Abstract
Objective
Excessive consumption of added sugars has been linked to the rise in obesity and associated metabolic abnormalities. Non-nutritive sweeteners (NNSs) offer a potential solution to reduce sugar intake, yet their metabolic safety remains debated. This study aimed to systematically assess the long-term metabolic effects of commonly used NNSs under both normal and obesogenic conditions.
Methods
To ensure consistent sweetness level and controlling for the acceptable daily intake (ADI), eight weeks old C57BL/6 male mice were administered with acesulfame K (ace K, 535.25 mg/L), aspartame (411.75 mg/L), sucralose (179.5 mg/L), saccharin (80 mg/L), or steviol glycoside (Reb M, 536.25 mg/L) in the drinking water, on the background of either regular or high-fat diets (in high fat diet 60% of calories from fat). Water or fructose-sweetened water (82.3.gr/L), were used as controls. Anthropometric and metabolic parameters, as well as microbiome composition, were analyzed following 20-weeks of exposure.
Results
Under a regular chow diet, chronic NNS consumption did not significantly affect body weight, fat mass, or glucose metabolism as compared to water consumption, with aspartame demonstrating decreased glucose tolerance. In diet-induced obesity, NNS exposure did not increase body weight or alter food intake. Exposure to sucralose and Reb M led to improved insulin sensitivity and decreased weight gain. Reb M specifically was associated with increased prevalence of colonic Lachnospiracea bacteria.
Conclusions
Long-term consumption of commonly used NNSs does not induce adverse metabolic effects, with Reb M demonstrating a mild improvement in metabolic abnormalities. These findings provide valuable insights into the metabolic impact of different NNSs, aiding in the development of strategies to combat obesity and related metabolic disorders.
Keywords: Non-nutritive sweeteners, Obesity, Insulin sensitivity, Glucose metabolism, Microbiome, Reb M
Highlights
-
•
NNS does not result in over-feeding, weight gain or impaired glucose metabolism.
-
•
RebM consumption improves insulin sensitivity and attenuates weight gain in obese mice.
-
•
RebM increases the prevalence of colonic Lachnospiracea bacteria.
-
•
Long-term consumption of commonly used NNSs does not induce adverse metabolic effects.
1. Introduction
Excessive dietary consumption of added sugars in both soft drinks and processed foods has been attributed to the epidemic proportions of obesity and its associated metabolic abnormalities in recent decades [1,2]. Thus, the use of non-nutritive sweeteners (NNS), that provide a sweet taste without a substantial contribution to caloric intake, has been advocated as a potential approach to mitigate the excessive use of sugars. Yet, currently there is a controversy regarding the metabolic safety of NNSs and whether or not they may induce, rather than attenuate, some of the common obesity-mediated metabolic abnormalities [[3], [4], [5], [6], [7]].
While association and cohort studies demonstrate a correlation between adverse metabolic parameters and NNS consumption [[8], [9], [10], [11]], most randomized controlled trials have failed to demonstrate a cause and effect relationship between consumption of NNS and increase in body weight, adiposity, or parameters of the metabolic syndrome [[10], [11], [12]]. Multiple potential biases, the difficulty to isolate the specific effects of NNS of the entire nutrition, inconsistencies in choosing comparators, short duration of studies and the use of NNS as an integral group of compounds, have all been suggested as plausible explanations for this apparent discrepancy in results. Regarding the latter, the currently approved sweeteners in wide use are saccharin, aspartame, sucralose, ace-K, neotame and advatame, in addition to plant-based sweeteners such as steviol glycosides, monk fruit and thaumatin. These compounds are profoundly distinct in their chemical structure, their absorption (or lack of) and metabolism. Perhaps the only characteristic shared by all of these compounds is their ability to induce a sweet taste [13]. As such, one could expect that their potential metabolic effects, being adverse or beneficial, may also differ extensively.
Indeed, hundreds of studies were performed in different animal models in order to test the chronic effects of NNS [[14], [15], [16]]. Yet, these studies differ in species and strain, duration of intervention, concentration, route of delivery, background, the various NNS and control used as well as measured outcomes. As such, conflicting evidence are similarly obtained from pre-clinical studies [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. Therefore, the aim of this study is to systematically assess the long-term metabolic effects of commonly used NNS, both in the setting of a healthy diet, and under obesogenic conditions. We studied the effects of supplementing either chow diet or high-fat diet fed mice with acesulfame K (ace K), aspartame, sucralose, saccharin or steviol glycoside (Reb M) compared with either water or fructose-sweetened water. We observed that chronic consumption of NNS does not result in over-eating, weight gain or metabolic alternations. Of note, consumption of Reb M resulted in mild attenuation of weight gain and improvement in insulin sensitivity in obese mice.
2. Materials and methods
2.1. Animal care and use
Eight weeks old [C57BL/6] male mice were purchased from Envigo (Israel). Upon arrival to Sheba Medical Center animal facility, mice were randomly divided into seven experimental groups, and each group was housed in 2 separate cages (70 mice total; 5 mice per cage). Mice were kept in a 12 h light/dark cycle. After one week of acclimation, the dietary interventions using either water, fructose or any one of the NNSs mentioned below was initiated for up to 20 weeks by supplementing drinking water with the corresponding sweetener. Mice were exposed to ad libitum food with either a standard chow (2018 Teklad Global 18% Protein Rodent Diets, Inotiv) or a high fat diet (D12492 60% calories from fat, Research Diets), as indicated in the various experiments.
2.2. Stability tests for sugars and NNSs
Fructose and NNSs solutions were dissolved in water, followed by pasteurization at 85 °C for 30 s and immediate cooling on ice. Fructose was purchased from Galam (Israel); Ace-K, aspartame and saccharin were purchased from Sigma–Aldrich; Sucralose was purchased from Techno (Fujian) Food Ingredients co. LTD and Reb M was purchased from Layn Natural ingredients (China). Concentration of all solutions was calculated to mimic the degree of sweetness of Coca-cola© (i.e. equivalent to sucrose at a concentration of 106 mg/mL) and did not exceed the upper limit of the allowed daily consumption. The specific concentration of each solution is described in Supplementary Table 1. Stability post-pasteurization was assessed using high-performance liquid chromatography (HPLC). Fructose analysis utilized the E 2695 Waters HPLC system with a refractive index detector (RI 2414) and 20 μL injector, employing a Waters Sugar Pack Column and an isocratic mobile phase of 0.01% CaEDTA in deionized distilled water. Similarly, stability evaluations for sucralose, Ace-K, aspartame, saccharin, and Reb M were carried out utilizing specific HPLC systems and columns, each with tailored isocratic mobile phases. All samples were rigorously filtered through a 0.45 μm syringe filter prior to analysis, ensuring the precision of the results obtained.
2.3. Blood glucose and body weight
Mice were monitored weekly for body weight and glucose. Blood glucose from tail vein was measured in the light cycle, following 6 h of food withdrawal using Statstrip Xpress® glucometer.
2.4. Body composition analysis
Percentages of lean and fat mass were measured using nuclear magnetic resonance spectroscopy with Minispec LF50 (Bruker optics, Billerica, MA).
2.5. Metabolic cages
PhenoMaster system (TSE-Systems) was used to measure all metabolic parameters. Each mouse was singly-housed in a cage for 60 h (a total of 8 mice from each group). The system automatically and simultaneously monitored drink and food consumption as well as activity and calorimetric parameters. Based on the food and fluid intake, a calculation of total caloric intake was performed using the following values: fructose 0.32 kcaL/ml, chow diet 3.1 kcal/gr, HFD 5.21 kcal/gr. NNSs were considered as having 0 kcaL/ml (Supplementary Table 2). Metabolic studies using the PhenoMaster system were conducted between weeks 10–13 (chow diet) and weeks 12–15 (HFD).
2.6. Glucose and insulin tolerance tests
Animals were subjected to an intraperitoneal (i.p.) glucose injection (1 gr/kg) following 16 h food withdrawal. Insulin tolerance tests were performed by intraperitoneal injection of human regular insulin (0.75 IU/kg or 1.5 IU/kg for chow and HFD, respectively) following 6 h of food withdrawal. For both assays, blood glucose levels were measured from tail vein at the indicated time points. Mice on chow diet were subjected to ITT on week 15 and GTT was performed on week 17. Mice on HFD were subjected to ITT on week 17 and GTT on week 19.
2.7. Histological analysis
Liver and epididymal white adipose tissue samples were dissected, washed in PBS and fixed overnight in formalin. Tissue processing, sectioning, and staining with hematoxylin and eosin was performed by the Sheba Medical Center histopathology core. NAFLD score was evaluated by a specialized pathologist based on the scoring described by Liang W. et al. [43]. Evaluation was done using Olympus light microscope BX43, Olympus digital camera DP21 with Olympus cellSens Entry 1.13 software.
