Abstract
Autophagic activity in isolated rat hepatocytes is strongly suppressed by OA (okadaic acid) and other PP (protein phosphatase)-inhibitory toxins as well as by AICAR (5-aminoimidazole-4-carboxamide riboside), a direct activator of AMPK (AMP-activated protein kinase). To investigate whether AMPK is a mediator of the effects of the toxin, a phosphospecific antibody directed against the activation of phosphorylation of the AMPK α (catalytic)-subunit at Thr172 was used to assess the activation status of this enzyme. AICAR as well as all the toxins tested (OA, microcystin-LR, calyculin A, cantharidin and tautomycin) induced strong, dose-dependent AMPKα phosphorylation, correlating with AMPK activity in situ (in intact hepatocytes) as measured by the AMPK-dependent phosphorylation of acetyl-CoA carboxylase at Ser79. All treatments induced the appearance of multiple, phosphatase-sensitive, low-mobility forms of the AMPK α-subunit, consistent with phosphorylation at several sites other than Thr172. The flavonoid naringin, an effective antagonist of OA-induced autophagy suppression, inhibited the AMPK phosphorylation and mobility shifting induced by AICAR, OA or microcystin, but not the changes induced by calyculin A or cantharidin. AMPK may thus be activated both by a naringin-sensitive and a naringin-resistant mechanism, probably involving the PPs PP2A and PP1 respectively. Neither the Thr172-phosphorylating protein kinase LKB1 nor the Thr172-dephosphorylating PP, PP2C, were mobility-shifted after treatment with toxins or AICAR, whereas a slight mobility shifting of the regulatory AMPK β-subunit was indicated. Immunoblotting with a phosphospecific antibody against pSer108 at the β-subunit revealed a naringin-sensitive phosphorylation induced by OA, microcystin and AICAR and a naringin-resistant phosphorylation induced by calyculin A and cantharidin, suggesting that β-subunit phosphorylation could play a role in AMPK activation. Naringin antagonized the autophagy-suppressive effects of AICAR and OA, but not the autophagy suppression caused by cantharidin, consistent with AMPK-mediated inhibition of autophagy by toxins as well as by AICAR.
Keywords: AMP-activated protein kinase (AMPK), hepatocyte, naringin, okadaic acid, phosphorylation, toxin
Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxamide riboside; AMPK, AMP-activated protein kinase; CaMK-II, Ca2+/calmodulin-dependent protein kinase II; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MC, microcystin-LR; OA, okadaic acid; pACC, phosphorylated ACC; PP, protein phosphatase; pSer108, phosphorylated Ser108; TBS, Tris-buffered saline
INTRODUCTION
Autophagy is a process used by eukaryotic cells to degrade their own cytoplasm under conditions of nitrogen starvation or stress, to obtain amino acids and other small molecules, which are needed for the maintenance of essential cell functions. Autophagy is executed by specialized intracellular membrane cisternae called phagophores, which excise and sequester pieces of cytoplasm, forming closed vacuoles called autophagosomes. The autophagosomes may fuse with endosomes to form intermediary vacuoles called amphisomes and, eventually, the vacuolar contents of sequestered cytoplasm are delivered to lysosomes for degradation [1].
Studies of autophagy (Atg) mutations [2] in yeast have indicated that a functional phagophore requires the protein Atg8 (LC3-II in mammalian cells), which is covalently bound to phosphatidylethanolamine in the phagophore membrane with the help of a ubiquitylation-like conjugation system. An Atg12–Atg5 conjugate, formed by a second conjugation system, seems to be associated with the phagophore during the autophagic sequestration process; however, on vacuole closure (autophagosome formation), Atg12–Atg5 detaches, whereas Atg8 remains [3]. Other proteins, mostly variants of cytosolic enzymes, have been found to be selectively associated with autophagosomal membranes in rat liver cells, but it is not clear whether these have an autophagic function or just represent degradation intermediates [4].
