Abstract
Introduction
Persistent inflammation, immunosuppression, and catabolism syndrome (PICS) has been proposed as an endotype of chronic critical illness (CCI). The aim of this systematic review is to synthesise the available evidence of risk factors, biomarkers, and biological mechanisms underlying PICS.
Methods
MEDLINE, CENTRAL, and EMBASE were searched on June 2, 2023. Our population of interest was adult intensive care unit survivors. The exposure group was patients with PICS and the comparator group was patients with no PICS, CCI, or rapid recovery. Mean differences were pooled for each biomarker using a random effects DerSimonian–Laird method. Risk of bias assessment was done using the Newcastle–Ottawa Scale.
Results
Six papers were included. Five were single-centre retrospective cohort studies, and one was a prospective cohort study, with sample sizes ranging from 22 to 391 patients. Two studies showed an increased incidence of PICS with age, and two studies showed an association between PICS and Charlson Comorbidity Index scores. PICS was associated with requiring mechanical ventilation in four studies. Meta-analysis showed a 34.4 mg L−1 higher C-reactive protein (95% confidence interval [CI] 12.7–56.2 mg L−1; P<0.01), a 4.4 g L−1 lower albumin (95% CI 0.5–8.3 g L−1; P<0.01), and a 0.36×109 L−1 lower lymphocyte count (95% CI 0.25–0.47×109 L−1; P=0.01) in the PICS compared with the non-PICS group. There are a large variety of other potential biomarkers but limited validation studies. The overall quality of evidence is limited, and these results should be interpreted accordingly.
Conclusions
While older patients and those with co-morbidities could be at greater risk for PICS, acquired risk factors, such as injury severity, are potentially more predictive of PICS than intrinsic patient characteristics. There are many potential biomarkers for PICS, but limited validation studies have been conducted. Persistent myeloid-derived suppressor cell expansion, the continual release of danger-associated molecular patterns and pathogen-associated molecular patterns propagating inflammation, and bioenergetic failure are all mechanisms underlying PICS that could offer potential for novel biomarkers and therapeutic interventions.
Clinical trial registration
International Prospective Register of Systematic Reviews (PROSPERO; CRD42023427749).
Keywords: catabolism, chronic critical illness, immunosuppression, intensive care, persistent inflammation, PICS, post-intensive care syndrome
Editor's key points.
-
•
Persistent inflammation, immunosuppression, and catabolism syndrome (PICS) has been proposed as an endotype of chronic critical illness (CCI).
-
•
This systematic review and meta-analysis identified a large variety of potential biomarkers but limited validation studies, and the overall quality of evidence was limited.
-
•
While older patients and those with co-morbidities could be at greater risk for PICS, acquired risk factors, such as injury severity, are potentially more predictive of PICS than intrinsic patient characteristics.
-
•
Further studies are necessary to identify and validate potential biomarkers for PICS that will enhance diagnosis and therapeutic interventions.
About 7.6% of critically ill patients develop chronic critical illness (CCI),1 defined as an intensive care unit (ICU) length of stay (LOS) of at least 14 days with evidence of persistent organ dysfunction,2 and 30–50% of these patients show evidence of persistent inflammation, immunosuppression, and catabolism syndrome (PICS).3 PICS consists of a self-perpetuating cycle of organ failure, inflammation, immunosuppression leading to recurrent infections, metabolic derangement, and muscle wasting.4 The syndrome offers a mechanistic paradigm for this clinical phenotype that can be observed after a wide range of critical illnesses.5,6 These patients commonly require long-term rehabilitation and frequent readmissions, and ultimately suffer an indolent death.7
There are many overlapping clinical and pathological features between CCI and PICS, although the causal relationship is unclear, with at least three possibilities.8 Firstly, PICS represents a pathophysiological mechanism of CCI,6 implying an onset before CCI. Secondly, PICS is a consequence of ongoing organ dysfunction in CCI,7 implying PICS does not occur without CCI. Thirdly, PICS is an endotype of CCI,9 implying other endotypes exist (Fig. 1). Understanding the pathophysiological basis of PICS is necessary to develop targeted treatment. Understanding risk factors and validation of diagnostic biomarkers will allow early identification and either prevention or prompt initiation of treatment.
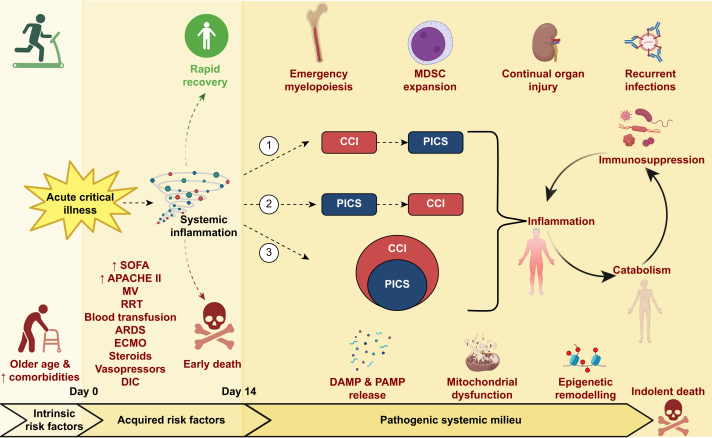
Fig 1.
Summary diagram of the risk factors and pathophysiological mechanisms for PICS. An acute critical illness can lead to rapid recovery, early death, or CCI/PICS. Intrinsic risk factors before day 0 and acquired risk factors between days 0 and 14 for the development of CCI/PICS are shown. The three possible causal relationships between CCI and PICS are also shown. 1) PICS could be a consequence of ongoing organ dysfunction in CCI. 2) PICS could be a pathophysiological mechanism of CCI. 3) PICS could be an endotype of CCI. Finally, the diagram shows the pathogenic systemic milieux that have been linked to PICS and contribute to the self-perpetuating cycle of inflammation, immunosuppression, and catabolism, that can lead to indolent death. APACHE II, acute physiologic and chronic health evaluation; ARDS, acute respiratory distress syndrome; CCI, chronic critical illness; DAMP, danger-associated molecular pattern; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; MDSC, myeloid-derived suppressor cell; MV, mechanical ventilation; PAMP, pathogen-associated molecular pattern; PICS, persistent inflammation, immunosuppression, and catabolism syndrome; RRT, renal replacement therapy; SOFA, Sequential Organ Failure Assessment. Created with BioRender.com.