2.8. Blood samples
At the end of the experiment, following terminal sacrifice, blood was collected by cardiac puncture. Serum was separated from whole blood by centrifugation at 2700 rpm for 15 min at 4 °C and either kept in aliquots at −80° or used for measurement of liver enzymes (alanine and aspartate aminotransferase [ALT and AST, respectively]) as well as lipid profile (triglycerides, cholesterol, low-density lipoprotein [LDL], and high-density lipoprotein [HDL]).
Insulin, C-peptide, adiponectin and leptin concentrations were determined in plasma samples using ELISA according to the manufacturer's instructions (Ultra-Sensitive Mouse Insulin ELISA Kit [Crystal Chem]; Rat/Mouse C-Peptide 2 ELISA kit [Milipore]; Mouse Adiponectin/Acrp30 Quantikine ELISA Kit [R&D Systems] and Quantikine ELISA mouse Leptin Immunoassay kit [R&D Systems], respectively).
2.9. Hepatic triglycerides concentration
Frozen liver tissues (∼100 mg) were washed 3 times with cold PBS. Samples were homogenized in 1 mL of 5% NP-40/ddH2O solution using a Bullet blender tissue homogenizer. The samples were then incubated at 95 °C in a water bath for 2–5 min and then cool down to room temperature (=RT). This step was repeated to solubilize all triglycerides. After cooling to RT, samples were centrifuged for 2 min at top speed to remove any insoluble material. Clear supernatant was transferred into a clear tube and samples were analyzed for their triglyceride content at Sheba core laboratory. Values were normalized to mg protein.
2.10. Fecal sample handling, DNA extraction, and 16S amplicon sequencing and analyses
Fresh fecal samples were collected and stored at −80 °C until further processing. These samples were collected at the beginning of the experiment, after 4 weeks as well as following 16 weeks of intervention. DNA extraction and PCR amplification of the variable region 4 (V4) of the 16S rRNA gene using Illumina adapted universal primers 515 F/806R 39 was conducted using the direct PCR protocol [Extract-N-Amp Plant PCR kit (Sigma–Aldrich, Inc.)] as previously described [44,45]. Sequencing was performed on the Illumina MiSeq platform. Reads were processed in a data curation pipeline implemented in QIIME 2 version 2019.4 [46,47]. Reads were demultiplexed according to sample-specific barcodes. Quality control was performed by truncating reads after three consecutive Phred scores lower than 20. Reads with ambiguous base calls or shorter than 150 bp after quality truncation were discarded. Amplicon sequence variants (ASVs) detection was performed using Deblur [48]. Reads were then truncated to 150 bp and taxonomic classification was performed using a naive Bayes fitted classifier, trained on the August 2013 99% identity Greengenes 1 (gg1) and also on the 2022 Greengenes 2 (gg2) databases as indicated. All specific ASV sequences are given in the supplementary database tables and figures. Unweighted UniFrac was used as a measure of β-diversity = between sample diversity. The median reads/sample was 9001 (IQR 5335-12567). All samples were rarefied to 3000 reads for β diversity analysis, to avoid sample size effect. The resulting distance matrix was used to perform a principal coordinate analysis (PCoA). Initial differential abundance and heatmap generation were done using Calour version 2019.5.1 with default parameters [49], and multiple hypothesis correction (dsFDR<0.25) was applied. This analysis was complemented with MaAsLin2 (Multivariate Association with Linear Models) R package version 1.4.0 [50] which was used with default parameters to find ASVs significantly associated with NNS, fructose, and water while controlling for cage and mice as random variable, and timepoint as a fixed variable. A supplementary dataset 1 summarizes the ASVs and taxonomy per sample.
2.11. Quantification and statistical analysis
Data expressed as mean ± standard error of the mean (SEM). All groups were compared either to fructose -treated mice or to the water group. The differences between groups in the experimental data were tested by one-way analysis of variance (ANOVA). In cases where the data did not follow a normal distribution, determined through the Kolmogorov–Smirnov normality test, the nonparametric Kruskal–Wallis test was utilized, followed by Dunn's multiple comparisons post-test. For evaluating predefined comparisons at specific time points, a two-way repeated-measures analysis of variance (ANOVA) with Dunnett's multiple comparisons post-test was applied. Outliers were identified and excluded using the “identify outlier” function in GraphPad, employing the ROUT method. Statistical significance was defined as ∗p ≤ 0.05. Data analysis was performed using GraphPad Prism software version 9.4.1 (GraphPad Software, San Diego, California USA, www.graphpad.com).
2.12. Study approval
All experimental procedures were approved by Sheba Medical Center Institutional Animal Care and Use Committee (protocol ID: 1212-19-ANIM).
3. Results
The experimental design was based on 7 parallel groups of mice (n = 10 per group) with daily exposure to non-supplemented drinking water as control, or to NNS-supplemented water with either ace K, aspartame, sucralose, saccharin or Reb M. In an additional group of mice, fructose, commonly used in sweet beverages, was used to allow comparison between NNS and a nutritive sweetener (full experimental layout is summarized in Supplementary Fig. 1). Doses of the various sweeteners used were calculated based on relative sweetness of 106 mg/mL of sucrose (Supplementary Table 1) to ensure similar sweet sensation of all sweetened drinking solutions. To validate the stability of the NNS-supplemented water solutions, concentrations were measured daily by HPLC. As shown in Supplementary Table 1, all sweetener solutions maintained stable concentration for at least 5 days, with the exception of aspartame, demonstrating a significant degradation after 3 days. Therefore, the aspartame solution was freshly prepared 3 times a week.
3.1. Effects of long term consumption of NNS with chow diet
In order to evaluate whether consumption of NNS has direct long-term metabolic effects, we first provided the various NNS to mice on ad libitum normal chow diet. As demonstrated in Figure 1, chronic consumption of either one of the NNS, or of fructose, for up to 20 weeks, did not result in significant differences in body weight gain compared with unsweetened drinking water (Figure 1A,B and Supplementary Fig. 2C). In addition, no significant between-group difference was recorded in either lean or fat mass measured at the end of the intervention (Figure 1C,D, respectively).
Figure 1.
Effects of Long-Term Non-Nutritive Sweetener (NNS) Consumption on Metabolic Parameters under Regular Diet Conditions. A. Weekly body weight measurements (n = 9–10 mice/group). B. Body weight at week 18 (n = 9–10 mice/group). C and D. Lean (C) and fat (D) mass percentages measured by NMR at 19 weeks of age (n = 7–10 mice/group). E. Fasting blood glucose levels at week 18 (n = 9–10 mice/group). F. Intraperitoneal glucose-tolerance test at week 17 (n = 9–10 mice/group; 1 g/kg glucose) with corresponding area under the curve (AUC) calculation (G). H. Intraperitoneal insulin-tolerance test using regular human insulin (0.75 IU/kg body weight) at week 15 (n = 3–5 mice/group) with corresponding AUC calculation (I). J-Q. Mice were housed in metabolic cages for 60 h (n = 7–8 mice/group) and the following parameters were collected: cumulative drinking (J) and feeding (L), heat production (O) and respiratory exchange ratios (P,Q). Based on this data and as described in the methods section, daily average drinking (K) and feeding (M) as well as cumulative calories (N) were calculated.
Data are presented as means ± SEM. (A,F,H) Data analyzed by a two-way ANOVA, with Dunnett's multiple comparisons post-test. (B,D,E,G,K,M,N) Data analyzed by a one-way ANOVA, with a Dunnett's test for multiple comparisons. (C,I,O,Q) Data analyzed by Kruskal–Wallis test followed by Dunn's multiple comparisons test. AUC, area under curve. RER, respiratory exchange rate. ∗p < 0.05. In graph F, ∗ refers to the comparison between water and aspartame. (J,L,P) White and black rectangles on the x-axis represent light and dark phases, respectively. Grey bars denote dark phase. ∗p < 0.05, ∗∗p < 0.01.
While chronic exposure of mice to fructose resulted in significant increase in blood glucose levels even in the fasting condition (p < 0.01, compared to water), long-term exposure of any of the NNS did not result in hyperglycemia, and fasting blood glucose levels were comparable to values recorded in the water-only group (Figure 1E). Of note, compared to fructose, significant lower blood glucose levels were observed in mice drinking sucralose or Reb M (Figure 1E, Supplementary Fig. 2A). NNS consumption did not result in altered glucose tolerance, other than aspartame consumption, which resulted in a 16% increase in the area under the glucose curve compared with water (Figure 1F,G). Of note, no significant differences were observed in insulin sensitivity between groups (Figure 1H,I).
