Abstract
XT-I (xylosyltransferase I) is the initial enzyme in the post-translational biosynthesis of glycosaminoglycan chains in proteoglycans. To gain insight into the structure–function relationship of the enzyme, a soluble active form of human XT-I was expressed in High Five insect cells with an apparent molecular mass of 90 kDa. Analysis of the electrophoretic mobility of the protein under non-reducing and reducing conditions indicated that soluble XT-I does not form homodimers through disulphide bridges. In addition, the role of the cysteine residues was investigated by site-directed mutagenesis combined with chemical modifications of XT-I by N-phenylmaleimide. Replacement of Cys471 or Cys574 with alanine led to a complete loss of catalytic activity, indicating the necessity of these residues for maintaining an active conformation of soluble recombinant XT-I by forming disulphide bonds. On the other hand, N-phenylmaleimide treatment showed no effect on wild-type XT-I but strongly inactivated the cysteine mutants in a dose-dependant manner, indicating that seven intramolecular disulphide bridges are formed in wild-type XT-I. The inhibitory effect of UDP on the XT-I activity of C561A (Cys561→Ala) mutant enzyme was significantly reduced compared with all other tested cysteine mutants. In addition, we tested for binding to UDP-agarose beads. The inactive mutants revealed no significantly different nucleotide-binding properties. Our study demonstrates that recombinant XT-I is organized as a monomer with no free thiol groups and strongly suggests that the catalytic activity does not depend on the presence of free thiol groups, furthermore, we identified five cysteine residues which are critical for enzyme activity.
Keywords: cysteine, functional characterization, glycosaminoglycan, proteoglycan, site-directed mutagenesis, xylosyltransferase I
Abbreviations: DTT, dithiothreitol; GAG, glycosaminoglycan; XT-I, xylosyltransferase I; rXT-I, recombinant XT-I; wt, wild-type; for brevity, the single-letter system for amino acids has been used, C561, for example means Cys561
INTRODUCTION
Proteoglycans are macromolecules composed of GAG (glycosaminoglycan) chains covalently bound to a protein core. The different types of proteoglycans are distinguished by their various core proteins and by structures of the polysaccharide chains [1]. GAGs are increasingly implicated as important regulators of many biological processes such as cell adhesion and differentiation, cytokine action and modulation of enzyme catalysis, and owe their activities to interactions with various components of cell surfaces and extracellular matrices through specific saccharide sequences [2–5]. GAG chain synthesis is supposed to be strictly regulated, since GAG structures vary considerably during normal embryonic development, growth and aging [6–8]. In addition, GAG chains differ in size and number under pathological conditions, leading to the alteration of the structural and functional properties of the tissues.
In GAG biosynthesis, the disaccharide units are formed by alternative monosaccharide addition from corresponding UDP-sugars to the non-reducing end of the elongating chain [9]. The GAGs chondroitin, dermatan and heparan sulphates are initiated by the formation of a common carbohydrate sequence, GlcAβ1,3Galβ1,3Galβ1,4Xylβ-, bound to specific serine residues in the core protein to form the so-called GAG protein linkage region [10].
XT-I (xylosyltransferase I; EC 2.4.2.26) is the chain-initiating enzyme involved in the biosynthesis of GAG-containing proteoglycans [11,12]. The enzyme catalyses the transfer of D-xylose from UDP-D-xylose to specific serine residues of the core protein and is a regulatory factor in chondroitin sulphate biosynthesis [10]. XT-I activity was found to be present in the cisternae of the rough endoplasmic reticulum of various species [13], and we have shown that the enzyme is secreted from the endoplasmic reticulum into the extracellular space together with chondroitin sulphate proteoglycans [14,15]. However, the processes resulting in the release of XT-I from the endoplasmic reticulum or the Golgi compartments and the role of XT-I in the extracellular matrix are not yet known.
Analyses of the primary structure revealed that XT-I is a member of the CAZy glycosyltransferase family 14 and, therefore, is homologous with β1,6-N-acetylglucosaminyltransferases involved in O-glycan and poly-N-acetyllactosamine branching [16]. XT-I differs from the other CAZy family 14 enzymes first of all by size, being approx. 800 amino acids long, and also in the position of the cysteine residues. The members of this glycosyltransferase family exhibit a common homologous region of approx. 300 amino acids with a degree of identity of approx. 30%. A putative involvement of this common region in the binding of the UDP-sugar donors and in the catalytic mechanism would be expected.
XT-I has a type II transmembrane protein topology, as do all Golgi-associated glycosyltransferases cloned so far. As generally seen for these enzymes, a short cytoplasmic segment of XT-I is connected to a transmembrane domain followed by a large intraluminal domain consisting of a stem region and catalytic domain. We have shown in previous studies that, in addition to the XT-I gene, higher organisms share another gene coding for XT-II, a protein highly homologous with XT-I [17]. However, the catalytic activity of XT-II is not yet known. Furthermore, Drosophila melanogaster [18] and Caenorhabditis elegans [19] have only one XT orthologue.
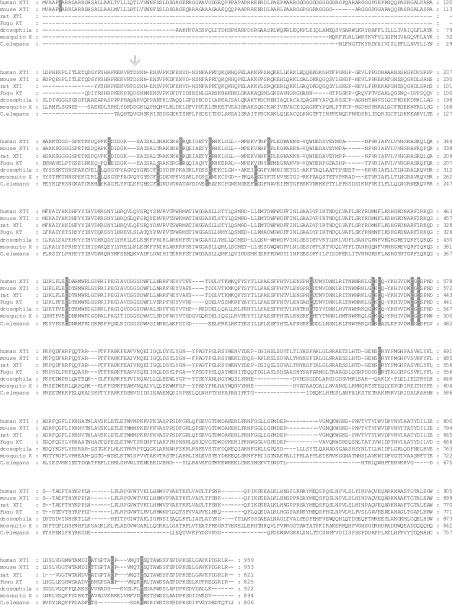
Among the conserved residues in the XT-I, including human, Mus musculus, Rattus norvegicus, Anopheles gambiae, D. melanogaster, C. elegans and Fugu rubripes, are 15 cysteine residues. In the mammalian XT-I, 14 of the cysteine residues are completely conserved, although not all of these residues are shared with other species such as flies and worms. Interestingly, these 14 cysteine residues found in the protein sequence are also conserved in XT from F. rubripes. Conserved cysteine residues occur in many glycosyltransferases [20–22], and intra- and inter-molecular disulphide bonds have been demonstrated [23]. Disulphide bonds can facilitate or stabilize protein folding, although cysteine residues could also be important for the activity of glycosyltransferase [24,25].
To provide further insight into the structure and function of XT-I, we generated an expression system in High Five insect cells for the expression of wt (wild-type) and mutant forms of XT-I. In the present study, we analysed the importance of the cysteine residues for the enzymic activity of the human XT-I for the first time. We mutated all 14 cysteine residues to determine which of the conserved residues are critical for enzymic activity. We found that two of the 14 cysteine mutants showed a complete loss of function, indicating the necessity of these residues for enzymic activity.
