Abstract
Hexavalent chromium [Cr(VI)] is an established human lung carcinogen, but the carcinogenesis mechanism is poorly understood. Chromosome instability, a hallmark of lung cancer, is considered a major driver of Cr(VI)-induced lung cancer. Unrepaired DNA double-strand breaks are the underlying cause, and homologous recombination repair is the primary mechanism preventing Cr(VI)-induced DNA breaks from causing chromosome instability. Cell culture studies show acute Cr(VI) exposure causes DNA double-strand breaks and increases homologous recombination repair activity. However, the ability of Cr(VI)-induced DNA breaks and repair impact has only been reported in cell culture studies. Therefore, we investigated whether acute Cr(VI) exposure could induce breaks and homologous recombination repair in rat lungs. Male and female Wistar rats were acutely exposed to either zinc chromate particles in a saline solution or saline alone by oropharyngeal aspiration. This exposure route resulted in increased Cr levels in each lobe of the lung. We found Cr(VI) induced DNA double-strand breaks in a concentration-dependent manner, with females being more susceptible than males, and induced homologous recombination repair at similar levels in both sexes. Thus, these data show this driving mechanism discovered in cell culture indeed translates to lung tissue in vivo.
Keywords: hexavalent chromium, homologous recombination repair, chromosome instability, DNA double-strand break, lung
Graphical Abstract
Highlights.
Particulate Cr(VI) induced DNA double-strand breaks in rat lung after acute exposure.
Particulate Cr(VI) activated homologous recombination repair in rat lung after acute exposure.
Particulate Cr(VI) induced more DNA double-strand breaks in females than males, but break repair remained similar between the sexes.
Hexavalent chromium [Cr(VI)] compounds have been widely used for more than 200 years in metallurgical applications, pigment production, leather tanning, and wood preservative production (Barnhart 1997). Cr(VI) is classified as a known human carcinogen (Group 1A) by the International Agency for Research on Cancer (IARC) and numerous other agencies (IARC 1990; Kondo et al. 2003). Data from multiple levels of study (i.e. epidemiology, experimental animal, and cell culture) all show water-insoluble particulate Cr(VI) compounds are more carcinogenic than water-soluble counterparts (Hayes et al. 1979; Alderson et al. 1981; NIOSH 2006). Workers exposed to Cr(VI) had a high incidence of bronchial cancer (Langård and Norseth 1975; Langård and Vigander 1983). Pathological examination revealed Cr(VI) particles deposited at the bifurcations of the bronchi, which was the main site of tumor formation induced by Cr(VI) (Ishikawa et al. 1994b).
Although Cr(VI) has been listed as a human lung carcinogen for decades, the carcinogenic mechanism of Cr(VI) remains poorly understood. Chromosome instability, a hallmark of lung cancer, is a key driver of Cr(VI)-induced lung cancer (Masuda and Takahashi 2002; Wise et al. 2018). Cr(VI)-induced structural chromosome instability has been reported in epidemiological studies of chromate workers and in extensive cell culture studies (Wise et al. 2002, 2003, 2006; Maeng et al. 2004; Xie et al. 2005, 2009; Halasova et al. 2008, 2012; Qin et al. 2014). Unrepaired or improper repair of DNA double-strand breaks is a major mechanism causing structural instability of chromosomes. Numerous cell culture studies show Cr(VI) induces the formation of DNA double-strand breaks (Ha et al. 2004; Wakeman et al. 2004; Xie et al. 2005, 2009; Qin et al. 2014; DeLoughery et al. 2015; Wise et al. 2016). Error-free repair of DNA double-strand breaks by the homologous recombination repair pathway prevents Cr(VI)-induced chromosome instability and neoplastic transformation (Bryant et al. 2006; Stackpole et al. 2007; Xie et al. 2007). Recent studies show acute Cr(VI) exposure increases homologous recombination repair in response to elevated DNA double-strand breaks in human lung cells (Xie et al. 2009; Qin et al. 2014; Browning et al. 2016). However, these results were found in cell culture experiments, and the effect of Cr(VI) in vivo is unknown.
To date, Cr(VI)-induced DNA double-strand breaks and homologous recombination repair in the lung in vivo have not been reported. Studying Cr(VI)-induced DNA double-strand breaks and homologous recombination repair in experimental animal models is a critical translational gap in the Cr(VI) carcinogenesis framework. Thus, in this project, we investigated whether these key lesions and repair activity were induced by Cr(VI) in lungs in vivo. Because both DNA double-strand breaks and homologous recombination repair activity increase in cultured lung cells after acute exposure, we tested our hypothesis that acute exposure to particulate Cr(VI) induces DNA double-strand breaks and activates homologous recombination repair in lung tissue of exposed rats.
Materials and methods
Chemicals and reagents
Zinc chromate (CAS no. 13530-65-9; 99.7% purity) was purchased from Pfaltz and Bauer (lot no. Z00277, Waterbury, CT). Phosphate-buffered saline (PBS) 1× without calcium or magnesium was purchased from Corning, Inc. (Corning, NY). Prolong Diamond Antifade Reagent with DAPI was purchased from Life Technologies (Grand Island, NY). Tween-20, nitric acid, 10% neutral buffered formalin (16004-128), Sudan black B (0593), micro cover glass, and tissue cassettes were purchased from VWR International (Radnor, PA). Ethanol (2716) was purchased from Decon Labs (King of Prussia, PA). Paraffin (39602004) was purchased from Leica Biosystems (Deer Park, IL). Gill’s hematoxylin (GHS116) and eosin (HT110116) were purchased from Sigma-Aldrich (St Louis, MO). Xylene was purchased from EMD Millipore (Billerica, MA). Permount mounting medium (17986-01) was purchased from Electron Microscopy Sciences (Hatfield, PA). The TrueBlack blocking buffer and background suppressor (23012) were purchased from Biotium (Fremont, CA).
Animal models of zinc chromate exposure
12-week-old male and female Wistar rats (Envigo, Indianapolis, IN) were maintained under standard laboratory conditions. All animals were housed under a 12:12 h light-dark cycle and provided food and water ad libitum. Animal procedures were approved by the Institutional Animal Care and Use Committee of University of Louisville (#18272), and followed the appropriate guidelines set forth by the American Veterinary Medical Association. Zinc chromate suspensions were prepared as described previously (Wise et al. 2022). The zinc chromate suspension was diluted with sterile normal saline to prepare the dosage concentrations of 0.4, 0.8 or 1.6 mg/ml, which given the 0.5 µl saline/kg body weight delivery volume, is equivalent to 0.2, 0.4, or 0.8 mg zinc chromate/kg body weight. These doses were based on previously reported Cr(VI) studies in mice adjusted for the larger body size of rats (Beaver et al. 2009a, 2009b) and our previous study in rats (Wise et al., 2022), as explained in the discussion below and in those previous papers, these doses are relevant to human exposures.
Rats were exposed to zinc chromate particles in saline solution or saline alone by oropharyngeal aspiration following our published methods (Wise et al. 2022). Briefly, rat nostrils were pinched, and the tongue held extruded. Either saline or zinc chromate in saline was placed on the back of the throat. Then the nostrils and tongue were held until the animal took 6 to 7 deep breaths. Then the nostrils and tongue were released, and the animal returned to its cage. Twenty-four hours after single administration animals were euthanized, and the lungs were isolated.
