Abstract
Exogenously added ROS (reactive oxygen species) cause generalized oxidation of cellular components, whereas endogenously generated ROS induced by physiological stimuli activate discrete signal transduction pathways. Compartmentation is an important aspect of such pathways, but little is known about its role in redox signalling. We measured the redox states of cytosolic and nuclear Trx1 (thioredoxin-1) and mitochondrial Trx2 (thioredoxin-2) using redox Western blot methodologies during endogenous ROS production induced by EGF (epidermal growth factor) signalling. The glutathione redox state was measured by HPLC. Results showed that only cytosolic Trx1 undergoes significant oxidation. Thus EGF signalling involves subcellular compartmental oxidation of Trx1 in the absence of a generalized cellular oxidation.
Keywords: epidermal growth factor (EGF), glutathione, redox signalling, thioredoxin-1, thioredoxin-2
Abbreviations: AMS, 4-acetoamido-4′-maleimidylstilbene-2,2′-disulphonic acid; ASK1, apoptosis signal-regulating kinase-1; DCFH-DA, dichlorofluorescein diacetate; DTT, dithiothreitol; Eh, redox potential; E′0, midpoint potential; EGF, epidermal growth factor; EGFR, EGF receptor; IAA, iodoacetic acid; Prx, peroxiredoxin; ROS, reactive oxygen species; TBS, Tris-buffered saline; Trx, thioredoxin
INTRODUCTION
ROS (reactive oxygen species), such as H2O2 and O2•− (superoxide) anion, are generated by numerous toxic reactions and as by-products of oxidative phosphorylation during respiration. Although these reactive species are known to have deleterious effects on macromolecules, they also play a key role in cell signalling processes [1]. These processes are involved in inflammation, cell proliferation, angiogenesis, apoptosis and aging [2]. Quantitative differences in ROS levels are responsible, at least in part, for regulating such events, but selectivity is also likely to involve subcellular compartmentation and variations in specificity of signalling components. Numerous cytokine- and growth-factor-induced signal transduction mechanisms are redox-sensitive, and there is accumulating evidence to suggest that ligand-stimulated ROS generation plays a role in these complex signalling cascades.
Binding of EGF (epidermal growth factor) to its receptor stimulates its tyrosine kinase activity, initiating a phosphorylation cascade that results in the activation of DNA replication and cell division [3]. An increase in H2O2 production accompanies the binding of EGF to its receptor [4]. PI3K (phosphoinositide 3-kinase) activation and subsequent Rac1-mediated induction of NADPH oxidase may also be involved in this process [5]. Specificity in ROS signalling would appear to be necessary; however, there is limited evidence to support specificity in oxidation by ROS or reduction by thiol–disulphide systems.
We have previously devised methodologies for parallel measurement of redox states of the nuclear and cytoplasmic Trx1 (thioredoxin-1) pools and the intracellular GSH pool [6]. While the GSH/GSSG couple provides a major cellular redox buffer, Trxs serve a more specific function in regulating redox-sensitive proteins [7,8]. Trx1 is a 12 kDa oxidoreductase, which has a highly conserved dithiol motif (-Cys-Gly-Pro-Cys-) at its active site. It is involved in the catalytic function of ribonucleotide reductase and the redox regulation of transcription factors such as NF-κB (nuclear factor κB), AP-1 (activator protein 1) and Nrf2 [9–11]. Trx1 can localize to both the nucleus and the cytoplasm. Previous studies show that identification of both oxidized and reduced forms of Trx can be achieved through the introduction of negative charges on thiol groups, via reaction with IAA (iodoacetic acid) and separation by native gel electrophoresis [12]. MS analysis established the identity of the fully reduced form and the two oxidized forms of Trx1 [13]. Experiments from other studies demonstrate that the antibody provided equivalent detection of the different redox forms of Trx1 [14]. Trx2 localizes to the mitochondria and shares some sequence identity with Trx1, including the conserved active-site sequence [15]. While Trx2 does appear to interact with mitochondrial ROS, no clear role has been found for it in oxidant-mediated signalling. Methodologies have been established for the detection of in vivo Trx2 redox status [16,17].
