Abstract
Background
Alzheimer’s disease (AD) is a neurodegenerative disorder with increasing prevalence due to population aging. Eggs provide many nutrients important for brain health, including choline, omega-3 fatty acids, and lutein. Emerging evidence suggests that frequent egg consumption may improve cognitive performance on verbal tests, but whether consumption influences the risk of Alzheimer’s dementia and AD is unknown.
Objectives
To examine the association of egg consumption with Alzheimer’s dementia risk among the Rush Memory and Aging Project cohort.
Methods
Dietary assessment was collected using a modified Harvard semiquantitative food frequency questionnaire. Participants’ first food frequency questionnaire was used as the baseline measure of egg consumption. Multivariable adjusted Cox proportional hazards regression models were used to investigate the associations of baseline egg consumption amount with Alzheimer’s dementia risk, adjusting for potential confounding factors. Subgroup analyses using Cox and logistic regression models were performed to investigate the associations with AD pathology in the brain. Mediation analysis was conducted to examine the mediation effect of dietary choline in the relationship between egg intake and incident Alzheimer’s dementia.
Results
This study included 1024 older adults {mean [±standard deviation (SD)] age = 81.38 ± 7.20 y}. Over a mean (±SD) follow-up of 6.7 ± 4.8 y, 280 participants (27.3%) were clinically diagnosed with Alzheimer’s dementia. Weekly consumption of >1 egg/wk (hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.34, 0.83) and ≥2 eggs/wk (HR: 0.53; 95% CI: 0.35, 0.81) was associated with a decreased risk of Alzheimer’s dementia. Subgroup analysis of brain autopsies from 578 deceased participants showed that intakes of >1 egg/wk (HR: 0.51; 95% CI: 0.35, 0.76) and ≥2 eggs/wk (HR: 0.62; 95% CI: 0.44, 0.90) were associated with a lower risk of AD pathology in the brain. Mediation analysis showed that 39% of the total effect of egg intake on incident Alzheimer’s dementia was mediated through dietary choline.
Conclusions
These findings suggest that frequent egg consumption is associated with a lower risk of Alzheimer’s dementia and AD pathology, and the association with Alzheimer’s dementia is partially mediated through dietary choline.
Keywords: Alzheimer’s disease, egg, dementia, diet, choline, cognition
Introduction
Alzheimer’s disease (AD) is among the leading causes of death and is the most common cause of cognitive decline in older adults. The prevalence of diagnosed AD cases increases exponentially with age. In 2020, the estimated burden of AD to United States healthcare costs alone was reported to be $305 billion [[1], [2], [3]]. An estimated 6.5 million Americans aged ≥65 y are living with AD, and this prevalence has been projected to increase to 13.8 million by 2060 [4]. The neuropathology of AD is an imbalance between the production and clearance of amyloid-β (Aβ) peptides in the brain that results in the accumulation and aggregation of Aβ plaques, soluble Aβ oligomers, and intraneuronal Aβ cause injury to synapses. The toxicity of Aβ is dependent on the presence of microtubule-associated protein tau and hyperphosphorylated forms that aggregate and deposit in the brain as neurofibrillary tangles. This persistent accumulation ultimately leads to cholinergic neurodegeneration and resultant dementia [5]. Genetically, the ϵ4 allele of the apolipoprotein E (ApoE) gene, which has been shown to be involved with the aggregation of Aβ plaques, is the strongest risk factor for late-onset AD [[6], [7], [8], [9]]. The human ApoE gene exists as 3 polymorphic alleles—ϵ2, ϵ3, and ϵ4—with an estimated worldwide frequency of 8.4%, 77.9%, and 13.7%, respectively [10]. The frequency of the ϵ4 allele has been estimated to be ∼40% among patients with AD [10]. ApoE-ϵ4 has been shown to be involved in the aggregation of Aβ plaques and is 1 of the major risk factors for early AD onset [[11], [12], [13]].
Effective dietary interventions have the potential to decrease the burden of AD from both a healthcare and quality-of-life standpoint. Egg yolks are a rich dietary source of choline, omega (ω)-3 fatty acids, lutein, and other nutrients thought to affect cognitive health [[14], [15], [16]]. They are the top food source of choline [17], an essential nutrient that is a precursor to the neurotransmitter acetylcholine and a critical component to many phospholipids that comprise cell membranes [18]. The neuroprotective actions of dietary choline intake have recently been reviewed by experts in the field [[19], [20], [21]]. Dietary intake of choline has been hypothesized to rescue this loss in cholinergic function [19]. At the same time, evidence suggests that ω-3 fatty acids have the potential to modulate a number of molecular and cellular processes that affect brain and visual health and inflammatory reactions [22]. Further, Mapstone et al. [23] demonstrated that concentrations of 10 validated lipids (8 of which contain choline and ω-3 fatty acids) in the peripheral blood predict phenoconversion to either amnestic mild cognitive impairment or AD within a 2–3 y timeframe. At the same time, eggs are a practical dietary intervention strategy for older adults, owing to their increased palatability compared with other animal-sourced foods [24].