The aim of this systematic review is to present the available evidence of potential risk factors, biomarkers, and biological mechanisms underlying PICS. We focus on PICS rather than the broader syndromes of CCI, persistent critical illness, or post-intensive care syndrome as PICS potentially offers a mechanistic paradigm and comprises a more defined group of patients who are likely to respond to similar targeted interventions.
Methods
This systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) a priori (CRD42023427749) on May 19, 2023. It is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines (Supplementary File 1).10,11
Search strategy
We used the following bibliographic databases: MEDLINE (via Ovid), CENTRAL, and EMBASE (via Ovid). The search was done on June 2, 2023 and the full search strategies for each database can be found in Supplementary File 2.
Inclusion and exclusion criteria
All relevant original research was included. There was no limit on publication dates. Unpublished studies, preprints, conference abstracts without subsequent study publication, studies not in English, review articles, editorials, and case reports were excluded. We excluded studies on paediatric patients (<16 yr old) and animal models. We used the original definition of PICS as ICU stay ≥10 days or prolonged hospitalisation >14 days, C-reactive protein (CRP) >1.5 mg L−1, total lymphocyte count <0.80×109 L−1, and serum albumin <3.0 g dl−1, creatinine height index <80%, weight loss >10%, BMI <18 kg m−2 during hospital admission, or retinol binding protein <10 μg dl−1.6 However, to ensure all potentially relevant studies were included, we opted for a lower CRP threshold of >0.5 mg L−1 as proposed.9 The comparator/control group included patients with CCI, rapid recovery, or no evidence of PICS. The outcomes of interest were broad and included any possible risk factors, biomarkers, or biological factors for PICS. We anticipated this might have included, but not been limited to patient baseline characteristics, interventions received during ICU admission, vital signs, blood results, imaging, muscle biopsy results, and multi-omic data.
Selection and data extraction
Duplications were removed and two authors (KRC, EEB) independently screened all records (title and abstract) for inclusion/exclusion against the prespecified criteria above. Full text reports were retrieved for the included studies and the same two authors independently screened the texts, documenting reasons for any exclusions. Any disagreement was resolved through involvement of a third author (ZP). Data were extracted from the final included papers by one author (KRC) using Microsoft Excel™ (Microsoft Corp., Redmond, WA, USA) and checked by a second author (EEB). The extracted data included study details, patient characteristics, potential risk factors, blood results and biomarkers, and details on possible mechanisms of PICS, including biopsy or multi-omic data if present.
Data synthesis
Biomarkers reported in different units across the included studies were transformed to the most frequently reported unit for each biomarker (CRP mg L−1, albumin g L−1, lymphocyte count ×109 L−1). In studies with a sample size >25, median values reported with inter-quartile ranges were converted to mean values and standard deviation using the Box–Cox method.12 Mean differences were pooled for each biomarker using a random effects DerSimonian–Laird method. Between-study heterogeneity was evaluated using the I2 test; we considered heterogeneity as I2 >50%. Forest plots were generated for study-specific effect sizes along with 95% confidence intervals (CIs) and pooled effects. A P-value ≤0.05 was considered statistically significant. Meta-analysis of data was performed using the statistical software package Review Manager 5.4 (RevMan 5.4.1).
Quality and risk of bias assessment
Two authors (KRC, EEB) carried out a quality assessment using the GRADE tool and a risk of bias assessment using the Newcastle–Ottawa Scale for cohort studies.13 The risk of bias as a result of missing results was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreement was resolved through discussion and consensus.
Results
Search results
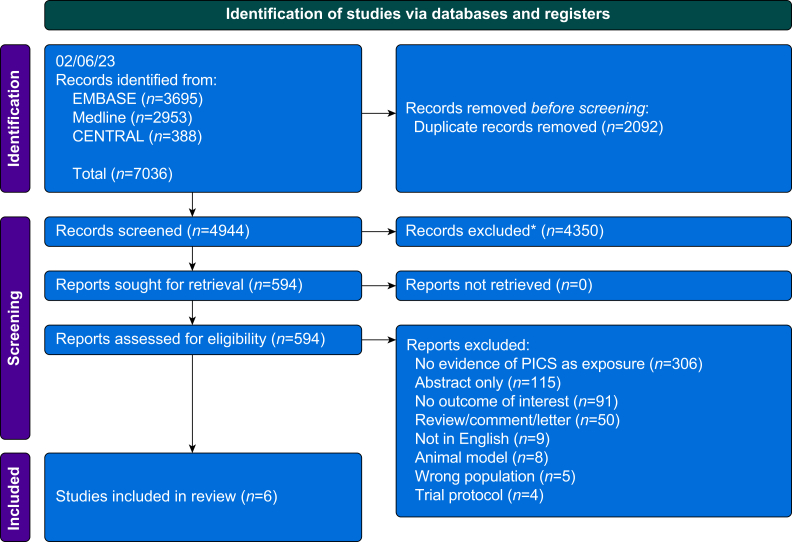
We identified 7036 articles across three databases (Fig. 2). After removal of duplicates (n=2092), 4944 records were screened, of which 4350 were excluded as not relevant. The remaining 594 papers were compared with the inclusion and exclusion criteria, of which six papers met the inclusion criteria.8,14, 15, 16, 17, 18 The study details are summarised in Table 1. Five out of the six are single-centre retrospective cohort studies, and the remaining one is a prospective cohort study.8 Four of the studies were based in China,8,15,16,18 one in Japan,17 and one in the Netherlands.14
Fig 2.