In addition to the conjugation reactions, phagophore formation is dependent on a lipid kinase complex that includes the phosphoinositide 3-kinase Atg6 (beclin-1 in mammalian cells) and a ‘switching complex’ that includes the protein kinase Atg1. Atg1, which appears to regulate yeast autophagy independent of its protein kinase activity [5], has no mammalian homologue, but several other kinases have been suggested to be involved in the regulation of autophagy both in yeast and mammalian cells [6]. For example, AMPK (AMP-activated protein kinase), a Ser/Thr kinase, has been suggested to play a role in the negative control of hepatocellular autophagy [7]; surprisingly, its yeast homologue, Snf1, has the opposite effect [8]. Pho85, a cyclin-activated Ser/Thr kinase, antagonizes Snf1 and suppresses autophagy in yeast [8]. Furthermore, the rapamycin-sensitive protein/lipid kinase TOR inhibits autophagy both in yeast and mammalian cells [9,10] and may partly mediate the autophagy-suppressive effect of amino acids [11]. In yeast, TOR has been shown to promote the phosphorylation and inactivation of Atg13, an Atg1-stimulatory protein factor [12]. Other protein kinases that have been implicated in the regulation of autophagic activity include PKA (cAMP-dependent protein kinase) [13], MAPK (mitogen-activated protein kinase) [14], stress-activated protein kinases [15,16], PKB (protein kinase B)/Akt [17], p70 S6 kinase [1,9,16], CaMKs (Ca2+/calmodulin-dependent protein kinases) [18,19] and protein-tyrosine kinases [20]. eIF2α (eukaryotic initiation factor 2α) kinases appear to be required for the long-term maintenance of autophagic capacity in both yeast and mammalian cells, probably through phosphorylation of a transcriptional activator (Gcn4) of autophagy genes [21].
In rat hepatocytes, overphosphorylation induced by various PP (protein phosphatase)-inhibitory toxins has been shown to inhibit autophagic activity completely [22]. Dose–response considerations indicated the involvement of PP2A, whereas autophagy-inhibitory protein kinases have remained elusive. Although the autophagy-suppressive effect of OA (okadaic acid), an algal toxin, is effectively antagonized by CaMK-II inhibitors [22], the flavonoid naringin, which does not inhibit CaMK-II [16], is an equally effective antagonist [23], indicating an enzyme other than CaMK-II as the mediator of the toxin effect. In a search for this naringin-sensitive protein kinase, we examined the effects of various toxins and of naringin on AMPK [7]. The results, parts of which have been reported in a preliminary form [16], show that AMPK can be phosphorylation-activated by naringin-sensitive as well as by naringin-resistant mechanisms, depending on the toxin administered. This differential naringin sensitivity is also observed at the level of autophagic sequestration. The suppression of hepatocellular autophagy during toxic stress may thus be mediated by complex signalling events upstream of AMPK.
EXPERIMENTAL
Reagents
OA and MC (microcystin-LR) were obtained from Alexis Biochemicals (Läufelfingen, Switzerland); calyculin A, cantharidin and tautomycin were from Calbiochem (San Diego, CA, U.S.A.) and AICAR (5-aminoimidazole-4-carboxamide riboside) was from Toronto Research Chemicals (North York, ON, Canada). Polyclonal rabbit antibodies against AMPK α- and β-subunits, LKB1, Thr172-phosphorylated AMPKα, Ser108-phosphorylated AMPKβ, Ser79-phosphorylated ACC (acetyl-CoA carboxylase) as well as horseradish peroxidase-linked anti-rabbit IgG antibody were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). A polyclonal antibody against PP2C was obtained from Oxford Biomedical Research (Oxford, MI, U.S.A.). Rainbow molecular-mass standards (RPN 756) and the ECL® Western blotting detection kit were from Amersham Biosciences (Little Chalfont, Bucks., U.K.). SDS, acrylamide and bisacrylamide were obtained from Bio-Rad Laboratories (Hercules, CA, U.S.A.). Dry milk powder was from Nestlé (Vevey, Switzerland) and nitrocellulose membranes were from Osmonics (Westborough, MA, U.S.A.). Methanol and acetic acid were from Merck (Whitehouse Station, NJ, U.S.A.), metrizamide was from Nycomed Pharma AS (Oslo, Norway) and alkaline phosphatase (molecular biology grade) was from Roche Applied Science (Penzberg, Germany). Other biochemicals were purchased from Sigma (St. Louis, MO, U.S.A.).
Animals and cells
Hepatocytes were isolated from 18 h-starved male Wistar rats (250–300 g; Harlan UK Ltd, Shaws Farm, Oxon, U.K.) by two-step collagenase perfusion [24], purified by differential centrifugation and resuspended in suspension buffer fortified with 2 mM Mg2+ and 15 mM pyruvate [24]. For immunoblotting studies, 0.4 ml aliquots of cell suspension, each containing approx. 30 mg of cells (wet mass), were incubated for 60 min at 37 °C in shaking centrifuge tubes. In autophagy experiments, 2 ml aliquots of cell suspension, containing approx. 20 mg of cells, were incubated for 120–140 min at 37 °C in 5 cm Nunclon Petri dishes precoated with albumin to prevent attachment of the cells to the substratum [25].