To our knowledge, no prior studies have investigated the association between egg consumption and the risk of Alzheimer’s dementia or AD among a community-dwelling cohort of older adults in the United States. Therefore, the primary aim of this research was to investigate the association between habitual egg consumption and Alzheimer’s dementia risk using data from participants enrolled in the Rush Memory And Aging Project (RMAP). We further assessed the consistency of findings in a subgroup of deceased cohort participants with brain autopsies for pathological AD findings.
Methods
Participant characteristics
The RMAP is an ongoing longitudinal prospective cohort study that enrolled 2152 participants without apparent dementia from >40 retirement communities and residential facilities throughout northeastern Illinois between 1997 and 2020 [24]. The RMAP was funded by the National Institute on Aging, informed consent was obtained from participants, and the protocol for the cohort data collection was approved by the institutional review board of Rush University Medical Center [25]. All participants agreed to annual clinical evaluations and brain donation at the time of death. Dietary assessment was introduced to the study in 2004; since then, 1064 participants have completed ≥1 food frequency questionnaire (FFQ). For this study, we excluded 7 participants with missing egg consumption data, 1 with missing age information, and 15 with Alzheimer’s dementia diagnosis at baseline (i.e., when the first FFQ was completed). We further excluded 17 participants with missing covariate data. Our final analytical sample included 1024 participants (Figure 1A). In the subgroup analysis, we included only the 578 deceased participants with postmortem brain autopsies (Figure 1B).
FIGURE 1.
Study population criteria. (A) Primary analysis (Alzheimer’s dementia). (B) Subgroup analysis [Alzheimer’s disease (AD)]. FFQ, food frequency questionnaire; RMAP, Rush Memory and Aging Project.
Alzheimer’s dementia diagnosis
At each annual assessment, all participants underwent a 3-stage clinical Alzheimer’s dementia diagnosis assessment, which included computerized scoring, clinical judgment by a neuropsychologist, and a diagnostic classification by a clinician based on criteria of the Joint Working Group of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association. Twenty-one cognitive tests were performed on all participants. Eleven of these test results could be summarized into 5 cognitive domains to generate an impairment score, in which the different cutoff scores were used based on educational attainment [26,27]. Other primary dementia, without clinical evidence of Alzheimer’s dementia, were not included.
AD pathology
Classification of AD postmortem was assessed based on the number of neurofibrillary tangles and neuritic plaques present within collected brain samples [25]. The brain autopsy methods and details on pathological evaluations of AD pathology were described previously [25]. Counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles based on silver stains from 5 regions of the brain were used to generate a global measure of AD pathology, as described previously in the scientific literature [28].
Dietary intake
Dietary assessment was collected using a modified Harvard semiquantitative FFQ that has been validated for our aging participants [29]. The FFQ ascertains the usual frequency of intake of >137 foods and dietary supplements over the previous 12 mo. Egg intake was assessed at 5 different amounts of consumption: never or <1 egg/mo, 1–3 eggs/mo, 1 egg/wk, 2–4 eggs/wk, and ≥5 eggs/wk. Due to the limited sample size, we combined 2–4 eggs/wk and ≥5 eggs/wk into an intake amount of ≥2 eggs/wk. Other items on the FFQ (e.g., eggs contained within desserts or mixed dishes) that may contain eggs as a component were not accounted for in these analyses. Participants’ first FFQ was used as the baseline measure of food intake, which includes eggs (our primary exposure) and other covariates (dark green leafy vegetables, strawberries, and seafood).
Choline intake was estimated by combining the data from FFQs with the USDA Food Composition Databases, as previously described [30]. Dietary choline intakes were estimated from major food sources of choline, including but not limited to eggs, seafood, milk, meat and poultry products, and plant-based sources. Total choline intake was calculated as a sum of free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin. The mean choline intake amount was calculated by averaging total choline intake across all follow-up time points as a measure of long-term choline intake amount for mediation analysis.
Covariates
Based on previous literature, various dietary and nondietary factors were selected as covariates to assess the association between egg consumption and AD risk. Dietary intake amounts of total energy (in kilocalories per day), leafy green vegetables, strawberries, and seafood were based on the baseline FFQ responses. Leafy green vegetable consumption included 3 food items (spinach, kale/collard/greens, and lettuce), and seafood intake included tuna sandwich, fish sandwich, fish as a main dish, and shrimp/lobster/crab. Nondietary factors, including age (in years), were computed based on the self-reported date of birth and the date of baseline FFQ; education was the self-reported years of education. ApoE-ϵ4 genotyping was performed using genetic sequencing [31]. Participation in cognitive activities was computed as the mean of frequency rating of 7 activities, such as reading, writing letters, visiting the library, and playing games, based on a 5-point scale. Physical activity (in hours per week) was calculated based on self-reported minutes spent over the past 2 wk on 5 activities, including walking for exercise, yard work, calisthenics, biking, and water exercise. Vascular risk was defined by several conditions, including hypertension, diabetes, myocardial infarction, and stroke. Hypertension was self-reported data. Myocardial infarction was self-reported or indicated by the use of cardiac glycosides [e.g., digoxin (Lanoxin)] as medication. Diabetes was also either a self-reported medical diagnosis or indicated by the current use of diabetes medication. History of stroke was based on self-reported questions, cognitive testing, interviews with participants, and neurological examination (when available).