EXPERIMENTAL
Materials
High Five insect cells (Trichoplusia ni, BTI-TN-5B1-4) were purchased from Invitrogen (Groningen, The Netherlands), Insect Xpress medium was obtained from Cambrex (Apen, Germany) and aqua ad injecta from Braun (Melsungen, Germany). Chondroitin 6-sulphate, heparan sulphate, UDP, UDP-agarose beads, heparin from porcine intestinal mucosa, heparin-acrylic beads, N-phenylmaleimide and the Bicinchoninic Acid Protein Assay kit were purchased from Sigma (Deisenhofen, Germany). Cell culture flasks, serological pipettes and sterile tubes were purchased from Becton Dickinson (Heidelberg, Germany). The vector pMIB-V5-His and the horseradish peroxidase-coupled anti-V5 antibodies were from Invitrogen. The QuikChange site-directed mutagenesis kit was from Stratagene (La Jolla, CA, U.S.A.). Pfu DNA polymerase was obtained from PeqLab (Erlangen, Germany), and Taq polymerase and Hotstar Taq polymerase were from Qiagen (Hilden, Germany). Qiaprep Spin Plasmid kit and the PCR purification kit were also obtained from Qiagen. Precast polyacrylamide gels, buffers, and NuPAGE electrophoresis system XCell II Mini-Cell and Blot Module were from Novex (San Diego, CA, U.S.A.). Ultrafiltration cells, YM1 membranes, Microcon 3000 tubes and PVDF membranes (Immobilon P) were purchased from Millipore (Eschborn, Germany). UDP-[14C]xylose (9.88 kBq/nmol) was obtained from DuPont (Bad Homburg, Germany), 25 mm diameter nitrocellulose discs were from Sartorius (Göttingen, Germany), and the scintillation mixture and liquid-scintillation counter LS5000TD were obtained from Beckman Coulter (Fullerton, CA, U.S.A.). The digital imaging system was from Raytest (Straubenhardt, Germany), and DNA sequencing was performed on an automated DNA sequencing system from PerkinElmer (Foster City, CA, U.S.A.). All other chemicals were of analytical grade and were obtained from Merck (Darmstadt, Germany).
Generation and cloning of epitope-tagged human XT-I cDNA
Synthetic oligonucleotide primers including 5′-HindIII and 3′-XbaI restriction sites (upper primer: 5′-gcaagcttagacagcaacaacgag-3′, lower primer 5′-gagtctagacctgagccggccatc-3′, restriction sites are underlined) were designed based on the XT-I cDNA sequence [17]. The primers were used to amplify the XT-I cDNA (2434 bp) from pCG227 [17], using standard PCR conditions with Pfu DNA polymerase. The resulting PCR product was digested with HindIII and XbaI and inserted into the HindIII–XbaI site of the plasmid pMIB/V5-A resulting in pCG255-1. The constructed pCG255-1 plasmid encodes a soluble form of an XT-I/V5-epitope fusion protein and was transformed into chemically competent Escherichia coli TOP10 cells. Clones containing the correct vector were identified by single and double restriction digestion, followed by agarose gel analysis, and confirmed by double-strand DNA sequencing. DNA sequencing was performed on an ABI 310 DNA sequencing system with the dye-terminator cycle sequencing kit. Multiple clones were sequenced to compensate for misreading.
Expression of rXT-I (recombinant XT-I) in High Five insect cells
High Five insect cells were grown at 27 °C in Insect Xpress medium supplemented with L-glutamine and 10 μg/ml gentamicin. The cells were cultured as monolayers and subcultured once a week. Transient expression experiments of the rXT-I were performed in 3 ml of High Five insect cell cultures (2×106 cells) seeded in a 60 mm tissue culture plate, transfected with recombinant pMIB-V5-His-XT-I vector and incubated for 96 h at 27 °C. The culture media containing the recombinant protein were collected and clarified by centrifugation at 1500 g for 5 min. The supernatant was enriched 50-fold by ultrafiltration using Microcon 3000 tubes, and the XT-I/V5-epitope fusion proteins were detected by Western-blot analysis. A stable High Five/pCG255-1 insect cell clone expressing rXT-IΔ1-148-V5-His was generated as described elsewhere in detail [26].
Site-directed mutagenesis and expression of mutants
By site-directed mutagenesis 14 cysteine residues were replaced with alanine using the QuikChange site-directed mutagenesis kit. For mutations of individual cysteine residues, mutant oligonucleotides had base-pair mismatches compared with that of wt XT-I cDNA introducing the chosen mutation. For Cys257 and Cys920 the codon TGT was changed to GCT, for all other 12 cysteine residues TGC was changed to GCC. The sequence of the sense mutation primers is indicated in Table 1. Mutated DNA was double-stranded sequenced to confirm the single codon change and to ensure that no additional changes were introduced. The various mutants were individually expressed in High Five insect cells as described above. At least two vectors encoding each XT-I variant and two independent transfection experiments were performed for the analysis of these mutants to ensure reproducibility of the results.
Table 1. Sequences of oligonucleotides used for mutagenesis.
The primers used for the site-directed mutagenesis of pCG255-1, which codes for the soluble rXT-IΔ1-148-V5-His, and the resulting alterations in the amino acid sequence of rXT-I are shown. All sequences shown are for sense strands.
| Primer | Oligonucleotide sequence (5′–3′) | Mutation |
|---|---|---|
| C_XT1U356 | ATGACCAGCCCCCTAAGGCTGACATCTCAG | p.C257A |
| C_XT1U416 | CTAAGTCCAAGCACGCCCGCCAGGAGATTG | p.C276A |
| C_XT1U443 | TTGGGGAGACTTACGCCCGCCACAAGTTAG | p.C285A |
| C_XT1U493 | GTGACTCGGTTCGCCCCCCTCGAGGGTAAAG | p.C301A |
| C_XT1U998 | ATCGGCTCTTCCTGGAGGCCGACGCTCAC | p.C471A |
| C_XT1U1212 | GGAGAACAGCCCCCACGCCGACACCATGG | p.C542A |
| CysXT1U1270 | AATCGCAAGCTGGGCGCCAAGTGCCAGTA | p.C561A |
| CysXT1U1277 | AGCTGGGCTGCAAGGCCCAGTACAAGCACA | p.C563A |
| CysXT1U1305 | CATCGTGGACTGGGCCGGCTGCTCCCCCAA | p.C572A |
| CysXT1U1310 | TGGACTGGTGCGGCGCCTCCCCCAATGACT | p.C574A |
| CysXT1U1611 | GGATGGGGAGAACAGCGCCCGATACTACC | p.C675A |
| CysXT1U2341 | ATGTGGACTGCCATGGACATCGCTGCCACGGG | p.C920A |
| CysXT1U2367 | CACGGGCCCCACAGCCGCCCCGGTCATGC | p.C927A |
| CysXT1U2383 | TGCCGGTCATGCAGACCGCCAGCCAGACG | p.C933A |
Western-blot analysis
Western blotting to PVDF membrane was performed using a semi-dry electroblotting apparatus. A 16 μl volume of concentrated High Five cell culture supernatant was added to 6.25 μl of sample buffer [1.00 M Tris/HCl, 1.17 M sucrose, 0.28 M SDS, 2.08 mM EDTA, 0.88 mM Serva Blue G250, 0.70 mM Phenyl Red and 0.10 M DTT (dithiothreitol), pH 8.5], size fractionated by SDS/PAGE on a 4–12% (w/v) Bis-Tris polyacrylamide gel with Mops running buffer (1.00 M Mops, Tris, 69.3 mM SDS and 20.5 mM EDTA, pH 7.7) and electrophoretically transferred on to PVDF membrane for immunodetection. Non-specific antibody-binding sites were blocked with 2% (w/v) BSA in 0.1 M Tris/HCl (pH 7.2) for 1 h at room temperature (25 °C). The membrane was incubated with anti-V5 antibodies coupled with horseradish peroxidase at 1:1000 dilution, and bound antibodies were detected using 4-chloro-1-naphthol. Non-reducing gels (8–16% Tris-glycine) were loaded and run in the absence of 2-mercaptoethanol. Prestained protein standards were used to calibrate the gels.