To assess the effects of NNS exposure on fluid and food consumption, mice were individually housed in metabolic cages. Of note, exposure of mice to the various NNS did not result in a decrease in fluid consumption compared to water, indicating that mice were adequately exposed to the interventions and that there was no taste aversion to either of the interventions (Figure 1J). Reb M exposure resulted in statistically significant increase in daily fluid consumption compared to water-only control group by 28% (p = 0.03, Figure 1K). Regarding food intake, as previously reported [51], adding fructose to the drinking water resulted in a significant decrease of 28% (p = 0.02) in total and daily food intake compared with water (Figure 1L,M). Addition of any of the NNS did not result in a significant increase in food intake compared with water (Figure 1M). Calculation of total caloric intake results in a comparable total caloric intake between groups (Figure 1N). In addition, no significant between-group differences were observed in energy expenditure or in respiratory exchange ratio (RER) (Figures 1O-Q), with a tendency towards increased fat oxidation (over glucose) in mice exposed to NNS compared with fructose-treated mice. While levels of triglycerides, HDL-cholesterol, ALT and AST were comparable between groups (Supplementary Figs. 3B, C, E and F, respectively), mice consuming sucralose demonstrated a significantly lower levels of total cholesterol (by 23%) and LDL-cholesterol (by 22%) (Supplementary Figs. 3A and D, respectively) compared to fructose.
3.2. Effects of long-term consumption of NNS in diet-induced obesity
Next, we aimed at evaluating the effects of long-term NNS consumption in the setting of diet-induced obesity. Similar to the experiments described above with regular chow diet, mice exposed to high-fat diet (HFD; 60% kcal from fat) were supplemented with NNS in the drinking water for 20 weeks. As depicted in Figure 2A and Supplementary Fig. 2D, fructose exposure resulted in the highest weight gain over the intervention period. This difference was statistically significant compared to mice drinking Reb M or sucralose. In the NNS group, exposure to none of the sweeteners resulted in increased body weight compared to water-only group, with mice drinking either sucralose or Reb M demonstrating the lowest degree of weight gain during the 20-week dietary intervention. This relative mild difference in total body weight did not translate to significant between group differences in fat or lean body mass (Figure 2B,C, respectively).
Figure 2.
Effects of Long-Term Non-Nutritive Sweetener (NNS) Consumption in Diet-Induced Obesity. A. Weekly body weight measurements (n = 9–10 mice/group). B and C. Fat (B) and lean (C) mass percentages measured by NMR at 19 weeks of age (n = 8–10 mice/group). D-K. Mice were housed in metabolic cages for 60 h (n = 6–8 mice/group) and the following parameters were collected: cumulative drinking (D) and feeding (F), heat production (H), activity (I) and respiratory exchange ratios (J,K). Based on this data and as described in the methods section, daily average drinking (E) and cumulative calories (G) were calculated.
Data are presented as means ± SEM. (A) Data analyzed by a two-way ANOVA, with Dunnett's multiple comparisons post-test. (B,C,E,H,I,K) Data analyzed by a one-way ANOVA, with a Dunnett's test for multiple comparisons. (G) Data analyzed by Kruskal–Wallis test followed by Dunn's multiple comparisons test. RER, respiratory exchange rate. (D,F,J) White and black rectangles on the x-axis represent light and dark phases, respectively. Grey bars denote dark phase. In graph A, ∗ refers to the comparison between sucralose and fructose/water (black/blue, respectively). # Refers to the comparison between Reb M and fructose/water (black/blue, respectively). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005; #p < 0.05, ##p < 0.01.
To assess for potential effects of NNS on either food intake or energy expenditure we studied HFD-fed mice from the various treatment groups in metabolic cages (Figure 2D–K). Both fluid consumption and food consumption were similar between groups (Figure 2D–F), resulting in comparable total caloric intake between fructose, NNS- and water-drinking mice (Figure 2G). Similarly, no significant differences were recorded in energy expenditure or in physical activity (Figure 2H–I). RER displayed a normal nocturnal pattern in all groups with no significant differences observed with the various dietary interventions (Figure 2J–K).
Similar to the observations with regular chow diet, mice drinking fructose supplemented water demonstrated numerically the highest fasting blood glucose levels compared to all other groups. Yet this value did not reach statistical significance (p = 0.09) except for the comparison to Ace-K. In contrast, exposure to any of the NNS tested did not result in a significant increase in blood glucose compared to water (Figure 3A, Supplementary Fig. 2B).
Figure 3.
Glucose Metabolism and Hepatic Evaluation in Response to Long-Term NNS Consumption under obesity. A. Fasting blood glucose levels at week 18 (n = 9–10 mice/group). B. Intraperitoneal insulin-tolerance test using regular human insulin (1.5 IU/kg body weight) at week 17 (n = 8–10 mice/group) with corresponding AUC calculation (C). D. Intraperitoneal glucose-tolerance test at week 19 (n = 9–10 mice/group; 1 g/kg glucose) with corresponding AUC calculation (E). Lipid profiles (n = 5–7 mice/group), including total cholesterol (F), triglycerides (G), HDL-Cholesterol (H), and LDL-Cholesterol (I), were measured at the end of the experiment. Pathologic evaluation, including steatosis (J) and inflammation (K) scores, was performed on liver tissues (n = 9–10 mice/group). (L) Triglyceride concentrations in liver samples (n = 9–10 mice/group). Serum ALT (M) and AST (N) levels were also measured at the end of the experiment (n = 5–7 mice/group).
Data are presented as means ± SEM. (A,C,F–I,L,M,N) Data analyzed by a one-way ANOVA, with a Dunnett's test for multiple comparisons. (B,D) Data analyzed by a two-way ANOVA, with Dunnett's multiple comparisons post-test. (E) Data analyzed by a one-way ANOVA, with a Kruskal–Wallis test for multiple comparisons. (J,K) Data analyzed by Kruskal–Wallis with Dunn's test for multiple comparisons. In graph B, ∗ refers to the comparison between sucralose and fructose/water (black/blue, respectively). # Refers to the comparison between Reb M and fructose/water (black/blue, respectively). ∗p < 0.05 ∗∗p < 0.01, ∗∗∗p < 0.005, #p < 0.05, ##p < 0.01. AUC, area under curve.TG, Triglyceride.
To further assess the effects of long-term exposure to NNS on glucose metabolism, both insulin tolerance and glucose tolerance were evaluated. As shown in Figure 3B,C, mice exposed to fructose exhibited the highest degree of insulin resistance compared to mice exposed to any of the NNS. NNS consumption resulted in numerically, yet not statistically, improvement in insulin resistance (excluding saccharin). More specifically, in comparison to fructose, the AUC of the ITT was numerically improved in Ace-K (p = 0.08) and in aspartame (p = 0.06), and statistically improved in sucralose (p = 0.01) and in Reb M (p = 0.03). In addition, long-term exposure to any of the NNS did not result in impaired insulin sensitivity when compared to the water-drinking control group (Figure 3B). Of note, under these conditions, exposure to either sucralose or Reb M resulted in improved insulin sensitivity when compared to the water-only control group, with Reb M demonstrating improved response to insulin at all time points from 45 to 120 min post insulin administration. Similarly, addition of fructose to the drinking water of HFD-fed mice resulted in the worse glucose tolerance. Exposure to all of the NNS tested did not alter glucose responsiveness as compared to water-only or fructose groups, with Reb M and sucralose groups exhibiting numerically the lowest post load glucose excursions (Figure 3D,E). Of note, the mild deterioration in glucose tolerance observed with aspartame exposure in lean mice, was not evident in obese, glucose intolerant mice.
As depicted in Figure 3F–I, mice consuming NNS demonstrated no alterations in levels of total cholesterol, fasting triglycerides, HDL- and LDL-cholesterol. To assess for a potential effect of NNS exposure on the development and progression of non-alcoholic fatty liver disease (NAFLD), pathologic evaluation was performed by a certified pathologist who was blinded to the treatment allocation. While no significant between-group differences were observed in the steatosis score (Figure 3J), the lowest inflammation score was recorded in mice exposed to water and Reb M. Mice drinking either aspartame or sucralose had 3.5-fold increase in their inflammation score compared to the score of water group (Figure 3K). Analysis of hepatic triglycerides concentration revealed that triglyceride content was similar between NNS and water-drinking groups (Figure 3L). In addition, mice drinking NNS had similar levels of ALT and AST compared with water-drinking mice (Figure 3M,N), with fructose group demonstrating the highest numeric AST levels, with p-value reaching statistically significance specifically when compared to sucralose and saccharin (Figure 3N).