Statistical analysis
Continuous variables are described as the mean (±SD), and dichotomous variables as the number and percentage of the population. Because all of the continuous variables in our analysis were not normally distributed, we used the Kruskal-Wallis test for comparisons between groups. The Pearson χ2 test was used to compare groups for categoric variables. We calculated the time interval (in years) between baseline and time of clinical diagnosis or death, whichever occurred first, as the time variable for the time-to-event analyses to examine associations between egg intake with incident Alzheimer’s dementia using both partially adjusted (model 1) and multivariate-adjusted (model 2) Cox proportional hazard models. Model 1 was adjusted for age, sex, and years of education. Model 2 was adjusted for model 1 plus BMI (in kg/m2), smoking status, physical activity, participation in cognitive activities, vascular risk, ApoE status, and intake of dark green leafy vegetables, strawberries, seafood, and total energy. Results are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). The same Cox models and additional logistic regression models were employed in our subanalysis that assessed the association between egg intake and pathological diagnosis of AD from postmortem brain samples. To examine the mediation effect of dietary choline in the relationship between egg intake with incident Alzheimer’s dementia, we conducted mediation analysis using the Med4way package in Stata with mean choline intake across all follow-up time points as the mediator [32]. Med4way allows estimating the 4-way decomposition of the overall effect of an exposure on an outcome, in the presence of a mediator with which the exposure may interact, using parametric regression models. Specifically, in our mediation analysis, Weibull accelerated failure time regression was used to investigate the relationship between egg intake (exposure) and survival time of Alzheimer’s dementia (outcome, i.e., time free of AD diagnosis) with the same covariates in model 2. Multivariate linear regression models were used to examine the linear associations between egg intake (exposure) and dietary choline intake (mediator) with the same covariates in model 2.
The analyses were performed using R version 4.2.1 and RStudio 2022.12.0+353 (RStudio, PBC) and STATA 17.0 SE (StataCorp LLC).
Results
Baseline characteristics of the 1024 study participants included in our analyses are shown in Table 1. Their mean (±SD) age was 81.4 ± 7.2 y. A total of 766 participants (74.8%) were female, and 843 (82.3%) carried the ApoE-ϵ4 allele. Significant differences in age, sex, education, BMI, physical activity, total energy intakes, and mean choline intakes were present across egg intake quartiles.
TABLE 1.
Participant baseline characteristics by quartile of egg intake
| Characteristic | Overall (N = 1024) | Egg consumption |