Synthesis of recombinant bikunin
Recombinant bikunin was expressed in E. coli strain BL21(DE3) as described previously [27]. The purified protein was then used as acceptor in the XT-I activity assay.
Assay for enzyme activity
The method for the determination of XT-I activity is based on the incorporation of [14C]D-xylose with recombinant bikunin as acceptor. The reaction mixture for the assay contained, in a total volume of 100 μl, 50 μl of XT-I solution, 25 mM Mes (pH 6.5), 25 mM KCl, 5 mM KF, 5 mM MgCl2, 5 mM MnCl2, 1.0 μM UDP-[14C]D-xylose and 1.5 μM recombinant bikunin [28]. After incubation for 1.25 h at 37 °C, the reaction mixtures were placed on nitrocellulose discs. After drying, the discs were washed once for 10 min with 10% (w/v) trichloroacetic acid and three times with 5% trichloroacetic acid solution. Incorporated radioactivity was quantified after the addition of 3.5 ml of scintillation mixture using an LS5000TD liquid-scintillation counter. The enzyme activity was expressed in units [1 unit=1 (μmol of incorporated xylose)·min−1]. The linear range of the XT-I activity assay was determined as 0.02–3.5 m-units/l [28]. To measure within the linear range, samples were diluted with PBS supplemented with 1% human serum albumin. The assay linearity and the dilution procedure were validated using an enriched human XT-I solution, which was prepared as described previously [29].
Commercial preparations were used for the investigation of the influence of GAGs and other effectors on the XT-I activity: heparin from porcine intestinal mucosa, chondroitin 6-sulphate from shark cartilage, heparan sulphate and UDP. Before initiating the reaction, the samples with the enzyme were preincubated for 5 min at room temperature. The XT-I activity was then assayed as described above.
Determination of Michaelis–Menten constant (Km) and Vmax
For the determination of the Km values, various concentrations of the recombinant bikunin were incubated with diluted XT-I solution and UDP-[14C]xylose under assay conditions as described above. The recombinant bikunin with a short leader sequence and one xylosylation site per molecule has a molecular mass of 17.52 kDa.
Km and Vmax were calculated on the basis of non-linear regression analysis.
Chemical modification
Reduction of disulphide bridges was performed by the incubation of diluted supernatant from High Five insect cells expressing wt or mutant XT-I with increasing concentrations of DTT (0–40 mM) for 10 min at room temperature. The mixture was assayed under standard conditions as described above.
Diluted supernatant from High Five insect cells expressing wt or mutant XT-I was incubated in the presence of an increasing concentration of N-phenylmaleimide (0–20 mM) for 5 min at 25 °C for cysteine-directed reagent inhibition studies. The enzymic assays were then performed as described above. All assays were performed at least in duplicate.
Measurement of total protein concentration
Protein measurement was determined by the Bicinchoninic Acid Protein Assay kit using BSA as a standard.
Binding to UDP-beads
A 30 μl aliquot of fresh UDP-beads was washed with 100 μl of PBS to remove stabilizing reagents and contaminants, and was then resuspended in 30 μl of PBS. The UDP-beads were then mixed with 50 μl of 50-fold enriched culture supernatant from High Five insect cells expressing the XT-I variants containing 5 mM MnCl2 in the presence or absence of 25 mM UDP or 5 mM EDTA. The reaction mixtures were incubated at 4 °C for 1 h and washed twice with 100 μl of PBS. The beads were then centrifuged at 1000 g for 1 min and boiled in sample buffer (1.00 M Tris/HCl, 1.17 M sucrose, 0.28 M SDS, 2.08 mM EDTA, 0.88 mM Serva Blue G250, 0.70 mM Phenyl Red and 0.10 M DTT, pH 8.5) for 10 min. The samples were then loaded on to an SDS/PAGE system and electrophoresed as described above. The bound XT-I-V5-His fusion proteins were then detected after Western blotting using a V5-antibody.
Binding to heparin beads
A 20 μl aliquot of heparin-acrylic beads was washed twice with PBS to remove unbound heparin molecules and resuspended in 20 μl of PBS. A 150 μl volume of cell culture supernatant containing the XT-I mutants was added and incubated for 1 h at 25 °C under rotation. The beads were then separated by centrifugation at 1000 g for 1 min, and the XT-I activity of the supernatant was measured and compared with the untreated specimen. All samples were properly diluted with PBS supplemented with 1% human serum albumin to measure within the linear range of the XT-I activity assay. The relative amount of XT-I bound to the heparin beads was derived from the remaining activity of the XT-I mutant in the supernatant and the corresponding untreated control. To exclude a potential inhibition of the XT-I variants in the XT-I activity assay by heparin released from the heparin beads, a 20 μl aliquot of heparin beads was incubated with 100 μl of PBS for 1 h. The beads were then centrifuged at 1000 g for 1 min, and the pelleted acrylic beads were removed. A 20 μl volume of culture supernatant containing XT-I was added to the supernatant, and the XT-I activity was monitored and compared with appropriate controls.
In a second experiment, the binding of rXT-I and the mutants to heparin-acrylic beads was repeated. After centrifugation at 1000 g for 1 min, the supernatant was removed and the beads were washed twice with 100 μl of PBS. The beads were boiled in sample buffer for 10 min and the samples were then loaded for SDS/PAGE and electrophoresed as described above. The bound rXT-I and cysteine mutants were then detected after Western blotting using a V5-antibody.
Analysis of XT-I amino acid sequences
Homology analysis of the amino acid sequences of the XT-I was performed with the ClustalX and Genedoc software packages using the following sequences: human XT-I (GenBank® accession no. AJ277441) [17], M. musculus XT-I (GenBank® accession no. AJ291750), R. norvegicus XT-I (GenBank® accession no. AJ295748) [17], A. gambiae (Ensembl gene ENSANGG00000003504), F. rubripes XT-I (Ensembl gene SINFRUG00000122544), D. melanogaster XylT (GenBank® accession no. NM_139448) and C. elegans (GenBank® accession no. AJ496235). If an amino acid sequence was not available, the corresponding cDNA or genomic DNA sequence was used to derive the amino acids.
Statistical analysis
Results are expressed as means±S.D. and statistical analysis was performed using t test; P values of 0.05 or less were considered significant.
RESULTS
Alignment of XT sequences
The alignment of the amino acid sequences of XT-I from human, mouse, rat, drosophila, C. elegans, mosquito and pufferfish showed that 15 cysteine residues are conserved among these species (Figure 1). The five cysteine residues near the N-terminus of the different proteins are less conserved than Cys471, Cys542, Cys561, Cys563, Cys572, Cys574, Cys920 and Cys933, which are completely conserved.