Our previous studies showed that externally supplied oxidant resulted in the simultaneous oxidation of nuclear Trx1, cytosolic Trx1 and cellular GSH [13]. Recent research has implicated Trx-dependent Prxs (peroxiredoxins) in the regulation of signalling-induced hydrogen peroxide [18]. Trx1 has been shown to undergo oxidation in response to EGF [19]; however, oxidation in subcellular compartments has not been examined. Our present results show that cytosolic Trx1 is oxidized without oxidation of cellular GSH/GSSG, nuclear Trx1 or Trx2. The results therefore show that localized and specific oxidation of Trx1 occurs during ROS signalling in response to EGF stimulation.
EXPERIMENTAL
Cell culture and subcellular fractionation
Human keratinocytes (HaCaT) were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% foetal bovine serum and antibiotics (penicillin/streptomycin). Cells were treated with EGF (200 ng/ml) (Sigma, St. Louis, MO, U.S.A.) or H2O2 (0.5 mM) (Baker Chemical Co., Phillipsburg, NJ, U.S.A.) and were then washed once with ice-cold PBS or ice-cold TBS (Tris-buffered saline). For nuclear isolation, cells were collected in 10 mM Hepes, pH 7.8, 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 0.2 mM NaF and 0.2 mM Na3VO4·6H2O) with protease inhibitors (5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin and 0.4 mM PMSF) and IAA (50 mM) [14]. Cell suspensions were incubated on ice for 5 min, followed by the addition of Nonidet P40 (final concentration 0.6%, v/v). After centrifugation at 12000 g, the nuclear and cytosolic fractions were separated and analysed by redox Western blotting [13].
Measurement of ROS production
ROS (principally H2O2) generation was measured with DCFH-DA (dichlorofluorescein diacetate) (Molecular Probes, Eugene, OR, U.S.A.) as described previously [20]. Cells were plated 24 h before assay in a 96-well plate (4×104/well). The DCFH-DA dye was loaded by incubation at a concentration of 100 μM in KRH (Krebs–Ringer–Hepes) buffer for 30 min at 37 °C, and treated for 5 min with EGF or H2O2. Fluorescence was measured using a fluorescence microplate reader (Packard Fluorocount; GMI, Albertville, MN, U.S.A.) with excitation at 485 nm and emission at 530 nm.
Western blotting
EGFR (EGF receptor) activation was analysed by standard Western blotting using an antibody that detects only phosphorylated EGFR (Tyr-1068). Cells were treated with EGF (200 ng/ml) and washed once in ice-cold TBS. Cell extracts were lysed in 20 mM Hepes, pH 7.8, 350 mM KCl, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA and 1% (v/v) Nonidet P40 with protease inhibitors (leupeptin, aprotinin, pepstatin and PMSF). Protein samples (15 μg) were separated by SDS/7.5% PAGE, transferred on to a PVDF membrane and probed for phosphorylated (Tyr-1068) EGFR (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.).
