Abstract
Background
Research suggests omega-3 polyunsaturated fatty acids (PUFAs) exert favorable effects on several biological processes involved in the development and progression of atherosclerotic cardiovascular disease (ASCVD). However, studies examining the relationship between omega-3 PUFAs and peripheral artery disease (PAD) are scarce.
Objectives
We evaluated the associations between omega-3 PUFAs and incident PAD in a meta-analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) and Atherosclerosis Risk in Communities (ARIC) study cohorts.
Methods
Omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were measured at baseline for all MESA (n = 6495) and Minnesota ARIC participants (n = 3612). Incident clinical PAD events (MESA n = 106; ARIC n = 149) identified primarily through ICD discharge codes were assessed through follow-up of each cohort. Associations between omega-3 PUFAs (EPA, DHA, and EPA+DHA) and incident PAD were modeled in MESA and ARIC as quartiles and continuously using Cox proportional hazards regression, respectively. A fixed-effects meta-analysis was conducted to evaluate associations in the 2 cohorts combined.
Results
In the fully adjusted model, in 10,107 participants, no significant associations were observed between EPA, DHA, or EPA+DHA, and incident PAD modeled as quartiles or continuously for either MESA or ARIC cohorts separately or in the meta-analysis after a follow-up of approximately 15 y.
Conclusion
This study is consistent with previous literature indicating that the beneficial effects of omega-3 PUFAs on the markers of ASCVD may not translate to a clinically meaningful decrease in PAD risk.
Keywords: Omega-3 PUFAs, PAD, fatty acids, MESA, ARIC, EPA, DHA
Introduction
Peripheral artery disease (PAD) is characterized by the presence of obstructive atherosclerotic plaque in the major conduit arteries in the lower extremities. In the Reduction of Atherothrombosis for Continued Health registry, it was found that 15% of individuals with clinical atherosclerotic disease had PAD and, more importantly, half of these individuals had isolated PAD [1]. This amounts to an estimated more than 12 million individuals in the United States living with PAD [2]. Individuals with PAD, whether isolated or not, experience a three- to six-fold higher rate of cardiovascular mortality compared with those without PAD [3]. In addition, these individuals are at a much higher risk for adverse limb outcomes that are directly attributable to their PAD. It is estimated that 5% of those with PAD who experience leg pain with exertion will require amputation within 5 y, whereas over 20% of those with PAD and evidence of resting limb ischemia will require an amputation within 1 y [4]. The average annual medical expenditure for individuals with PAD is estimated to be nearly 3 times higher compared with those without PAD ($11,553 compared with $4219) [5]. Although prevention and treatment focus on the modification of cardiovascular disease risk factors, individuals with PAD are relatively undertreated, and dietary and lifestyle recommendations are primarily based on studies of coronary heart disease (CHD) [6,7].
Accumulating evidence suggests that omega-3 PUFAs exert favorable effects on several biological processes involved in the development and progression of atherosclerotic cardiovascular disease (ASCVD). In particular, the marine omega-3 PUFAs, EPA, and DHA have been shown to have various anti-inflammatory, antiatherosclerotic, and antithrombotic effects [[8], [9], [10]]. Moreover, higher dietary intake of omega-3 PUFAs and a higher omega-3 to omega-6 PUFA ratio have been shown to be associated with lower incident cardiovascular disease (CVD) events, including CHD and ischemic stroke, and lower odds of carotid atherosclerosis [[11], [12], [13]]. Despite this, as well as previous associations with CVD risk factors and outcomes, studies examining associations between circulating omega-3 PUFA levels and PAD are limited, particularly in large ethnically diverse cohorts.
To address this important research gap, we evaluated whether plasma phospholipid omega-3 PUFAs (EPA, DHA, and EPA+DHA) were associated with incident clinical PAD events in individuals free of clinical ASCVD at baseline in 2 cohorts: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that omega-3 PUFAs, EPA and DHA, would be inversely associated with incident PAD.
Methods
MESA population
The primary aim and study design of MESA have been previously described, and detailed information is available online at http://www.mesa-nhlbi.org [14]. Briefly, MESA consists of 6814 adult males and females aged 45–84 y without evidence of overt CVD at the time of recruitment (2000–2002). The cohort of self-reported White, Black, Chinese, and Hispanic subjects was recruited from 6 communities across the United States (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN). Each field site was recruited from locally available sources, which included lists of residents, lists of dwellings, and telephone exchanges. In the last few months of the recruitment period, supplemental sources (lists of Medicare beneficiaries from the Centers for Medicare and Medicaid Services and referrals by participants) were used to ensure adequate numbers of minorities and elderly subjects.