Gel electrophoresis and immunoblotting
Incubations were stopped by adding 2 ml of ice-cold TBS (Tris-buffered saline; 20 mM Tris and 0.1% Tween 20, pH 7.6) to each tube, followed by centrifugation of the cells at 600 gav. for 4 min in the cold (4 °C); the washing was repeated once. The cells were lysed for 30 min on ice in 1 ml of lysis buffer containing 0.4% (w/v) SDS, 5 mM EDTA, 5 mM EGTA, 10 mM sodium pyrophosphate and 20 mM Tris (pH 7.2). The resulting whole-cell extracts were diluted with an equal volume of double-strength SDS gel-loading buffer [single-strength: 0.5% (v/v) SDS, 2.4 mM sodium deoxycholate, 5% (v/v) mercaptoethanol, 0.5% (v/v) Igepal CA-630, ∼0.05% (w/v) Bromophenol Blue, 6.7% (v/v) glycerol and 60 mM Tris/HCl, pH 6.8] and boiled for 5 min at 95 °C. After measuring the protein contents of the extracts by the method of Bradford [26], using the BCA protein assay kit from Pierce (Rockford, IL, U.S.A.), samples containing 20 μg of protein were separated by SDS gel electrophoresis for approx. 40 min at 200 V, generally in 10% (w/v) polyacrylamide gels containing 0.1% SDS. The gels to be used for immunoblotting against the high-molecular-mass enzyme pACC (phosphorylated ACC) contained 6% polyacrylamide and 6 M urea. Molecular-mass standards were included in all gels.
The separated proteins were transferred on to nitrocellulose blotting membranes using a semi-dry transfer unit or on to PVDF membranes using a Mini Trans-Blot transfer unit (both instruments from Bio-Rad Laboratories). Towbin's blotting buffer [192 mM glycine, 20% (v/v) methanol and 25 mM Tris, pH 8.3] was used with both instruments, except for pACC immunoblotting, where the methanol was omitted. The membranes were blocked with 5% (w/v) dry milk in TBS-T (TBS containing 0.2% Tween 20) and washed three times with TBS-T at room temperature (22 °C). For detection, the membranes were first incubated overnight at 4 °C with the respective antibodies, generally diluted 1:1000 with TBS-T containing 5% (w/v) BSA. The antibody against phospho-AMPKα was diluted with BSA-free TBS-T, and the antibody against pACC was diluted with TBS-T containing 2% dry milk. After washing three times with TBS-T, the membranes were incubated for 30 min at room temperature with anti-rabbit horseradish peroxidase (diluted 1:1000 in TBS-T), washed three more times with TBS-T and finally visualized by chemiluminescence using the ECL®/ECL+Western detection kit (Amersham Biosciences).
Alkaline phosphatase treatment
To dephosphorylate phosphoproteins, 15 μl of lysed cell extract (containing approx. 100 μg of protein) was mixed with 1.5 μl of alkaline phosphatase (2 units/μl; prepared by diluting the stock solution to 1:10 using the dephosphorylation buffer provided by the supplier) and incubated for 3 h at 37 °C. This concentration of alkaline phosphatase (∼200 units/ml) appeared to provide maximal dephosphorylation of phosphorylated AMPK, as higher concentrations (up to 700 units/ml) were no longer effective.
Measurement of autophagy
Autophagy was measured as the net sequestration of an endogenous cytosolic enzyme, LDH (lactate dehydrogenase), into sedimentable autophagic vacuoles (mostly autolysosomes) during 2 h of incubation at 37 °C, with the proteinase inhibitor leupeptin (0.3 mM) added to prevent intralysosomal LDH degradation [27]. After incubation, the cells were sedimented at 600 g for 4 min in the cold (4 °C), resuspended in 4 ml of ice-cold unbuffered (electrolyte-free) 10% (w/v) sucrose and centrifuged again. The resuspension and centrifugation were repeated once more and, finally, the cell pellet was resuspended in 0.5 ml of 10% sucrose, briefly warmed to 37 °C and electrodisrupted by a single high-voltage (2 kV/cm) pulse. The disrupted cells were centrifuged through a metrizamide/sucrose cushion, and the amount of LDH in the resulting cytosol-free sediment was measured and expressed as a percentage (autophagically sequestered per hour) of the total cellular LDH in the sample [27].
RESULTS
Stimulation of AMPK phosphorylation by AICAR but not by amino acids
Autophagic activity in isolated rat hepatocytes has been shown to be strongly suppressed by adenosine, AICAR and various adenosine analogues [7,28]. The suppression of autophagy can be eliminated by the adenosine kinase inhibitor, 5-iodotubercidin, suggesting mediation by AMP and AMP analogues and a possible involvement of AMPK [7,28]. Hepatocytic autophagy can also be inhibited by amino acid mixtures [11], as well as by OA and other algal toxins [22], but the mechanisms of action of these agents are not known.