While insulin levels did not differ among mice (Figure 4A), levels of C-peptide in mice exposed to Reb M were the lowest and significantly lower than mice drinking fructose (Figure 4B). This observation is in agreement with the improved insulin sensitivity observed in this group. Circulating levels of adiponectin and leptin were comparable between the various groups (Figure 4C,D).
Figure 4.
Levels of glycemic markers and adipokines following long term consumption of NNSs under HFD. The levels of the following parameters were evaluated at the end of experiment in plasma samples: A. Insulin (n = 7–10 mice/group). B. C-peptide (n = 8–10 mice/group). C. Adiponectin (n = 9–10 mice/group). D. Leptin (n = 3–4 mice/group). Data are presented as means ± SEM. (A–C) Data analyzed by a one-way ANOVA, with Dunnett's multiple comparisons post-test. (D) Data analyzed by Kruskal–Wallis with Dunn’s test for multiple comparisons. ∗p < 0.05.
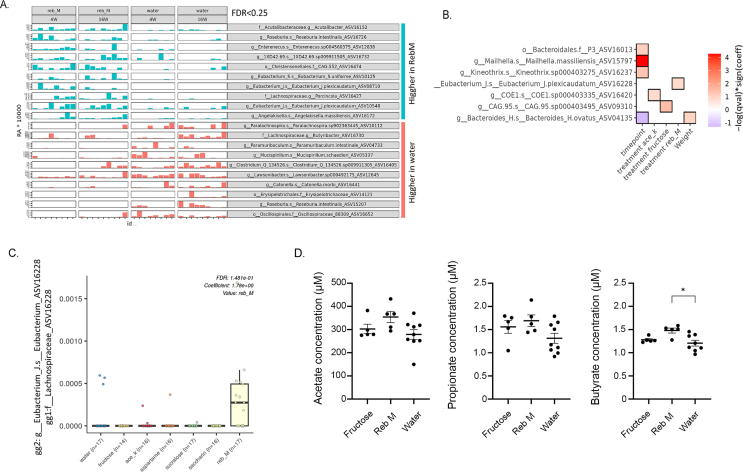
Given the suggested role for NNS-induced changes in microbiome on glucose metabolism, we next analyzed fecal microbiome of HFD-fed mice consuming fructose, water or any of the NNS at three different time points: before initiation of dietary intervention, during intervention and towards the end of the intervention. Using 16S rRNA, a total of 169 samples were sequenced (Supplementary dataset 1 summarizes ASVs and taxonomy). PCoA analysis visually demonstrated the major effects of age and diet on changes in microbiome (Figure 5A,B, respectively). Further taxonomic analysis at the class level also strengthens the above observations (Figure 5C). The transition to HFD dramatically increased the class-level diversity of the microbiome with increased frequency of some classes (Figure 5C–D, e.g Clostridia and Epsilonproteobacteria) and a decrease in Bacteroidia, but with reduced diversity at the ASV-levels (Figure 5D). Overall, the major alterations in the microbiome were attributed to the switch from a regular chow diet to HFD, observed already at week 4, and maintained during the 16 weeks period. Alpha diversity was not significantly different between NNS intervention and water groups, and PCoA of samples post-intervention is shown (Supplementary Fig. 4). To further investigate the effects of NNS exposure on the microbial composition per each of the NNS interventions, we studied the differential abundance between the bacterial composition (at 4 and 16 weeks) against bacterial composition seen with no NNS (water only) intervention. This analysis revealed that exposure to Reb M had the strongest effect on the microbiome composition with a substantial portion of bacterial ASVs increased (n = 34) and with a relatively small number of bacteria ASVs decreased (n = 10) which is of specific interest given the relative improvement in insulin sensitivity observed with Reb M exposure (Figure 6A showing the top 10 increase and decreased ASVs bacteria and the full list can be found in Supplementary dataset 2). To complement this, a multivariate association with linear models (MaAsLin) analysis was performed. This analysis, accounting for cage-specific effects, revealed that exposure to Reb M was linked with an increase in the Lachnospiracea family of bacteria (ASV 16228, Figure 6B–C, Supplementary Table 3, p < 0.001 FDR<0.15), which has been attributed in previous publications to improved insulin sensitivity [[52], [53], [54]]. Evaluation of plasma levels of the short-chain fatty acids, acetate, propionate, and butyrate in serum samples from mice drinking water, fructose, or Reb M revealed a mild significant increase in butyrate levels in mice drinking Reb M compared to water (Figure 6D).
Figure 5.
Microbial composition in mice with NNSs under HFD. Unweighted UniFrac PCoA plot representing diet-induced obesity mice before (baseline) and after 4 and 16 weeks of HFD colored by timepoints (A) or weight (B) with indicated PC1 and PC2 explaining 28% and 6.5% of the overall variation. C. Taxa bar plot at the class level per NNS and timepoint as indicated. The full taxonomy and ASVs can be found in Supplementary dataset 1. D. Shannon and Simpson diversity at the class- and ASV-levels are shown for baseline (before) and after 4 and 16 weeks of HFD.
Figure 6.
Reb M exposure association with specific fecal microbial composition and with blood short-chain fatty acid metabolite. A. Bar plot showing the top 10 increasing and top 10 decreasing ASVs (with genegene2 annotation) between water and RebM. Samples are stratified by group and ordered by time point (full list in Supplementary Dataset 2). Relative abundance (RA) values were multiplied by 10,000 to improve visualization. B. Multivariate Association with Linear Models (Maaslin) was applied to associate between microbial abundance and NNS exposure after controlling for cage, animal, and timepoint. Taxonomic classification of the numbered ASV is indicated. The heat-map indicates associations with q < 0.15 and p < 0.001. The red color indicates positive, and blue indicates negative association with continuous values, and increased or decreased (respectively) from the reference value (ref) in the case of dichotomous conditions. C. Specific association of significant Lachnospiraceae/Eubacterium [greengene 1 (gg1) or gg2 respectively, ASV16228, from panel B] is shown (see also supplementary dataset 1 for ASV sequence and annotations). D. Levels of Acetate (left panel), propionate (middle) and butyrate (right) as measured using gas chromatography–mass spectrometry (GC-MS) in plasma of mice drinking fructose (n = 5), Reb M (n = 5) or water (n = 8–9). Data analyzed by Kruskal–Wallis with Dunn's test for multiple comparisons. ∗p < 0.05.
4. Discussion
The prevalence of NNS and low-calorie sweeteners (LCSs) in Western diets is notable, extending well beyond products designed for individuals with diabetes and encompassing a wide array of reduced-calorie foods such as soft drinks, dairy items, baked goods, and confectionery [55]. Despite the acknowledgment of their safety by regulatory agencies such as the FDA and EFSA, considerable debate surrounds their long-term health implications. A long-lasting controversy is especially evident concerning the long-term metabolic effects of NNS, where conflicts arise between findings from cohort and observational studies and those from randomized controlled trials (RCTs) [10,11]. Most observational data suggest that routine consumption of NNS may be associated with a long-term increase in BMI and elevated risk of cardiometabolic diseases, primarily obesity and type 2 diabetes [8,10,11]. More specifically, an influence of specific artificial sweeteners on glucose tolerance through modification of gut microbiota [56,57], alterations in gut hormone secretion [58] or by interference with the neurological circuits of food reward [59] have been suggested as potential mechanisms. Yet, interventional studies in humans have largely failed to demonstrate the clinical relevance of these suggested mechanisms [[60], [61], [62], [63], [64]]. The RCT performed recently by Suez et al. [57] shed some light on the personalized effect of NNS consumption on microbiome as well as downstream effects on the host glucose tolerance. While some NNS demonstrated impairment in glucose tolerance, the use of stevia (Reb M) seems to have resulted in the lowest median AUC of the glucose tolerance test. In addition, as pointed out by the authors, the NNS in their study were administered as sachets containing a mixture of glucose and a given NNS, which could have influenced the results. Indeed, both the microbiome and glycemic responses seem to differ when administered as commercial NNS sachets (containing carbohydrates as fillers) [32,65] compared with the purified forms, as was used in our study, and in NNS-sweetened commercial beverages [26,64,[66], [67], [68]]. Longer, controlled studies in humans, with single NNS will likely provide an insight on the potential long-term metabolic effects of these findings. In addition, NNSs' ability to perhaps indirectly lower blood glucose by reduced carbohydrate intake adds complexity to the real-world relevance of these suggested mechanisms. Moreover, various NNSs were reported to differ in their metabolic effects, which may be mediated independently of their common activation of the sweet taste receptors [57,69]. Of note, while most RCTs do not support impaired metabolic effects of NNSs consumption, they have also failed to clearly support the intended benefits of NNSs on weight management. In this regard, the impairment in glucose tolerance observed with some NNS by Suez et al. continues to be of concern. Thus, while a causal role between sugar-sweetened beverages and deleterious metabolic effects in humans has been relatively well established [[70], [71], [72], [73]], studies have failed to present concrete evidence to establish a link between NNSs consumption and either beneficial or harmful consequences on glucose homeostasis, weight management or type 2 diabetes [10,[74], [75], [76], [77], [78]]. Therefore, the goal of the current study was to sort out the long-term metabolic effects of each of the NNSs separately, under healthy and obese conditions, and in comparison to both water and caloric/nutritive sweetener, in order to dissect out potential NNS-specific effects.