P value | |||
|---|---|---|---|---|---|---|
| Never or <1 /mo(N = 111) | 1–3/mo (N = 259) | 1/wk(N = 227) | ≥2/wk (N = 427) | |||
| AD diagnosis, no. (%) | ||||||
| Yes | 280 (27.3) | 36 (32.4) | 92 (35.5) | 55 (24.2) | 97 (22.7) | 0.002 |
| No | 744 (72.7) | 75 (67.6) | 167 (64.5) | 172 (75.8) | 330 (77.3) | |
| Age, y, mean (SD) | 81.38 (7.20) | 81.27 (7.08) | 82.74 (6.98) | 81.10 (7.16) | 80.74 (7.31) | 0.006 |
| Sex, no. (%) | ||||||
| Female | 766 (74.8) | 82 (73.9) | 210 (81.1) | 184 (81.1) | 290 (67.9) | <0.001 |
| Male | 258 (25.2) | 29 (26.1) | 49 (18.9) | 43 (18.9) | 137 (32.1) | |
| Education, y, mean (SD) | 14.87 (2.93) | 14.95 (2.93) | 14.45 (2.91) | 14.96 (3.05) | 15.07 (2.86) | 0.048 |
| BMI, kg/m2, no. (%) | ||||||
| Normal | 354 (34.6) | 52 (46.9) | 108 (41.7) | 84 (37.0) | 110 (25.8) | <0.001 |
| Overweight | 377 (36.8) | 31 (27.9) | 82 (31.7) | 89 (39.2) | 175 (41.0) | |
| Obese | 242 (23.6) | 22 (19.8) | 54 (20.9) | 45 (19.8) | 121 (28.3) | |
| Underweight or missing | 51 (5.0) | 6 (5.4) | 15 (5.8) | 9 (4.0) | 21 (4.9) | |
| Smoking status, no. (%) | ||||||
| Never smoked | 590 (57.6) | 67 (60.4) | 145 (56.0) | 132 (58.2) | 246 (57.6) | 0.282 |
| Former smoker | 403 (39.4) | 41 (36.9) | 100 (38.6) | 89 (39.2) | 173 (40.5) | |
| Current smoker | 31 (3.0) | 3 (2.7) | 14 (5.4) | 6 (2.6) | 8 (1.9) | |
| Physical activity, h/d, mean (SD) | 3.30 (3.71) | 3.09 (4.67) | 2.86 (3.33) | 3.29 (3.01) | 3.63 (3.97) | <0.001 |
| Cognitive activities, frequency, mean (SD) | 3.21 (0.67) | 3.13 (0.7) | 3.15 (0.65) | 3.25 (0.63) | 3.25 (0.69) | 0.073 |
| ApoE-ϵ4 high risk, no. (%) | ||||||
| No | 181 (17.7) | 25 (22.5) | 52 (20.1) | 36 (15.9) | 68 (15.9) | 0.282 |
| Yes | 843 (82.3) | 86 (77.5) | 207 (79.9) | 191 (84.1) | 359 (84.1) | |
| Cardiovascular condition, (%) | ||||||
| Hypertension | 57 | 51 | 58 | 56 | 58 | 0.588 |
| Diabetes | 15 | 10 | 13 | 12 | 18 | 0.077 |
| Myocardial infarction | 13 | 11 | 16 | 15 | 11 | 0.271 |
| Stroke | 11 | 8 | 14 | 12 | 9 | 0.151 |
| Dietary nutrients intake | ||||||
| Mean choline intake, mg/d, mean (SD) | 300.6 (86.7) | 238.9 (80.0) | 268.1 (81.1) | 297.8 (75.9) | 337.8 (79.5) | <0.001 |
| Total energy, kcal/d, mean (SD) | 1739.43 (545.10) | 1474.90 (493.75) | 1621.75 (523.38) | 1757.56 (532.03) | 1869.95 (539.09) | <0.001 |
Abbreviations: AD, Alzheimer’s disease; ApoE, apolipoprotein E; BMI, body mass index; SD, standard deviation.
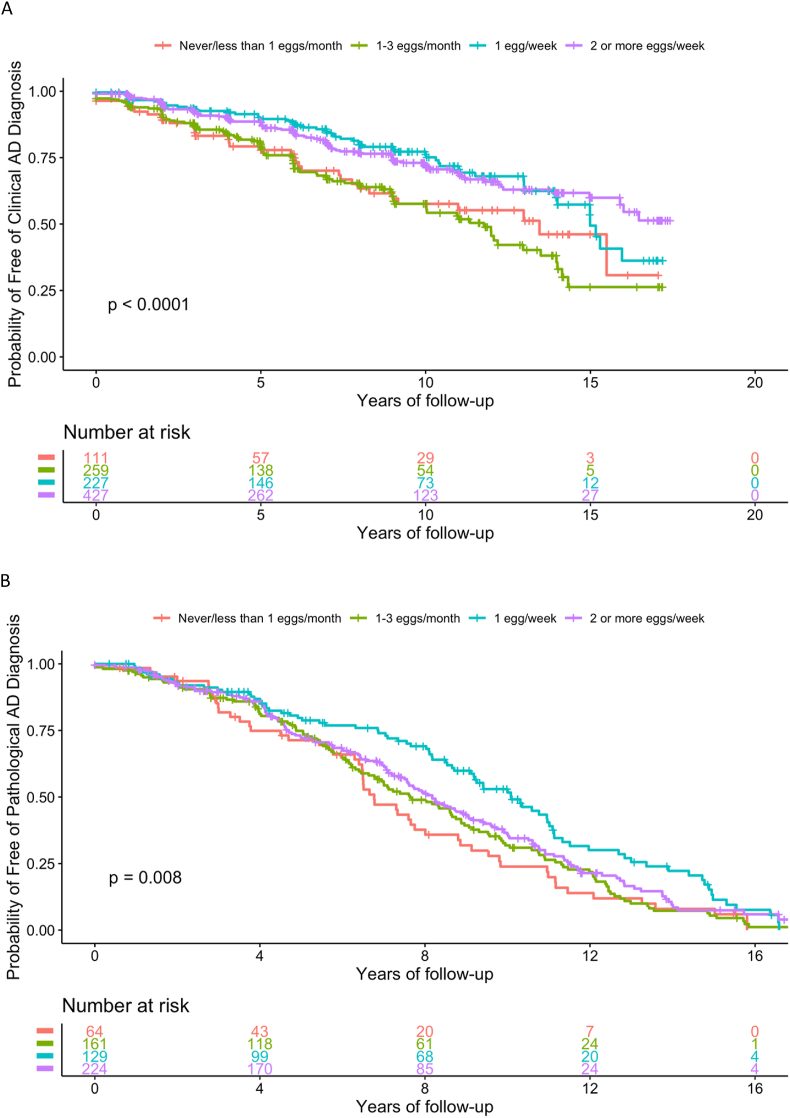
Over a mean (±SD) follow-up of 6.7 ± 4.8 y, 280 participants (27.3%) were diagnosed with Alzheimer’s dementia. In the multivariate-adjusted model (model 2), consumption of 1 egg/wk (HR: 0.53; 95% CI: 0.34, 0.83) and ≥2 eggs/wk (HR: 0.53; 95% CI: 0.35, 0.81) was associated with a lower rate of clinical diagnosis compared with consumption of <1 egg/wk (Table 2; Figure 2A). Results were similar in the partially adjusted model (model 1; Table 2).
TABLE 3.