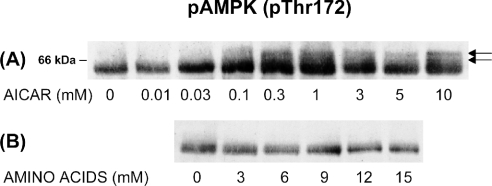
AMPK is activated allosterically by AMP; however, in addition, its activity is absolutely dependent on phosphorylation at Thr172 by an upstream protein kinase [29], recently identified as LKB1 [30,31]. The availability of a commercial, phosphospecific antibody that detects phosphorylation of AMPK at Thr172 has allowed a closer examination of the effects of autophagy suppressants on AMPK activation. As shown in Figure 1(A), treatment of freshly isolated rat hepatocytes with AICAR induced a dosedependent phosphorylation of AMPK at Thr172, indicating activation of the enzyme. Probably, the direct binding of AMP to AMPK alters its susceptibility towards phosphorylation by LKB1, although it has also been suggested that the AMPK-phosphorylating enzyme itself could be allosterically activated by AMP [29]. Some basic AMPK phosphorylation could usually be detected, but it varied, probably reflecting various degrees of hypoxia sustained during hepatocyte preparation. This variable background probably accounted for the variability (in the range 0.03–1 mM) in the AICAR concentrations needed to obtain a detectable effect.
Figure 1. Stimulation of AMPK phosphorylation by AICAR.
Freshly isolated rat hepatocytes were incubated for 1 h at 37 °C with (A) AICAR or (B) a physiological amino acid mixture, at the concentrations indicated. Cell extracts were immunoblotted with an antibody against the Thr172-phosphorylated AMPK α-subunit. The position of a 66 kDa marker protein is indicated.
In addition to increasing the pThr172 immunoreactivity of the single AMPK band seen in unstimulated cells, AICAR treatment induced immunoreactivity at two adjacent bands of lower mobility (Figure 1A, arrows). Since Thr172 phosphorylation could be detected in all three bands, additional structural or conformational changes associated with AMPK activation must be responsible for the mobility shift. Phosphorylation at additional sites is quite probable, particularly since both Thr258 and Ser485 in the AMPK α-subunit have been shown to be subject to phosphorylation, apparently by the same enzyme [32]. Probably, binding of the AICAR-derived AMP analogue increases the susceptibility of AMPK towards phosphorylation at several sites.
Unlike AICAR, a physiological mixture of amino acids had no effect on AMPK phosphorylation (Figure 1B). The mixture has previously been shown to inhibit autophagy maximally at approx. 12 mM, the ‘single-strength’ (1×) concentration corresponding to the amino acid levels in portal blood after a protein-rich meal [33]. Thus the autophagy-suppressive effect of amino acids may not be mediated by AMPK.
Activation of AMPK by OA, microcystin and other toxins
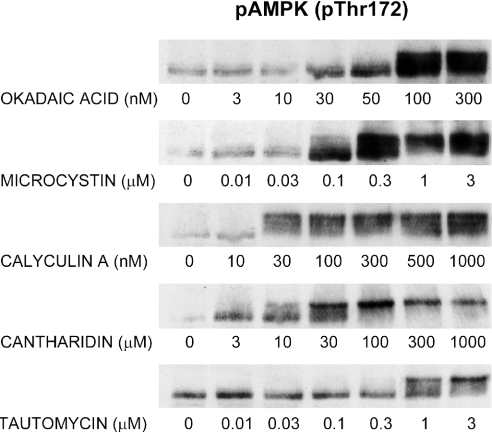
To see if other autophagy suppressants might stimulate AMPK phosphorylation, the effects of various PP-inhibitory toxins were examined. OA and other algal toxins have previously been shown to suppress hepatocytic autophagy almost completely, with dose characteristics suggesting the involvement of PP2A [22]. As shown in Figure 2, the toxins OA, MC, calyculin A, cantharidin and tautomycin all stimulated AMPK phosphorylation in a dose-dependent manner. OA and calyculin A were the most potent stimulants, with detectable effects at 30 nM and maximal effects at 100–300 nM. The effect of microcystin could be detected at 100 nM, whereas tautomycin and cantharidin required micromolar concentrations to produce detectable effects. The toxins induced a more pronounced mobility shift when compared with AICAR; at the highest toxin concentrations, the AMPK form of lowest mobility was predominant. It should be noted that effective toxin concentrations vary considerably from experiment to experiment (partly due to the variable AMPK pre-activation alluded to above), necessitating some caution when performing inter-experimental comparisons.