Regarding metabolic safety of NNSs, the concerns raised have been primarily attributed to potential interference with reward and hedonic pathways following sensation of the sweet taste without the distal sensing of nutrients and energy which may lead to compensatory hyperphagia [79]. Evaluation of these potential deleterious effects of NNS could be best assessed when consumption of each of the various NNS is compared to water-only. Here we demonstrate that in the setting of regular chow diet, exposure to NNS does not result in compensatory hyperphagia or alterations in food intake patterns, nor does it affect energy expenditure. As such, when compared to plain drinking water, NNS consumption does not lead to weight gain or metabolic abnormalities. In these settings, exposure to the caloric-sweetener fructose, at the same level of sweetness, does lead to a compensatory decrease in food intake, which does not seem to mitigate weight accumulation. In addition, exposure to fructose also resulted in an increase in glucose levels and impairment in glucose tolerance, which were more prominent under obese conditions. These deleterious effects of fructose are prevented when NNSs are used, resulting in glycemic indices that are comparable to water-only control. Therefore, it is unlikely that activation of the sweet taste receptor per se, in the absence of accompanying calories, mediates, directly or indirectly, compensatory over-eating, weight gain and glucose intolerance. It is worth mentioning that assessment of food intake and energy exposure was limited to ∼3 days of the prolonged dietary intervention, and could have missed alterations in caloric intake and/or energy expenditure early or late during the intervention. The mild decrease in glucose tolerance observed in lean mice exposed to aspartame is of interest, yet it was not observed in obese mice, nor was it accompanied by an increase in fasting glucose, alterations in C-peptide levels, or in insulin resistance. In addition, a recent study in humans did not demonstrate any impairment in glucose tolerance, at least after two weeks of daily aspartame exposure [57]. Regarding the potential efficacy, which is the potential use of NNS as an approach to decrease body weight and to improve metabolic alterations, this is best assessed when NNSs substitute a regular consumption of caloric sweeteners (in our study, the comparison of fructose to NNS). In this regard, in a recent statement, the WHO declared that in humans, there is not enough evidence to support such an approach [80]. In this study, for the most part, NNSs exposure exhibited improved metabolic outcomes when compared to fructose consumption, with Reb M exposure likely exhibiting the greatest effect. Of interest, the improved glycemic parameters observed with Reb M, when compared to either fructose or water consumption, suggest alternative mechanism(s) by which this glycoside derivative exerts its metabolic effects, which cannot be attributed simply to the absence of caloric value of this compound.
The observation that mice consuming the steviol glycoside Reb M exhibited an improved insulin sensitivity under either lean or obese condition is in agreement with previous publications, that highlighted potential metabolic benefits of various extracts of Stevia. Stevia glycosides were reported to exert an anti hyperglycemic effects, along with improvement of various metabolic parameters in some rodent models [[81], [82], [83], [84], [85], [86]] but not in all [87]. The potential mechanisms by which steviol glycosides improve glucose homeostastasis have not been fully elucidated. Yet, the stevioside of Stevia has been reported to exert an anti-inflammatory response by lowering the level of proinfammatory cytokines, and/or modulation of the IκBα/NF-κB and MAPK pathways [[88], [89], [90]]. In addition, stevia extracts have been implicated in improving the anti-oxidative capacity [[91], [92], [93]]. In a recent study, administration of stevia extracts to db/db mice was demonstrated to attenuate the marked hyperglycemia and insulin resistance by regulating mitochondrial function and increasing muscle fiber size [42]. Some of these metabolic beneficial effects were also reported in humans, either with or without diabetes [[94], [95], [96]]. Of note, a recent study in mice elegantly demonstrated that the effects of dietary butyrate on appetite suppression and amelioration of diet-induced obesity and improvement in insulin sensitivity are mediated by selective proliferation of Lachnospiraceae bacterium [97]. In accordance, the mild improvement in insulin sensitivity observed with Reb M consumption in our study, was associated not only with increased abundance of Lachnospiraceae, but also with increased circulating levels of butyrate. Of interest, the observations connecting Lachnospiraceae to improved metabolic health were also reported in humans. In the PRIMED-Plus randomized controlled trial, dietary intervention with Mediterranean diet was associated with increased occurrence of the Lachnospiraceae family in the gut, positively correlating with both adherence to the diet and metabolic health [53]. Similarly, the reduction of cardiometabolic risk observed with the Mediterranean diet was strongly associated with increased colonic abundance of Lachnospiraceae [54]. In another randomized control trial in humans, consumption of fried meat resulted in decreased abundance of Lachnospiraceae which was associated with muscle insulin resistance and increased in inflammatory cytokines and biomarkers [98]. Similar association was also recently reported in a pediatric population [99]. Yet, it is worth mentioning that the microbial changes observed with Reb M exposure may not necessarily be mechanistically linked to the relatively mild improvement in metabolic parameters observed in our study.
In summary, our study demonstrates that when systematically studied, NNSs do not have any recognizable deleterious, long-term metabolic effects. Activation of the sweet taste, by itself, does not seem to activate food seeking behavior or to increase caloric intake, and therefore, does not result in significant increase in body weight or metabolic abnormalities. In addition, the stevia derivative Reb M may improve insulin sensitivity, further highlighting the importance of evaluating each one of the NNSs separately. Additional health-related concerns raised with the use of NNSs have not been assessed in this study and should be carefully assessed in designated experiments in a similar fashion.
CRediT authorship contribution statement
Moran Rathaus: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Conceptualization. Loziana Azem: Writing – original draft, Investigation, Formal analysis, Conceptualization. Rinat Livne: Writing – review & editing, Investigation. Sophie Ron: Writing – review & editing, Investigation. Idit Ron: Writing – review & editing, Investigation. Rotem Hadar: Writing – review & editing, Investigation. Gilat Efroni: Writing – review & editing, Investigation. Amnon Amir: Writing – review & editing, Investigation. Tzipi Braun: Writing – review & editing, Investigation. Yael Haberman: Writing – review & editing, Investigation. Amir Tirosh: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
None.
Acknowledgements
We would like to thank the entire members of the Tirosh lab for thoughtful discussions. This work was supported by a research grant from Gat (27795) and in part by the Israel Science Foundation (2070/22). Both to A.T. The funding agencies had no involvement in the execution of the study, analysis and interpretation of the data, the decision to publish the manuscript and drafting of the manuscript. Supplementary Fig. 1 was created in BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.101985.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Summary of experimental layout. HFD, high fat diet; ITT, insulin tolerance test; GTT, glucose tolerance test.
Fasting blood glucose and weight gain values of all groups through all the experimental intervention. (A) glucose, chow diet (B) glucose, high fat diet. (C) weight gain, chow diet (D) weight gain, high fat diet.
Lipid profile and liver enzymes in mice consumption NNSs under Regular Diet. (A) Cholesterol (n=6-10 mice/group), (B) triglycerides (C) HDL-Cholesterol and LDL-Cholesterol (D), were measured at the end of the experiment. Serum ALT (E) and AST (F) levels were also measured at the end of the experiment (n=5-7 mice/group). Data are presented as means ± SEM. (A,B,F) Data analyzed by a one-way ANOVA, with a Dunnett’s test for multiple comparisons. (C-E) Data analyzed by Kruskal-Wallis with Dunn's test for multiple comparisons. *p<0.05, ∗∗p<0.01.
Alpha diversity and PCoA by treatment. A. Boxplot of alpha diversity values, using ASV-based Faith’s phylogenetic and Shanon diversities, by treatment at the indicated timepoint. No significant differences were noted between water and the different NNS interventions. B. Unweighted UniFrac PCoA plot colored by NNS interventions group as indicated.