PRISMA flow diagram of the included and excluded articles from this systematic review. PICS, persistent inflammation, immunosuppression, and catabolism syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
A summary of the main study details for the included papers. CCI, chronic critical illness; CKD, chronic kidney disease; ECF, enterocutaneous fistula; ICU, intensive care unit; PICS, persistent inflammation, immunosuppression, and catabolism syndrome; SLE, systemic lupus erythematosus.
| Reference | Year | Setting | Study type | Aims | Population | Exposure | Exposure n | Control | Control n | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hesselink and colleagues14 | 2020 | University Medical Center Utrecht, Netherlands | Single-centre retrospective cohort | Investigate incidence of PICS after trauma, determine the clinical course, and test postulated markers to identify PICS patients | Trauma patients ≥14 days in ICU | Clinical PICS or PICS markers positive | 22 | CCI with no PICS | 78 | 1. ≥16 yr old 2. ICU stay ≥14 days |
1. ICU admission for other reasons than critical illness 2. Isolated neurotrauma |
| Hu and colleagues15 | 2014 | Jinling Hospital, Nanjing University, China | Single-centre retrospective cohort | Examine the prevalence and characteristics of PICS and its impact on the outcome of ECF patients | Patients with ECF admitted to ICU | ECF+PICS | 53 | ECF+no PICS | 70 | 1. Patients with ECF admitted to ICU | 1. Terminal malignant tumour 2. Transferred to another hospital |
| Li and colleagues16 | 2023 | Jinling Hospital, Nanjing University, China | Single-centre retrospective cohort | Analyse the clinical characteristics and prognosis of SLE complicated with PICS, and the risk factors affecting prognosis | SLE patients on ICU >14 days | SLE+PICS | 61 | SLE+no PICS | 35 | 1. SLE 2. ICU LOS >14 days |
1. Age <18 or >70 yr 2. Regular dialysis started in CKD5 3. Loss to follow-up |
| Suzuki and colleagues17 | 2022 | Toho University Omori Medical Center, Japan | Single-centre retrospective cohort | Investigate the relationship between blood transfusions and PICS | ICU patients who survived 14 days or more of admission | PICS | 391 | No PICS | 762 | 1. Tertiary emergency patients admitted to the ICU | 1. Age <18 yr 2. Death within 14 days of admission to the ICU. 3. Acute deterioration in hospital 4. Scheduled to undergo surgery |
| Zhong and colleagues18 | 2021 | Ruijin Hospital, China | Single-centre retrospective cohort | Find the predictors for PICS in ICU surgical septic patients | Surgical ICU patients | PICS | 47 | No PICS | 122 | 1. ≥18 yr old 2. Admitted to ICU after major surgery or within 48 h after the diagnosis of sepsis |
1. Pregnant or lactating patients 2. Severe immune deficiency 3. Death within 14 days of sepsis diagnosis |
| Zhou and colleagues8 | 2023 | Suzhou Municipal Hospital and Suzhou Ninth People's Hospital, China | Prospective cohort | Investigate and compare the clinical features and prognosis of CCI/PICS | ICU patients with LOS ≥14 days | PICS, CCI+PICS |
17 50 |
CCI only, No CCI or PICS |
71, 30 |
1. ≥18 yr old 2. ICU LOS ≥14 days |
1. ICU LOS <14 days 2. Long-term use of immunosuppressants, corticosteroids, or both 3. Congenital immunodeficiency, acquired immunodeficiency, or both 4. Pregnancy 5. No consent |
The populations between studies differed, including trauma, surgical, emergency, and general medical patients. Two of the study populations had specific conditions of systemic lupus erythematosus (SLE)16 and an enterocutaneous fistula (ECF).15 The sample sizes ranged from 22 to 391 patients. The control groups differed and two studies had CCI patients as a control,8,14 whereas the other four were based on patients without PICS, who might not have met criteria for CCI.15, 16, 17, 18 The prospective cohort study differed in that it compared four groups of patients: PICS only, PICS + CCI, CCI only, and no PICS or CCI.8 There were differences in the PICS definition used but they all met the prespecified definition.6,9 Hesselink and colleagues14 provided a clinical and biomarker definition for PICS and we used results from their biomarker positive cohort as this matched our inclusion criteria.
Risk factors
Patient characteristics
All six studies reported age: two demonstrating associations with an increased incidence of PICS,15,17 and four did not.8,14,16,18 All six studies reported no association between sex and PICS.8,14, 15, 16, 17, 18 Two out of six studies reported BMI results: one reporting a lower BMI as a risk factor for PICS (17.7 [0.21] vs 18.4 [0.15], P<0.01),15 whereas the other did not (22.2 [19.5–26.2] vs 23.0 [20.2–25.0], P=0.99).18 Five out of six studies reported APACHE II (acute physiologic and chronic health evaluation) scores. Two studies reported an association between APACHE II scores and PICS,17,18 and two found no differences between groups.8,16 Hu and colleagues15 reported higher APACHE II scores in the PICS compared with the non-PICS group (11.3 [0.87] vs 8.14 [0.73], P<0.01) but gave different values for the non-PICS group in their table (9.20 [0.72]) with a non-significant P-value of 0.08.15 Two out of six studies reported Charlson Comorbidity Index scores to be associated with PICS.17,18 Two out of six studies reported SOFA (Sequential Organ Failure Assessment) scores to be associated with PICS.8,18
One study reported PICS to be associated with acute respiratory distress syndrome (ARDS) (34.3% vs 8.6%, P<0.01).16 PICS is associated with use of steroids (13.9% vs 4.8%, P<0.01), immunosuppressants (1.3% vs 0.1%, P=0.02), vasopressors (53.2% vs 13.2%, P<0.01), extracorporeal membrane oxygenation (ECMO) (6.1% vs 0.4%, P<0.01), and shock on day 14 (39.4% vs 10.4%, P<0.01).17
Complications