Figure 2. Stimulation of AMPK phosphorylation by various toxins.
Hepatocytes were incubated for 1 h at 37 °C with OA, MC, calyculin A, cantharidin or tautomycin at the concentrations indicated. Cell extracts were immunoblotted with an antibody against Thr172-phosphorylated AMPKα.
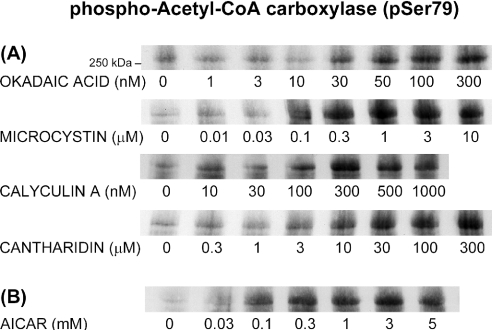
To check whether phosphorylation of AMPK at Thr172 really correlated with AMPK activity, the phosphorylation of cellular ACC in toxin-treated hepatocytes was examined. ACC is specifically phosphorylated by AMPK at Ser79 [34], making this phosphosite a convenient indicator for the activity of AMPK in intact cells. As shown in Figure 3(A), ACC phosphorylation was strongly stimulated by all the toxins tested (OA, MC, calyculin A and cantharidin), confirming the assumption that AMPK phosphorylation at Thr172 is accompanied by AMPK activation. ACC phosphorylation was also clearly stimulated by the established AMPK activator, AICAR (Figure 3B). The ability of the toxins to stimulate AMPK phosphorylation and AMPK activity would be compatible with a role for AMPK in mediating their autophagy-suppressive effects.
Figure 3. Toxin- and AICAR-induced phosphorylation of ACC, an AMPK substrate.
Hepatocytes were incubated for 1 h at 37 °C with (A) OA, MC, calyculin A or cantharidin or (B) AICAR, at the concentrations indicated. Cell extracts were immunoblotted with an antibody against Ser79-phosphorylated ACC. The position of a 250 kDa marker protein is indicated.
Differential naringin sensitivity of toxin-induced AMPK phosphorylation
The autophagy-suppressive effect of OA has been shown to be antagonized effectively by naringin, a grapefruit flavonoid believed to inhibit a protein kinase involved in the negative regulation of autophagy [23]. Several other OA effects on rat hepatocytes are also naringin-sensitive, such as the inhibition of endocytosis [23], the disruption of keratin and plectin cytoskeletal networks [23,35,36] and the induction of apoptotic cell death [35].
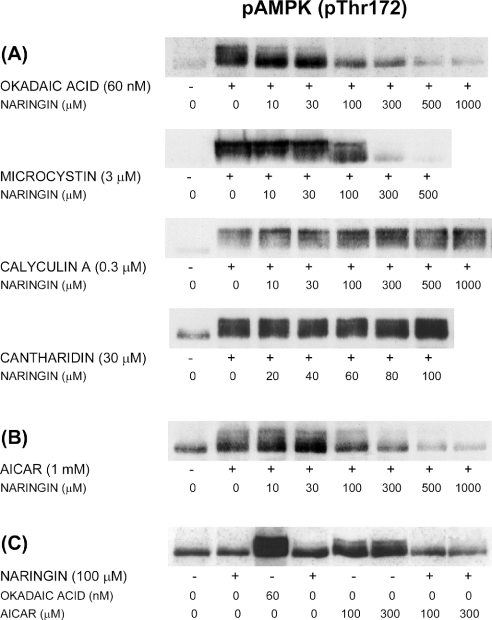
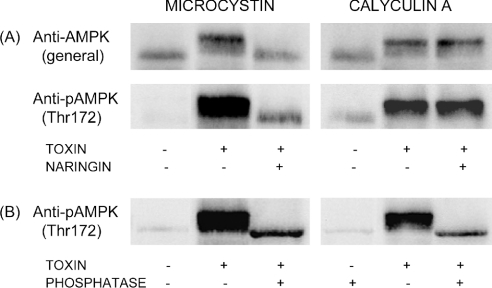
As shown in Figure 4(A), naringin strongly antagonized the effect of OA on AMPK (Thr172) phosphorylation, largely preventing the formation of phosphorylated low-mobility forms. Similarly, the AMPK phosphorylation induced by MC was virtually abolished by naringin. In contrast, naringin did not antagonize AMPK phosphorylation induced by calyculin A or cantharidin. These results parallel our previous observations on hepatocytic plectin phosphorylation, which suggested the involvement of two phosphorylation mechanisms: one naringin-sensitive mechanism probably involving inhibition of PP2A by microcystin or low concentrations of OA and one naringin-resistant mechanism probably involving PP1 inhibition by calyculin A, cantharidin or tautomycin [16]. The hypothesis is based in large measure on the known relative specificities of the toxins towards PP1 and PP2A in intact cells and cell-free systems.
