty L., Fort P. S26 ribosomal protein RNA: an invariant control for gene regulation experiments in eucaryotic cells and tissues. Nucleic Acids Res. 1993;21:1498. doi: 10.1093/nar/21.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson R. W., Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 33.Ide T., Shimano H., Yahagi N., Matsuzaka T., Nakakuki M., Yamamoto T., Nakagawa Y., Takahashi A., Suzuki H., Sone H., et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat. Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]