Five out of six studies reported PICS to be associated with longer ICU LOS,8,14, 15, 16,18 but not hospital LOS (three studies).14,15,18 PICS was associated with a higher incidence of sepsis in three studies.14,16,17 Another study showed no difference between PICS and non-PICS for early sepsis (75.5% vs 60.0%, P=0.08) but a higher incidence of late sepsis (after day 7 of admission).15 One study reported a higher incidence of ICU acquired infection with PICS,18 whereas another found no differences in secondary infections between groups.8 Two studies showed that PICS was associated with pneumonia,14,15 one with catheter-related bloodstream infections,15 and one showed no difference between infection sites.16
PICS was associated with requiring mechanical ventilation in four studies.14, 15, 16, 17 Three out of six studies reported conflicting incidences of renal replacement therapy (RRT): two studies demonstrated an association,14,17 whereas one study on patients with SLE did not.16 Similar conflicting relationships are seen with reported surgical procedures in two studies, one demonstrating a higher association17 and one not.14 Infusion of blood products, specifically red blood cells, plasma, and platelets, was shown to be associated with PICS.17 No association between PICS and instigation of a massive transfusion protocol was seen in another study.14
Blood results and biomarkers
Pooled analysis
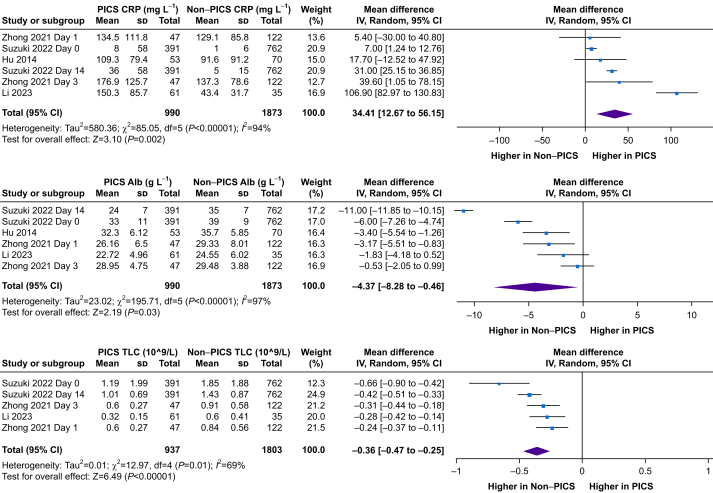
Meta-analysis of pooled studies showed a 34.4 mg L−1 higher CRP (n=2863; 95% CI 12.7–56.2 mg L−1; P<0.01), a 4.4 g L−1 lower albumin (n=2863, 95% CI 0.5–8.3 g L−1; P<0.01), and a 0.36×109 L−1 lower lymphocyte count (n=2810; 95% CI 0.25–0.47 ×109 L−1; P=0.01) in the PICS compared with the non-PICS group (Fig. 3).
Fig 3.
Forest plots of studies reporting biomarker outcomes demonstrated as a weighted mean difference using frequentist meta-analysis. Alb, albumin; CI, confidence interval; CRP, C-reactive protein; df, degrees of freedom; PICS, persistent inflammation, immunosuppression, and catabolism syndrome; sd, standard deviation; TLC, total lymphocyte count.
Individual studies
One study differentiated patients who met PICS criteria by biomarkers, and those with clinical evidence. Out of the 22 patients in the PICS marker positive group, eight had clinical evidence of PICS, giving a sensitivity and specificity of these PICS markers (lymphocytes, CRP, and albumin) as 44% and 77%, respectively.14 No difference in lymphocyte or CRP levels was seen between groups.14 Two studies showed no difference in CRP levels between groups,15,18 in contrast to two other studies showing a higher CRP with PICS.16,17 One other study reported higher CRP levels in the PICS and CCI group (67.7 mg L−1 [32.9–138.8 mg L−1]) compared with the PICS only group (14.1 mg L−1 [5.6–78.4 mg L−1]) and the non-PICS and non-CCI group (21.4 mg L−1 [9.7–59.1 mg L−1]) on day 21 but no differences on days 1 and 14.8
Reduced total lymphocyte counts and levels of CD3, CD4, CD8, and CD20 were associated with PICS.16 PICS was associated with lower total lymphocyte counts on admission, day 3, day 14, and day 21.8,17,18 In a univariate logistic regression analysis, there were differences between PICS and non-PICS in CRP on day 14, lymphocyte and albumin levels on days 1 and 14, and neutrophil, albumin, and CRP levels on day 21, but in multivariate analyses, lymphocyte levels were not significant.8 The only included study to report the neutrophil:lymphocyte ratio (NLR) found it was higher on day 3 in PICS compared with non-PICS (16.9 [10.3–26.4] vs 10.5 [7.2–17.9], p<0.01) but not on day 1.18 Li and colleagues16 reported no difference in albumin between groups.16 No difference in albumin between groups was seen on day 18 or day 3.18 However, four other studies showed an association between PICS and low albumin levels,15 on admission,17,18 and days 14 and 21.8
PICS was associated with raised inflammatory markers, namely procalcitonin (2.19 μg L−1 [0.58–9.18 μg L−1] vs 0.68 μg L−1 [0.26–1.97 μg L−1], P<0.01) and interleukin-6 (IL-6) (224 ng L−1 [64.2–618 ng L−1] vs 39.1 ng L−1 [22.7–84.6 ng L−1], P<0.01).16 Lower haemoglobin level and platelet count were associated with PICS, and lower urea and creatinine.16 Another study similarly showed a reduced haemoglobin level with PICS, but higher creatinine levels.17 The same study showed further associations of PICS with higher lactate levels and disseminated intravascular coagulation.17
Risk of bias assessment
Using the Newcastle–Ottawa Scale, three studies were at moderate risk of bias14, 15, 16 and three studies as low risk8,17,18 (Supplementary File 3). Using the GRADE assessment tool, the included studies were deemed very low quality for the outcomes of risk factors and biomarkers for PICS (Supplementary File 4). Our pooled analysis of lymphocyte count is missing data from Hu and colleagues15 because of unavailable results. The missing data are unlikely to be because of the magnitude or direction of the results because lymphocyte count was not used as a specific outcome in the study, however selective non-reporting of results cannot be ruled out indicating a high risk of bias. Funnel plots were not used because of the low number of studies.
Discussion
Persistent inflammation, immunosuppression, and catabolism syndrome is seen after a wide range of critical illnesses and accounts for significant morbidity and mortality.5,6 As its components are potentially reversible, it is a classification system that potentially offers mechanistic insight for targeted intervention. However, the definitions for PICS vary in the literature.19 Despite PICS being first described >10 yr ago, this systematic review has highlighted the lack of studies comparing this specific cohort of patients with early death, rapid recovery, or CCI cohorts. Furthermore, there are no randomised controlled trials targeting patients with PICS specifically.