Figure 4. Differential naringin sensitivity of AMPK phosphorylation induced by algal toxins or AICAR.
Hepatocytes were incubated for 1 h at 37 °C with (A) OA (60 nM), MC (3 μM), calyculin A (0.3 μM) or cantharidin (30 μM), (B) AICAR (1 mM), at various concentrations of naringin, or (C) naringin (100 μM) alone or in the presence of OA or AICAR, as indicated. Cell extracts were immunoblotted with an antibody against Thr172-phosphorylated AMPK.
Consistent with its effects on AMPK phosphorylation, naringin antagonized the stimulatory effects of OA and microcystin on phosphorylation of the AMPK substrate ACC, whereas the stimulatory effects of calyculin A and cantharidin were unaffected (results not shown). Both the naringin-sensitive and the naringin-resistant toxin mechanisms thus clearly regulate AMPK activity. Naringin alone had little or no effect on the basal (unstimulated) level of AMPK phosphorylation (Figure 4C).
AICAR-induced AMPK phosphorylation is also naringin-sensitive
Interestingly, the AMPK phosphorylation induced by AICAR was also strongly antagonized by naringin (Figure 4B). This effect of naringin could reflect a direct binding to AMPK, e.g. in competition with AMP, resulting in decreased susceptibility of the enzyme to phosphorylation. Alternatively, naringin could interfere with regulatory interactions between the AMPK subunits or affect, directly or indirectly, the enzymes involved in AMPK phosphorylation and dephosphorylation. Flavonoids are structurally similar to adenine nucleotides and inhibit a variety of protein kinases by competing with ATP for binding to the catalytic site [37–39]. The ability of naringin to suppress the direct AMPK activation by AICAR seems to suggest that the naringin-sensitive, putatively PP2A-mediated toxin mechanism discussed above is more proximal to AMPK when compared with the naringin-resistant, putatively PP1-mediated mechanism.
Low-mobility forms of AMPK could not be detected before toxin treatment
The low-mobility AMPK forms detected by the anti-pAMPK-(pThr172) antibody in toxin- or AICAR-treated cells could be the result of a treatment-induced mobility shift, or else they could represent pre-existing molecular forms that become detectable as the result of treatment-induced Thr172 phosphorylation. To distinguish between these two possibilities, immunoblotting was performed with a general anti-AMPK antibody capable of detecting unphosphorylated as well as phosphorylated AMPK α-subunits. As shown in Figure 5(A), a mobility shift was detected with this general antibody (upper lanes) as well as with the phosphospecific antibody (lower lanes) after treatment with either microcystin or calyculin A. Similar shifts (not shown) were observed with OA, cantharidin or AICAR. The mobility shifts induced by microcystin (Figure 5A), OA or AICAR were naringin-sensitive, whereas the shifts induced by calyculin A (Figure 5A) or cantharidin were not. Results obtained with the general anti-AMPK antibody thus mimic those obtained with the phosphospecific antibody, showing that the low-mobility forms of AMPK do not pre-exist in detectable amounts in control cells, but are formed as a result of the treatment with AICAR or phosphorylation-inducing toxins.
Figure 5. Toxin-induced, naringin-sensitive AMPK mobility shifts as detected by general or phosphospecific anti-AMPK antibodies.
(A) Hepatocytes were incubated for 1 h at 37 °C with MC (3 μM) or calyculin A (0.5 μM) in the absence or presence of naringin (100 μM). Cell extracts were immunoblotted with a general antibody against the AMPK α-subunit (upper lanes) or with a phosphospecific antibody against Thr172-phosphorylated AMPK (lower lanes). (B) Extracts (containing ∼6 mg of protein/ml) from hepatocytes incubated with or without MC or calyculin A as in (A) were treated for 3 h at 37 °C with alkaline phosphatase (150 units/ml) as indicated.
To check whether the toxin-induced low-mobility AMPK forms might represent phosphorylated enzyme species, the cell extracts were pretreated with alkaline phosphatase before gel electrophoresis. The phosphatase treatment completely eliminated the low-mobility bands induced by microcystin or calyculin A (Figure 5B), indicating that they indeed represented AMPK α-subunits modified by toxin-induced phosphorylation at sites additional to Thr172. Some immunoreactivity remained in the high-mobility band after phosphatase treatment of extracts from toxin-treated cells, perhaps indicating that the pThr172 site was more resistant to dephosphorylation compared with the other sites.