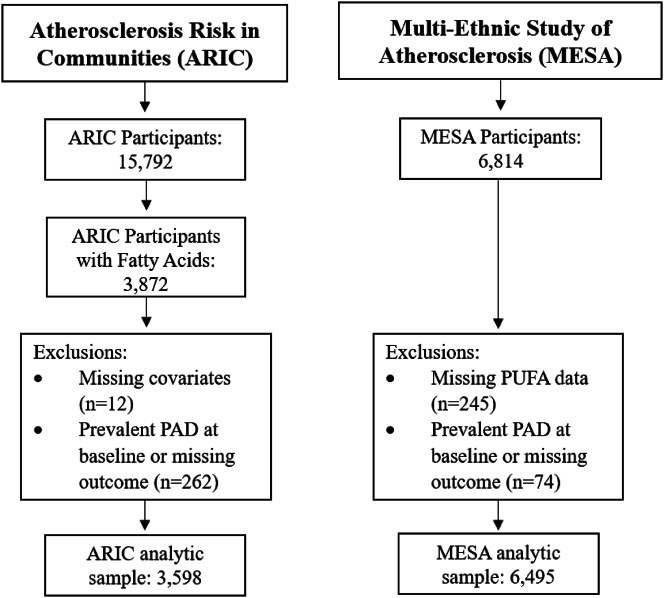
Institutional review board approval was obtained at all MESA sites, and all participants gave informed consent. Participants with missing plasma EPA or DHA (N = 245), and those with prevalent clinical PAD at baseline or missing PAD incidence outcome (N = 74) were excluded, resulting in a study sample of 6495 participants (Figure 1). The median follow-up time was 14.05 y (mean = 12.39 y) and examinations took place on average every 2 y to total 5 examinations for follow-up PAD diagnosis.
FIGURE 1.
Scheme of inclusion/exclusion criteria for Atherosclerosis Risk in Communities (ARIC) and Multi-Ethnic Study of Atherosclerosis (ARIC) cohorts used in the analysis.
ARIC population
The ARIC study is a population-based study aimed at investigating the etiology of atherosclerosis and its clinical sequelae, and the longitudinal impact of variation in cardiovascular disease risk factors and detailed information has been previously published [15]. Briefly, ARIC consists of participants from 4 United States communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD). Participants were between 45 and 64 y of age at baseline (1987–1989) and have been followed with serial in-person assessments and hospitalization surveillance. The ARIC study protocol was approved by each field center’s institutional review board, and after written informed consent was obtained. Out of the total sample examined at baseline (n = 15,792), only a subset of individuals from the Minnesota clinical site had plasma phospholipid fatty acids measured at baseline (n = 3872). For this analysis, we excluded participants missing covariates of interest (N = 12), and participants missing PAD status or with prevalent PAD (defined as ABI ≤ 0.9, self-reported intermittent claudication, or a history of leg artery revascularization) at baseline (N = 262), resulting in an analytic sample of 3598 participants (Figure 1). We used a median follow-up time of 15 y (mean = 13.85 y) to be consistent and comparable with the MESA cohort.
Polyunsaturated fatty acids method for MESA and ARIC
In both MESA and ARIC, fasting blood was collected in EDTA-anticoagulant vacuum tubes and stored at −70°C until the time of analysis. EDTA plasma from the first examination was used for the extraction of plasma phospholipid fatty acid profiles using previously described methods [16,17]. Briefly, plasma phospholipids were separated by TLC and converted to FAMEs by boron trifluoride catalysis. Then, the methyl esters were separated and measured on a model 5890 gas chromatograph (Hewlett Packard) equipped with a capillary column and flame-ionization detection. Plasma phospholipid fatty acids were identified by comparing each fatty acid peak’s retention time to the retention times of fatty acid synthetic standards of known compositions. The relative amount of each fatty acid (as a percentage of all plasma phospholipid fatty acids) was computed by the integration of the area under the peak and the division of the result by the total area for all plasma phospholipid fatty acids (×100). Data from the chromatogram were transferred electronically to a computer for analysis. Each fatty acid was expressed as a percentage of the total fatty acids in plasma phospholipids. For each fatty acid, the limit of detection was 0.03%. The interassay coefficient of variation for MESA was EPA: 8.3% and DHA: 6.8%. The reliability coefficients for ARIC was EPA: 0.31 and DHA: 0.85.
Clinical PAD—MESA
Incident PAD events from the MESA study were collected through 2015, and diagnostic criteria have been previously published [18]. During follow-up, incident clinical PAD was identified by a self-report of a PAD diagnosis by the participant at 1) MESA clinic visits, 2) annual follow-up phone calls, or 3) participant notification. This self-report triggered the collection of relevant medical records, which were compiled for adjudication. Two physician members of the MESA mortality and morbidity review committee independently classified events. The full committee made final classifications if there were disagreements.
Physician adjudicators subclassified clinical PAD as 1) lower extremity claudication, 2) atherosclerosis of arteries of the lower extremities, or 3) arterial embolism and/or thrombosis of the lower extremities. Criteria for clinical PAD were met by 1) ultrasonographically or angiographically demonstrated obstruction or ulcerated plaque (≥50% of the diameter or ≥75% of the cross-sectional area) demonstrated on the ultrasound or angiogram of the iliac arteries or below, 2) the absence of pulse by Doppler in any major vessel of the lower extremities, 3) exercise test that is positive for lower extremity claudication, 4) surgery, angioplasty, or thrombolysis for peripheral vascular disease, 5) amputation of one or more toes or part of the lower extremity because of ischemia or gangrene, or 6) exertional leg pain relieved by rest in combination with either physician-diagnosed claudication diagnosed or an ankle–arm blood pressure ratio ≤0.8.
Clinical PAD—ARIC
Incident PAD events from the ARIC study were collected for first 15 y of follow-up. Incident PAD events were identified according to the International Classification of Diseases, Ninth Revision (ICD-9) hospital discharge codes, as previously published [19]. PAD-related hospitalizations were identified according to the following ICD-9 codes: atherosclerosis of native arteries of the extremities, unspecified (440.20); atherosclerosis of native arteries of the extremities with intermittent claudication (440.21); atherosclerosis of native arteries of the extremities with rest pain (440.22); atherosclerosis of native arteries of the extremities with ulceration (440.23); atherosclerosis of native arteries of the extremities with gangrene (440.24); other atherosclerosis of native arteries of the extremities (440.29); atherosclerosis of bypass graft of the extremities (440.3); atherosclerosis of other specified arteries (440.8); peripheral vascular disease, unspecified (443.9); and leg artery revascularization (38.18, 39.25, 39.29, 39.50).