Comparison between clinical and pathological diagnosis
| Autopsied participants (N = 578) | Clinical diagnosis |
||
|---|---|---|---|
| No | Yes | ||
| Pathology diagnosis | No | 164 | 371 |
| Yes | 2082 | 1692 | |
Time-to-event was calculated as follows: age of clinical diagnosis—age when entering the cohort.
Time-to-event was defined as follows: age of death—age when entering the cohort.
TABLE 2.
Associations of egg intake with Alzheimer’s dementia risk and Alzheimer’s disease pathology
| Baseline egg consumption | Model 11 |
Model 22 |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Full study population (N = 1024) | ||||
| Never or <1/mo | 1.00 | Reference | 1.00 | Reference |
| 1–3/mo | 1.02 (0.69, 1.51) | 0.918 | 0.94 (0.62, 1.43) | 0.780 |
| 1/wk | 0.60 (0.39, 0.92) | 0.018 | 0.53 (0.34, 0.83) | 0.006 |
| ≥2/wk | 0.63 (0.43, 0.93) | 0.020 | 0.53 (0.35, 0.81) | 0.003 |
| Deceased population with brain autopsies (N = 578)3 | ||||
| Never or <1/mo | 1.00 | Reference | 1.00 | Reference |
| 1–3/mo | 0.85 (0.61, 1.17) | 0.313 | 0.72 (0.49, 1.04) | 0.076 |
| 1/wk | 0.61 (0.43, 0.86) | 0.005 | 0.51 (0.35, 0.76) | 0.001 |
| ≥2/wk | 0.80 (0.58, 1.10) | 0.174 | 0.62 (0.44, 0.90) | 0.01 |
Abbreviations: BMI, body mass index; CI, 95% confidence interval; HR, hazard ratio.
Model 1 adjusted for age, sex, and years of education.
Model 2 adjusted for age, sex, years of education, BMI, smoking status, physical activity, participation in cognitive activities, vascular risk, apolipoprotein E status, dark green leafy vegetables, strawberries, seafood, and total energy intake.
See Supplemental Table 2 for the results of logistic regression models.
FIGURE 2.
Unadjusted Kaplan-Meier curves divided into 4 groups based on egg consumption. (A) Among the 1024 Rush Memory and Aging Cohort (RMAP) participants. (B) Among the 578 deceased RMAP participants with brain autopsies. AD, Alzheimer’s disease.
In the subgroup analyses of 578 participants with postmortem brain samples, 208 of 372 (55.9%) without a clinical diagnosis of Alzheimer’s dementia were pathologically diagnosed with AD postmortem, and 37 of 206 participants (17.9%) with a clinical diagnosis were not pathologically diagnosed with AD postmortem (Supplemental Tables 1 and 3). Time-to-event analysis showed that consuming ≥1 egg/wk was associated with a reduced risk of pathological diagnosis of AD using the National Institute on Aging-Reagan criteria in both the partially (HR: 0.61; 95% CI: 0.43, 0.86) and multivariate (HR: 0.51; 95% CI: 0.35, 0.76) adjusted models. The highest frequency of egg consumption (≥2 eggs/wk) was only significant in the multivariate-adjusted model (HR: 0.62; 95% CI: 0.44, 0.90) (Table 2; Figure 2B). Similar results are shown using logistic regression analyses (Supplemental Table 2), except that the odds of having a pathological diagnosis of AD in the brain did not reach statistical significance when comparing the highest frequency of egg consumption (≥2 eggs/wk) to the lowest intake amount (odds ratio: 0.53; 95% CI: 0.24, 1.15).
Mediation analysis showed a significant total effect in that consuming >2 eggs/wk was associated with a 34% longer time to develop Alzheimer’s dementia (excess relative risk: 0.34, 95% CI: 0.01, 0.68, P = 0.047) compared to never or <1 egg consumption per month, and 39.2% (0.135/0.344) of the total effect was due to pure indirect effect of dietary choline intake on Alzheimer’s dementia (Table 4).
TABLE 4.