Risk factors
The included studies show conflicting results for intrinsic risk factors for PICS, such as age. Other studies have shown that older patients are more likely to develop CCI.20, 21, 22 Furthermore, older patients had more persistent abnormalities in PICS biomarkers 14 days post-sepsis.23 The acquired risk factors, such as type and severity of injury, are potentially more important in predicting PICS, suggesting that PICS is more than a manifestation of frailty and previous co-morbidities. This is supported by an analysis on 102 patients with haemorrhagic shock using k-means clustering, which identified a hyperinflammatory endotype associated with the highest incidence of CCI, infections, and LOS, and two rapid recovery endotypes. There were no differences in baseline characteristics, such as age, sex, co-morbidities, and BMI, between the three endotypes but the hyperinflammatory endotype was associated with a higher injury severity and blood transfusion requirements.24 This is supported by some of the included studies showing PICS is associated with higher APACHE II scores,17,18 SOFA scores,8,18 ARDS,16 mechanical ventilation,14, 15, 16, 17 RRT,14,17 blood transfusions,17 ECMO,17 and use of steroids, immunosuppressants, and vasopressors17 (Fig. 1). Reliable biomarkers could be a more objective way of accurately predicting risk and making early diagnosis.
Blood tests and biomarkers
C-reactive protein, lymphocyte, and albumin levels are part of the diagnostic criteria for PICS. Our meta-analysis confirmed the expected differences in these biomarkers between PICS and non-PICS, albeit with high levels of heterogeneity. Conflicting results among the individual studies might be as a result of differences in the timing of biomarker measurement, which requires standardisation, or because of the nonspecific nature of these biomarkers.14 Trajectories, as opposed to single absolute values, might be helpful in diagnosing PICS. Group-based trajectory modelling noted a persistent lymphopaenia endotype to have the highest incidence of PICS.25
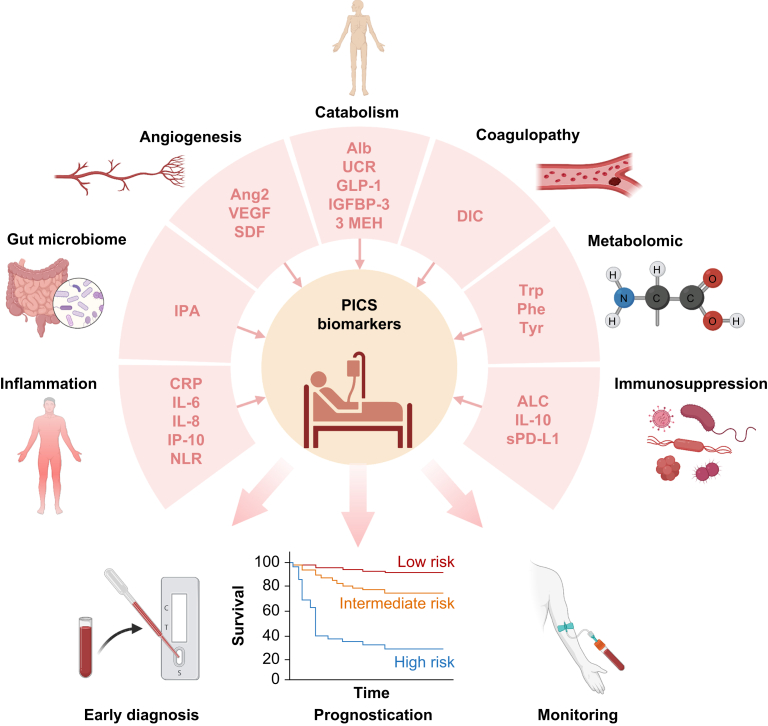
There is a growing interest in studying other biomarkers for PICS (Fig. 4). CCI patients have higher biomarker levels of inflammation (IL-6, IL-8, interferon gamma-induced protein [IP-10], monocyte chemoattractant protein 1), immunosuppression (IL-10, soluble programmed death ligand-1), stress metabolism (glucagon-like peptide 1 [GLP-1]), catabolism markers (plasma insulin-like growth factor binding protein 3 and urinary 3-methylhistidine excretion) and angiogenesis (angiopoietin-2, vascular endothelial growth factor [VEGF], VEGF receptor-1, stromal cell-derived factor).26,27 Both the NLR and monocyte:lymphocyte ratio reflect immune activation and inflammation in critical illness and correlate with clinical severity.28, 29, 30
Fig 4.
Summary diagram of potential biomarkers for PICS. This illustration highlights a range of biomarkers, organised by pathophysiological mechanism, and their potential utility in the early diagnosis, prognostication, and monitoring of PICS. 3 MEH, urinary 3-methylhistidine; Alb, albumin; ALC, absolute lymphocyte count; Ang2, angiopoietin-2; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; GLP-1, glucagon-like peptide-1; IGFBP-3, insulin-like growth factor binding protein 3; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IP-10, interferon gamma-induced protein; IPA, indole-3-propionate; NLR, neutrophil:lymphocyte ratio; Phe, phenylalanine; PICS, persistent inflammation, immunosuppression, and catabolism syndrome; SDF, stromal cell-derived factor; sPD-L1, soluble programmed death ligand-1; Trp, tryptophan; Tyr, tyrosine; UCR, urea: creatinine ratio; VEGF, vascular endothelial growth factor. Created with BioRender.com.
Most, but not all, included studies showed PICS is associated with low albumin levels. Other more specific biomarkers of catabolism have been studied. For example, elevation of GLP-1 at 24 h is an independent predictor for development of CCI. GLP-1 remains high at day 21 in sepsis survivors with CCI, suggesting it might be a biomarker for the non-resolving catabolic state that occurs in PICS.31 An elevated urea:creatinine ratio (UCR) occurs with muscle wasting and provides a biochemical signature for PICS.32 The UCR was significantly increased in persistent critical illness (PerCI) compared with the control group.33 The UCR also performed better at discriminating catabolism than albumin levels.34
Anaemia and coagulopathy have both been implicated in CCI and PICS in animal and human studies.35, 36, 37 Further research is needed to establish the role that coagulopathy and microthrombi formation could play in the clinical trajectory of PICS. None of the included studies looked at lipid levels as potential biomarkers for PICS, but several studies have for CCI. One such study revealed a hypolipoprotein cluster, characterised by lower lipoprotein levels, higher SOFA scores, increased endothelial dysfunction (intercellular adhesion molecule-1 [ICAM-1]), and a higher incidence of CCI.38 Another study demonstrated an early loss of circulating lipids followed by a delayed and selective increase in predominately diacylglycerols (DAG), triglycerides (TAG), and phosphatidylethanolamines (PE) in patients with PerCI.39 The decline in circulating lipids could reflect the uptake and catabolism of lipids to meet the energy requirements of critical illness. The increase of DAG, TAG, and PE could reflect enhanced lipogenesis and ongoing systemic inflammation and metabolic stress.39 Lipid levels or other metabolic markers could therefore be potential biomarkers in PICS.