Figure 1. Alignment of XT sequences.
The coding sequences of seven different species are shown including human (human_XT-I), mouse (mouse_XT-I), rat (rat_XT-I), drosophila, worm (C. elegans), mosquito and pufferfish (Fugu_XT). The highly conserved cysteine residues are indicated by grey shading, and the first amino acid of the recombinant soluble enzyme expressed in this study is marked by an arrow. All 14 cysteine residues were mutated individually.
Expression of soluble human XT-I
A soluble form of the human XT-I lacking the cytoplasmic tail and transmembrane domain was expressed in High Five insect cells with high activity. Maximal production of the enzyme occurred between days 4 and 6 of post-transfection. After SDS/PAGE, a single sharp band was detected on Western blots using antibodies specific for the V5-epitope expressed at the C-terminal end of the recombinant protein (Figure 2B, lane 1). The XT-I activity of the culture medium conditioned by High Five/pCG255 cells was determined as 220 m-units/l, which is approx. 200-fold higher in comparison with the XT-I activity secreted by JAR choriocarcinoma cells [30] and to the XT-I activity in human blood [15].
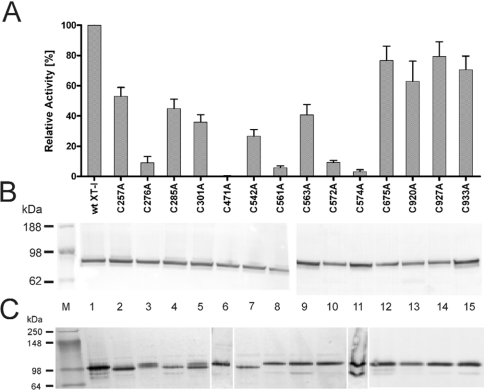
Figure 2. XT-I activity of rXT-I and the cysteine mutants.
(A) Relative activities of wt XT-I and cysteine mutants. The bar chart indicates the percentage XT-I activity relative to the rXT-I wt with error bars representing the S.D. (B) Western blots of 50 times concentrated media containing wt XT-I (lane 1) and cysteine mutants (lanes 2–15) under reducing conditions (lane M, multicolour prestained molecular mass standard). Proteins were visualized using anti-V5 antibodies. (C) Immunoblot analysis of expressed rXT-I with cysteine for alanine mutants under non-reducing conditions. Samples of 50-fold concentrated High Five cell culture supernatant were analysed on an 8–16% Tris-glycine gel and detected with anti-V5 antibodies as described in the Experimental section.
Site-directed replacement of cysteine residues
The rXT-I is a soluble enzyme lacking the cytoplasmic tail, the transmembrane domain and consequently also the first cysteine residue Cys6. We investigated the role of 14 cysteine residues in disulphide bond formation and in catalytic activity by constructing mutants in which the cysteine residues were individually replaced by alanine. The alanine-substituted mutants were found to be expressed in insect cells at similar levels compared with the wt protein and were characterized by SDS/PAGE. When the proteins were analysed under reducing conditions, the rXT-I and all 14 cysteine mutants gave a single band of approx. 90 kDa (Figure 2B). When samples where analysed by PAGE under non-reducing conditions (Figure 2C), the alanine-substituted mutants were found to be fractionated with slightly different mobility. C276A and C301A mutants migrated with a double band near 100 kDa. C257A, C285A and C542A mutants migrated with an apparent molecular mass of approx. 5 kDa less than the wt protein, indicating that the loss of disulphide bonds induces a misfolding of the mutant enzymes resulting in a slightly higher mobility under native conditions. C675A, C920A, C927A and C933A showed no variation and occurred as a single band like the wt protein in Western blot. Interestingly, the C574A mutant showed a second band approx. 20 kDa less than the wt enzyme.
Characterization of rXT-I and cysteine mutants for catalytic activity
The activities of the mutant proteins were determined in standard XT-I activity assays as described in the Experimental section. rXT-I was always expressed in parallel with the mutants as a positive control. In all experiments, the wt XT-I and cysteine mutants were expressed at comparable levels. Two of the 14 cysteine mutants, C471A and C574A, showed a complete loss of function (Figure 2A). The C276A, C561A and C572A mutations resulted in a loss of enzymic activity of more than 91%, indicating the necessity of these residues for the enzymic activity. Interestingly, alterations of cysteine residues located near the C-terminus of the protein, C675A, C920A, C927A and C933A, had no significant influence on XT-I activity, even though Cys920 and Cys933 are highly conserved in mammals, flies and worms. Taken together, these results suggest that five cysteine residues are critical for the activity of rXT-I. The kinetic analysis of the wt secretory XT-I and mutants revealed a slight change in Km values. The apparent kinetic parameters for wt and mutant XT-I are presented in Table 2.
Table 2. Kinetic constants for the wt rXT-I and its cysteine mutants.
Michaelis–Menten constants (Km) and maximal reaction rates (Vmax) of rXT-I and mutants with recombinant bikunin as acceptor protein. XT-activity was assayed as described in the Experimental section. Enzyme activity is expressed as micromoles of [14C]xylose incorporated into bikunin per minute. Results are average for two independent assays, n.d., not determined.
| Mutant | Km (μM) | Vmax (pmol·h−1) | Vmax/Km (pmol·h−1·μM−1) |
|---|---|---|---|
| wt XT-I | 1.0 | 882 | 900 |
| p.C257A | 5.3 | 514 | 398 |
| p.C276A | n.d. | n.d. | – |
| p.C285A | 2.8 | 938 | 488 |
| p.C301A | 1.4 | 693 | 396 |
| p.C471A | n.d. | n.d. | – |
| p.C542A | 1.6 | 509 | 310 |
| p.C561A | n.d. | n.d. | – |
| p.C563A | 11.9 | 1400 | 436 |
| p.C572A | n.d. | n.d. | – |
| p.C574A | n.d. | n.d. | – |
| p.C675A | 1.4 | 1900 | 1357 |
| p.C920A | 1.2 | 1600 | 1333 |
| p.C927A | 2.0 | 1300 | 650 |
| p.C933A | 1.0 | 1168 | 1229 |
Effect of disulphide reducing agents on rXT-I enzyme activity
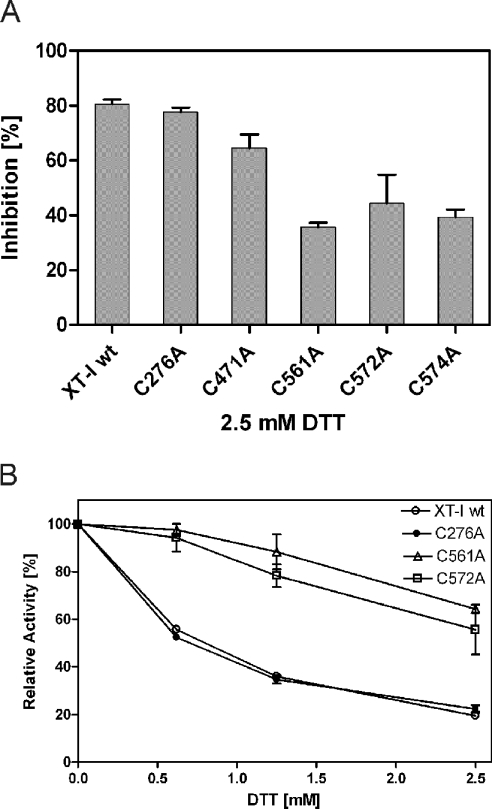
The role of disulphide bonds in maintaining enzyme function was further investigated by treating the wt XT-I with DTT. Results obtained for wt XT-I indicated that DTT produced a loss of activity of approx. 95% at the highest dose used (40 mM). In addition, we wanted to investigate whether the inactive mutants could be activated with DTT. We incubated the C276A, C471A, C561A, C572A and C574A mutant enzymes with increasing concentrations of DTT and analysed them for activity. None of the inactive mutants tested could be activated with DTT. The addition of 2.5 mM DTT inhibited the C276A mutant by 80%, comparable with the wt XT-I protein (Figure 3A). C471A, C561A, C572A and C574A mutants showed a decreased inhibition. It is noteworthy that the C561A and C572A mutants were not as equally sensitive to DTT inactivation as the wt protein and C276A mutant (Figure 3B).