Stability of sweeteners solutions. Solutions were freshly prepared and their concentration was measured for five consecutive days using HPLC. ∗Due to technical limitations, sucralose was measured only twice as indicated.
Summary of the amount of each sweetener in drinking water, as well as the average amount and calories consumed per day.
Multivariate association with linear models (MaAsLin) results. Association between microbial abundance and NNS exposure after controlling for cage, animal and time point. gg1, Greengenes1 ASV annotation 2013; gg2, Greengenes 2 ASV annotation 2022.
Summary of ASVs and taxonomy per sample.
Comparison between each NNS/fructose and water.
Data availability
Data will be made available on request.
References
- 1.Guideline: sugars intake for adults and children. World Health Organization; Geneva: 2015. [PubMed] [Google Scholar]
- 2.Huang Y., Chen Z., Chen B., Li J., Yuan X., Li J., et al. Dietary sugar consumption and health: umbrella review. BMJ. 2023;381 doi: 10.1136/bmj-2022-071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan T.A., Lee J.J., Ayoub-Charette S., Noronha J.C., McGlynn N., Chiavaroli L., et al. WHO guideline on the use of non-sugar sweeteners: a need for reconsideration. Eur J Clin Nutr. 2023;77:1009–1013. doi: 10.1038/s41430-023-01314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinosa A., Mendoza K., Laviada-Molina H., Rangel-Méndez J.A., Molina-Segui F., Sun Q., et al. Effects of non-nutritive sweeteners on the BMI of children and adolescents: a systematic review and meta-analysis of randomised controlled trials and prospective cohort studies. Lancet Global Health. 2023;11(Suppl 1):S8. doi: 10.1016/S2214-109X(23)00093-1. [DOI] [PubMed] [Google Scholar]
- 5.Rogers P.J., Appleton K.M. The effects of low-calorie sweeteners on energy intake and body weight: a systematic review and meta-analyses of sustained intervention studies. Int J Obes. 2021;45:464–478. doi: 10.1038/s41366-020-00704-2. [DOI] [PubMed] [Google Scholar]
- 6.Lohner S., Kuellenberg de Gaudry D., Toews I., Ferenci T., Meerpohl J.J. Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst Rev. 2020;5 doi: 10.1002/14651858.CD012885.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laviada-Molina H., Molina-Segui F., Pérez-Gaxiola G., Cuello-García C., Arjona-Villicaña R., Espinosa-Marrón A., et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev. 2020;21 doi: 10.1111/obr.13020. [DOI] [PubMed] [Google Scholar]
- 8.Fowler S.P., Williams K., Resendez R.G., Hunt K.J., Hazuda H.P., Stern M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 9.Sylvetsky A.C., Figueroa J., Zimmerman T., Swithers S.E., Welsh J.A. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr. Obes. 2019;14 doi: 10.1111/ijpo.12535. [DOI] [PubMed] [Google Scholar]
- 10.Azad M.B., Abou-Setta A.M., Chauhan B.F., Rabbani R., Lys J., Copstein L., et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can Med Assoc J. 2017;189:E929–E939. doi: 10.1503/cmaj.161390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylvetsky A.C., Rother K.I. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity. 2018;26:635–640. doi: 10.1002/oby.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walbolt J., Koh Y. Non-nutritive sweeteners and their associations with obesity and type 2 diabetes. J. Obes. Metab. Syndr. 2020;29:114–123. doi: 10.7570/jomes19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basson A.R., Rodriguez-Palacios A., Cominelli F. Artificial sweeteners: history and new concepts on inflammation. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.746247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan C.B., Hashemi Z., Subhan F.B. The impact of low and no-caloric sweeteners on glucose absorption, incretin secretion, and glucose tolerance. Appl Physiol Nutr Metabol. 2017;42:793–801. doi: 10.1139/apnm-2016-0705. [DOI] [PubMed] [Google Scholar]
- 15.Shankar P., Ahuja S., Sriram K. Non-nutritive sweeteners: review and update. Nutrition. 2013;29:1293–1299. doi: 10.1016/j.nut.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Liauchonak I., Qorri B., Dawoud F., Riat Y., Szewczuk M.R. Non-nutritive sweeteners and their implications on the development of metabolic syndrome. Nutrients. 2019;11 doi: 10.3390/nu11030644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian X., Chi L., Gao B., Tu P., Ru H., Lu K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collison K.S., Makhoul N.J., Zaidi M.Z., Saleh S.M., Andres B., Inglis A., et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gul S.S., Hamilton A.R.L., Munoz A.R., Phupitakphol T., Liu W., Hyoju S.K., et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl Physiol Nutr Metabol. 2017;42:77–83. doi: 10.1139/apnm-2016-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsutomi K., Masaki T., Shimasaki T., Gotoh K., Chiba S., Kakuma T., et al. Effects of a nonnutritive sweetener on body adiposity and energy metabolism in mice with diet-induced obesity. Metab Clin Exp. 2014;63:69–78. doi: 10.1016/j.metabol.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Olivier-Van Stichelen S., Rother K.I., Hanover J.A. Maternal exposure to non-nutritive sweeteners impacts progeny's metabolism and microbiome. Front Microbiol. 2019;10:1360. doi: 10.3389/fmicb.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otero-Losada M., Cao G., Mc Loughlin S., Rodríguez-Granillo G., Ottaviano G., Milei J. Rate of atherosclerosis progression in ApoE-/- mice long after discontinuation of cola beverage drinking. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Q., Cai L., Jia H., Zhu X., Chen L., Deng S. Low intake of digestible carbohydrates ameliorates duodenal absorption of carbohydrates in mice with glucose metabolism disorders induced by artificial sweeteners. J Sci Food Agric. 2019;99:4952–4962. doi: 10.1002/jsfa.9727. [DOI] [PubMed] [Google Scholar]
- 24.Bailey C.J., Day C., Knapper J.M., Turner S.L., Flatt P.R. Antihyperglycaemic effect of saccharin in diabetic ob/ob mice. Br J Pharmacol. 1997;120:74–78. doi: 10.1038/sj.bjp.0700871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parlee S.D., Simon B.R., Scheller E.L., Alejandro E.U., Learman B.S., Krishnan V., et al. Administration of saccharin to neonatal mice influences body composition of adult males and reduces body weight of females. Endocrinology. 2014;155:1313–1326. doi: 10.1210/en.2013-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano J., Smith K.R., Crouch A.L., Sharma V., Yi F., Vargova V., et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome. 2021;9:11. doi: 10.1186/s40168-020-00976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collison K.S., Makhoul N.J., Zaidi M.Z., Al-Rabiah R., Inglis A., Andres B.L., et al. Interactive effects of neonatal exposure to monosodium glutamate and aspartame on glucose homeostasis. Nutr Metab. 2012;9:58. doi: 10.1186/1743-7075-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q.-P., Browman D., Herzog H., Neely G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippaert K., Pironet A., Mesuere M., Sones W., Vermeiren L., Kerselaers S., et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat Commun. 2017;8 doi: 10.1038/ncomms14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosales-Gómez C.A., Martínez-Carrillo B.E., Reséndiz-Albor A.A., Ramírez-Durán N., Valdés-Ramos R., Mondragón-Velásquez T., et al. Chronic consumption of sweeteners and its effect on glycaemia, cytokines, hormones, and lymphocytes of GALT in CD1 mice. BioMed Res Int. 2018;2018 doi: 10.1155/2018/1345282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holvoet P., Rull A., García-Heredia A., López-Sanromà S., Geeraert B., Joven J., et al. Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: a transcriptomic and metabolomic study. Food Chem Toxicol. 2015;77:22–33. doi: 10.1016/j.fct.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 33.Uebanso T., Ohnishi A., Kitayama R., Yoshimoto A., Nakahashi M., Shimohata T., et al. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients. 2017;9 doi: 10.3390/nu9060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian X., Tu P., Chi L., Gao B., Ru H., Lu K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem Toxicol. 2017;107:530–539. doi: 10.1016/j.fct.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian X., Chi L., Gao B., Tu P., Ru H., Lu K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol. 2017;8:487. doi: 10.3389/fphys.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morissette A., de Wouters d'Oplinter A., Andre D.M., Lavoie M., Marcotte B., Varin T.V., et al. Rebaudioside D decreases adiposity and hepatic lipid accumulation in a mouse model of obesity. Sci Rep. 2024;14:3077. doi: 10.1038/s41598-024-53587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glendinning J.I., Hart S.A., Lee H., Maleh J., Ortiz G., Sang Ryu Y., et al. Low calorie sweeteners cause only limited metabolic effects in mice. Am J Physiol Regul Integr Comp Physiol. 2019 doi: 10.1152/ajpregu.00245.2019. [DOI] [PubMed] [Google Scholar]