Pathophysiology
There is a limited number of biomarker studies on patients with PICS. Developing our understanding of the pathophysiology of PICS will help inform future work on potential novel, disease-specific biomarkers relating to immunosuppression, inflammation, or catabolism.
Immunosuppression
Emergency myelopoiesis in response to tissue injury results in rapid release of mature myeloid populations leading to store depletion and release of immature populations.40 In a murine model, 7 days post-sepsis, 90% of the bone marrow were myeloid cells, mostly immature and functionally immunosuppressive.41 The increased myeloid cell production is favoured at the expense of lymphopoiesis and erythropoiesis, leading to immunosuppression and anaemia, respectively.42 Emergency myelopoiesis leads to expansion of a heterogenous group of inducible immature myeloid cells, named myeloid-derived suppressor cells (MDSCs).43, 44, 45 MDSCs have a role in protecting host innate immunity46 and mediating inflammation through production of reactive oxygen species (ROS), nitric oxide, tumour necrosis factor α, regulated on activation, normal T-cell expressed and secreted (RANTES), and macrophage inflammatory protein 1β (MIP-1β).41,47 MDSCs have been shown to become more immunosuppressive with time.42,48
After sepsis, MDSCs remain elevated for 6 weeks, and suppress T-cell proliferation and IL-2 production after 2 weeks.49 Circulating MDSCs post-sepsis are associated with longer ICU LOS, secondary infections, and poor functional outcomes.42,50,51 MDSC expansion persisted for 28 days in septic patients, with inhibited T cell proliferation in vitro and reduced TH1 and TH2 cytokine release.42 Emergency myelopoiesis is impaired in the older population.3 Aged mice have a higher mortality from secondary infection after failure of bone marrow progenitors to initiate and complete an emergency myelopoietic response, rather than from an exaggerated inflammatory insult.52 Older patients are also less able to resolve their initial inflammatory response.52 Unresolved inflammation leads to continued pathological expansion and activation of MDSCs, which further trigger inflammation and immunosuppression.5
Inflammation
Elevation of inflammatory markers can persist for at least 28 days after sepsis.53 Alarmins are likely to mediate persistent inflammation in PICS. Pattern-recognition receptors (PRRs) bind to alarmins from two sources.5 Exogenous pathogen-associated molecular patterns (PAMPs) arise from reactivation of latent viral infections or nosocomial infections. PAMPs trigger dendritic cell maturation for antigen processing, MHC expression, and migration to lymph nodes to stimulate T cells.54 Secondly, endogenous alarmins from damaged cells release danger-associated molecular patterns (DAMPs),55 such as nuclear DNA, high mobility group box 1 (HMGB1), and S100, which are raised in septic patients throughout their admission56, 57, 58 and contribute to persisting low-grade inflammation in PICS.9 The inflammation results in oxidative stress and subsequent mitochondrial dysfunction and bioenergetic failure that further aggravates the inflammatory pathways. This leads to a self-perpetuating cycle of inflammation and organ injury.5,59
Catabolism
Patients with CCI and PICS experience prolonged muscle wasting and cachexia, resulting from a disruption of the balance between muscle protein synthesis and breakdown.5 Muscle loss can reach up to 2–3% per day and is most rapid in the acute phase, but, in prolonged critical illness this catabolic state continues for up to 30 days.60, 61, 62 Muscle biopsies in critically ill patients show intramuscular inflammation and myocyte necrosis with both neutrophil and macrophage infiltration.61 Systemic inflammation acts as an anti-anabolic signal, decreasing muscle protein synthesis, and increasing insulin resistance, leading to impaired glucose utilisation and muscle atrophy.62 Mitochondrial dysfunction reduced mitochondrial numbers and density, and mitochondrial swelling has been reported in critical illness.63,64 The resultant bioenergetic crisis leads to myocyte necrosis and the intramuscular inflammation observed in biopsies.62 This is supported by a study on ventilated patients with ICU-acquired weakness showing ∼50% reduction of aerobic ATP synthesis in skeletal muscle compared with controls, linked to depletion of mitochondrial complexes III and IV.64
Practical implications
Given our ageing population and the increased provision of critical care services, patients with PICS are a growing cohort. This review has several practical implications. For academics, the low number of included studies has highlighted the need for a standardised population and definitions in order to conduct further clinical studies. For clinicians, it has summarised our current understanding of risk factors and biomarkers of PICS that have potential to better risk stratify patients, prognosticate, and diagnose patients at earlier stages. Furthermore, the pathophysiological summary of PICS demonstrates the potential therapeutic targets for intervention. For policymakers, the absolute pooled values can be used in future work to help define precise, evidence-based cut-off values for PICS that will standardise data collection. This can aid with assessing the prevalence and incidence of the condition and allocating appropriate resources to help survivors of critical illness with PICS to recover.
Limitations
There are only a small number of low-quality studies comparing patients with PICS against CCI or rapid recovery patients. The risk of bias in the included studies was low to moderate and there was a possibility of selective non-reporting of data limiting the ability to draw conclusions from our evidence synthesis. Furthermore, the six included studies used varying definitions of PICS and two of the studies were on specific cohorts of patients. Meta-analysis of biomarkers was performed but required transformation of data possibly introducing uncertainty in the data. Four of the studies were in China, which could limit the external validity of the results when considering other healthcare settings. Five of the studies were single centre, adding to this issue. All the studies were observational, limiting the ability to make any casual inferences from the data. Another challenge is the lack of pre-admission data on this cohort of patients, as it is difficult to establish whether PICS is induced by the critical illness insult or a manifestation of frailty and other co-morbidities. Future research could consider studying surgical patients admitted to ICU as these patients will likely have the relevant preadmission data for comparison as part of their perioperative assessment. The limited available studies on PICS means that the results of this systematic review should be interpreted with appropriate circumspection.