Figure 3. Effect of DTT on rXT-I enzyme activity.
(A) The inhibitory effect of DTT on the activity of wt enzyme and on C276A, C471A, C561A, C572A and C574A expressed in a parallel experiment is indicated. None of the tested mutants could be activated with DTT. (B) The variation in relative activity as a function of the concentration of DTT for wt XT-I, C276A, C561A and C572A mutants.
A number of other glycosyltransferases have been shown to form dimers linked through disulphide bonds [31–35]. rXT-I was therefore analysed by SDS/PAGE under both reducing and non-reducing conditions. The secreted rXT-I protein gave only a single distinct band of molecular mass 90 kDa under reducing conditions (Figure 2B). When the sample was prepared and subjected to PAGE in the absence of 2-mercaptoethanol, the unreduced protein also showed a single band with a molecular mass of 100 kDa as visualized by Western blots (Figure 2C). These results indicate that the secreted active form of the enzyme was a monomer rather than a dimeric or multimeric protein.
N-phenylmaleimide sensitivity of wt XT-I and cysteine mutant enzymes
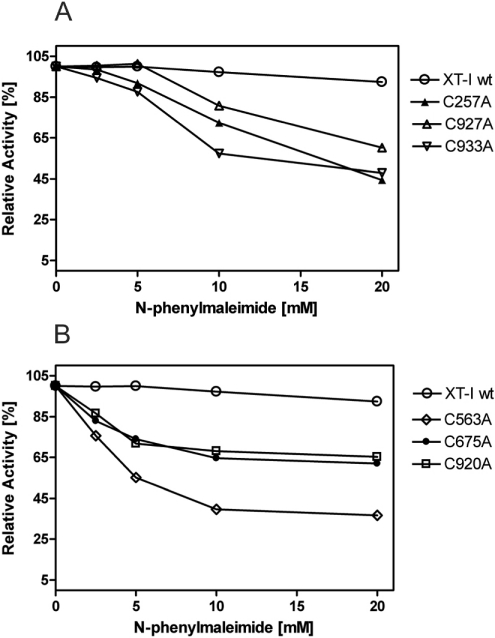
We examined the possible role of cysteine residues in the functionality of XT-I by chemical modification using a specific thiol reagent. N-phenylmaleimide treatment showed no effect on wt XT-I at the highest dose used (20 mM) (Figure 4), but strongly inactivated the cysteine mutants with increasing concentrations (0–20 mM). The results obtained indicate that there are no free thiol groups in wt XT-I and strongly suggest that the catalytic activity of XT-I does not depend on the presence of free thiol groups.
Figure 4. Sensitivity of human XT-I to N-phenylmaleimide.
Supernatant from High Five insect cells expressing rXT-I was incubated with N-phenylmaleimide. (A, B) The variation in activity as a function of the concentration of thiol-specific agents.
Effect of mutations on binding to UDP and UDP-beads
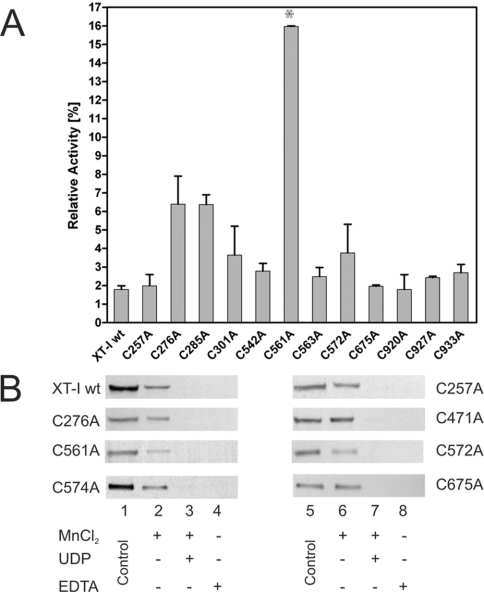
Since the XT-I enzyme contains a number of highly conserved cysteine residues throughout the sequence, several effectors were tested for their effects on enzyme activity. UDP is a competitive inhibitor of many glycosyltransferases [36,37] including the human XT-I (J. Kuhn, C. Götting and K. Kleesiek, unpublished work). Thus we analysed the role of the cysteine residues on the nucleotide sugar binding. The inhibition of the enzymic activity by UDP at different concentrations was investigated for the generated active XT-I mutants. The addition of 0.25 mM UDP inhibited the wt XT-I activity by 98% (Figure 5A), whereas the addition of 1.5 mM UDP resulted in a loss of activity of more than 99%. Most of the cysteine mutants did not have any significant influence on the reduction of the relative XT-I activity after the addition of UDP. However, the inhibition of the enzyme activity by 0.25 and 0.5 mM UDP was significantly reduced in C561A mutants (Figure 5A).
Figure 5. Inhibition of the enzymic activity by UDP for the generated cysteine mutants.
(A) wt XT-I and cysteine mutants were incubated in the presence of 0.25 mM UDP and the activity assays were performed as described in the Experimental section. The relative activity is given in percentage. The enzyme activity of each mutant without the addition of an effector was set at 100%. The C561A mutant showed an increased enzyme activity in comparison with the wt XT-I (*, P<0.05). (B) UDP-beads were incubated with the samples, centrifuged, washed, boiled in sample buffer and electrophoresed as described in the Experimental section. The detection of wt XT-I bound to UDP-beads by immunoblot using horseradish peroxidase-coupled anti-V5 antibodies (lanes 1 and 5: control: culture media containing the rXT-I or mutant enzyme; lanes 2–4, 6–8, protein bound to the beads after the incubation in the presence of either 5 mM MnCl2, 5 mM EDTA, 25 mM UDP or a combination thereof). For lanes 2–4 and 6–8, the media were in 5-fold excess of the beads to enable detection of the rXT-I mutants. A specific single band was detected in all experiments.