Sample collection and preparation of lung sections
After euthanasia, animals were exsanguinated and perfused with cold PBS, lungs were removed. Lungs from 3 animals in each group per sex were separated into lobes for metal analysis. The right lungs from 7 animals in each group per sex were inflated with 10% neutral buffered formalin as described in our previous method (Wise et al. 2022). After fixation with 10% neutral buffered formalin for 24 h, lung samples were stored in 75% ethanol for at least 1 h and submitted for paraffin embedding. After embedding, lung tissues were sectioned at 5 microns on a rotary microtome and affixed to slides for histology/immunohistochemistry.
Hematoxylin and eosin staining
Hematoxylin and eosin (H&E) staining provides a detailed representation of tissues with high resolution for histopathological studies, thus allowing the identification of tissue abnormalities. Lung sections were deparaffinized with xylene and rehydrated with an ethanol gradient to distilled water. Lung sections were then stained sequentially with Gill’s hematoxylin and eosin. After dehydration with an ethanol gradient, coverslips were mounted with the Permount mounting medium. The stained lung sections were scanned using a 3DHISTECH digital slide scanner (Pannoramic DESK DW II).
Histological analysis
The images of all rat lung tissue sections were reviewed using CaseViewer (ver. 2.4.0.119028) to identify tissue abnormalities and analyze the amounts of macrophages and other immune inflammatory cells in H&E-stained rat lung tissue sections. We analyzed inflammation and determined the degree of inflammation according to our published scoring system (Wise et al. 2022). Each slide was scored based on the number and size of abnormal aggregation of macrophages or inflammatory cells according to the scoring system.
Tissue metal analysis by ICP-MS
Inductively coupled plasma mass spectrometry (ICP-MS) was performed following a previously published method (Wise et al. 2022). In brief, tissues were digested in 70% trace-element free nitric acid. Then the samples were heated at 95 °C for 1 h using a DiGiPrep MS programmable digestion block followed by adding 5 drops of 30% H2O2, then heated for 1 h. Samples were cooled and brought to 10 ml with 2% UHP grade HNO3, then vortexed at high speed. The material was allowed to settle, then filtered with a 0.45-micron filter and analyzed using an PerkinElme NexION 300D ICP-MS in the Integrative Molecular Analysis Core (IMAC) at the University of New Mexico in direct mode with anhydrous ammonia in the Dynamic Reaction Cell (DRC) to avoid mass interferences.
Immunofluorescent staining
Histone H2A.X is phosphorylated at Ser 139 in response to DNA double-strand breaks forming gamma-H2A.X (Fernandez-Capetillo et al. 2004). The number of phosphorylated H2A.X foci forming at double-strand break sites represents the number of DNA double-strand breaks (Rogakou et al. 1999; Sedelnikova et al. 2002). Therefore, the formation of gamma-H2A.X foci is considered a quantitative marker of DNA double-strand breaks. We detected the formation of gamma-H2A.X foci in Cr(VI)-exposed rat lungs by immunofluorescence.
Homologous recombination repair is the faithful repair of DNA double-strand breaks using undamaged sister chromatids as a template to maintain the integrity of the genome (van Gent et al. 2001). Recombinase RAD51 is the key repair protein in homologous recombination repair, as it binds single-stranded DNA and forms nuclear filaments that play a key role in homologous recombination by guiding strand exchange and homology searching (Li and Heyer 2008; San Filippo et al. 2008). RAD51 foci are used as indicators of RAD51 function and cell culture studies show acute exposure to particulate Cr(VI) activates homologous recombination repair by increasing RAD51 foci (Qin et al. 2014; Browning et al. 2016). Therefore, we used immunofluorescent RAD51 foci formation to assess homologous recombination repair.
Lung sections were deparaffinized with xylene followed by being rehydrated by incubation with an ethanol gradient. 1 mM EDTA (pH 8.0) was used for antigen retrieval, which was performed in a steamer for 30 min, and slides were cooled at room temperature for 45 min. Slides were then rinsed 3 times for 5 min each with Tris-buffered saline containing 0.025% Triton X-100. Slides were then blocked with TrueBlack blocking buffer for 15 min at room temperature and incubated with the primary antibody diluted with TrueBlack background suppressor overnight at 4 °C. Anti-gamma-H2A.X antibody (Cell Signaling, 2577S) was used at 1:750, anti-RAD51 (Thermo Fisher, PA5-27195) at 1:10,000, anti-cytokeratin (Sigma-Aldrich, C2931) at 1:100, and anti-PMCA ATPase (Invitrogen, MA3-914) at 1:500. Next, slides were rinsed 3 times for 5 minutes each with Tris-buffered saline with 0.025% Triton X-100. Goat anti-rabbit Alexa Fluor 488 (Life Technologies, A11034; 1:500) and Goat anti-mouse Alexa Fluor 555 (Life Technologies, A21422; 1:500) were added to slides and incubated for 60 min at room temperature. Slides were then rinsed 3 times for 5 min with Tris-buffered saline. Next, slides were immersed in 1% Sudan black B with 70% ethanol for 20 min, followed by 3 washes with Tris-buffered saline for 5 min. The coverslips were mounted with DAPI. Nuclear foci were scored in 100 cells per concentration by confocal microscopy.
Confocal microscopy and image analysis
Confocal images were captured under an A1 confocal inverted Nikon Eclipse Ti microscope equipped with 20× and 100× Plan Fluor objectives. The Z-stack images of the alveolar area and the bronchiole area were respectively obtained under the 100× oil objective with 3× digital magnification using the NIS-Elements software (ver. AR 5.20.02). The step sizes of gamma-H2A.X foci and RAD51 foci Z-stack images are 1 and 0.75 µm, respectively. After being denoised, the high-magnification images were analyzed for foci counting. For the analysis of gamma-H2A.X foci, the denoised high-magnification images were processed in a maximum image projections (MAXIP) step, and the MAXIP images were analyzed for foci counting. For RAD51 foci, the denoised high-magnification images were analyzed layer by layer.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 9.4.0. All data were expressed as mean ± standard error of the mean (SEM). In the animal study, simple linear regression was used to compare dose responses. One-way ANOVA was used when comparing single factor variates. Two-way ANOVA was used when comparing different treatments, lung regions, lung lobes and sexes. When comparing the means of 2 variables without interaction, 2-way ANOVA with Dunnett multiple comparisons test was performed. If one of the main effects or interaction were significant, then Bonferroni’s adjusted post hoc t-tests were performed. When comparing the difference between the individual chromium levels within different sex groups, a Student t-test also was included to test the statistics. Statistical significance was determined as a P-value less than 0.05. We also included P values less than 0.1 to provide a more complete picture of the results. Because in some cases, these results are significantly different (i.e. P < 0.1), which have not yet reached the statistical level of P < 0.05, but as the number of samples increases, such results may be reached in some cases, yet this condition is described as not significantly different from the control, which is an erroneous conclusion. Therefore, we provide the reader with this option to consider the implications of the results.
Results
Metal accumulation in lung after oropharyngeal aspiration
We measured Cr levels in rat lung tissue by ICP-MS. After acute exposure to Cr(VI), we found Cr accumulation in lung tissue (Fig. 1A). Cr levels in the lungs increased in a dose-dependent manner (linear regression analysis: R2 = 0.6795, P < 0.0001) (Fig. 1B). After acute exposure to 0, 0.2, 0.4, and 0.8 mg/kg zinc chromate, the mean Cr levels in whole lung tissue were 0.120, 3.295, 6.147, and 16.145 mg Cr/kg lung tissue, respectively. The Cr level was significantly higher in the group treated with zinc chromate at 0.8 mg/kg compared with the group treated with saline (Fig. 1A).