Redox Western blotting
For Trx1 redox analysis, cells (106/well) were washed once with ice-cold PBS immediately after treatment. Although this procedure could allow some artifactual oxidation, this step was viewed as essential for the removal of secreted Trx1, which is largely oxidized. Cells were immediately lysed in 6 M guanidinium chloride, 50 mM Tris/HCl, pH 8.3, 3 mM EDTA, 0.5% Triton-X-100 containing 50 mM IAA (Sigma) [13]. After 30 min at 37 °C, the excess IAA was removed using Microspin G-25 columns (Amersham Biosciences, Little Chalfont, Bucks., U.K.), and Trx1 redox states were separated by native PAGE. The gel was electroblotted on to a nitrocellulose membrane and probed with a goat anti-(human Trx1) antibody (American Diagnostica, Stamford, CT, U.S.A.) and with an Alexa-Fluor-680-conjugated anti-goat secondary antibody (Molecular Probes). Bands corresponding to Trx1 were visualized using the Odyssey scanner (LI-COR, Lincoln, NE, U.S.A.). Bands were quantified using the Odyssey version 1.1 software. Integrated intensities of oxidized and reduced Trx1 were used in the Nernst equation to calculate Eh (redox potential) values with E′0 (midpoint potential)=−254 mV at pH 7.4 and at 25 °C [13]. Experimental evidence from our previous studies has established that the immunodetection of carboxymethylated Trx1 under these conditions provides a means to measure Trx1 Eh, and that the signals are linear over the range of values analysed [13]. MS techniques were used to unequivocally identify the three redox states of Trx1 [13]. Direct comparisons of immunoblot and Coomassie-Blue-stained gels with pure Trx1 showed that the immunoreactivity of the anti-Trx1 polyclonal antibody is not adversely affected by the carboxymethylation of Trx1 thiol groups [14].
For Trx2 redox analysis, cells were treated with 200 ng/ml EGF for 0–30 min or 0.5 mM tBH (t-butyl hydroperoxide) (Sigma) for 5 min, then were washed once with ice-cold PBS. Cells were precipitated with ice-cold trichloroacetic acid (10%) for 30 min at 4 °C. Samples were centrifuged at 12000 g for 10 min, resuspended in 100% acetone and incubated at 4 °C for 30 min. Following centrifugation at 12000 g for 10 min, acetone was removed, and protein pellets were dissolved in lysis/derivatization buffer [20 mM Tris/HCl, pH 8, 15 mM AMS (4-acetoamido-4′-maleimidylstilbene-2,2′-disulphonic acid) (Molecular Probes)] and incubated at room temperature (22 °C) for 3 h. Trx2 redox forms were separated on a SDS/15% polyacrylamide gel in the presence of non-reducing loading buffer. Immunoblotting was performed as described above, but utilized rabbit anti-Trx2 as the primary antibody. This antibody was obtained by injecting recombinant human Trx2, which lacks the N-terminal mitochondrial localization sequence, into New Zealand White rabbits for antiserum production (Covance, Princeton, NJ, U.S.A.). The purified antibody showed specific binding to human Trx2, but no cross-reactivity with Trx1 by Western blot analysis (results not shown). IRDye™-800-conjugated anti-rabbit (Rockland Immunochemicals, Gilbertsville, PA, U.S.A.) was used as the secondary antibody. Integrated intensities of oxidized and reduced Trx2 were used with the Nernst equation to calculate Eh values with E′0=−330 mV at pH 7.6 (mitochondrial pH) and at 25 °C. Purified Trx2 was obtained from Lab Frontiers (Seoul, South Korea). Trx2 was placed in deoxygenated redox buffer (100 mM potassium phosphate buffer and 1 mM EDTA, pH 7.0 or 7.6) containing different concentrations of DTTred (reduced dithiothreitol) and DTTox (trans-1,2-dithiane-4,5-diol or oxidized DTT). The E′0 for DTT is −330 mV, and concentrations of DTTred and DTTox were used to create different Eh values ranging from −270 to −360 mV. Samples were incubated for 3 h and then derivitized with AMS as described above, resolved by non-reducing SDS/PAGE and stained with Coomassie Blue for protein detection. E′0 values were determined where approx. 50% of Trx2 was both reduced and oxidized. E′0 values were determined as −292 mV and −330 mV at 25 °C at pH 7.0 and 7.6 respectively (results not shown).
Previous studies have demonstrated that the separation of Trx2 redox forms by alkylation with AMS provides a means to measure the in vivo redox state of Trx2 [17]. To validate quantification of the Trx2 redox Western methodology further, we compared immunoblots and Coomassie-Blue-stained gels directly with pure Trx2 and found that the anti-Trx2 polyclonal antibody immunoreactivity is not adversely affected by the alkylation of Trx2 thiol groups. We have verified that immunodetection of the Trx2 redox state was linear over the range of values used for analysis.