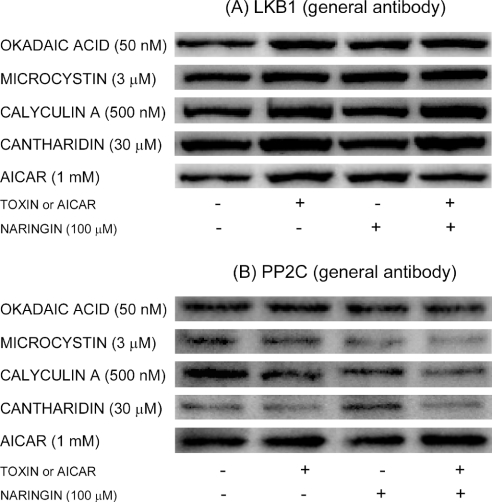
Lack of effect of toxins and AICAR on the mobility of LKB1 or PP2C
The AMPK α-subunit is phosphorylated at Thr172 by the protein kinase LKB1 [30,31] and thought to be dephosphorylated at this site by PP2C [40,41]. Both of these enzymes are, therefore, potential mediators of the toxin effects on Thr172 phosphorylation. Immunoblotting with general antibodies against LKB1 and PP2C (phosphospecific antibodies being unavailable at this time) gave good staining in the expected gel positions of both LKB1 (at 53 kDa; this enzyme bands higher than its nominal molecular mass of 49 kDa) and PP2C (at 42 kDa, which corresponds closely to the molecular masses of the α- and β-subunits, both of which react with this polyclonal antibody) respectively. However, none of the enzymes exhibited any detectable mobility shifting after treatment of hepatocytes with toxins or AICAR, be it in the absence or presence of naringin (Figure 6). Although phosphorylations that do not alter the mobility of the enzymes cannot be excluded, the results do not support a role for either LKB1 or PP2C as toxin targets.
Figure 6. Lack of effect of toxins, AICAR and naringin on LKB1 and PP2C electrophoretic mobility.
Hepatocytes were incubated for 1 h at 37 °C with AICAR or toxins at the concentrations indicated, in the presence or absence of naringin (100 μM). Cell extracts were immunoblotted with a general antibody against (A) LKB1 (bands located at approx. 53 kDa) or (B) PP2C (bands located at approx. 42 kDa). No mobility shifts are indicated.
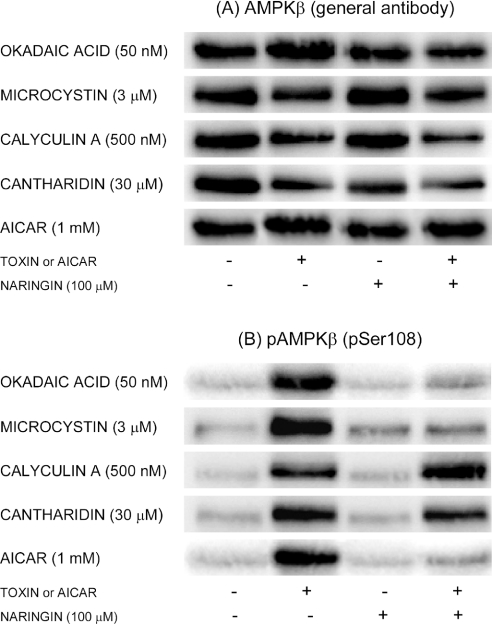
Toxin- and AICAR-induced mobility shifting and phosphorylation of the AMPK β-subunit
Since phosphorylation of the regulatory AMPK β-subunit at Ser108 has been suggested to be important for AMPK enzymic activity [42], the possibility that this subunit could be affected by toxin treatment was examined. As shown in Figure 7(A), immunoblotting with a general antibody against AMPKβ indicated a slight upward mobility shift of a band at approx. 35 kDa (the nominal molecular mass of AMPKβ is 30 kDa) after treatment with toxins or AICAR. Antagonistic effects of naringin were too marginal to be interpreted reliably.
Figure 7. Effects of toxins, AICAR and naringin on electrophoretic mobility and phosphorylation of the AMPK β-subunit.
Hepatocytes were incubated for 1 h at 37 °C with AICAR or toxins at the concentrations indicated, in the presence or absence of naringin (100 μM). Cell extracts were immunoblotted with (A) a general antibody against AMPKβ or (B) a phosphospecific antibody against AMPKβ phosphorylated at Ser108 (pAMPKβ); in both cases, the bands are located at approx. 35 kDa in the gels. The mobility shifts in (A) are, in most cases, best seen as a slight increase in the band front (bottom) in the second column relative to the first and third columns.