- 38.Wang X., Guo L., Zheng L., Zhao W., Li L. Natural sweetener glycyrrhetinic acid monoglucuronide improves glucose homeostasis in healthy mice. J Agric Food Chem. 2024;72:3483–3494. doi: 10.1021/acs.jafc.3c06151. [DOI] [PubMed] [Google Scholar]
- 39.Cyphert E.L., Liu C., Morales A.L., Nixon J.C., Blackford E., Garcia M., et al. Effects of long-term high dose aspartame on body mass, bone strength, femoral geometry, and microbiota composition in a young and aged cohort of male and female mice. bioRxiv. 2024 doi: 10.1101/2024.01.02.573970. [DOI] [Google Scholar]
- 40.Pino-Seguel P., Moya O., Borquez J.C., Pino-de la Fuente F., Díaz-Castro F., Donoso-Barraza C., et al. Sucralose consumption ameliorates high-fat diet-induced glucose intolerance and liver weight gain in mice. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.979624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zani F., Blagih J., Gruber T., Buck M.D., Jones N., Hennequart M., et al. The dietary sweetener sucralose is a negative modulator of T cell-mediated responses. Nature. 2023;615:705–711. doi: 10.1038/s41586-023-05801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han J.-Y., Park M., Lee H.-J. Stevia (Stevia rebaudiana) extract ameliorates insulin resistance by regulating mitochondrial function and oxidative stress in the skeletal muscle of db/db mice. BMC Complement. Med. Ther. 2023;23:264. doi: 10.1186/s12906-023-04033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang W., Menke A.L., Driessen A., Koek G.H., Lindeman J.H., Stoop R., et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bier A., Braun T., Khasbab R., Di Segni A., Grossman E., Haberman Y., et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10 doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Segni A., Braun T., BenShoshan M., Farage Barhom S., Glick Saar E., Cesarkas K., et al. Guided protocol for fecal microbial characterization by 16S rRNA-amplicon sequencing. J Vis Exp. 2018 doi: 10.3791/56845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amir A., McDonald D., Navas-Molina J.A., Kopylova E., Morton J.T., Zech Xu Z., et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems. 2017;2 doi: 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z.Z., Amir A., Sanders J., Zhu Q., Morton J.T., Bletz M.C., et al. Calour: an interactive, microbe-centric analysis tool. mSystems. 2019;4 doi: 10.1128/mSystems.00269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13 doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jürgens H., Haass W., Castañeda T.R., Schürmann A., Koebnick C., Dombrowski F., et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 52.Udayappan S., Manneras-Holm L., Chaplin-Scott A., Belzer C., Herrema H., Dallinga-Thie G.M., et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. Npj Biofilms and Microbiomes. 2016;2 doi: 10.1038/npjbiofilms.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muralidharan J., Moreno-Indias I., Bulló M., Lopez J.V., Corella D., Castañer O., et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am J Clin Nutr. 2021;114:1148–1158. doi: 10.1093/ajcn/nqab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galié S., García-Gavilán J., Camacho-Barcía L., Atzeni A., Muralidharan J., Papandreou C., et al. Effects of the mediterranean diet or nut consumption on gut microbiota composition and fecal metabolites and their relationship with cardiometabolic risk factors. Mol Nutr Food Res. 2021;65 doi: 10.1002/mnfr.202000982. [DOI] [PubMed] [Google Scholar]
- 55.Martyn D., Darch M., Roberts A., Lee H.Y., Yaqiong Tian T., Kaburagi N., et al. Low-/No-Calorie sweeteners: a review of global intakes. Nutrients. 2018;10 doi: 10.3390/nu10030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suez J., Korem T., Zilberman-Schapira G., Segal E., Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microb. 2015;6:149–155. doi: 10.1080/19490976.2015.1017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suez J., Cohen Y., Valdés-Mas R., Mor U., Dori-Bachash M., Federici S., et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022;185:3307–3328.e19. doi: 10.1016/j.cell.2022.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Brown R.J., Rother K.I. Non-nutritive sweeteners and their role in the gastrointestinal tract. J Clin Endocrinol Metab. 2012;97:2597–2605. doi: 10.1210/jc.2012-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: neuroscience 2010. Yale J Biol Med. 2010;83:101–108. [PMC free article] [PubMed] [Google Scholar]
- 60.Griffioen-Roose S., Smeets P.A.M., Weijzen P.L.G., van Rijn I., van den Bosch I., de Graaf C. Effect of replacing sugar with non-caloric sweeteners in beverages on the reward value after repeated exposure. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maersk M., Belza A., Holst J.J., Fenger-Grøn M., Pedersen S.B., Astrup A., et al. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr. 2012;66:523–529. doi: 10.1038/ejcn.2011.223. [DOI] [PubMed] [Google Scholar]
- 62.Almiron-Roig E., Navas-Carretero S., Castelnuovo G., Kjølbæk L., Romo-Hualde A., Normand M., et al. Impact of acute consumption of beverages containing plant-based or alternative sweetener blends on postprandial appetite, food intake, metabolism, and gastro-intestinal symptoms: results of the SWEET beverages trial. Appetite. 2023;184 doi: 10.1016/j.appet.2023.106515. [DOI] [PubMed] [Google Scholar]
- 63.Stamataki N.S., Crooks B., Ahmed A., McLaughlin J.T. Effects of the daily consumption of stevia on glucose homeostasis, body weight, and energy intake: a randomised open-label 12-week trial in healthy adults. Nutrients. 2020;12 doi: 10.3390/nu12103049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmad S.Y., Friel J.K., MacKay D.S. The effect of the artificial sweeteners on glucose metabolism in healthy adults: a randomized, double-blinded, crossover clinical trial. Appl Physiol Nutr Metabol. 2020;45:606–612. doi: 10.1139/apnm-2019-0359. [DOI] [PubMed] [Google Scholar]
- 65.Romo-Romo A., Aguilar-Salinas C.A., Brito-Córdova G.X., Gómez-Díaz R.A., Almeda-Valdes P. Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial. Am J Clin Nutr. 2018;108:485–491. doi: 10.1093/ajcn/nqy152. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad S.Y., Friel J., Mackay D. The effects of non-nutritive artificial sweeteners, aspartame and sucralose, on the gut microbiome in healthy adults: secondary outcomes of a randomized double-blinded crossover clinical trial. Nutrients. 2020;12 doi: 10.3390/nu12113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y., Keogh J.B., Clifton P.M. Consumption of a beverage containing aspartame and acesulfame K for two weeks does not adversely influence glucose metabolism in adult males and females: a randomized crossover study. Int J Environ Res Publ Health. 2020;17 doi: 10.3390/ijerph17239049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson P., Santibañez R., Aguirre C., Galgani J.E., Garrido D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br J Nutr. 2019;122:856–862. doi: 10.1017/S0007114519001570. [DOI] [PubMed] [Google Scholar]
- 69.Higgins K.A., Mattes R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109:1288–1301. doi: 10.1093/ajcn/nqy381. [DOI] [PubMed] [Google Scholar]