We, furthermore, tested the binding of soluble rXT-I, and active and inactive cysteine mutants to UDP-agarose beads (Figure 5B). For each experiment, crude enzyme preparations were incubated with UDP-beads and, after washing, the bound enzyme was detected by Western blotting using anti-V5 antibodies. As expected, the wt enzyme bound to UDP beads in the presence of Mn2+, but binding was inhibited by 25 mM UDP. No binding to the UDP-beads was observed after the addition of 5 mM EDTA, indicating the importance of Mn2+ ions for the binding of rXT-I to UDP. Similar results were obtained using the cysteine mutants. Even using our inactive mutants, we found no significantly different binding properties.
Inhibition of XT-I activity by heparin and binding of rXT-I to immobilized heparin
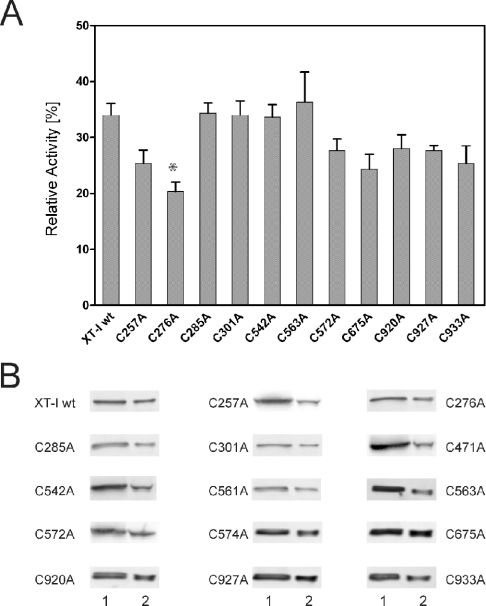
We have shown in previous studies that heparin is a potent inhibitor of human XT-I and that the enzyme strongly binds to the heparin matrix during heparin affinity chromatography [17,26,30]. In the present study, we used the generated XT-I mutants to investigate the inhibitory effects of heparin. Because of their low activity, C471A, C561A and C574A were not studied further. The addition of heparin to wt XT-I at a concentration of 1 unit/ml (specific activity, 204 units/mg of protein) resulted in >70% reduced enzyme activity (Figure 6A), whereas heparin concentrations of 100 units/ml led to a complete loss of function (results not shown). The 11 mutants tested could be strongly inhibited by heparin at concentrations ranging from 1 to 100 units/ml. However, the inhibition of the enzyme activity of C276A was significantly increased (Figure 6A).
Figure 6. Effect of heparin on rXT-I enzyme activity and binding of rXT-I mutants to heparin beads.
(A) Enzyme assays were performed as described in the Experimental section. Heparin in a concentration of 1 unit/ml was added directly to the incubation mixture. The effect of heparin on the activity of wt XT-I and cysteine mutants is indicated (*, P<0.05). (B) The detection of soluble rXT-I and mutants bound to the beads by anti-V5 antibodies. Heparin-acrylic beads were incubated with the samples, centrifuged, washed, boiled in sample buffer and electrophoresed as described in the Experimental section. Most of the enzyme present in the sample was bound to the heparin beads. Lane 1, control; equal amounts of protein were added to each control lane and to the heparin beads. Lane 2, boiled heparin beads.
The binding of rXT-I and the cysteine mutants to heparin was also investigated using heparin-acrylic beads. Heparin beads were incubated with the crude enzyme preparations, and the remaining enzyme activity in the supernatant was determined. Proper controls, as described in the Experimental section, were performed to ensure that no heparin released from the beads during the incubation period led to false negative results. The maximum binding for the wt enzyme was 85% of the input enzyme. All XT-I mutants efficiently bound to the heparin beads, and no significant difference between the binding properties of each mutant was observed (results not shown).
To analyse the heparin binding characteristics of all mutants, including those that had lost most of their enzyme activity, we used Western blotting for visualization of the amount of enzyme bound to the beads. As expected, most of the rXT-I enzyme present in the sample was bound to the heparin beads and was not released by washing steps. Interestingly, similar results were obtained using the cysteine mutants (Figure 6B) and no significantly different binding properties were observed.
Effect of chondroitin 6-sulphate and heparan sulphate on cysteine mutant enzymes
The inhibiton of the enzymic activity of wt XT-I enzyme by chondroitin 6-sulphate and heparan sulphate has been shown in previous studies [38]. To investigate a potential inhibitory effect of GAGs on the enzymic activity of cysteine mutant proteins, chondroitin 6-sulphate and heparan sulphate were added to samples, and the XT-activity was determined. The effector concentrations used covered a range from 10 to 1000 μg/ml. The addition of 10 μg/ml chondroitin 6-sulphate inhibited the wt XT-I activity by 40%, whereas the addition of 1000 μg/ml chondroitin 6-sulphate resulted in a loss of activity of more than 80%. Similar results were obtained using the cysteine mutants. No significantly different inhibitory effects were observed using these mutants.
Large amounts of heparan sulphate (1000 μg/ml) also revealed up to 80% inhibition of wt XT-I activity. All cysteine mutant enzymes were equally sensitive to heparan sulphate inhibition as the wt protein (results not shown).
DISCUSSION
XTs, type II transmembrane proteins involved in proteoglycan biosynthesis, catalyse the transfer of D-xylose from UDP-D-xylose to specific serine residues of the core protein to form the so-called GAG–protein linkage region. Three different relevant mammalian cDNAs from human, mouse and rat sources have been cloned [17]. In addition, the search for a homologue of mammalian XTs in D. melanogaster and C. elegans revealed potential XTs in the genomes of both organisms. These proteins have extensive amino acid sequence homology including nine completely conserved cysteine residues (Figure 1) in the globular catalytic domain. In total, the full-length mammalian XT contains 15 cysteine residues, and the recombinant secreted enzyme studied here contains 14 of these cysteine residues, due to the lack of Cys6, which is present in the cytoplasmic tail.
The presence of 14 cysteine residues, which are absolutely conserved in mammalian XT-I, suggests that they play an important role in the enzymic function. Results from the non-reducing gel indicate that the secreted form of soluble truncated rXT-I exists as an active monomer. Therefore any disulphide bond in this enzyme is intramolecular. Loss of activity at high concentrations of reducing reagents is in agreement with the thesis that disulphide bonds are required to stabilize the enzyme in an active conformation.
We investigated the role of 14 cysteine residues in disulphide bond formation and in catalytic activity by constructing mutants in which the cysteine residues were individually replaced by alanine. We were able to produce equal levels of the wt XT-I and cysteine mutant XT-I forms in the High Five insect expression system. When mutants were tested for activity, our results suggested that five cysteine residues are critical for the production and maintenance of an active conformation of XT-I. The fact that the substitution of the conserved cysteine residues to alanine has little effect on the kinetics of XT-I suggests that the invariant cysteine residues tested may not be directly involved in the substrate binding.
To analyse further the active conformation of XT-I, we investigated the consequences of DTT and N-phenylmaleimide treatment. The number of cysteine residues in wt XT-I indicates the possible presence of a maximum of seven disulphide bonds with no free thiol groups. Although reduction of the enzyme with DTT abolishes enzyme activity, agents that react with free cysteine residues had no effect on the activity of wt XT-I (Figure 4). This result suggests that the thiol-specific reagent does not react non-specifically with other amino acid residues in the protein. As expected N-phenylmaleimide strongly inactivated the cysteine mutants with increasing concentrations but, interestingly, the sensitivity for the reagent was not equal. Enzymes with mutations in positions Cys257, Cys927 or Cys933 showed a decreased effect on N-phenylmaleimide treatment, probably due to their location near the N- or C-terminus of the protein. Since five of the cysteine to alanine mutants were inactive, they could not be tested for the effect of thiol reagents. However, our results indicate that there are seven disulphide bonds in wt XT-I and no free thiol group.