Mediation analysis with mean choline intake as the mediator in the association of egg intake with Alzheimer’s dementia risk
| Excess relative risk | Egg consumption |
|||
|---|---|---|---|---|
| Never or <1/mo | 1–3/mo | 1/wk | ≥2/wk | |
| Total effect | Reference | 0.069 (–0.216, 0.353) | 0.380 (-0.005, 0.765) | 0.344 (0.005, 0.682) |
| P = 0.636 | P = 0.053 | P = 0.047 | ||
| Pure indirect effect | Reference | 0.022 (–0.012, 0.056) | 0.044 (–0.005, 0.093) | 0.135 (0.025, 0.245) |
| P = 0.202 | P = 0.079 | P = 0.016 | ||
| Controlled direct effect | Reference | 0.028 (–0.238, 0.293) | 0.304 (–0.046, 0.654) | 0.279 (0.071, 0.628) |
| P = 0.839 | P = 0.089 | P = 0.118 | ||
| Reference interaction | Reference | –0.001 (–0.007, 0.005) | –0.004 (–0.014, 0.005) | 0.037 (–0.017, 0.091) |
| P = 0.698 | P = 0.384 | P = 0.184 | ||
| Mediated interaction | Reference | 0.020 (–0.012, 0.056) | 0.036 (–0.068, 0.141) | –0.107 (–0.252, 0.038) |
| P = 0.389 | P = 0.499 | P = 0.147 | ||
Discussion
In this prospective cohort study of noninstitutionalized community-dwelling older adults in the United States, we found that consuming ≥1 egg/wk was associated with a 47.0% reduced risk of Alzheimer’s dementia. To our knowledge, this is the first longitudinal cohort study to investigate the association of egg intake with clinically diagnosed Alzheimer’s dementia as a primary outcome, as well as with AD pathology in human brains. Our findings are somewhat supported by those from the Kuopio Ischemic Heart Disease Risk Factor Study (KIHDRFS) [33]. The KIHDRFS prospective cohort study reported egg intake to be associated with better performance on neuropsychological tests of the frontal lobe and executive functioning, which is consistent with smaller clinical studies [[34], [35], [36]] and animal models [37] that examined the effects of higher choline intake on cognitive function with aging. Choline modulates the expression of key genes related to memory, learning, and cognitive functions via epigenetic mechanisms [38]. Although the KIHDRFS showed null effects (with borderline significance) of egg intake on incident dementia or Alzheimer’s dementia, there are several differences between that study and our analysis of the RMAP. First, the mean age of participants enrolled in the RMAP was substantially higher. In addition, the RMAP cohort consisted of both males and females (the KIHDRFS only enrolled males), and >3-quarters of the participants were females. Age and females are widely known to be correlated with an increased incidence of AD [[1], [2], [3], [4]]. The primary outcome of the RMAP is a diagnosis of Alzheimer’s dementia, ascertained clinically on an annual basis and AD pathologically postmortem. A higher number of confirmed cases in the RMAP may also contribute to greater statistical power to detect the effect of a single component of the diet. Our findings are also supported by those from the Biopsychosocial Religion and Health Study, in which participants with limited egg consumption (∼1 egg/wk) exhibited a slower rate of memory decline compared with participants who consumed few to no eggs [39].
The neuroprotective actions of ω-3 fatty acids and choline have been demonstrated to exhibit synergistic actions in the brain, which may explain why eggs as whole foods are consistently shown to decrease AD risk. There is substantial evidence of a pathologic shortage of both choline and DHA in the brains of patients with AD [40]. The brain can only synthesize a limited amount of choline and ω-3 fatty acids; therefore, most must enter the brain through the blood. PUFAs (e.g., DHA) are transported across the blood-brain barrier in the form of choline-containing phospholipids such as lysophosphatidylcholine. Studies have shown that adults with low serum lysophosphatidylcholine concentrations are at greater risk of developing cognitive decline and AD [41]. It is also somewhat challenging to correct choline shortfalls in older adults through diet or supplementation because brain uptake from the plasma decreases with aging [42], even though recent work demonstrates that phosphatidylcholine–DHA-containing species in the blood and plasma can be greatly increased through co-supplementation [40]. Results from the Framingham Heart Study show the highest concentrations of phosphatidylcholine–DHA to be associated with a significant reduction in risk of all-cause dementia [43]. Patients with AD have been shown to have lower concentrations of 8 choline-containing phospholipid species (and 2 noncholine-containing species) compared with healthy controls; these 10 lipids derived from the peripheral blood have been validated to predict mild cognitive impairment or AD in a 2- to 3-y timeframe with 90% accuracy [23].
Our study has several limitations. First, we used the baseline amount of nutrient intake to match the baseline egg intake because, in this cohort of older adults, as reported previously, the diet stayed similar over the course of follow-up [44]. Moreover, the FFQ only contained 1 question related to egg intake (with only 6 levels of frequencies), limiting an accurate estimation of exposure. Finally, our study is limited by the short mean 6.7-y follow-up period and thus may be prone to reverse causality. The strengths of this study include well characterized community cohort, its prospective longitudinal design, structural and standardized clinical ascertainment of Alzheimer’s dementia annually, standardized neuropathologic confirmation of AD in postmortem brains, and the number of cases that allowed for good statistical power. Lastly, dietary assessment using a comprehensive and validated FFQ for this specific population is another strength.
In conclusion, these findings suggest that more frequent egg consumption is associated with a lower risk of Alzheimer’s dementia, and this association is partially mediated through the effect of dietary choline on Alzheimer’s dementia. More frequent egg consumption is also associated with a lower risk of AD pathology in the brain in the subgroup analyses. Once replicated in other prospective cohorts and confirmed by clinical trials, these findings may have important public health implications for reducing the population’s risk of AD.
Acknowledgments
We are thankful to the participants of the Rush Memory and Aging Project and the staff of the Rush Alzheimer's Disease Center for data collection.