Fig. 1.
Cr accumulates in whole lungs after acute exposure. This figure shows Cr levels in the lung increased with dose after acute zinc chromate exposure. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) Cr levels in whole lungs. B) Cr levels shown with a linear regression line. This figure shows a linear correlation between these 4 variables: saline, 0.2, 0.4, and 0.8 mg/kg zinc chromate. The P-value is <0.0001. C) Cr levels in left and right lungs. D) Cr levels in individual lung lobes and trachea.
We investigated the specific distribution of zinc chromate particles in both the left and right lungs of rats. We found Cr levels increased in a dose-dependent manner in both the left and right lungs, after acute zinc chromate exposure (Fig. 1C). After acute exposure to 0, 0.2, 0.4, and 0.8 mg/kg zinc chromate, the average Cr levels in left lungs were 0.011, 0.359, 1.091, and 1.163 mg Cr/kg lung tissue, respectively. In the right lungs, after 24-hour exposure to 0, 0.2, 0.4, and 0.8 mg/kg zinc chromate, the average Cr levels were 0.072, 3.423, 5.773, and 14.556 mg Cr/kg lung tissue, respectively. The elevation of Cr levels in the right lung was statistically significant, after 24-h 0.4 and 0.8 mg/kg zinc chromate exposures (P = 0.0115 and P < 0.0001, respectively). Compared with Cr levels in the left lung, there was a statistically significant increase in Cr deposition in the right lung (2-way ANOVA, P < 0.0001). Cr levels were significantly higher in the right lung than in the left lung, after 24-h 0.4 and 0.8 mg/kg zinc chromate exposures (P < 0.0001 and P < 0.0001, respectively).
The left lung of the rat has only 1 lobe, and the right lung has 4 lobes, including the superior, middle, inferior, and accessory lobes. We further determined the distribution of Cr in each lung lobe and the trachea. Cr levels increased in a dose-dependent manner in each individual lung lobe after acute zinc chromate exposure (Fig. 1D). However, the increase in Cr concentration in the trachea was significantly less pronounced than in lung lobes after acute exposure. This outcome indicates Cr was inhaled and deposited more distally in the lungs.
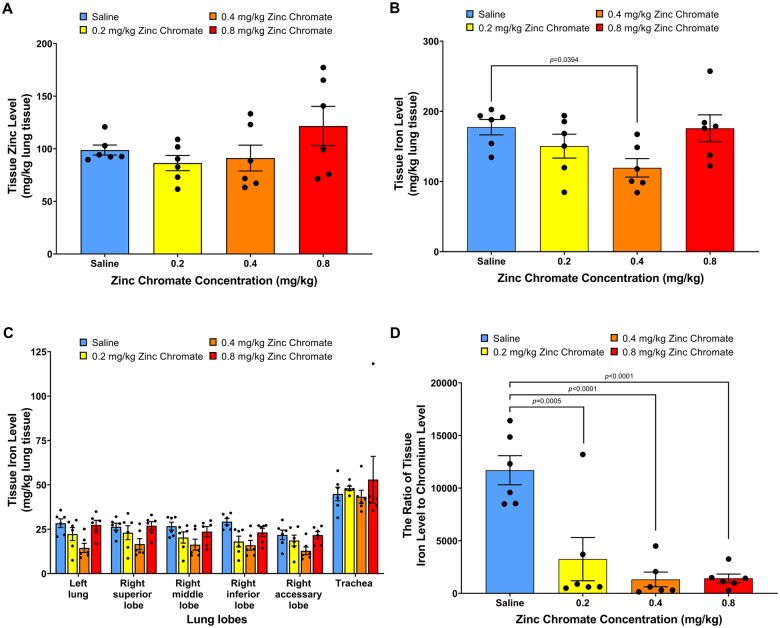
Because we treated rats with zinc chromate, we also measured zinc levels in the lungs. Zinc is an essential trace element. We found zinc levels in lung tissue did not increase with dose after acute zinc chromate exposure (Fig. 2A). Combined with Cr data in the whole lung, a dose-dependent accumulation of Cr was confirmed in the lung. We also investigated iron levels in rat lungs. We found iron levels did not increase but instead slightly decreased in rat lung tissue after acute exposure to zinc chromate (Fig. 2B and C). Iron and chromium can be antagonistic in the body (Staniek and Wójciak 2018). We therefore investigated whether Cr(VI) exposure affected functional iron levels in rat lungs. We used the ratio of iron content to Cr content to assess functional iron levels in rat lungs. We found functional iron levels were significantly reduced after acute zinc chromate exposure (Fig. 2D). Specifically, after acute exposure to 0, 0.2, 0.4, and 0.8 mg/kg zinc chromate, iron to Cr ratios in rat lungs were 11,701, 3,254, 1,326, and 1,428, respectively. These data suggest Cr exposure may impact iron levels in the lung.
Fig. 2.
Zinc levels are unaffected but the iron/chromium rations are decreased in lungs after acute exposure. This figure shows zinc levels were unaffected with dose after acute zinc chromate exposure. It also shows functional iron levels in the lung were reduced. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) Zinc levels in whole lungs. B) Iron levels in whole lungs. C) Iron levels in individual lung lobes. D) Functional iron lung levels.
Cr(VI) induces inflammation in rat lungs after acute exposure
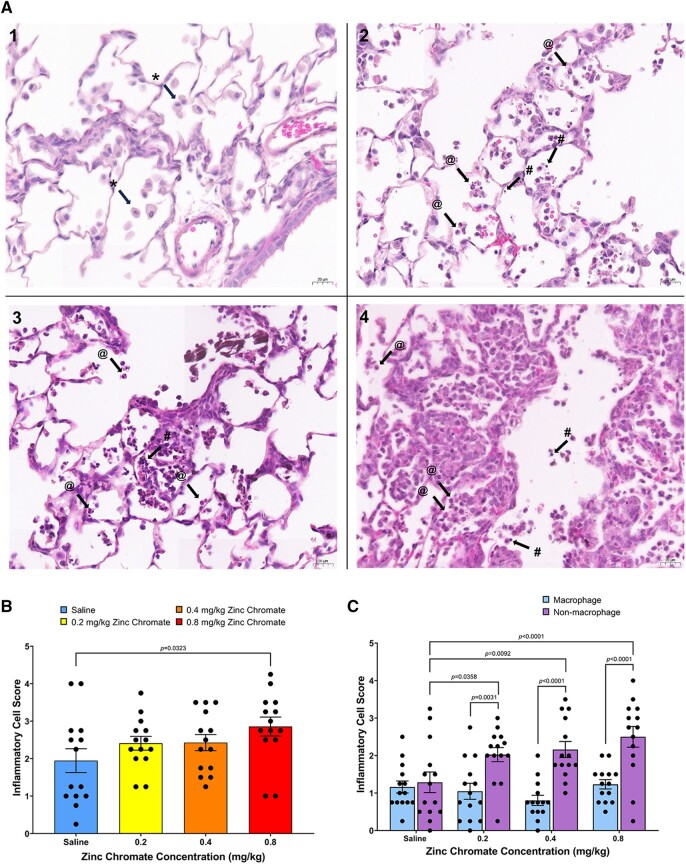
Repeated exposure to Cr(VI) induces lung damage and inflammation. A study found a single nasal aspiration exposure to zinc chromate particles in mice caused lung tissue damage and inflammation that persisted for up to 21 days (Beaver et al. 2009b). We therefore investigated Cr(VI)-induced lung histological changes by H&E staining according to our published method (Wise et al. 2022). We found infiltration of inflammatory cells in the alveolar area after acute Cr(VI) exposure. Inflammatory cells accumulated around the alveoli and terminal airways (Fig. 3A). In the group exposed to saline, the main cell type aggregated in the alveolar region was macrophages. In lungs exposed to zinc chromate, various inflammatory cells were gathered around the alveolar area and terminal airways, mainly neutrophils (Fig. 3A). Cr(VI)-induced inflammatory cells aggregated around the alveolar area and terminal airways showed a slightly increasing trend after acute exposure (Fig. 3B).