Conclusions
Although older patients and those with co-morbidities may be at greater risk for persistent inflammation, immunosuppression, and catabolism syndrome (PICS), acquired risk factors, such as injury severity, are potentially more predictive of PICS than intrinsic patient characteristics. There are many potential biomarkers for PICS, but limited validation studies have been conducted. Persistent myeloid-derived suppressor cell expansion, the continual release of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) propagating inflammation, and bioenergetic failure are all significant mechanisms underlying PICS that could offer potential for novel biomarkers and therapeutic interventions.
Authors' contributions
Conception: KRC, ZP
Supervision: ZP
Data extraction: KRC, EEB
Data analysis and interpretation: all authors
Drafting the original manuscript: KRC
Revising subsequent drafts: all authors
Approved the final version of the article to be published: all authors
Declaration of interest
ZP has received honoraria for consultancy from Nestlé, Nutricia, Faraday Pharmaceuticals, and Fresenius-Kabi and speaker fees from Baxter, Fresenius-Kabi, Nutricia, and Nestlé. The other authors declare that they have no conflicts of interest.
Handling Editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2024.03.038.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kahn J.M., Le T., Angus D.C., et al. The epidemiology of chronic critical illness in the United States. Crit Care Med. 2015;43:282–287. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loftus T.J., Mira J.C., Ozrazgat-Baslanti T., et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mira J.C., Brakenridge S.C., Moldawer L.L., Moore F.A. Persistent inflammation, immunosuppression and catabolism syndrome. Crit Care Clin. 2017;33:245–258. doi: 10.1016/j.ccc.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins R.B., Raymond S.L., Stortz J.A., et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Immunol. 2018;9:1511. doi: 10.3389/fimmu.2018.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darden D.B., Kelly L.S., Fenner B.P., Moldawer L.L., Mohr A.M., Efron P.A. Dysregulated immunity and immunotherapy after sepsis. J Clin Med. 2021;10:1742. doi: 10.3390/jcm10081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentile L.F., Cuenca A.G., Efron P.A., et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. The journal of trauma and acute care surgery. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal M.D., Kamel A.Y., Rosenthal C.M., Brakenridge S., Croft C.A., Moore F.A. Chronic critical illness: application of what we know. Nutr Clin Pract. 2018;33:39–45. doi: 10.1002/ncp.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q., Qian H., Yang A., Lu J., Liu J. Clinical and prognostic features of chronic critical illness/persistent inflammation immunosuppression and catabolism patients: a prospective observational clinical study. Shock. 2023;59:5–11. doi: 10.1097/SHK.0000000000002035. [DOI] [PubMed] [Google Scholar]
- 9.Mira J.C., Gentile L.F., Mathias B.J., et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath S., Zhao X., Steele R., Thombs B.D., Benedetti A., Collaboration D.E.S.D. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–2537. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G.A., Shea B., O’Connell D., et al. 3rd symposium on systematic reviews: beyond the basics. 3-5 July 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford, UK. [Google Scholar]
- 14.Hesselink L., Hoepelman R.J., Spijkerman R., et al. Persistent inflammation, immunosuppression and catabolism syndrome (PICS) after polytrauma: a rare syndrome with major consequences. J Clin Med. 2020;9:191. doi: 10.3390/jcm9010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu D., Ren J., Wang G., et al. Persistent inflammation-immunosuppression catabolism syndrome, a common manifestation of patients with enterocutaneous fistula in intensive care unit. J Trauma Acute Care Surg. 2014;76:725–729. doi: 10.1097/TA.0b013e3182aafe6b. [DOI] [PubMed] [Google Scholar]
- 16.Li Z., Hu W., Wang Y., Xu S., Zhou Y., Li S. Persistent inflammation-immunosuppression-catabolism syndrome in patients with systemic lupus erythematosus. Int Urol Nephrol. 2023;55:1757–1765. doi: 10.1007/s11255-023-03479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki G., Ichibayashi R., Masuyama Y., et al. Association of red blood cell and platelet transfusions with persistent inflammation, immunosuppression, and catabolism syndrome in critically ill patients. Sci Rep. 2022;12:629. doi: 10.1038/s41598-021-04327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong M., Pan T., Sun N.N., et al. Early prediction for persistent inflammation-immunosuppression catabolism syndrome in surgical sepsis patients. Int J Gen Med. 2021;14:5441–5448. doi: 10.2147/IJGM.S331411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadda K.R., Puthucheary Z. Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): a review of definitions, potential therapies, and research priorities. Br J Anaesth. 2024;132:507–518. doi: 10.1016/j.bja.2023.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguiar F.P., Westphal G.A., Dadam M.M., Mota E.C.C., Pfutzenreuter F., Franca P.H.C. Characteristics and predictors of chronic critical illness in the intensive care unit. Rev Bras Ter Intensiva. 2019;31:511–520. doi: 10.5935/0103-507X.20190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mira J.C., Cuschieri J., Ozrazgat-Baslanti T., et al. The epidemiology of chronic critical illness after severe traumatic injury at two level-one trauma centers. Crit Care Med. 2017;45:1989–1996. doi: 10.1097/CCM.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanzant E.L., Lopez C.M., Ozrazgat-Baslanti T., et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:21–29. doi: 10.1097/TA.0b013e3182ab1ab5. ; discussion 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mankowski R.T., Anton S.D., Ghita G.L., et al. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression, and catabolism syndrome (PICS) after sepsis. J Gerontol A Biol Sci Med Sci. 2022;77:188–196. doi: 10.1093/gerona/glab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brakenridge S.C., Wang Z., Cox M., et al. Distinct immunologic endotypes are associated with clinical trajectory after severe blunt trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2021;90:257–267. doi: 10.1097/TA.0000000000003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei F., Song W., Wang L., et al. Lymphocyte trajectories are associated with prognosis in critically ill patients: a convenient way to monitor immune status. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.953103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darden D.B., Brakenridge S.C., Efron P.A., et al. Biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) in chronic critical illness (CCI) after surgical sepsis. Ann Surg. 2021;274:664–673. doi: 10.1097/SLA.0000000000005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stortz J.A., Mira J.C., Raymond S.L., et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84:342–349. doi: 10.1097/TA.