Author contributions
The authors’ responsibilities were as follows – YP, TCW, MC: designed research; YP, TCW, TK, PA, DAB, MC: conducted research; YP, TCW, TK, PA, DAB, MC: analyzed data; YP, TCW, TK, PA, DAB, MC: wrote the article; TCW, MC: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
TCW is the Editor-in-chief of the Journal of Dietary Supplements, Deputy Editor-in-chief of the Journal of the American Nutrition Association, and Nutrition Section Editor of Annals of Medicine. He receives royalties from the Academy of Nutrition and Dietetics for his editorship of the Health Professionals Guide for Dietary Supplements. He has received competitive unrestricted research grants from the American Pulse Association, Balchem Corporation, Egg Nutrition Center, Florida Citrus, National Dairy Council, National Pork Board, Nestle Health Sciences, New Capstone, Inc., Oak Ridge Institute for Science and Education, The Simply Good Foods Company, and Reach Global Strategies. He has received scientific consulting fees from the Academy of Nutrition and Dietetics, Haleon, National Pork Board, and The Beer Institute. He has received speaker honoraria from the American Society for Nutrition, Berry Health Benefits Symposium, International Food Additives Council, Amazentis, Tea Institute, Forbes Health, National Dairy Council, Balchem, Corporation, San Diego State University, and University of Missouri. He is a member of the scientific advisory boards for AHARA, Beli, Deerland Probiotics & Enzymes, Forbes Health Advisory Board, and Produce for Better Health Foundation. He was an expert panel member of the Academy of Nutrition and Dietetics Working Group on Flavan-3-ols and is an unpaid coalition member of the Brightseed Bioactives Coalition. He has received in-kind nutrient analysis services from Eurofins United States Food. He is an unpaid senior fellow at the Center for Magnesium Education & Research. He has an up-to-date International Committee of Medical Journal Editors (ICMJE) disclosure of interest statement available on his website, www.drtaylorwallace.com. MC is Nutrition Section Editor of Annals of Medicine. All other authors report no conflicts of interest.
Funding
TCW and MC have received past research support from the Egg Nutrition Center. Funding for the Rush University qualifications of choline intake was provided through an unrestricted investigator-initiated grant from the Egg Nutrition Center to Think Healthy Group, LLC. The study data was supported by grants from the National Institutes of Health (R01AG017917[DAB]). None of the funding sources had any role in data anlaysis, interpretations, or manuscript preparation. MAP data can be requested at http://www.radc.rush.edu. The authors and sponsor strictly adhered to the American Society for Nutrition’s guiding principles for private funding for food science and nutrition research.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending (e.g., application and approval).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2024.05.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gauthier S., Webster C., Servaes S., Morais J.A., Rosa-Neto P. Alzheimer’s Disease International; London, England: 2022. World Alzheimer Report 2022: life after diagnosis: navigating treatment, care and support. [Google Scholar]
- 2.Kelley B.J., Petersen R.C. Alzheimer’s disease and mild cognitive impairment. Neurol Clin. 2007;25(3):577–609. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care. 2020;26(Suppl 8):S177–S183. doi: 10.37765/ajmc.2020.88482. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 6.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namboori P.K., Vineeth K.V., Rohith V., Hassan I., Sekhar L., Sekhar A., et al. The ApoE gene of Alzheimer’s disease (AD), Funct. Integr. Genomics. 2011;11(4):519–522. doi: 10.1007/s10142-011-0238-z. [DOI] [PubMed] [Google Scholar]
- 10.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278(16):1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Li Q., Wang J. Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc. Natl. Acad. Sci. U S A. 2011;108(36):14813–14818. doi: 10.1073/pnas.1106420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frieden C., Garai K. Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A. 2012;109(23):8913–8918. doi: 10.1073/pnas.1207022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong N., Weisgraber K.H. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J Biol. Chem. 2009;284(10):6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace T.C., Fulgoni V.L. Usual choline intakes are associated with egg and protein food consumption in the United States. Nutrients. 2017;9(8):839. doi: 10.3390/nu9080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irawan A., Ningsih N., Hafizuddin, Rusli R.K., Suprayogi W.P.S., Akhirini N., et al. Supplementary n-3 fatty acids sources on performance and formation of omega-3 in egg of laying hens: a meta-analysis. Poult. Sci. 2022;101(1) doi: 10.1016/j.psj.2021.101566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimalaratne C., Wu J. Hen egg as an antioxidant food commodity: a review. Nutrients. 2015;7(10):8274–8293. doi: 10.3390/nu7105394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services, US Department of Agriculture . 8th ed. 2015-2020. Dietary guidelines for Americans. [Google Scholar]
- 18.Wallace T.C., Blusztajn J.K., Caudill M.A., Klatt K.C., Zeisel S.H. Choline: the neurocognitive essential nutrient of interest to obstetricians and gynecologists. J Diet Suppl. 2020;17(6):733–752. doi: 10.1080/19390211.2019.1639875. [DOI] [PubMed] [Google Scholar]