Fig. 3.
Cr(VI) induces infiltration of inflammatory cells in lung tissue after acute exposure. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) H&E staining of lung tissue. *with arrows, @with arrows, and #with arrows, respectively indicate examples of macrophages, neutrophiles, and lymphocytes. Magnifications are 400×, scale bar = 20um. A.1) Saline exposure—macrophages accumulated in the alveolar region after exposure to saline. A.2) 0.2 mg/kg zinc chromate exposure—neutrophils and lymphocytes accumulated in the alveolar region. A.3) 0.4 mg/kg zinc chromate exposure—many neutrophils and lymphocytes accumulated in the alveolar region. A.4) 0.8 mg/kg zinc chromate exposure—a lot of neutrophils and lymphocytes accumulated in the alveolar region, with pulmonary interstitial edema and capillary congestion. B) Cr(VI)-induced inflammatory cell aggregation in the lung. C) Cr-induced nonmacrophage inflammatory cell aggregation in the lung.
Because the types of inflammatory cells aggregated after acute exposure differed between saline and Cr(VI) exposures, we investigated macrophage aggregation and nonmacrophage aggregation separately. We found macrophage aggregation did not differ between groups after acute exposure, whereas nonmacrophage inflammatory cells increased significantly with increasing zinc chromate doses after acute Cr(VI) exposure (Fig. 3C). These data suggest the inflammatory response to acute Cr(VI) exposure is dominated by the aggregation of non-macrophages, including neutrophils and lymphocytes.
Cr induces DNA double-strand breaks and activates homologous recombination repair in lung tissue
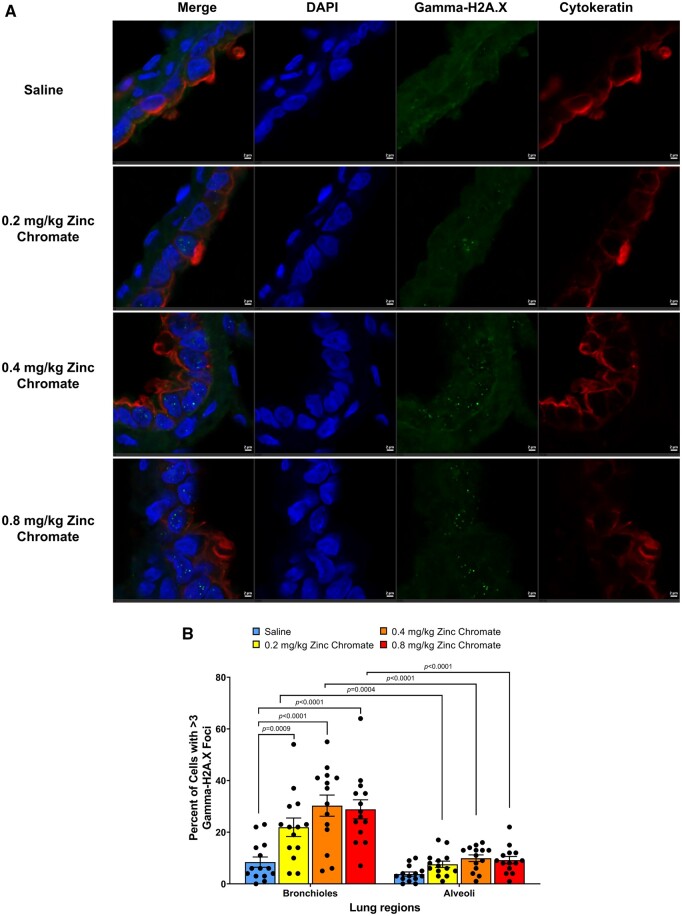
Cr(VI) induced DNA double-strand breaks in the lung in a dose-dependent manner after acute zinc chromate exposure, particularly targeting the bronchiolar region (Fig. 4A and B). Specifically, 0.2, 0.4, and 0.8 mg/kg zinc chromate increased the percent of cells with gamma-H2A.X foci in the bronchioles from 8.43 in controls to 21.93%, 30.29%, and 28.86% in treated groups, respectively (Fig. 4B). There were statistically significant increases of gamma-H2A.X foci in bronchioles after exposure to 0.2, 0.4, and 0.8 mg/kg zinc chromate compared with control (P = 0.0009, P < 0.0001, and P < 0.0001, respectively) (Fig. 4B). By contrast, in the alveoli, the levels of breaks were more muted. Specifically, after 0, 0.2, 0.4, and 0.8 mg/kg zinc chromate, the percent of cells with gamma-H2A.X foci in the alveoli were 3.79%, 7.57%, 9.93%, and 9.21%, respectively (Fig. 4B). The formation of Cr(VI)-induced breaks was statistically more pronounced in bronchioles than in alveoli (2-way ANOVA, P < 0.0001).
Fig. 4.
Cr(VI) induces DNA double-strand breaks in the lung after acute exposure. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) Representative images of gamma-H2A.X foci captured using confocal microscopy. First column, merged image; second column, DAPI staining; third column, gamma-H2A.X foci staining; fourth column, staining for cytokeratin, a marker of epithelial cells. Scale bar = 2 um. B) Quantification of Cr(VI)-induced DNA double-strand breaks measured as gamma-H2A.X foci.