UDP is a strong competitive inhibitor of many glycosyltransferases, including the XT-I. At a low concentration of 0.25 mM the XT-I activity was reduced by 98% in comparison with the controls. The inhibition kinetics of rXT-I by UDP was comparable with that of the native human XT-I isolated from the culture supernatant of JAR choriocarcinoma cells (J. Kuhn, unpublished work). In most of the cysteine mutants, inhibition by UDP was not significantly altered. Interestingly, the mutant C561A exhibited a significantly reduced response to UDP. Cys561 is localized in a region of approx. 300 amino acids with a putative involvement in the binding of UDP-sugar donors [16]. However, the significance of these results has to be clarified in further studies. We could show in the present study that the binding of rXT-I and the cysteine mutants to UDP-beads was bivalent ion dependent and was inhibited by EDTA and by the soluble competitor UDP. No difference in the binding properties to immobilized UDP was observed between the wt and the active and inactive mutants (Figure 5B). Our results indicate that the binding of nucleotide is not dependent on the conserved cysteine residues nor do the alterations induce a misfolding of the UDP-binding site.
Heparin is a potent inhibitor of the human XT-I, and the enzyme strongly binds to a heparin matrix during heparin affinity chromatography [17,26,30]. In the present study, we could demonstrate that the mutants tested could be strongly inhibited by heparin, and the sensitivity for the reagent was equal for most cysteine mutants compared with the wt XT-I. However, the inhibition of the enzyme activity of mutant C276A was significantly increased (Figure 6A). Therefore we have examined the binding properties to immobilized heparin between the wt XT-I and the mutants. We used enzyme activity to quantify binding characteristics and Western blotting to visualize the amount of bound enzyme. Taken together, these experiments revealed that all mutants were efficiently bound to immobilized heparin with no significantly different binding properties (Figure 6B). This indicates that the conserved cysteine residues are not directly involved in the heparin binding, and that the alterations do not induce a misfolding of the heparin-binding site.
XT-I was previously shown to be a regulatory factor of the chondroitin sulphate biosynthesis [10]. To investigate a potential inhibitory effect of GAGs on the enzymic activity of cysteine mutant proteins, we applied chondroitin 6-sulphate and heparan sulphate to enzyme samples, and the activity was determined. As we have shown in previous studies, the addition of high doses of chondroitin 6-sulphate or heparan sulphate was necessary to reduce the wt XT-I enzyme activity by more than 80% [38]. No difference in the inhibitory effect between the wt and the mutants was observed. Our findings suggest that the binding sites for the tested GAGs are not abolished in the cysteine mutants, and that the alterations do not lead to a misfolding of the mutant enzymes with a strong effect on the binding. Furthermore, an artificial inhibition of XT-I activity due to a lack of manganese and magnesium ions in the samples after the addition of the polyanionic effector molecules could be excluded, as no restoration of XT-I activity was observed after the supplementation of additional cations.
In the present study, we analysed for the first time the relationship between the structure and function of XT-I. In conclusion, we demonstrate the critical role of distinguished cysteine residues on the enzyme activity of XT-I. Additional investigations of the enzyme protein structure by X-ray crystallography may support these findings and reveal the catalytic mechanism of human XT-I.
Acknowledgments
We thank A. Adam for excellent technical assistance and G. Delany for linguistic advice. This work was supported by the Deutsche Forschungsgemeinschaft, grant no. BR1226/5-2.
References
- 1.Kjellen L., Lindahl U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 2.Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem. J. 1986;236:1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruoslahti E. Proteoglycans in cell regulation. J. Biol. Chem. 1989;264:13369–13372. [PubMed] [Google Scholar]
- 4.Salmivirta M., Lidholt K., Lindahl U. Heparan sulfate: a piece of information. FASEB J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 5.Aikawa J., Esko J. D. Molecular cloning and expression of a third member of the heparan sulfate/heparin GlcNAc N-deacetylase/N-sulfotransferase family. J. Biol. Chem. 1999;274:2690–2695. doi: 10.1074/jbc.274.5.2690. [DOI] [PubMed] [Google Scholar]
- 6.Sweet E. B. E., Thonar E. J.-M. A., Marsh J. Age-related changes in proteoglycan structure. Arch. Biochem. Biophys. 1979;198:439–448. doi: 10.1016/0003-9861(79)90518-6. [DOI] [PubMed] [Google Scholar]
- 7.Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits form human articular cartilage. J. Biol. Chem. 1980;264:13369–13372. [PubMed] [Google Scholar]
- 8.Kitagawa H., Tsutsumi K., Tone Y., Sugahara K. Developmental regulation of the sulfation profile of chondroitin sulfate chain in the chicken embryo brain. J. Biol. Chem. 1997;272:31377–31381. doi: 10.1074/jbc.272.50.31377. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl U., Rodén L. Carbohydrate-peptide linkages in proteoglycans of animal, plant and bacterial origin. In: Gottschalk A., editor. Glycoproteins. New York: Elsevier; 1972. pp. 491–517. [Google Scholar]
- 10.Rodén L. Structure and metabolism of connective tissue proteoglycans. In: Lennarz W. J., editor. The Biochemistry of Glycoproteins and Proteoglycans. New York: Plenum; 1980. pp. 269–314. [Google Scholar]
- 11.Schwartz N. B. Regulation of chondrotin sulfate synthesis. Effect of β-xylosides on synthesis of chondroitin sulfate proteoglycan, chondrotin sulfate chains, and core protein. J. Biol. Chem. 1977;252:6316–6321. [PubMed] [Google Scholar]
- 12.Kearns A. E., Campbell S. C., Westley J., Schwartz N. B. Initiation of chondroitin sulfate biosynthesis: a kinetic analysis of UDP-D-xylose:core protein β-D-xylosyltransferase. Biochemestry. 1991;30:7477–7483. doi: 10.1021/bi00244a016. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann H. P., Schwartz N. B., Roden L., Prockop D. J. Location of xylosyltransferase in the cisternae of the rough endoplasmic reticulum of embryonic chick cartilage cells. Connect. Tissue Res. 1984;12:151–164. doi: 10.3109/03008208408992780. [DOI] [PubMed] [Google Scholar]
- 14.Kähnert H., Paddenberg R., Kleesiek K. Simultaneous secretion of xylosyltransferase and chondroitin sulphate proteoclycan in chondrocyte culture. Eur. J. Clin. Chem. Clin. Biochem. 1991;29:624–625. [Google Scholar]