- 19.Blusztajn J.K., Slack B.E., Mellott T.J. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8) doi: 10.3390/nu9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bortz J., Klatt K.C., Wallace T.C. Perspective: estrogen and the risk of cognitive decline: a missing choline(rgic) link? Adv. Nutr. 2022;13(2):376–387. doi: 10.1093/advances/nmab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd J.M., Jasbi P., Winslow W., Serrano G.E., Beach T.G., Klein-Seetharaman J., et al. Inflammation and the pathological progression of Alzheimer’s disease are associated with low circulating choline levels. Acta. Neuropathol. 2023;146(4):565–583. doi: 10.1007/s00401-023-02616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surette M.E. The science behind dietary omega-3 fatty acids. CMAJ. 2008;178(2):177–180. doi: 10.1503/cmaj.071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mapstone M., Cheema A.K., Fiandaca M.S., Zhong X., Mhyre T.R., MacArthur L.H., et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014;20(4):415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett D.A., Schneider J.A., Buchman A.S., Mendes de Leon C., Bienias J.L., Wilson R.S. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 25.Bennett D.A., Schneider J.A., Buchman A.S., Barnes L.L., Boyle P.A., Wilson R.S. Overview and findings from the Rush Memory and Aging Project. Curr. Alzheimer. Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett D.A., Schneider J.A., Arvanitakis Z., Kelly J.F., Aggarwal N.T., Shah R.C., et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 27.Schneider J.A., Arvanitakis Z., Leurgans S.E., Bennett D.A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett D.A., Schneider J.A., Tang Y., Arnold S.E., Wilson R.S. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet. Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 29.Morris M.C., Tangney C.C., Bienias J.L., Evans D.A., Wilson R.S. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am. J Epidemiol. 2003;158(12):1213–1217. doi: 10.1093/aje/kwg290. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Agriculture . 2023. Nutrient database for standard reference. Washington, DC, USA. [Google Scholar]
- 31.Buchman A.S., Boyle P.A., Wilson R.S., Beck T.L., Kelly J.F., Bennett D.A. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis. Assoc. Disord. 2009;23(1):63–69. doi: 10.1097/WAD.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Discacciati A., Bellavia A., Lee J.J., Mazumdar M., Valeri L. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int. J Epidemiol. 2018 doi: 10.1093/ije/dyy236. [DOI] [PubMed] [Google Scholar]
- 33.Ylilauri M.P., Voutilainen S., Lönnroos E., Mursu J., Virtanen H.E., Koskinen T.T., et al. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Alzheimer disease: the Kuopio ischaemic Heart Disease Risk Factor Study1,2. Am. J. Clin. Nutr. 2017;105(2):476–484. doi: 10.3945/ajcn.116.146753. [DOI] [PubMed] [Google Scholar]
- 34.Caudill M.A., Strupp B.J., Muscalu L., Nevins J.E., Canfield R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J. 2018;32(4):2172–2180. doi: 10.1096/fj.201700692RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poly C., Massaro J.M., Seshadri S., Wolf P.A., Cho E., Krall E., et al. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J Clin. Nutr. 2011;94(6):1584–1591. doi: 10.3945/ajcn.110.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leermakers E.T., Moreira E.M., Kiefte-de Jong J.C., Darweesh S.K., Visser T., Voortman T., et al. Effects of choline on health across the life course: a systematic review. Nutr. Rev. 2015;73(8):500–522. doi: 10.1093/nutrit/nuv010. [DOI] [PubMed] [Google Scholar]
- 37.Gámiz F., Gallo M. A systematic review of the dietary choline impact on cognition from a psychobiological approach: insights from animal studies. Nutrients. 2021;13(6):1966. doi: 10.3390/nu13061966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekdash R.A. Neuroprotective effects of choline and other methyl donors. Nutrients. 2019;11(12):2995. doi: 10.3390/nu11122995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee G.J., Oda K., Morton K.R., Orlich M., Sabate J. Egg intake moderates the rate of memory decline in healthy older adults. J Nutr. Sci. 2021;10:e79. doi: 10.1017/jns.2021.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumel B.S., Doraiswamy P.M., Sabbagh M., Wurtman R. Potential neuroregenerative and neuroprotective effects of uridine/choline-enriched multinutrient dietary intervention for mild cognitive impairment: a narrative review. Neurol. Ther. 2021;10(1):43–60. doi: 10.1007/s40120-020-00227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semba R.D. Perspective: the potential role of circulating lysophosphatidylcholine in neuroprotection against Alzheimer disease. Adv. Nutr. 2020;11(4):760–772. doi: 10.1093/advances/nmaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen B.M., Renshaw P.F., Stoll A.L., Wurtman R.J., Yurgelun-Todd D., Babb S.M. Decreased brain choline uptake in older adults. An in vivo proton magnetic resonance spectroscopy study, JAMA. 1995;274(11):902–907. doi: 10.1001/jama.1995.03530110064037. [DOI] [PubMed] [Google Scholar]
- 43.Schaefer E.J., Bongard V., Beiser A.S., Lamon-Fava S., Robins S.J., Au R., et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 2006;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal P., Leurgans S.E., Agrawal S., Aggarwal N.T., Cherian L.J., James B.D., et al. Association of Mediterranean-DASH intervention for neurodegenerative delay and Mediterranean diets with Alzheimer disease pathology. Neurology. 2023;100(22):e2259–e2268. doi: 10.1212/WNL.0000000000207176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending (e.g., application and approval).