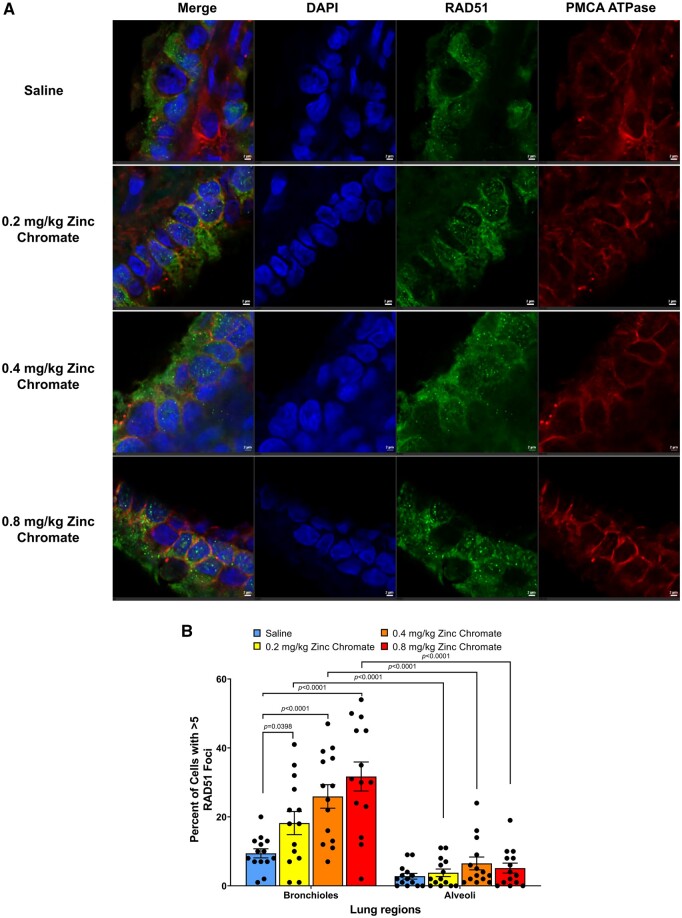
Consistent with the formation of breaks, Cr(VI) induced homologous recombination repair of the breaks in the lung in a dose-dependent manner after acute zinc chromate exposure, which was enhanced in the bronchiolar region (Fig. 5A and B). Specifically, 0.2, 0.4, and 0.8 mg/kg zinc chromate increased the percent of cells with RAD51 foci in the bronchioles from 9.43 in controls to 18.21%, 25.93%, and 31.71% in treated groups, respectively (Fig. 5B). RAD51 foci formation in bronchioles were significantly increased after exposure to 0.2, 0.4 and 0.8 mg/kg zinc chromate (P = 0.0398, P < 0.0001, and P < 0.0001, respectively) (Fig. 5B). By contrast, in the alveoli, there was no increase in homologous recombination repair consistent with the muted levels of DNA double-strand breaks. Cr(VI)-induced homologous recombination repair was significantly different in the bronchioles compared with the alveoli (2-way ANOVA, P < 0.0001).
Fig. 5.
Cr(VI) induces homologous recombination repair in the lung after acute exposure. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) Representative images of RAD51 foci captured using confocal microscopy. First column, merged image; second column, DAPI staining; third column, RAD51 foci staining; fourth column, staining for PMCA ATPase, a marker of the cell membrane. Scale bar = 2um. B) Quantification of Cr(VI)-induced homologous recombination repair measured as RAD51 foci.
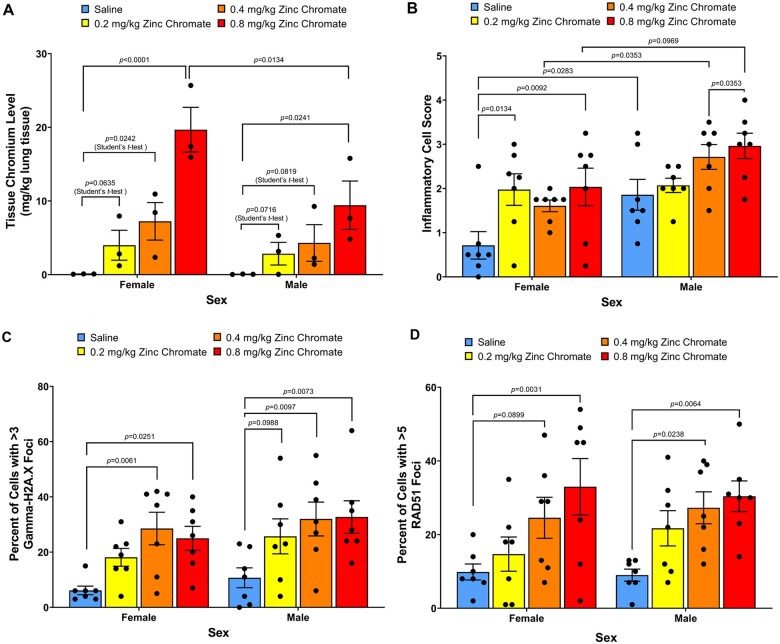
Sex differences in Cr level, Cr-induced inflammation, Cr-induced DNA double-strand breaks, and homologous recombination repair activity in rat lungs
There are sex differences in the incidence of lung cancer among nonsmoking patients; women who have never smoked have higher rates of lung cancer than men who have never smoked (Belani et al. 2007). Therefore, we considered sex differences in our study. We found greater impacts in female animals. Females had higher Cr levels (Fig. 6A; 2-way ANOVA, P = 0.035) and a greater aggregation of nonmacrophage immune cells (Fig. 6B; 2-way ANOVA, P = 0.0004). For DNA double-strand breaks, Cr(VI) induced similar levels of DNA double-strand breaks in female and male bronchioles (Fig. 6C; 2-way ANOVA, P = 0.0991). For DNA double-strand break repair, homologous recombination repair was similarly elevated in females and males (Fig. 6D).
Fig. 6.
Cr(VI) induces similar DNA double-strand breaks and homologous recombination repair responses in females compared with males. Error bars = Standard error of the mean. The P-values of statistical differences are shown in the graphs. A) Cr levels in the right lung. Cr levels in the lungs increased in a dose-dependent manner in the female group and male group, respectively. The lower doses (0.2 and 0.4) in both sex groups did not show significant differences from the control, but when we used Student t-test to analyze the data, there were significant differences. B) Nonmacrophage inflammatory cell aggregation. C) DNA double-strand breaks measured as gamma-H2A.X foci in bronchioles. D) Homologous recombination repair measured as RAD51 foci in bronchioles.
Discussion
The mechanisms of carcinogenesis for Cr(VI), a well-known lung cancer carcinogen, remain poorly understood. Much of the research on the carcinogenic mechanism of Cr(VI) so far has focused on cell culture experiments. Mechanistic outcomes in lung tissue in vivo are largely unexplored and it is urgent and crucial to translate key mechanisms that have been discovered in cell culture experiments to animal models. Cr(VI)-induced chromosome instability is a key driving force in Cr(VI) carcinogenesis and in lung cancer in general. Cell culture studies show a key genotoxic outcome for Cr(VI) is the production of DNA double-strand breaks (Ha et al. 2004; Wakeman et al. 2004; Xie et al. 2005, 2009; Reynolds et al. 2007; Qin et al. 2014; DeLoughery et al. 2015). Our study translates this key outcome to lung tissue and is the first to consider Cr(VI) induction of DNA double-strand breaks and homologous recombination repair in lung tissue in vivo.
We successfully translated particulate Cr(VI)-induced DNA double-strand breaks to rat lungs after acute exposure. The particulate Cr(VI)-induced DNA double-strand breaks increased in a dose-dependent manner. This outcome is consistent with cell culture studies showing Cr(VI) induces DNA double-strand breaks as a key mechanistic lesion (Ha et al. 2004; Wakeman et al. 2004; Xie et al. 2005, 2009; Reynolds et al. 2007; Qin et al. 2014; DeLoughery et al. 2015). We also successfully translated particulate Cr(VI)-induced homologous recombination repair to rat lungs, showing a dose-dependent increase in repair after acute exposure. Our findings are consistent with data from cell culture studies, which report homologous recombination repair is induced and RAD51 increased after acute Cr(VI) exposure (Bryant et al. 2006; Qin et al. 2014; Browning et al. 2016). Cr(VI) producing these breaks in vivo strengthens the observations in cell culture of the importance of these lesions for Cr(VI) carcinogenicity and deepens the observations in cell culture that homologous recombination repair is the key pathway for Cr(VI)-induced DNA double-strand breaks.