Determination of GSH/GSSG redox state
GSH and GSSG were measured by HPLC as S-carboxymethyl N-dansyl derivatives using γ-glutamylglutamate as an internal standard, and Eh values were calculated using the Nernst equation assuming 5 μl cell volume per mg of protein and E′0=−264 mV at pH 7.4 and at 25 °C [6].
Statistical analysis
A paired Student's t test was used to compare Eh values in control and EGF-treated samples. P<0.05 was considered to be significant.
RESULTS AND DISCUSSION
Phosphorylation of EGFR and ROS generation occur in EGF-treated human keratinocytes
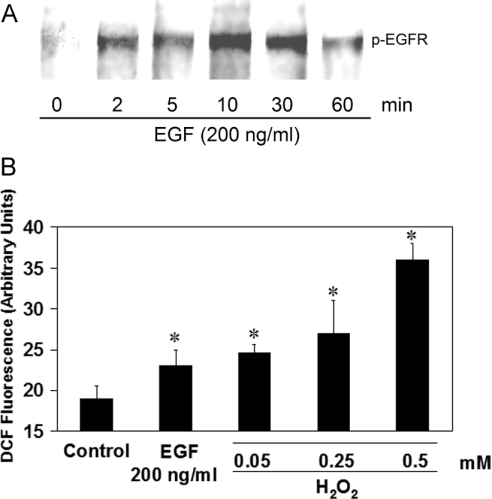
Using an antibody specific for the Tyr-1068 phosphorylated form of the EGFR, we examined EGFR stimulation for 1 h post-EGF treatment (200 ng/ml) (Figure 1A). Activation occurred after 2 min and was maintained over 30 min, decreasing slightly at 60 min. Further verification of EGF stimulation in HaCaT cells was carried out by monitoring the level of ROS output after ligand binding. This was done using a DCF (dichlorofluorescein) assay (Figure 1B). A significant increase (17%) in ROS production in EGF-treated samples was observed. Significant increases in ROS were also seen in H2O2-treated positive controls. Both the initiation of kinase activity and the production of ROS after EGF stimulation confirm activation of the EGFR in this human keratinocyte cell line.
Figure 1. Activation of EGFR in HaCaT cells.
(A) Western blot of phosphorylated EGFR (top) showing a time-dependent response to EGF (200 ng/ml). (B) DCFH-DA assay for the detection of ROS. An increase in dichlorofluorescein (DCF) fluorescence corresponds to an increase in ROS. Fluorescence measured after 5 min is given in relative fluorescence units. Results are means±S.E.M for at least three separate experiments. Asterisks (*) denote a statistically significant difference (P<0.05) as compared with the control.
Redox Western blot analysis of cytoplasmic and nuclear Trx1 and Trx2 pools in EGF-treated keratinocytes
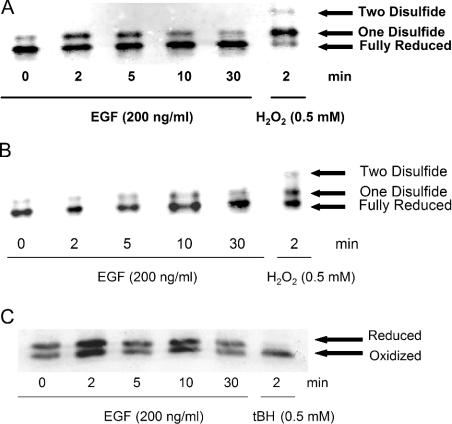
Redox Western analysis was used to determine the relative amounts of reduced and oxidized Trx1 and Trx2 in EGF-treated human keratinocytes. Thiol alkylation by IAA introduces extra negative charges, thereby giving rise to three distinct Trx1 bands: a fully reduced, a one-disulphide and a two-disulphide band [13]. In untreated controls, Trx1 was predominantly in the reduced state in both cytosolic (Figure 2A, 0 min) and nuclear (Figure 2B, 0 min) fractions. There was a clear difference in the pattern of oxidation of Trx1 between cytoplasm and nucleus in response to EGF. Maximal oxidation occurred after 2 min in the cytoplasm. The oxidized band was observed up to 30 min after treatment in cytoplasmic fractions. The two-disulphide band was only visible in H2O2-treated positive controls (Figures 2A and 2B). In contrast with Trx1, which has two dithiol motifs, Trx2 has a single dithiol. Therefore Trx2 produces only two bands in redox Western analysis (Figure 2C). Thiol alkylation by AMS caused a mass shift in the reduced form, thereby yielding the reverse redox orientation to IAA-derivatized Trx1. Trx2 did not undergo oxidation in response to EGF signalling during the time frame examined here.