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 29.Mirna M., Schmutzler L., Topf A., Hoppe U.C., Lichtenauer M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B., Han Y., Chen X., et al. Association of monocyte-to-lymphocyte and neutrophil-to-lymphocyte ratios with persistent critical illness in patients with severe trauma. J Trauma Nurs. 2022;29:240–251. doi: 10.1097/JTN.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 31.Brakenridge S.C., Moore F.A., Mercier N.R., et al. Persistently elevated glucagon-like peptide-1 levels among critically ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J Am Coll Surg. 2019;229:58–67.e1. doi: 10.1016/j.jamcollsurg.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haines R.W., Zolfaghari P., Wan Y., Pearse R.M., Puthucheary Z., Prowle J.R. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019;45:1718–1731. doi: 10.1007/s00134-019-05760-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z., Ho K.M., Gu H., Hong Y., Yu Y. Defining persistent critical illness based on growth trajectories in patients with sepsis. Crit Care. 2020;24:57. doi: 10.1186/s13054-020-2768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugg C., Strohle M., Treml B., Bachler M., Schmid S., Kreutziger J. ICU-acquired hypernatremia is associated with persistent inflammation, immunosuppression and catabolism syndrome. J Clin Med. 2020;9:3017. doi: 10.3390/jcm9093017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmichael E.D., Apple C.G., Kannan K.B., et al. Chronic critical illness in patients with sepsis is associated with persistent anemia, inflammation, and impaired functional outcomes. Am Surg. 2022;89:2563–2571. doi: 10.1177/00031348221104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K., Ogura K., Nakano H., et al. Disseminated intravascular coagulopathy is associated with the outcome of persistent inflammation, immunosuppression and catabolism syndrome. J Clin Med. 2020;9:2662. doi: 10.3390/jcm9082662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winer L.K., Beckmann N., Veile R.A., Goodman M.D., Caldwell C.C., Nomellini V. Consumptive coagulopathy is associated with organ dysfunction during PICS. Am J Physiol Lung Cell Mol Physiol. 2019;316:L946–L952. doi: 10.1152/ajplung.00521.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guirgis F.W., Black L.P., Henson M., et al. A hypolipoprotein sepsis phenotype indicates reduced lipoprotein antioxidant capacity, increased endothelial dysfunction and organ failure, and worse clinical outcomes. Crit Care. 2021;25:341. doi: 10.1186/s13054-021-03757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J., Cyr A., Gruen D.S., et al. Lipidomic signatures align with inflammatory patterns and outcomes in critical illness. Nat Commun. 2022;13:6789. doi: 10.1038/s41467-022-34420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenner B.P., Darden D.B., Kelly L.S., et al. Immunological endotyping of chronic critical illness after severe sepsis. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.616694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delano M.J., Scumpia P.O., Weinstein J.S., et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathias B., Delmas A.L., Ozrazgat-Baslanti T., et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265:827–834. doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 45.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youn J.I., Gabrilovich D.I. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noel J.G., Osterburg A., Wang Q., et al. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28:684–693. doi: 10.1097/shk.0b013e31805362ed. [DOI] [PubMed] [Google Scholar]
- 48.Brudecki L., Ferguson D.A., McCall C.E., El Gazzar M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun. 2012;80:2026–2034. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollen M.K., Stortz J.A., Darden D., et al. Myeloid-derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit Care. 2019;23:355. doi: 10.1186/s13054-019-2628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerin E., Orabona M., Raquil M.A., et al. Circulating immature granulocytes with T-cell killing functions predict sepsis deterioration. Crit Care Med. 2014;42:2007–2018. doi: 10.1097/CCM.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 51.Janols H., Bergenfelz C., Allaoui R., et al. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96:685–693. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 52.Nacionales D.C., Szpila B., Ungaro R., et al. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol. 2015;195:2396–2407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins R.B., Stortz J.A., Holden D.C., et al. Persistently increased cell-free DNA concentrations only modestly contribute to outcome and host response in sepsis survivors with chronic critical illness. Surgery. 2020;167:646–652. doi: 10.1016/j.surg.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 55.Kang J.W., Kim S.J., Cho H.I., Lee S.M. DAMPs activating innate immune responses in sepsis. Ageing Res Rev. 2015;24(Pt A):54–65. doi: 10.1016/j.arr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Achouiti A., Foll D., Vogl T., et al. S100A12 and soluble receptor for advanced glycation end products levels during human severe sepsis. Shock. 2013;40:188–194. doi: 10.1097/SHK.0b013e31829fbc38. [DOI] [PubMed] [Google Scholar]
- 57.Ingels C., Derese I., Wouters P.J., Van den Berghe G., Vanhorebeek I. Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: impact of glycemic control with insulin and relation with clinical outcome. Shock. 2015;43:109–116. doi: 10.1097/SHK.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 58.Timmermans K., Kox M., Scheffer G.J., Pickkers P. Plasma nuclear and mitochondrial dna levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. 2016;45:607–612. doi: 10.1097/SHK.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 59.Singer M. Metabolic failure. Crit Care Med. 2005;33:S539–S542. doi: 10.1097/01.ccm.0000186080.13402.96. [DOI] [PubMed] [Google Scholar]
- 60.Flower L., Puthucheary Z. Muscle wasting in the critically ill patient: how to minimise subsequent disability. Br J Hosp Med (Lond) 2020;81:1–9. doi: 10.12968/hmed.2020.0045. [DOI] [PubMed] [Google Scholar]
- 61.Puthucheary Z.A., Rawal J., McPhail M., et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 62.Flower L., Summers C., Puthucheary Z. Springer Cham; Switzerland: 2023. Management of dysregulated immune response in the critically ill; pp. 255–262. [Google Scholar]
- 63.Schefold J.C., Wollersheim T., Grunow J.J., Luedi M.M., Z'Graggen W.J., Weber-Carstens S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. 2020;11:1399–1412. doi: 10.1002/jcsm.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiroutkova K., Krajcova A., Ziak J., et al. Mitochondrial function in skeletal muscle of patients with protracted critical illness and ICU-acquired weakness. Crit Care. 2015;19:448. doi: 10.1186/s13054-015-1160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.