- 15.Götting C., Sollberg S., Kuhn J., Weilke C., Huerkamp C., Brinkmann T., Krieg T., Kleesiek K. Serum xylosyltransferase: a new biochemical marker of the sclerotic process in systemic sclerosis. J. Invest. Dermatol. 1999;112:919–924. doi: 10.1046/j.1523-1747.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson I. B. H. The never-ending story of peptide O-xylosyltransferase. Cell. Mol. Life Sci. 2004;61:794–809. doi: 10.1007/s00018-003-3278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Götting C., Kuhn J., Zahn R., Brinkmann T., Kleesiek K. Molecular cloning and expression of human UDP-D-xylose:proteoglycan core protein β-D-xylosyltransferase and its first isoform XT-II. J. Mol. Biol. 2000;304:517–528. doi: 10.1006/jmbi.2000.4261. [DOI] [PubMed] [Google Scholar]
- 18.Wilson I. B. Functional characterisation of Drosophila melanogaster peptide O-xylosyltransferase, the key enzyme for proteoglycan chain initiation and member of the core 2/I N-acetylglucosaminyltransferase family. J. Biol. Chem. 2002;277:21207–21212. doi: 10.1074/jbc.M201634200. [DOI] [PubMed] [Google Scholar]
- 19.Hwang H. Y., Olson S. K., Brown J. R., Esko J. D., Horvitz H. R. The Caenorhabidits elegans genes sqv-2 and sqv-6, which are required for vulval morphogenesis, encode glydosaminoglycan galactosyltransferase II and xylosyltransferase. J. Biol. Chem. 2003;278:11735–11738. doi: 10.1074/jbc.C200518200. [DOI] [PubMed] [Google Scholar]
- 20.Holmes E. H., Yen T. Y., Thomas S., Josh R., Nguyen A., Long T., Gallet F., Maftah A., Julien R., Macher B. Human alpha 1,3/4 fucosyltransferases. Characterization of highly conserved cysteine residues and N-linked glycosylation sites. J. Biol. Chem. 2000;275:24237–24245. doi: 10.1074/jbc.M000888200. [DOI] [PubMed] [Google Scholar]
- 21.Boeggeman E. E., Balaji P. V., Sethi N., Masibay A. S., Quasba P. K. Expression of deletion constructs of bovine beta-1,4-galactosyltransferase in Escherichia coli: importance of Cys134 for its activity. Protein Eng. 1993;6:779–785. doi: 10.1093/protein/6.7.779. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Wong S. S., Fukuda M. N., Ju H., Liu Z., Tang Q., Appert H. E. Identification of functional cysteine residues in human galactosyltransferase. Biochem. Biophys. Res. Commun. 1994;204:701–709. doi: 10.1006/bbrc.1994.2516. [DOI] [PubMed] [Google Scholar]
- 23.Datta A. K., Chammas R., Paulson J. C. Conserved cysteines in the sialyltransferase sialylmotifs form an essential disulfide bond. J. Biol. Chem. 2001;276:15200–15207. doi: 10.1074/jbc.M010542200. [DOI] [PubMed] [Google Scholar]
- 24.Puthalakath H., Burke J., Gleeson P. A. Glycosylation defect in Lec1 Chinese hamster ovary mutant is due to a point mutation in N-acetylglucosaminyltransferase I gene. J. Biol. Chem. 1996;271:27818–27822. doi: 10.1074/jbc.271.44.27818. [DOI] [PubMed] [Google Scholar]
- 25.Angata K., Yen T. Y., El-Battari A., Macher B. A., Fukuda M. Unique disulfide bond structures found in ST8Sia IV polysialyltransferase are required for its activity. J. Biol. Chem. 2001;276:15369–15377. doi: 10.1074/jbc.M100576200. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn J., Müller S., Schnölzer M., Kempf T., Schön S., Brinkmann T., Schöttler M., Götting C., Kleesiek K. High-level expression and purification of human xylosyltransferase I in High Five insect cells as biochemically active form. Biochem. Biophys. Res. Commun. 2003;312:537–544. doi: 10.1016/j.bbrc.2003.10.157. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann T., Weilke C., Kleesiek K. Recognition of acceptor proteins by UDP-D-xylose proteoglycan core protein β-D-xylosyltransferase. J. Biol. Chem. 1997;272:11171–11175. doi: 10.1074/jbc.272.17.11171. [DOI] [PubMed] [Google Scholar]
- 28.Weilke C., Brinkmann T., Kleesiek K. Determination of xylosyltransferase activity in serum with recombinant human bikunin as acceptor. Clin. Chem. 1997;43:45–51. [PubMed] [Google Scholar]
- 29.Götting C., Kuhn J., Brinkmann T., Kleesiek K. Xylosylation of alternatively spliced isoforms of Alzheimer APP by xylosyltransferase. J. Protein Chem. 1998;17:295–302. doi: 10.1023/a:1022549121672. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn J., Götting C., Schnölzer M., Kempf T., Brinkmann T., Kleesiek K. First isolation of human UDP-D-xylose: proteoglycan core protein beta-D-xylosyltransferase secreted from cultured JAR choriocarcinoma cells. J. Biol. Chem. 2001;276:4940–4947. doi: 10.1074/jbc.M005111200. [DOI] [PubMed] [Google Scholar]
- 31.Ma J., Colley K. J. A disulfide-bonded dimer of the Golgi β-galactoside α2,6-sialyltransferase is catalytically inactive yet still retains the ability to bind galactose. J. Biol. Chem. 1996;271:7758–7766. doi: 10.1074/jbc.271.13.7758. [DOI] [PubMed] [Google Scholar]
- 32.Qian R., Chen D., Colley K. J. Location and mechanism of α2,6-sialyltransferase dimer formation. Role of cysteine residues in enzyme dimerisation, localisation, activity and processing. J. Biol. Chem. 2001;276:28641–28649. doi: 10.1074/jbc.M103664200. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Yen T. Y., Allende M. L., Joshi R. K., Cai J., Pierce W. M., Jaskiewicz E., Darling D. S., Macher B. A., Young W. W., Jr Disulfide bonds of GM2 synthase homodimers. Antiparallel orientation of catalytic domains. J. Biol. Chem. 2000;275:41476–41486. doi: 10.1074/jbc.M007480200. [DOI] [PubMed] [Google Scholar]
- 34.Ouzzine M., Gulberti S., Netter P., Magdalou J., Fournel-Gigleux S. Structure/function of the human Galβ1,3-glucuronyltransferase. Dimerization and funtional activity are mediated by two crucial cysteine residues. J. Biol. Chem. 2000;275:28254–28260. doi: 10.1074/jbc.M002182200. [DOI] [PubMed] [Google Scholar]
- 35.Ju T., Cummings R. D., Canfield W. M. Purification, characterization and subunit structure of rat core 1 β1,3-galactosyltransferase. J. Biol. Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- 36.Kleineidam R. G., Schmelter T., Schwarz R. T., Schauer R. Studies on the inhibition of sialyl- and galactosyltransferases. Glycoconj. J. 1997;14:57–66. doi: 10.1023/a:1018560931389. [DOI] [PubMed] [Google Scholar]
- 37.Grancharov K., Naydenova Z., Lozeva S., Golovinsky E. Natural and synthetic inhibitors of UDP-glucuronosyltransferase. Pharmacol. Ther. 2001;89:171–186. doi: 10.1016/s0163-7258(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 38.Götting C., Kuhn J., Tinneberg H. R., Brinkmann T., Kleesiek K. High xylosyltransferase activities in human follicular fluid and cultured granulosa-lutein cells. Mol. Hum. Reprod. 2002;12:1079–1086. doi: 10.1093/molehr/8.12.1079. [DOI] [PubMed] [Google Scholar]