Measurement of covariates
Standardized questionnaires for both MESA and ARIC were used at baseline to obtain age (years), sex (male/female), race/ethnicity, smoking history (current/former/never), alcohol consumption (g/wk), level of education, physical activity, and dietary intake. Education was categorized into “less than high school,” “high school/GED/Technical,” or “some college or more.” Physical activity was recorded as the participant-reported number of intentional exercise metabolic equivalent (MET)-min/wk. Diet quality was assessed using the Alternative Healthy Eating Index. Medication usage, including statin, antihypertensive, and antidiabetic use, was determined by standardized questionnaires (MESA) or visual inspection of medications at study visits and linkage to Medi-Span Therapeutic Classification codes (ARIC). Systolic and diastolic resting blood pressure measurements were taken in seated participants (mmHg). BMI was calculated as weight in kilograms divided by height in meters squared. Diabetes was defined as self-reported physician diagnosis of diabetes, ≥126 mg/dL fasting glucose, ≥200 mg/dL nonfasting glucose, or use of diabetes medications. Blood concentrations of total cholesterol, HDL cholesterol, and triglycerides were measured using standard methods [20,21]. LDL cholesterol was calculated by the Friedewald equation in those with triglycerides <400 mg/dL. Fasting glucose was measured in serum by rate reflectance spectrophotometry on a thin-film adaptation of the glucose oxidase method with a Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc.).
Statistical methods
Descriptive statistics for participant demographics, lifestyle, and clinical characteristics were tabulated. Omega-3 PUFAs were modeled continuously (log-transformed) and as study-specific quartiles (MESA: EPA quartile cut-points: 0.485%; 0.668%; 0.995%; DHA quartile cut-points: 2.721%; 3.591%; 4.688%; EPA+DHA quartile cut-points: 3.278%, 4.266%, 5.645%; ARIC: EPA quartile cut-points: 0.39%; 0.51%; 0.66%; DHA quartile cut-points: 2.22%; 2.67%; 3.27%; EPA+DHA quartile cut-points: 2.71%, 3.21%, 3.83%. Continuous associations were tabulated per log-unit increase. Cox proportional hazards regression was used to estimate hazard ratios (HR) for omega-3 PUFAs and incident PAD in MESA and ARIC separately. Proportional hazards assumptions were confirmed using time-dependent covariates in the Cox proportional hazards models. Participants’ follow-up was from baseline to the time of the event or end of follow-up, whichever came first. Participants were not censored during follow-up if they developed interim coronary heart or cerebrovascular disease. The model was adjusted for age (continuous), sex (female/male), and race/ethnicity (White, Black, Hispanic, Chinese), education (<High school, High school/GED/Technical, College), smoking status (never, former, current), alcohol intake (continuous, g/wk), systolic blood pressure (continuous), taking blood pressure medication (yes/no), total cholesterol (continuous), HDL-C (continuous), log-transformed triglycerides (continuous), taking lipid-lowering medication (yes/no), BMI (continuous), and diabetes status (present/absent). MESA analyses were additionally adjusted for race/ethnicity. The inclusion of fasting glucose (log-transformed) instead of and in addition to diabetes status did not change findings. Moderate/vigorous levels of physical activity (MET-min/wk) were not associated with PAD in either cohort and not included in the final study models. Data analysis was conducted using SAS 9.4 (SAS Institute Inc.), and a P value <0.05 was defined as statistical significance.
Fixed-effects meta-analysis was conducted to combine results from MESA and ARIC using Stata 17.0 (Stata Corp). I2 values were used to measure heterogeneity between studies.
Results
Baseline characteristics
There were 108 MESA participants and 149 ARIC participants with incident PAD after an approximate 15-y follow-up period among the 10,107 participants included in this analysis. Baseline demographic and biomarker characteristics categorized by PAD status are shown in Table 1. Individuals with incident PAD were older and more likely to be male, to have less education, to be current smokers, be on lipid-lowering or hypertension medication, and have a higher BMI and glucose.
TABLE 1.