- 70.Tran Q.D., Nguyen T.H.H., Le C.L., Hoang L.V., Vu T.Q.C., Phan N.Q., et al. Sugar-sweetened beverages consumption increases the risk of metabolic syndrome and its components in adults: consistent and robust evidence from an umbrella review. Clin. Nutr. ESPEN. 2023;57:655–664. doi: 10.1016/j.clnesp.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen M., Jarvis S.E., Tinajero M.G., Yu J., Chiavaroli L., Mejia S.B., et al. Sugar-sweetened beverage consumption and weight gain in children and adults: a systematic review and meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2023;117:160–174. doi: 10.1016/j.ajcnut.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Hu F.B. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14:606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik V.S., Pan A., Willett W.C., Hu F.B. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alsunni A.A. Effects of artificial sweetener consumption on glucose homeostasis and its association with type 2 diabetes and obesity. Int J Gen Med. 2020;13:775–785. doi: 10.2147/IJGM.S274760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toews I., Lohner S., Küllenberg de Gaudry D., Sommer H., Meerpohl J.J. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364 doi: 10.1136/bmj.k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pang M.D., Goossens G.H., Blaak E.E. The impact of artificial sweeteners on body weight control and glucose homeostasis. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.598340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang R., Noronha J.C., Khan T.A., McGlynn N., Back S., Grant S.M., et al. The effect of non-nutritive sweetened beverages on postprandial glycemic and endocrine responses: a systematic review and network meta-analysis. Nutrients. 2023;15 doi: 10.3390/nu15041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGlynn N.D., Khan T.A., Wang L., Zhang R., Chiavaroli L., Au-Yeung F., et al. Association of low- and No-calorie sweetened beverages as a replacement for sugar-sweetened beverages with body weight and cardiometabolic risk: a systematic review and meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilk K., Korytek W., Pelczyńska M., Moszak M., Bogdański P. The effect of artificial sweeteners use on sweet taste perception and weight loss efficacy: a review. Nutrients. 2022;14 doi: 10.3390/nu14061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Use of non-sugar sweeteners: WHO guideline. World Health Organization; Geneva: 2023. [PubMed] [Google Scholar]
- 81.Atteh J.O., Onagbesan O.M., Tona K., Decuypere E., Geuns J.M.C., Buyse J. Evaluation of supplementary stevia (Stevia rebaudiana, bertoni) leaves and stevioside in broiler diets: effects on feed intake, nutrient metabolism, blood parameters and growth performance. J Anim Physiol Anim Nutr. 2008;92:640–649. doi: 10.1111/j.1439-0396.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen J., Jeppesen P.B., Abudula R., Dyrskog S.E.U., Colombo M., Hermansen K. Stevioside does not cause increased basal insulin secretion or beta-cell desensitization as does the sulphonylurea, glibenclamide: studies in vitro. Life Sci. 2006;78:1748–1753. doi: 10.1016/j.lfs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Jeppesen P.B., Gregersen S., Poulsen C.R., Hermansen K. Stevioside acts directly on pancreatic beta cells to secrete insulin: actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metab Clin Exp. 2000;49:208–214. doi: 10.1016/s0026-0495(00)91325-8. [DOI] [PubMed] [Google Scholar]
- 84.Suanarunsawat T., Chaiyabutr N. The effect of stevioside on glucose metabolism in rat. Can J Physiol Pharmacol. 1997;75:976–982. [PubMed] [Google Scholar]
- 85.Shivanna N., Naika M., Khanum F., Kaul V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabetes Complicat. 2013;27:103–113. doi: 10.1016/j.jdiacomp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Khakpai F., Naseroleslami M., Moheb-Alian M., Ghanimati E., Abdollah-Pour F., Mousavi-Niri N. Intra-gastrically administration of Stevia and particularly Nano-Stevia reversed the hyperglycemia, anxiety, and memory impairment in streptozotocin-induced diabetic rats. Physiol Behav. 2023;263 doi: 10.1016/j.physbeh.2023.114100. [DOI] [PubMed] [Google Scholar]
- 87.Dyrskog S.E.U., Jeppesen P.B., Chen J., Christensen L.P., Hermansen K. The diterpene glycoside, rebaudioside A, does not improve glycemic control or affect blood pressure after eight weeks treatment in the Goto-Kakizaki rat. Rev Diabet Stud. 2005;2:84–91. doi: 10.1900/RDS.2005.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang T., Guo M., Song X., Zhang Z., Jiang H., Wang W., et al. Stevioside plays an anti-inflammatory role by regulating the NF-κB and MAPK pathways in S. aureus-infected mouse mammary glands. Inflammation. 2014;37:1837–1846. doi: 10.1007/s10753-014-9915-0. [DOI] [PubMed] [Google Scholar]
- 89.Cai T., Ye H., Jiang H., Lin C., Lou C., Wang W., et al. Stevioside targets the NF-κB and MAPK pathways for inhibiting inflammation and apoptosis of chondrocytes and ameliorates osteoarthritis in vivo. Int Immunopharm. 2023;115 doi: 10.1016/j.intimp.2023.109683. [DOI] [PubMed] [Google Scholar]
- 90.Mehmood A., Althobaiti F., Zhao L., Usman M., Chen X., Alharthi F., et al. Anti-inflammatory potential of stevia residue extract against uric acid-associated renal injury in mice. J Food Biochem. 2022;46 doi: 10.1111/jfbc.14286. [DOI] [PubMed] [Google Scholar]
- 91.Papaefthimiou M., Kontou P.I., Bagos P.G., Braliou G.G. Antioxidant activity of leaf extracts from Stevia rebaudiana bertoni exerts attenuating effect on diseased experimental rats: a systematic review and meta-analysis. Nutrients. 2023;15 doi: 10.3390/nu15153325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Altamimi J.Z., Alfaris N.A., Alshammari G.M., Alagal R.I., Aljabryn D.H., Yahya M.A. Isosteviol attenuates streptozotocin-mediated diabetic nephropathy in rats by upregulating and stimulating adenosine monophosphate-activated protein kinase. J Physiol Pharmacol. 2023;74 doi: 10.26402/jpp.2023.3.06. [DOI] [PubMed] [Google Scholar]
- 93.Kim E.-N., Trang N.M., Kang H., Kim K.H., Jeong G.-S. Phytol suppresses osteoclast differentiation and oxidative stress through Nrf2/HO-1 regulation in RANKL-induced RAW264.7 cells. Cells. 2022;11 doi: 10.3390/cells11223596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gregersen S., Jeppesen P.B., Holst J.J., Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metab Clin Exp. 2004;53:73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 95.Raghavan G., Bapna A., Mehta A., Shah A., Vyas T. Effect of sugar replacement with stevia-based tabletop sweetener on weight and cardiometabolic health among Indian adults. Nutrients. 2023;15 doi: 10.3390/nu15071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chowdhury A.I., Rahanur Alam M., Raihan M.M., Rahman T., Islam S., Halima O. Effect of stevia leaves (Stevia rebaudiana Bertoni) on diabetes: a systematic review and meta-analysis of preclinical studies. Food Sci Nutr. 2022;10:2868–2878. doi: 10.1002/fsn3.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z., Zhou E., Liu C., Wicks H., Yildiz S., Razack F., et al. Dietary butyrate ameliorates metabolic health associated with selective proliferation of gut Lachnospiraceae bacterium 28-4. JCI Insight. 2023;8 doi: 10.1172/jci.insight.166655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao J., Guo X., Wei W., Li R., Hu K., Liu X., et al. The association of fried meat consumption with the gut microbiota and fecal metabolites and its impact on glucose homoeostasis, intestinal endotoxin levels, and systemic inflammation: a randomized controlled-feeding trial. Diabetes Care. 2021;44:1970–1979. doi: 10.2337/dc21-0099. [DOI] [PubMed] [Google Scholar]
- 99.Sun J., Ma X., Yang L., Jin X., Zhao M., Xi B., et al. The number of metabolic syndrome risk factors predicts alterations in gut microbiota in Chinese children from the Huantai study. BMC Pediatr. 2023;23:191. doi: 10.1186/s12887-023-04017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of experimental layout. HFD, high fat diet; ITT, insulin tolerance test; GTT, glucose tolerance test.
Fasting blood glucose and weight gain values of all groups through all the experimental intervention. (A) glucose, chow diet (B) glucose, high fat diet. (C) weight gain, chow diet (D) weight gain, high fat diet.
Lipid profile and liver enzymes in mice consumption NNSs under Regular Diet. (A) Cholesterol (n=6-10 mice/group), (B) triglycerides (C) HDL-Cholesterol and LDL-Cholesterol (D), were measured at the end of the experiment. Serum ALT (E) and AST (F) levels were also measured at the end of the experiment (n=5-7 mice/group). Data are presented as means ± SEM. (A,B,F) Data analyzed by a one-way ANOVA, with a Dunnett’s test for multiple comparisons. (C-E) Data analyzed by Kruskal-Wallis with Dunn's test for multiple comparisons. *p<0.05, ∗∗p<0.01.
Alpha diversity and PCoA by treatment. A. Boxplot of alpha diversity values, using ASV-based Faith’s phylogenetic and Shanon diversities, by treatment at the indicated timepoint. No significant differences were noted between water and the different NNS interventions. B. Unweighted UniFrac PCoA plot colored by NNS interventions group as indicated.
Stability of sweeteners solutions. Solutions were freshly prepared and their concentration was measured for five consecutive days using HPLC. ∗Due to technical limitations, sucralose was measured only twice as indicated.
Summary of the amount of each sweetener in drinking water, as well as the average amount and calories consumed per day.
Multivariate association with linear models (MaAsLin) results. Association between microbial abundance and NNS exposure after controlling for cage, animal and time point. gg1, Greengenes1 ASV annotation 2013; gg2, Greengenes 2 ASV annotation 2022.
Summary of ASVs and taxonomy per sample.
Comparison between each NNS/fructose and water.
Data Availability Statement
Data will be made available on request.