Notably, we observed differences in particulate Cr(VI)-induced double-strand breaks in different lung regions. In the bronchioles, Cr(VI)-induced DNA double-strand breaks were prominent along with the increase in homologous recombination repair. By contrast, in the alveoli the breaks and the repair response were much lower compared with the bronchioles. These outcomes indicate the bronchiole region is the key target area for Cr(VI). Such a conclusion is consistent with particle inhalation rat models that indicate bronchiole bifurcation sites are where inhaled particles impact and persist (Levy et al. 1986; Takahashi et al. 2005). It is also consistent with key observations from Cr(VI)-induced tumors. Pathological studies of Cr(VI) workers that died of lung cancer show Cr(VI)-induced lung tumors originate from the bronchial epithelium with very little tumor formation in the alveolar region (Ishikawa et al. 1994a, 1994b; Kondo et al. 1997; Ewis et al. 2001, 2006). Moreover, the data show Cr accumulates at bronchial bifurcation sites, and Cr(VI)-induced tumors form at these bronchial bifurcation sites (Ishikawa et al. 1994a, 1994b). Fiberoptic bronchoscopy biopsies of Cr(VI) workers show premalignant lesions in the bronchial epithelium, and significantly increased accumulation of Cr with the progression of bronchial epithelial malignancy (Kondo et al. 2003). Thus, our findings support the conclusion that the bronchioles are the key target structure for Cr(VI) in the lung.
Sex differences are an important consideration in lung cancer. Women who have never smoked have a greater chance (1 in 5 women) of developing lung cancer than men who have never smoked (1 in 12 men) (Belani et al. 2007). However, sex differences in Cr(VI)-induced lung cancer are poorly understood. We also considered sex differences with Cr(VI)-induced DNA breaks and repair. The outcome is complex and difficult to ascertain the overall impact. We found on the surface that when we compare Cr(VI)-induced DNA double-strand breaks between female rats and male rats, the P-value is 0.0991, which suggests there is a biological difference between sex if the significance level used in 0.1 (meaning a 90% probability there is a statistically significant difference). There was no difference in repair levels to offset these increased breaks. This observation suggests there is a sex difference with more breaks in female animals.
In contrast, closer inspection reveals that the underlying difference in the levels of DNA double-strand breaks between the sexes was due to contrasting variability in break levels in the control animals and not due to an overt increase in breaks in female animals. Specifically, there was less variation among control female compared with control males and thus the females appear to have more breaks creating the apparent difference and raising some question about whether there is in fact a sex difference. On the surface, a conclusion of no difference would be consistent with our observations that Cr accumulates more in female rats. Thus, one might argue that on an internal dose comparison, female rats would have less breaks than male rats (we did not make such a graphical comparison because the Cr levels and the break levels were measured in different animals). However, if one based the comparison on internal dose than one would need to do the same for repair and there would then be less repair per internal dose in female animals compared with male animals. This would again suggest there is a sex difference with females more susceptible to repair inhibition than males as less Cr in males would have induced similar amounts of repair inhibition (we did not make such a graphical comparison because the Cr levels and the repair levels were measured in different animals). Thus, overall our data suggests that females may be more sensitive to Cr(VI)-induced breaks or Cr(VI)-induced repair, but at this juncture a firm conclusion about sex difference cannot be made. However, this remains an important consideration for future studies.
Another important factor to consider is how these animal doses compare with human exposures. The connection to occupational doses of Cr is quite apparent. One approach would be to consider the amount delivered to the lung, the rats in our study had a level of 0.004 to 2.7 µg/g in the left lung and 0.007 to 4.6 µg/g in the middle lobe of the right lung. Human Cr(VI) workers who died of Cr(VI)-induced lung cancer had Cr levels in their lungs ranging from 0.4 to 210 µg/g (Tsuneta et al. 1980, Kishi et al. 1987). Thus, the amount of Cr delivered to the lungs in our experiment are overall much lower than those found in the lungs of Cr(VI) exposed workers. Another approach would be to consider dose comparisons based on the total amount of Cr(VI) we placed in the throat of the animals, we gave 13.9 to 72.5 µg Cr(VI) to female rats and 20.5 to 96.3 µg Cr(VI) for males (the specific amount varying with the size of the animals as the administered dose was mg zinc chromate/kg body weight). At the current occupational exposure limit of 5 µg Cr(VI)/m3 of air set by the U.S. Occupational Safety and Health Administration (OSHA), we calculate an individual at work exposed to this amount for 8 h would inhale somewhere between 37.2 and 192 µg Cr depending on how much time was spent at rest or working hard (OSHA 2006; EPA 2011). (The calculation was performed as follows: Cr level ug/m3 × Ventilation rate (m3/time) = ug Cr; Ventilation rate comes from EPA 2011, for workers it is necessary to multiply the ventilation rate by a factor of 8 h as the unit is m3/h, so no additional factor needed.) Thus, without adjusting for differences among humans and rodents, the levels we used in the animals is below or within the range of occupational exposures meaning these exposures are relevant to occupational exposures.
Considering the relevance of the experimental exposures to environmental exposures is more complicated. One approach is to consider Cr levels in the lungs of nonoccupationally exposed individuals used the lung pathology study discussed above for Cr(VI)-exposed workers with lung cancer (Tsuneta et al. 1980, Kishi et al. 1987). These individuals had Cr levels in their lungs ranging from 0.07 to 1.01 µg/g, which is consistent with the levels in the rats in our study (0.004 to 2.7 µg/g in the left lung and 0.007 to 4.6 µg/g in the middle lobe of the right lung) indicating our exposure deliver an amount of Cr(VI) to the lung that overlaps nonoccupational (i.e. environmental) exposures. Another approach is to consider dose comparisons based on the amount of Cr(VI) we placed in the throat of the animals (13.9 to 96.3 µg) and reported environmental levels of Cr(VI) in the air, 0.005 to 0.525 µg Cr(VI)/m3 of air depending on whether the setting is rural or urban air (EPA 1984, 1990, IARC 1990; ATSDR 2012). At these levels, we calculate a person breathing these environmental amounts for 24 h would inhale 0.1 to 12 ug Cr(VI) in 24 h (EPA 2011). (The calculation was performed as follows: Cr level ug/m3 × Ventilation rate (m3/time) = ug Cr; ventilation rate comes from EPA 2011, for environmental exposures the rate is in m3/d, so no additional factor needed.) Thus, with no adjustment for extrapolation between humans and rodents, the lower end of the range is slightly higher than the environmental exposure range. However, there are adjustment factors for extrapolations from rodents to humans when using mg/kg doses (i.e. the dosing we used in our study). To find the human equivalent dose, one divides the animal dose by 6.2 (Rockville 2005). We know the actual amount of Cr(VI) we treated with at each mg/kg dose so If we apply this same adjustment factor to the total amount of Cr(VI) given to the animal (i.e. divide the animal levels by 6.2) then the human equivalent Cr(VI) dose would be 2.24 to 15.5 µg Cr(VI), well within the range of environmental exposures. We acknowledge applying the adjustment factor in precisely this manner offer some uncertainty, but overall we feel based on all 4 comparisons, it is reasonable to conclude the doses we used are consistent with human occupational exposure and environmental exposures.
We did observe a disparity in Cr levels between the right and left lung with the tight lung receiving much more Cr than the left. The explanation for this difference is uncertain. There are no studies comparing the dosimetry of Cr(VI) between oropharyngeal aspiration and chamber inhalation exposures. Moreover, the dosimetry of chamber inhalation studies is poorly understood. However, we note that when airborne particles are inhaled into the airways, the deposition of particles in the respiratory tract is known to occur in a nonuniform manner (Lippmann et al. 1980, Oberdörster 1988), Thus, it is not surprising that there are disparities in Cr levels in the lung and likely the differences are due to the localized physics in the lung and not due to aspiration versus inhalation. Indeed, uneven distribution of Cr in the lung was observed in the human Cr(VI) pathology study, which showed higher Cr levels in the upper lodes than the lower lobes (Tsuneta et al. 1980, Kishi et al. 1987).