Figure 2. Redox states of cytoplasmic and nuclear Trx1 and Trx2 pools.
(A, B) Time course of Trx1 oxidation in the cytoplasm (A) and the nucleus (B) following EGF (200 ng/ml) treatment from 0–30 min. Trx1 appears as three distinct bands. The two-disulphide form (top band) is only visible under highly oxidizing conditions. The oxidized band (middle) represents the one-disulphide form and the lower band is the fully reduced form. H2O2 was used as a positive control. (C) Time course of Trx2 redox state following EGF (200 ng/ml) treatment from 0–30 min. Separation of reduced and oxidized bands is based on a mass shift due to AMS alkylation of thiols. tBH (t-butyl hydroperoxide) was used as a positive control.
Differential Eh values in nuclear Trx1, cytoplasmic Trx1 and GSH pools in response to EGF
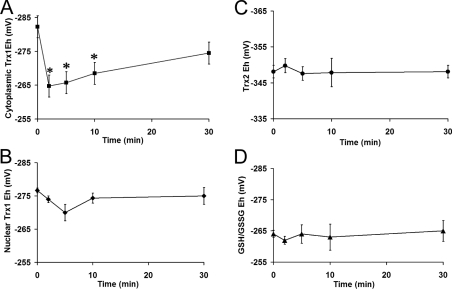
Steady-state Eh values were calculated using the Nernst equation (see the Experimental section). Cytoplasmic Trx1 showed a Eh value of −283±4 mV in unstimulated HaCaT cells, which is comparable with values obtained in other untreated cell lines [14]. Upon EGF stimulation (200 ng/ml), this pool underwent rapid oxidation of 21±5 mV (Figure 3A). Oxidation was sustained, and, by 30 min post-EGF treatment, Eh was −270±4 mV. Nuclear Trx1 (Figure 3B) did not show significant oxidation during the EGF time course.
Figure 3. EGF signalling differentially affects redox pools.
Changes in Eh values of (A) cytoplasmic Trx1, (B) nuclear Trx1, (C) Trx2 and (D) GSH in EGF-treated HaCaT cells over 30 min. Values were calculated using the Nernst equation (see the Experimental section). Results are means±S.E.M for at least three separate experiments. Asterisks (*) denote a statistically significant difference (P<0.05) as compared with the control
The E′0 for Trx2 is considerably less than that of Trx1 (−330 mV compared with −240 mV). Therefore, while redox Western data (Figure 2) show more of the oxidized form of Trx2 than Trx1 in untreated cells, analysis of the corresponding Eh values reveals that Trx2 is more reducing (−348±2 mV; Figure 3C). EGF stimulation did not have an effect on Trx2 Eh values.
The GSH redox couple did not undergo oxidation during EGF stimulation (Figure 3D). In untreated controls, the GSH/GSSG Eh was −264±3 mV and, after 30 min was −265±4 mV. These GSH/GSSG Eh values compare well with values from other studies in unstimulated cells [14,21]. Thus the data show that only the cytoplasmic Trx1 pool undergoes significant oxidation in response to EGF signalling.