Baseline characteristics of participants according to the incidence of clinical peripheral artery disease during follow-up in MESA and ARIC
| MESA |
ARIC |
|||||
|---|---|---|---|---|---|---|
| Noncases | Cases | P | Noncases | Cases | P | |
| Overall, n (%) | 6387 (98.3) | 108 (1.7) | 3463 (95.9) | 149 (4.1) | ||
| Age1 | 62.0 (53.0, 70.0) | 67.0 (59.0, 74.0) | <0.0001 | 53.0 (49.0, 58.0) | 58.0 (54.0, 61.0) | <0.0001 |
| Sex, n (% female) | 3409 (53.4) | 40 (37.0) | 0.0007 | 1792 (51.9) | 42 (28.2) | <0.0001 |
| Race/ethnicity, n (%) | 0.003 | |||||
| White | 2452 (38.4) | 52 (48.2) | 3563 (95.9) | 149 (4.1) | — | |
| Black | 1742 (27.3) | 37 (34.3) | — | — | ||
| Chinese-American | 785 (12.3) | 4 (3.7) | — | — | ||
| Hispanic | 1408 (22.0) | 15 (13.9) | — | — | ||
| Education, n (%) | 0.01 | 0.005 | ||||
| <High school | 1166 (18.3) | 25 (23.2) | 201 (5.8) | 18 (12.1) | ||
| High school/GED/Technical | 1613 (25.3) | 30 (27.8) | 1607 (46.6) | 71 (47.7) | ||
| College | 3608 (56.5) | 53 (49.1) | 1641 (47.6) | 60 (40.3) | ||
| Medication use, n (%) | ||||||
| Lipid-lowering medication | 1016 (15.9) | 26 (24.1) | 0.02 | 99 (2.9) | 12 (8.1) | 0.0003 |
| Hypertension medication | 2343 (36.7) | 65 (60.2) | <0.0001 | 758 (22.0) | 82 (55.0) | <0.0001 |
| Smoking status, n (%) | <0.0001 | <0.0001 | ||||
| Current | 816 (12.8) | 35 (32.4) | 723 (21.0) | 78 (52.4) | ||
| Former | 2321 (36.5) | 44 (40.7) | 1413 (41.0) | 56 (37.6) | ||
| Never | 3231 (50.7) | 29 (26.9) | 1313 (38.1) | 15 (10.1) | ||
| Alcohol use1 (g/wk) | 3.3 (0.3, 30.6) | 3.4 (0.2, 60.6) | 0.004 | 21.6 (0.0, 75.6) | 15.1 (0.0, 105.7) | 0.003 |
| Systolic blood pressure1 (mmHg) | 124.0 (111.0, 140.0) | 133.0 (118.0, 151.0) | <0.0001 | 116.0 (1.07.0, 128.0) | 124.0 (112.0, 135.0) | <0.0001 |
| Diastolic blood pressure1 (mmHg) | 71.5 (65.0, 78.5) | 72.5 (65.5, 80.0) | 0.17 | 73.0 (67.0, 80.0) | 74.0 (67.0, 80.0) | 0.55 |
| BMI1 (m/kg2) | 27.6 (24.5, 31.2) | 28.7 (25.3, 31.5) | 0.32 | 26.4 (23.8, 29.5) | 27.3 (24.9, 30.8) | 0.007 |
| Fasting glucose (mol/dL) | 90.0 (83.0, 99.0) | 94.5 (86.0, 114.5) | <0.0001 | 99.2 (93.4, 116.0) | 104.0 (98.0, 117.5) | <0.0001 |
| Diabetes, n (%) | 776 (12.2) | 30 (27.8) | <0.0001 | 235 (6.8) | 29 (19.5) | <0.0001 |
| Lipid profile1 | ||||||
| Total cholesterol (mg/dL) | 192.0 (171.0, 215.0) | 195.0 (171.5, 221.0) | 0.24 | 212.0 (187.0, 237.0) | 215.0 (189.0, 246.0) | 0.05 |
| HDL-C (mg/dL) | 48.0 (40.0, 59.0) | 44.5 (39.5, 55.0) | 0.004 | 49.1 (40.0, 62.6) | 38.5 (32.0, 48.1) | <0.0001 |
| Triglycerides (mg/dL) | 111.0 (77.0, 161.0) | 130.5 (93.5, 180.5) | 0.02 | 106.0 (76.5, 152.0) | 138.0 (102.0, 201.0) | <0.0001 |
| Diet quality score2 (N (%)) | 0.02 | 0.28 | ||||
| Quartile 1 | 1501 (23.5) | 35 (32.4) | 790 (22.8) | 42 (28.2) | ||
| Quartile 2 | 1556 (24.4) | 21 (19.4) | 892 (25.7) | 39 (26.2) | ||
| Quartile 3 | 1534 (24..0) | 24 (22.0) | 814 (23.5) | 30 (20.1) | ||
| Quartile 4 | 1541 (24.1) | 15 (13.9) | 921 (26.6) | 34 (22.8) | ||
| Missing | 255 (4.0) | 13 (12.0) | 47 (1.4) | 4 (2.7) | ||
| Phospholipid PUFA1 | ||||||
| EPA (% of total) | 0.67 (0.49, 1.00) | 0.65 (0.48, 0.89) | 0.91 | 0.51 (0.39, 0.66) | 0.46 (0.37, 0.61) | 0.95 |
| DHA (% of total) | 3.60 (2.72, 4.70) | 3.38 (2.70, 4.46) | 0.38 | 2.67 (2.22, 3.27) | 2.51 (2.08, 3.08) | 0.04 |
| EPA+DHA (% of total) | 4.27 (3.28, 5.65) | 4.07 (3.21, 5.42) | 0.50 | 3.20 (2.71, 3.82) | 3.01 (2.57, 3.59) | 0.08 |
| Dietary PUFAs | ||||||
| Total PUFAs (% kcal/d) | 5.79 (4.78, 6.91) | 5.73 (4.66, 7.23) | 0.74 | 4.94 (4.08, 5.93) | 4.98 (4.20, 612) | 0.23 |
| Total PUFAs (g/d) | 9.36 (6.16, 13.6) | 9.97 (6.72, 15.7) | 0.09 | 8.64 (6.118, 11.7) | 9.55 (6.72, 12.2) | 0.04 |
| EPA (g/d) | 0.03 (0.01, 0.05) | 0.02 (0.01, 0.04) | 0.06 | 0.05 (0.03, 0.10) | 0.05 (0.03, 0.10) | 0.64 |
| DHA (g/d) | 0.06 (0.03, 0.10) | 0.06 (0.03, 0.10) | 0.14 | 0.11 (0.06, 0.20) | 0.11 (0.07, 0.19) | 0.47 |
| EPA+DHA (g/d) | 0.09 (0.05, 0.15) | 0.08 (0.05, 0.15) | 0.10 | 0.16 (0.08, 0.31) | 0.16 (0.10, 0.30) | 0.53 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; GED, General Educational Development; MESA, Multi-Ethnic Study of Atherosclerosis; PAD, peripheral artery disease.