In this study, we established an oropharyngeal aspiration rat model to study particulate Cr(VI) carcinogenesis. We found Cr was inhaled into rat lungs and accumulated in each lung lobe after acute exposures. We observed the outcomes of Cr(VI)-induced DNA double-strand break and homologous recombination repair changes in rat lung tissues. Ours is the first study to apply oropharyngeal aspiration to a rat model for studying Cr(VI) exposure. This mode of administration has been used in 2 recent studies of mice considering the effects of chronic repeated calcium chromate exposure (Zeidler-Erdely et al. 2020; Wang et al. 2022). One study exposed A/J mice to iron sesquioxide or calcium chromate through oropharyngeal aspiration once per week for 26 wk and found increased incidence of tumors compared with the control group (Zeidler-Erdely et al. 2020). The other study exposed A/J mice to calcium chromate once per week for 26 wk via oropharyngeal aspiration and found total RNA m6A levels in chromate-exposed mouse lungs were significantly higher than in the vehicle group and METTL3 protein levels were drastically increased in chromate-caused mouse lung tumors (Wang et al. 2022). These 2 mouse studies combined with our rat study indicate oropharyngeal aspiration is a useful tool for studying Cr(VI) carcinogenesis. We successfully translated the outcomes found in cell culture studies to experimental animals through this model.
In conclusion, we successfully translated Cr(VI)-induced DNA double-strand break and DNA repair impacts from cells to experimental animals. Our results indicate acute particulate Cr(VI) exposure induces DNA double-strand breaks and activates homologous recombination repair in rat lungs, and particulate Cr(VI) exposure has differential impacts in male and female rats. Our research is a crucial step in revealing the carcinogenic mechanism of Cr(VI).
Acknowledgments
The authors would like to acknowledge members of the Cai Laboratory for their assistance with animal tissue collection, Hannah Jaggers, and Roman Isakov for assistance with tissue embedding.
Contributor Information
Haiyan Lu, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Sandra S Wise, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Rachel M Speer, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Tayler J Croom-Perez, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Jennifer H Toyoda, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Idoia Meaza, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Aggie Williams, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
John Pierce Wise, Jr, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Pediatric Research Institute, University of Louisville, Louisville, KY 40292, United States.
J Calvin Kouokam, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Jamie Young Wise, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Gary W Hoyle, Department of Environmental and Occupational Health Sciences, School of Public Health and Information Sciences, University of Louisville, Louisville, KY 40292, United States.
Cairong Zhu, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan 610044, China.
Abdul-Mehdi Ali, Earth and Planetary Sciences Department, The University of New Mexico, Albuquerque, NM 87131, United States.
John Pierce Wise, Sr, Wise Laboratory of Environmental and Genetic Toxicology, Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, United States.
Funding
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health [R35ES032876, R01ES016893, and P30ES030283 to J.P.W.Sr] and [T32ES011564 to J.P.W.Sr, R.M.S., and J.H.T.]. Animal work was performed under UofL IACUC approval #18272. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Conflicts of interest. None declared.
References
- Alderson MR, Rattan NS, Bidstrup L.. 1981. Health of workmen in the chromate-producing industry in Britain. Br J Ind Med. 38(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. 2012. Toxicological profile for chromium. Atlanta (GA: ): US Department of Health and Human Services, Public Health Service. [Google Scholar]
- Barnhart J. 1997. Occurrences, uses, and properties of chromium. Regul Toxicol Pharmacol. 26(1):S3–S7. [DOI] [PubMed] [Google Scholar]
- Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR.. 2009a. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol Appl Pharmacol. 235(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, Patierno SR.. 2009b. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ Health Perspect. 117(12):1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belani CP, Marts S, Schiller J, Socinski MA.. 2007. Women and lung cancer: epidemiology, tumor biology, and emerging trends in clinical research. Lung Cancer. 55(1):15–23. [DOI] [PubMed] [Google Scholar]
- Browning CL, Qin Q, Kelly DF, Prakash R, Vanoli F, Jasin M, Wise JP Sr. 2016. Prolonged particulate hexavalent chromium exposure suppresses homologous recombination repair in human lung cells. Toxicol Sci. 153(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant HE, Ying S, Helleday T.. 2006. Homologous recombination is involved in repair of chromium-induced DNA damage in mammalian cells. Mutat Res. 599(1-2):116–123. [DOI] [PubMed] [Google Scholar]
- DeLoughery Z, Luczak MW, Ortega-Atienza S, Zhitkovich A.. 2015. DNA double-strand breaks by Cr(VI) are targeted to euchromatin and cause ATR-dependent phosphorylation of histone H2AX and its ubiquitination. Toxicol Sci. 143(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. 1984. Health assessment document for chromium. Research Triangle Park (NC): Environmental Assessment and Criteria Office, U.S. Environmental Protection Agency. EPA 600/8-83-014F.
- EPA. 1990. Noncarcinogenic effects of chromium: update to health-assessment document. Final report. Research Triangle Park (NC): Environmental Protection Agency, Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, U.S. Environmental Protection Agency. EPA 600/8-87/048F.