The use of Eh values to quantify redox states allows for easy comparison between GSH and Trx1 responses to growth factor signalling. In the present study, we have shown that EGF-induced ROS give rise to significantly different oxidation profiles in the GSH, Trx1 and Trx2 redox pools. Previous investigations have indicated that exposure to an exogenous oxidant causes a significant increase in the Eh values of both nuclear and cytoplasmic Trx1 as well as of GSH [14]. The present data show that physiologically derived ROS take part in a very specific signalling mechanism that involves the preferential oxidation of cytoplasmic Trx1 over nuclear Trx1, Trx2 and GSH.
Conclusions
The specific oxidation of cytoplasmic Trx1 by EGF-induced ROS may be brought about by the internalization of the activated EGFR. Many tyrosine kinase receptors become internalized rapidly in endosomal bodies upon activation [22,23]. However, the relationship between oxidant signalling and receptor endocytosis has not been investigated. If EGF-signalling-induced ROS occurs in these discrete compartments, it may explain the specific oxidation of cytoplasmic Trx1 observed here. A further possibility is that a localized signal in the vicinity of the EGFR at the plasma membrane is responsible for precise compartmented signalling. There is some evidence that shows that Trx1 is associated with the plasma membrane [24–27]. Selective oxidation of the cytoplasmic/plasma-membrane-localized Trx1 pool might account for the absence of oxidation in the GSH redox pool.
The distinct pattern of oxidation in each of these antioxidant pools during EGF signalling suggests the involvement of Trx peroxidase or Prx, a family of thiol-specific antioxidant enzymes that are involved in the catalysis of hydroperoxides such as H2O2 and peroxynitrite [28]. Prxs regulate a cellular response to a rapid burst of peroxide during a signalling event [29]. This would account for a sharp oxidation in Trx1, since it is required for the reduction of Prxs upon its oxidation by ROS. Furthermore, it may be that Prxs have greater substrate specificity for growth-factor-signalling-induced ROS than glutathione peroxidase. This would, at least in part, explain the absence of glutathione oxidation that we observed.
The oxidation of cytoplasmic Trx1, as shown in the present study, provides a means for altering various redox-regulated processes during EGF signalling. One potential target for Trx1 is the Akt kinase, which is involved in the promotion of cell survival in response to stress [30]. ROS are capable of causing a decrease in Akt activity [31]. Trx1, due to its recognized role in ROS elimination, could have an impact upon cell survival signalling as well as growth factor signalling. Trx1 has an inhibitory effect on ASK1 (apoptosis signal-regulating kinase-1), a member of the MAPKKK (mitogen-activated protein kinase kinase kinase) family. Interestingly, there is some evidence to suggest that, during oxidative stress, ASK1 activation occurs through both GSH-dependent and GSH-independent pathways [32]. Oxidant-induced, Trx1-mediated activation of ASK1 appears to be independent of GSH. This agrees with our finding that ROS signalling and kinase signalling may be linked via specific oxidation of the cytoplasmic Trx1 redox pool.
In conclusion, the present findings show that ROS generation in response to EGF signalling in keratinocytes results in selective oxidation of cytoplasmic Trx1. However, whether this oxidation is simply a reflection of Trx1 oxidation as a consequence of Prx-dependent termination of a redox signal, or part of the signalling mechanism itself, remains to be established. Given the evidence for widespread function of Trxs, one may expect that both contribute to redox signalling control. Nonetheless, these results provide the first clear evidence that physiological redox signalling is specifically associated with oxidation of the Trx1 system and not the GSH system.
Acknowledgments
We thank Dr Yan Chen for assistance with Trx2 redox Western blot analysis. HaCaT cells were a gift from Bito Toshinori of the Kobe Graduate University School of Medicine, Kobe, Japan. This work was supported by National Institutes of Health (NIH) grant ES011195. W.H.W. was supported in part by NIH grant ES012260. J.M.H. was supported in part by NIH grant ES013015.