Median (25th percentile, 75th percentile).
Alternative Health Eating Index-2010; MESA quartile cut-points = 49.55, 55.465, 61.165; ARIC quartile cut-points = 47.5, 53.9, 58.8.
Associations of omega-3 PUFAs with Incident clinical PAD
A significant inverse relationship was observed between the 4th and 1st quartile of EPA (P = 0.03) and clinical PAD among ARIC participants in a minimally adjusted model for age, sex, and race/ethnicity (Supplemental Table 1). No association was observed for MESA participants with minimal adjustment. In the meta-analysis, a significant inverse relationship between the 4th and 1st quartile of EPA (P = 0.05) and clinical PAD was observed.
The fully adjusted associations between baseline EPA, DHA and EPA+DHA levels and clinical PAD incidence for MESA and ARIC are presented separately and as a meta-analysis in Table 2. Regardless of whether omega-3 PUFAs were modeled continuously or categorically and the covariates included in the model, no significant associations were observed between omega-3 PUFAs and incident clinical PAD in the MESA or ARIC cohort individually. A combined meta-analysis did not show a significant relationship between omega-3 PUFAs and incident PAD. The I2 values suggested no sign of heterogeneity between MESA and ARIC results in all models. No significant associations were observed between omega-3 PUFAs and incident clinical PAD in the MESA or ARIC cohort individually or as a combined meta-analysis when additionally adjusting for diet quality (Supplemental Table 2).
TABLE 2.
Association of baseline plasma omega-3 PUFA levels with incident clinical peripheral artery disease (PAD) in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Atherosclerosis Risk in Communities (ARIC) study with multivariate adjustment
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Continuous (per 1 log-unit) | |
|---|---|---|---|---|---|
| EPA | |||||
| Range | 0.000–0.440% | 0.441–0.593% | 0.594–0.840% | 0.841–14.46% | |
| MESA | |||||
| N (total/cases) | 1224/19 | 1391/25 | 1670/31 | 2210/33 | 6495/108 |
| HR (95% CI) | Ref | 1.09 (0.56, 2.14) | 1.07 (0.56, 2.06) | 0.97 (0.50, 1.87) | 0.91 (0.63, 1.30) |
| ARIC | |||||
| N (total/cases) | 1253/62 | 1152/48 | 839/31 | 368/8 | 3598/149 |
| HR (95% CI) | Ref | 0.87 (0.59, 1.29) | 0.79 (0.50, 1.25) | 0.50 (0.23, 1.04) | 0.76 (0.50, 1.15) |
| Meta-analysis | |||||
| N (total/cases) | 2580/76 | 2511/68 | 2505/66 | 2497/47 | 10,093/257 |
| HR (95% CI) | Ref | 0.92 (0.66, 1.29) P = 0.63 | 0.87 (0.60, 1.27) P = 0.48 | 0.73 (0.44, 1.20) P = 0.21 | 0.84 (0.64, 1.11) P = 0.22 |
| I2 | I2=0 | I2 = 0 | I2 = 40 | I2 = 0 | |
| DHA | |||||
| Range | 0.000–2.440% | 2.441–3.148% | 3.149–4.188% | 4.189–10.565% | |
| MESA | |||||
| N (total/cases) | 1129/20 | 1312/25 | 1751/28 | 2303/35 | 6495/108 |
| HR (95% CI) | Ref | 1.09 (0.55, 2.17) | 1.04 (0.53, 2.05) | 0.98 (0.49, 1.95) | 0.92 (0.73, 1.16) |
| ARIC | |||||
| N (total/cases) | 1368/70 | 1201/45 | 781/26 | 262/8 | 3598/149 |
| HR (95% CI) | Ref | 0.85 (0.58, 1.24) | 0.73 (0.53, 2.05) | 0.84 (0.40, 1.78) | 0.64 (0.36, 1.14) |
| Meta-analysis | |||||
| N (total/cases) | 2544/76 | 2523/74 | 2521/56 | 2505/51 | 10,093/257 |
| HR (95% CI) | Ref | 0.90 (0.65, 1.26) P = 0.54 | 0.87 (0.54, 1.41) P = 0.57 | 0.91 (0.55, 1.52) P = 0.72 | 0.87 (0.71, 1.08) P = 0.22 |
| I2 | I2 = 0 | I2 = 0 | I2 = 0 | I2 = 24 | |
| EPA+DHA | |||||
| Range | 0.000–2.965% | 2.966–3.733% | 3.734–4.975% | 4.976–22.098% | |
| MESA | |||||
| N (total/cases) | 1113/18 | 1274/27 | 1769/28 | 2339/35 | 6495/108 |
| HR (95% CI) | Ref | 1.33 (0.66, 2.68) | 1.10 (0.54, 2.24) | 1.06 (0.52, 2.17) | 0.80 (0.46, 1.40) |
| ARIC | |||||
| N (total/cases) | 1390/70 | 1233/59 | 758/24 | 231/6 | 3598/149 |
| HR (95% CI) | Ref | 0.85 (0.59, 1.24) | 0.72 (0.45, 1.16) | 0.66 (0.28, 1.54) | 0.61 (0.33, 1.14) |
| Meta-analysis | |||||
| N (total/cases) | 2539/79 | 2536/74 | 2516/55 | 2502/49 | 10,093/257 |
| HR (95% CI) | Ref | 0.94 (0.68, 1.30) P = 0.70 | 0.82 (0.55, 1.20) P = 0.32 | 0.87 (0.50, 1.51) P = 0.62 | 0.71 (0.47, 1.07) P = 0.10 |
| I2 | I2 = 18 | I2 = 0 | I2 = 0 | I2 = 0 | |
Cox proportional hazards regression was used to estimate hazard ratios for omega-3 PUFAs (log-transformed) and incident PAD in MESA and ARIC separately. Proportional hazards assumptions were confirmed using time-dependent covariates in the Cox proportional hazards models. Participants’ follow-up was from baseline to the time of the event or end of follow-up, whichever came first. Fixed-effect meta-analysis was conducted to combine results from MESA and ARIC. I2 values were used to measure heterogeneity between studies.