- EPA. 2011. Exposure factors handbook 2011 edition (final). Washington (DC: ): U.S. Environmental Protection Agency, EPA/600/R-09/052F. [Google Scholar]
- Ewis AA, Kondo K, Dang F, Nakahori Y, Shinohara Y, Ishikawa M, Baba Y.. 2006. Surfactant protein b gene variations and susceptibility to lung cancer in chromate workers. Am J Ind Med. 49(5):367–373. [DOI] [PubMed] [Google Scholar]
- Ewis AA, Kondo K, Lee J, Tsuyuguchi M, Hashimoto M, Yokose T, Mukai K, Kodama T, Shinka T, Monden Y, et al. 2001. Occupational cancer genetics: infrequent ras oncogenes point mutations in lung cancer samples from chromate workers. Am J Ind Med. 40(1):92–97. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A.. 2004. H2ax: the histone guardian of the genome. DNA Repair (Amst). 3(8-9):959–967. [DOI] [PubMed] [Google Scholar]
- Ha L, Ceryak S, Patierno SR.. 2004. Generation of s phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of atm in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 25(11):2265–2274. [DOI] [PubMed] [Google Scholar]
- Halasova E, Matakova T, Musak L, Polakova V, Letkova L, Dobrota D, Vodicka P.. 2012. Evaluating chromosomal damage in workers exposed to hexavalent chromium and the modulating role of polymorphisms of DNA repair genes. Int Arch Occup Environ Health. 85(5):473–481. [DOI] [PubMed] [Google Scholar]
- Halasova E, Matakova T, Musak L, Polakova V, Vodicka P.. 2008. Chromosomal damage and polymorphisms of DNA repair genes XRCC1 and XRCC3 in workers exposed to chromium. Neuro Endocrinol Lett. 29(5):658–662. [PubMed] [Google Scholar]
- Hayes RB, Lilienfeld AM, Snell LM.. 1979. Mortality in chromium chemical production workers: a prospective study. Int J Epidemiol. 8(4):365–374. [DOI] [PubMed] [Google Scholar]
- IARC. 1990. Chromium, nickel and welding. IARC Monogr Eval Carcin Risks Humans. 49:1–648. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E.. 1994a. Characteristics of chromate workers’ cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br J Cancer. 70(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E.. 1994b. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Res. 54(9):2342–2346. [PubMed] [Google Scholar]
- Kishi R, Tarumi T, Uchino E, Miyake H.. 1987. Chromium content of organs of chromate workers with lung cancer. Am J Ind Med. 11(1):67–74. [DOI] [PubMed] [Google Scholar]
- Kondo K, Hino N, Sasa M, Kamamura Y, Sakiyama S, Tsuyuguchi M, Hashimoto M, Uyama T, Monden Y.. 1997. Mutations of the p53 gene in human lung cancer from chromate-exposed workers. Biochem Biophys Res Commun. 239(1):95–100. [DOI] [PubMed] [Google Scholar]
- Kondo K, Takahashi Y, Ishikawa S, Uchihara H, Hirose Y, Yoshizawa K, Tsuyuguchi M, Takizawa H, Miyoshi T, Sakiyama S, et al. 2003. Microscopic analysis of chromium accumulation in the bronchi and lung of chromate workers. Cancer. 98(11):2420–2429. [DOI] [PubMed] [Google Scholar]
- Langård S, Norseth T.. 1975. A cohort study of bronchial carcinomas in workers producing chromate pigments. Br J Ind Med. 32(1):62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langård S, Vigander T.. 1983. Occurrence of lung cancer in workers producing chromium pigments. Br J Ind Med. 40(1):71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy LS, Martin PA, Bidstrup PL.. 1986. Investigation of the potential carcinogenicity of a range of chromium containing materials on rat lung. Br J Ind Med. 43(4):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Heyer WD.. 2008. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 18(1):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Yeates DB, Albert RE.. 1980. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 37(4):337–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng SH, Chung HW, Kim KJ, Lee BM, Shin YC, Kim SJ, Yu IJ.. 2004. Chromosome aberration and lipid peroxidation in chromium-exposed workers. Biomarkers. 9(6):418–434. [DOI] [PubMed] [Google Scholar]
- Masuda A, Takahashi T.. 2002. Chromosome instability in human lung cancers: Possible underlying mechanisms and potential consequences in the pathogenesis. Oncogene. 21(45):6884–6897. [DOI] [PubMed] [Google Scholar]
- NIOSH. 2006. The final standard on hexavalent chromium. Effective and practical protection for workers. Washington (DC): Health Occupational Safety and Health Administration.
- Oberdörster G. 1988. Lung clearance of inhaled insoluble and soluble particles. J Aerosol Med. 1(4):289–330. [Google Scholar]
- OSHA. 2006. Occupational exposure to hexavalent chromium. Final rule. Federal register. p. 10099–10385. [PubMed]
- Qin Q, Xie H, Wise SS, Browning CL, Thompson KN, Holmes AL, Wise JP Sr. 2014. Homologous recombination repair signaling in chemical carcinogenesis: prolonged particulate hexavalent chromium exposure suppresses the rad51 response in human lung cells. Toxicol Sci. 142(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Stoddard L, Bespalov I, Zhitkovich A.. 2007. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in g2 phase by mismatch repair. Nucleic Acids Res. 35(2):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockville M. 2005. Guidance for industry: estimating the maximum safe starting dose in adult healthy volunteer. Silver Spring (MD: ): USFDA. [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM.. 1999. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 146(5):905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo J, Sung P, Klein H.. 2008. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 77:229–257. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM.. 2002. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 158(4):486–492. [DOI] [PubMed] [Google Scholar]
- Stackpole MM, Wise SS, Goodale BC, Duzevik EG, Munroe RC, Thompson WD, Thacker J, Thompson LH, Hinz JM, Wise JP Sr. 2007. Homologous recombination repair protects against particulate chromate-induced chromosome instability in Chinese hamster cells. Mutat Res. 625(1–2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniek H, Wójciak RW.. 2018. The combined effects of iron excess in the diet and chromium(III) supplementation on the iron and chromium status in female rats. Biol Trace Elem Res. 184(2):398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kondo K, Ishikawa S, Uchihara H, Fujino H, Sawada N, Miyoshi T, Sakiyama S, Izumi K, Monden Y.. 2005. Microscopic analysis of the chromium content in the chromium-induced malignant and premalignant bronchial lesions of the rat. Environ Res. 99(2):267–272. [DOI] [PubMed] [Google Scholar]
- Tsuneta Y, Ohsaki Y, Kimura K, Mikami H, Abe S, Murao M.. 1980. Chromium content of lungs of chromate workers with lung cancer. Thorax. 35(4):294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent DC, Hoeijmakers JH, Kanaar R.. 2001. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2(3):196–206. [DOI] [PubMed] [Google Scholar]
- Wakeman TP, Kim WJ, Callens S, Chiu A, Brown KD, Xu B.. 2004. The ATM-SMC1 pathway is essential for activation of the chromium[VI]-induced s-phase checkpoint. Mutat Res. 554(1–2):241–251. [DOI] [PubMed] [Google Scholar]
- Wang Z, Uddin MB, Xie J, Tao H, Zeidler-Erdely PC, Kondo K, Yang C.. 2022. Chronic hexavalent chromium exposure upregulates the RNA methyltransferase METTL3 expression to promote cell transformation, cancer stem cell-like property, and tumorigenesis. Toxicol Sci. 187(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP Sr, Wise SS, Little JE.. 2002. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat Res. 517(1–2):221–229. [DOI] [PubMed] [Google Scholar]
- Wise SS, Aboueissa AE, Martino J, Wise JP Sr. 2018. Hexavalent chromium-induced chromosome instability drives permanent and heritable numerical and structural changes and a DNA repair-deficient phenotype. Cancer Res. 78(15):4203–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Liou L, Adam RM, Wise JP Sr. 2016. Hexavalent chromium induces chromosome instability in human urothelial cells. Toxicol Appl Pharmacol. 296:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP Sr. 2006. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutat Res. 610(1-2):2–7. [DOI] [PubMed] [Google Scholar]
- Wise SS, Lu H, Speer RM, Wise JP Jr., Young J, Toyoda JH, Meaza I, Croom-Perez TJ, Kouokam JC, Specht A, et al. 2022. Chromium distribution in an oropharyngeal aspiration model for hexavalent chromium in rats. Toxicol Appl Pharmacol. 457:116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SS, Schuler JH, Katsifis SP, Wise JP Sr. 2003. Barium chromate is cytotoxic and genotoxic to human lung cells. Environ Mol Mutagen. 42(4):274–278. [DOI] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Huang S, Peng C, Wise JP Sr. 2007. Neoplastic transformation of human bronchial cells by lead chromate particles. Am J Respir Cell Mol Biol. 37(5):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Young JL, Qin Q, Joyce K, Pelsue SC, Peng C, Wise SS, Jeevarajan AS, Wallace WT, et al. 2009. Zinc chromate induces chromosome instability and DNA double strand breaks in human lung cells. Toxicol Appl Pharmacol. 234(3):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise JP Sr. 2005. Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat Res. 586(2):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Falcone LM, Antonini JM, Fraser K, Kashon ML, Battelli LA, Salmen R, Trainor T, Grose L, Friend S, et al. 2020. Tumorigenic response in lung tumor susceptible A/J mice after sub-chronic exposure to calcium chromate or iron (III) oxide. Toxicol Lett. 334:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]