References
- 1.Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G., Cohen S. Epidermal growth factor. J. Biol. Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 4.Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 5.Meng T. C., Fukada T., Tonks N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 6.Jones D. P. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 7.Powis G., Montfort W. R. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 8.Schafer F. Q., Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 9.Matthews J. R., Wakasugi N., Virelizier J. L., Yodoi J., Hay R. T. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen J. M., Watson W. H., Jones D. P. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol. Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren A., Fagerstedt M. The in vivo distribution of oxidized and reduced thioredoxin in Escherichia coli. J. Biol. Chem. 1982;257:6926–6930. [PubMed] [Google Scholar]
- 13.Watson W. H., Pohl J., Montfort W. R., Stuchlik O., Reed M. S., Powis G., Jones D. P. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- 14.Watson W. H., Jones D. P. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 2003;543:144–147. doi: 10.1016/s0014-5793(03)00430-7. [DOI] [PubMed] [Google Scholar]
- 15.Spyrou G., Enmark E., Miranda-Vizuete A., Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J. Biol. Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Cai J., Murphy T. J., Jones D. P. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J. Biol. Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 17.Damdimopoulos A. E., Miranda-Vizuete A., Pelto-Huikko M., Gustafsson J. A., Spyrou G. Human mitochondrial thioredoxin: involvement in mitochondrial membrane potential and cell death. J. Biol. Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- 18.Rhee S. G., Chang T. S., Bae Y. S., Lee S. R., Kang S. W. Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 19.Gitler C., Zarmi B., Kalef E., Meller R., Zor U., Goldman R. Calcium-dependent oxidation of thioredoxin during cellular growth initiation. Biochem. Biophys. Res. Commun. 2002;290:624–628. doi: 10.1006/bbrc.2001.6214. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Joseph J. A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 21.Nkabyo Y. S., Ziegler T. R., Gu L. H., Watson W. H., Jones D. P. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wiley H. S., Herbst J. J., Walsh B. J., Lauffenburger D. A., Rosenfeld M. G., Gill G. N. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- 23.Sorkin A., Waters C. M. Endocytosis of growth factor receptors. BioEssays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 24.Hansson H. A., Holmgren A., Rozell B., Taljedal I. B. Immunohistochemical localization of thioredoxin and thioredoxin reductase in mouse exocrine and endocrine pancreas. Cell Tissue Res. 1986;245:189–195. doi: 10.1007/BF00218100. [DOI] [PubMed] [Google Scholar]
- 25.Rozell B., Hansson H. A., Luthman M., Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur. J. Cell Biol. 1985;38:79–86. [PubMed] [Google Scholar]
- 26.Sahaf B., Soderberg A., Spyrou G., Barral A. M., Pekkari K., Holmgren A., Rosen A. Thioredoxin expression and localization in human cell lines: detection of full-length and truncated species. Exp. Cell Res. 1997;236:181–192. doi: 10.1006/excr.1997.3699. [DOI] [PubMed] [Google Scholar]
- 27.Wollman E. E., Kahan A., Fradelizi D. Detection of membrane associated thioredoxin on human cell lines. Biochem. Biophys. Res. Commun. 1997;230:602–606. doi: 10.1006/bbrc.1996.6015. [DOI] [PubMed] [Google Scholar]
- 28.Wood Z. A., Schroder E., Robin Harris J., Poole L. B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 29.Wood Z. A., Poole L. B., Karplus P. A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 30.Scheid M. P., Woodgett J. R. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108–112. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 31.Martin D., Salinas M., Fujita N., Tsuruo T., Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J. Biol. Chem. 2002;277:42943–42952. doi: 10.1074/jbc.M201070200. [DOI] [PubMed] [Google Scholar]
- 32.Song J. J., Lee Y. J. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem. J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]