Adjustments for age, sex, race/ethnicity, education, smoking status, alcohol consumption status, systolic blood pressure, taking blood pressure medication, total cholesterol, HDL-C, log-transformed triglycerides, taking lipid-lowering medication, BMI, and diabetes status.
Discussion
In this multi-ethnic population, the omega-3 PUFAs, EPA, DHA, and EPA+DHA were not significantly associated with incident clinical PAD after adjusting for traditional risk factors after 15-y follow-up. Similar results were observed in both MESA and ARIC separately as well as in meta-analyses. However, individuals with the highest omega-3 PUFA levels generally had lower clinical PAD risk, albeit not statistically significant.
Evidence from cross-sectional studies supports a beneficial role for marine omega-3 PUFAs on clinical PAD. Studies of Japanese cohorts have reported lower circulating levels of EPA and DHA in PAD cases versus controls, and EPA levels were lower in Japanese CAD patients with PAD than in those without PAD with the level of EPA being a significant predictor for PAD [[22], [23], [24]]. Likewise, a study of 145 PAD cases and 34 controls found those with PAD had lower levels of erythrocyte omega-3 PUFAs than controls [25]. Location of PAD, for example infrainguinal lesions, have also been shown to be associated with levels of omega-3 PUFAs [26]. In the only other prior study evaluating incident PAD, a large Danish case-cohort study showed high adipose tissue levels of EPA, but not DHA, and EPA+DHA were associated with a lower incidence of PAD [27]. Adipose tissue is generally considered a representative marker of long-term habitual diets whereas phospholipids reflect more acute changes in the diet with a shorter incorporation rate of 3–5 wk, which may explain the difference in findings between our study and the Danish cohort. It is also worth noting the overall intake of omega-3 PUFAs is low across the US population, which may limit the ability of observational studies, such as MESA and ARIC, to detect an association between dietary and measured omega-3 PUFA levels with incident clinical PAD [28].
Similarly, small interventional studies examining omega-3 PUFA supplementation with a variety of PAD outcomes also suggests a beneficial effect. The OMEGA-PAD trial evaluated high-dose omega-3 PUFA supplementation (4.4 g/d oral) versus a placebo with vascular function and inflammation in 80 patients with PAD over a 1-mo period [29]. Although supplementation increased several anti-inflammatory mediators, including pro-resolving lipoxins, no significant improvements in clinical endpoints were observed [[30], [31], [32]]. In a randomized, double-blind, placebo-controlled trial of 70 patients with symptomatic PAD, omega-3 supplementation (4 g/d oral) improved endothelial function but did not have an effect on clinical endpoints including walking distance or flow-mediated dilation over a 3-mo treatment period [33]. Thus, although it appears high levels of omega-3 PUFAs do improve vascular biomarkers, this does not translate into clinically meaningful change in PAD symptoms. However, these studies have unusually short treatment time period (3 mo) and the null findings with PAD outcomes maybe because of the insufficient interventional timeframe of the studies.
In an attempt to elucidate dosage and/or duration, a few meta-analyses and large interventional trials of omega-3 PUFAs designed to evaluate multiple ASCVD outcomes with either higher dosage or longer duration have also provided conflicting results. A meta-analysis from 2018 examining lower doses of omega-3 PUFA supplements (no minimum daily dose was required but varied from 226 to 1800 mg/d for EPA and 0 to 1700 mg/d for DHA) but longer duration of the treatment period (of at least 1 y for eligibility) demonstrated no association with CHD or major vascular events [34]. Conversely, recent meta-analyses of omega-3 PUFAs reported that supplementation was associated with reduced risk of CHD events and CHD mortality but not CVD events [35]. However, the slope of the dose-effect relationship between omega-3 PUFAs and CVD events was negative and significantly nonzero (P < 0.01), suggesting higher dosages are associated with increased protection. The VITAL trial, a double-blind, placebo-controlled study designed to test the benefits and risks of supplementation with vitamin D and omega-3 PUFAs (1 g/d) over a median 5.3 y found omega-3 PUFAs did not lower incidence of major cardiovascular events compared with the placebo [36]. However, findings published by the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) provide compelling evidence for high dose and longer duration interventional studies [37]. Specifically, REDUCE-IT utilized a synthetic derivative of the omega-3 fatty acid EPA, icosapent ethyl (4 g/d), over a median of 4.9 y and demonstrated significant reductions in vascular events. Conversely, recent results from the Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) study, which evaluated effects of omega-3 carboxylic acid (mixture of EPA and DHA) at 4 g/d for a median of 41.3 mo, showed no significant reduction in vascular events compared with those receiving a corn oil placebo [38]. More studies are required for a better understanding of the contradictory results obtained from the REDUCE-IT and STRENGTH studies, and whether only EPA but not DHA at high dose and longer duration might result in significant reductions of vascular disease events.
This study has several strengths. The multi-ethnic cohort of MESA allows for observations that are more applicable to the general United States population. The addition of ARIC and the combined analysis strengthen the observations from MESA. Individual PUFAs were measured in plasma providing an objective measure of omega-3 fatty acid levels and avoiding issues inherent in calculating PUFA intake through questionnaires and dietary recall. However, some limitations exist. Fatty acid data were available only at baseline, so our results do not reflect possible changes in plasma phospholipid fatty acids during follow-up. Some heterogeneity exists between the 2 studies suggesting that the results may not be interpretable when combined. However, heterogeneity was relatively low (<50) and isolated to a few quartiles. Finally, we had a limited number of incident PAD cases which could have hindered our ability to detect associations and impacted our ability to further explore potential interactions. This may be because of the statin treatment over time or because of MESA and ARIC being generally healthier than the average population.
In conclusion, the present study confirmed previous observational studies in the United States population showing no association between omega-3 PUFAs and incident clinical PAD. However, a nonsignificant decreased risk of PAD with higher levels of omega-3 PUFAs was observed. Considering the positive findings from previous Japanese and Danish populations coupled with the beneficial effects observed from REDUCE-IT, we speculate the relationship between omega-3 PUFAs and PAD may be dose and duration dependent. Thus, high dietary intake of omega-3 PUFAs or high-dose omega-3 PUFA supplementation over a long duration may have a protective affect against incident PAD. Our results indicate a need for large pooled analyses with higher power as well as studies determining if higher duration and/or dosage of omega-3 PUFAs supplementation can decrease risk for incident clinical PAD.
Author contributions
The authors’ responsibilities were as follows—NLW, WG, MYT: contributed to the conception or design of the work; NLW, SON: drafted the manuscript; and all authors: contributed to the acquisition, analysis, or interpretation of the data for the work, critically revised the manuscript, agree to be accountable for all aspects of the work ensuring integrity and accuracy, and read and approved the final manuscript.
Funding
This research was supported by MESA contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgments
We thank the other investigators, the staff, and the participants of the MESA and ARIC studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. A full list of participating ARIC investigators and institutions can be found at https://sites.cscc.unc.edu/aric/.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.11.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bhatt D.L., Steg P.G., Ohman E.M., Hirsch A.T., Ikeda Y., Mas J.-L., et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 2.Allison M.A., Armstrong D.G., Goodney P.P., Hamburg N.M., Kirksey L., Lancaster K.J., et al. Health disparities in peripheral artery disease: a scientific statement from the American heart association. Circulation. 2023;148(3):286–296. doi: 10.1161/CIR.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacoub P.P., Abola M.T.B., Baumgartner I., Bhatt D.L., Creager M.A., Liau C.-S., et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) registry. Atherosclerosis. 2009;204(2):e86–e92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan A., Vemulapalli S., Patel M.R., Jones W.S. Lower extremity amputation in peripheral artery disease: improving patient outcomes, Vasc. Health Risk Manag. 2014;10:417–424. doi: 10.2147/VHRM.S50588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korhonen P.E., Seppälä T., Kautiainen H., Järvenpää S., Aarnio P.T., Kivelä S.-L. Ankle-brachial index and health-related quality of life. Eur. J. Prev. Cardiol. 2012;19(5):901–907. doi: 10.1177/1741826711420346. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsen M.U., O'Reilly E.J., Heitmann B.L., Pereira M.A., Bälter K., Fraser G.E., et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009;89(5):1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott M.M., Mehta S., Ahn H., Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J. Gen. Intern. Med. 1997;12(4):209–215. doi: 10.1046/j.1525-1497.1997.012004209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi: 10.1186/1476-511X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farzaneh-Far R., Harris W.S., Garg S., Na B., Whooley M.A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: the heart and soul study. Atherosclerosis. 2009;205(2):538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajaram S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014;100(Suppl 1):443S–448S. doi: 10.3945/ajcn.113.071514. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira Otto M.C., Wu J.H.Y., Baylin A., Vaidya D., Rich S.S., Tsai M.Y., et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013;2(6) doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umemoto N., Ishii H., Kamoi D., Aoyama T., Sakakibara T., Takahashi H., et al. Reverse association of omega-3/omega-6 polyunsaturated fatty acids ratios with carotid atherosclerosis in patients on hemodialysis. Atherosclerosis. 2016;249:65–69. doi: 10.1016/j.atherosclerosis.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Venø S.K., Bork C.S., Jakobsen M.U., Lundbye-Christensen S., McLennan P.L., Bach F.W., et al. Marine n-3 polyunsaturated fatty acids and the risk of ischemic stroke. Stroke. 2019;50(2):274–282. doi: 10.1161/STROKEAHA.118.023384. [DOI] [PubMed] [Google Scholar]
- 14.Bild D.E., Bluemke D.A., Burke G.L., Detrano R., Diez Roux A.V., Folsom A.R., et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Wright J.D., Folsom A.R., Coresh J., Sharrett A.R., Couper D., Wagenknecht L.E., et al. The ARIC (Atherosclerosis Risk In Communities) study: JACC Focus Seminar 3/8. J. Am. Coll. Cardiol. 2021;77(23):2939–2959. doi: 10.1016/j.jacc.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J., Schwichtenberg K.A., Hanson N.Q., Tsai M.Y. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin. Chem. 2006;52(12):2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 17.Ma J., Folsom A.R., Shahar E., Eckfeldt J.H. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Clin. Nutr. 1995;62(3):564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 18.Garg P.K., Buzkova P., Meyghani Z., Budoff M.J., Lima J., Criqui M., et al. Valvular calcification and risk of peripheral artery disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Eur. Heart J. Cardiovasc. Imaging. 2020;21(10):1152–1159. doi: 10.1093/ehjci/jez284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks C.W., Ding N., Kwak L., Ballew S.H., Kalbaugh C.A., Folsom A.R., et al. Risk of peripheral artery disease according to race and sex: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2021;324:52–57. doi: 10.1016/j.atherosclerosis.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrinello C.M., Grams M.E., Couper D., Ballantyne C.M., Hoogeveen R.C., Eckfeldt J.H., et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin. Chem. 2015;61(7):938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rikhi R., Hammoud A., Ashburn N., Snavely A.C., D Michos E., Chevli P., et al. Relationship of low-density lipoprotein-cholesterol and lipoprotein(a) to cardiovascular risk: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2022;363:102–108. doi: 10.1016/j.atherosclerosis.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautam M., Izawa A., Shiba Y., Motoki H., Takeuchi T., Okada A., et al. Importance of fatty acid compositions in patients with peripheral arterial disease. PLOS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujihara M., Fukata M., Odashiro K., Maruyama T., Akashi K., Yokoi Y. Reduced plasma eicosapentaenoic acid-arachidonic acid ratio in peripheral artery disease. Angiology. 2013;64(2):112–118. doi: 10.1177/0003319712437031. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura T., Yoshikawa D., Ishii H., Suzuki S., Kumagai S., Inoue Y., et al. Relation of omega-3 fatty acid and C-reactive protein to peripheral artery disease in patients with coronary artery disease. Heart Vessels. 2014;29(4):449–455. doi: 10.1007/s00380-013-0384-4. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez J.L., Zahner G.J., Spaulding K.A., Khetani S.A., Hills N.K., Gasper W.J., et al. Peripheral artery disease is associated with a deficiency of erythrocyte membrane n-3 polyunsaturated fatty acids. Lipids. 2019;54(4):211–219. doi: 10.1002/lipd.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki T., Nishibe T., Ohya Y., Inoue S., Ogino H. Infrainguinal lesion of peripheral artery disease and levels of ω-3 polyunsaturated fatty acids in peripheral artery disease. Ann. Vasc. Dis. 2018;11(1):96–100. doi: 10.3400/avd.oa.17-00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasota A.N., Grønholdt M.-L.M., Bork C.S., Lundbye-Christensen S., Overvad K., Schmidt E.B. Marine n-3 fatty acids and the risk of peripheral arterial disease. J. Am. Coll. Cardiol. 2018;72(14):1576–1584. doi: 10.1016/j.jacc.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Papanikolaou Y., Brooks J., Reider C., Fulgoni V.L., III U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014;13:31. doi: 10.1186/1475-2891-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grenon S.M., Owens C.D., Alley H., Chong K., Yen P.K., Harris W., et al. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vasc. Med. 2013;18(5):263–274. doi: 10.1177/1358863X13503695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grenon S.M., Owens C.D., Nosova E.V., Hughes-Fulford M., Alley H.F., Chong K., et al. Short-term, high-dose fish oil supplementation increases the production of omega-3 fatty acid-derived mediators in patients with peripheral artery disease (the OMEGA-PAD I Trial) J. Am. Heart Assoc. 2015;4(8) doi: 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller M.S., Zahner G.J., Gasper W.J., Harris W.S., Conte M.S., Hills N.K., et al. Relationship between the omega-3 index and specialized pro-resolving lipid mediators in patients with peripheral arterial disease taking fish oil supplements. J. Clin. Lipidol. 2017;11(5):1289–1295. doi: 10.1016/j.jacl.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez J.L., Gasper W.J., Khetani S.A., Zahner G.J., Hills N.K., Mitchell P.T., et al. Fish oil increases specialized pro-resolving lipid mediators in PAD (The OMEGA-PAD II trial) J. Surg. Res. 2019;238:164–174. doi: 10.1016/j.jss.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer A., Moert D., Schlager O., Matschuck M., Seidinger D., Koppensteiner R., et al. Effects of n-3 PUFA on endothelial function in patients with peripheral arterial disease: a randomised, placebo-controlled, double-blind trial. Br. J. Nutr. 2019;122(6):698–706. doi: 10.1017/S0007114519001582. [DOI] [PubMed] [Google Scholar]
- 34.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernasconi A.A., Wiest M.M., Lavie C.J., Milani R.V., Laukkanen J.A. Effect of omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and meta-regression of interventional trials. Mayo Clin. Proc. 2021;96(2):304–313. doi: 10.1016/j.mayocp.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380(1):23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt D.L., Gabriel Steg P., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls S.J., Michael Lincoff A., Garcia M., Bash D., Ballantyne C.M., Barter P.J., et al. Effect of high-dose omega-3 fatty acids vs. corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.