To ascertain better the effects of the tested agents on AMPKβ phosphorylation, immunoblotting with a phosphospecific antibody against Ser108-phosphorylated AMPKβ was performed. Strong immunostaining at 35 kDa was observed after treatment with toxins or AICAR. The phosphorylation was naringin-sensitive for OA, MC or AICAR, but naringin-resistant for calyculin A or cantharidin (Figure 7B). The effects of these agents on AMPKβ phosphorylation thus parallel their effects on AMPKα phosphorylation.
Autophagy-suppressive toxin effects are differentially naringin-sensitive
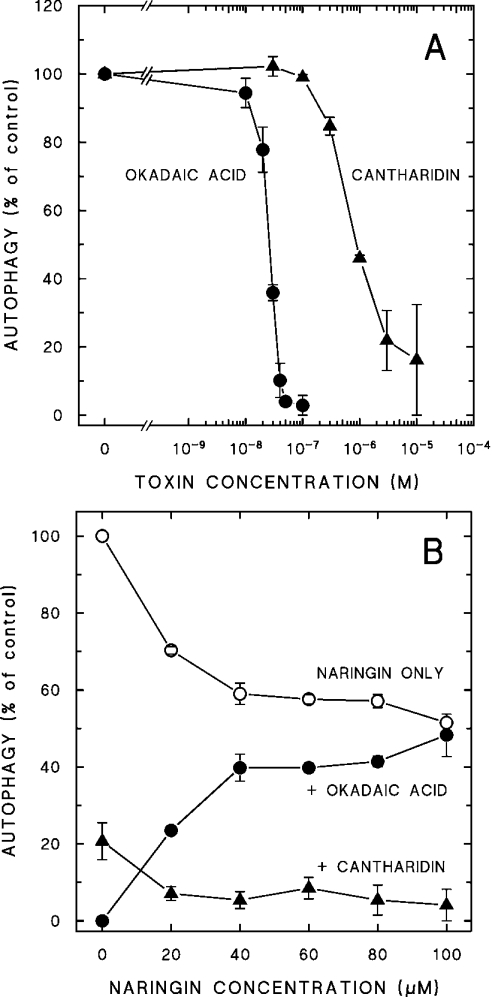
To see if the differential naringin sensitivity of the AMPK-activating toxins might be reflected at the level of autophagy, the autophagic activity of hepatocytes treated with either OA (naringin-sensitive) or cantharidin (naringin-resistant) was examined. As shown in Figure 8(A), both OA and cantharidin suppressed autophagy effectively, OA being approximately two orders of magnitude more potent than cantharidin. The autophagy-inhibitory effect of OA was effectively antagonized by naringin, but the inhibition by cantharidin was not (Figure 8B). Although naringin itself, for reasons not known, decreased the autophagic activity rather more than in previous experiments [23], there was clearly very little additional effect of OA at the highest concentrations of the flavonoid, whereas cantharidin was strongly autophagy-suppressive at all naringin concentrations. Thus, similar to AMPK phosphorylation, autophagy can apparently be modulated by either naringin-sensitive or naringin-resistant mechanisms, depending on the nature of the toxin applied. This observation is consistent with an involvement of AMPK in the down-regulation of hepatocellular autophagy under toxic stress.
Figure 8. Differential naringin sensitivity of autophagy-suppressive toxins.
(A) Hepatocytes were incubated for 2 h at 37 °C with OA (●) or cantharidin (▲) at the concentration indicated. Autophagic activity during this period was measured and expressed as a percentage of the control rate without toxins (3.3%/h). (B) Hepatocytes were incubated for 140 min at 37 °C with naringin alone at the indicated concentrations (○) or together with 40 nM OA (●) or 3 μM cantharidin (▲), added 20 min after naringin. Autophagic activity during the last 2 h of incubation was measured and expressed as percentage of the control rate without additions (2.9%/h). Each value is the mean±S.E. (or range) of 2–4 experiments. Some of the standard errors are hidden by the symbols.
Autophagy-suppressive effect of AICAR is naringin-sensitive
Since AMPK appears to be a mediator of naringin-sensitive as well as of naringin-resistant toxic stress, it would be of interest to examine to what extent the autophagy-suppressive effect of AICAR might be naringin-sensitive. As shown in Table 1, naringin eliminated the AICAR-induced suppression of autophagy as effectively as it eliminated the autophagy-suppressive effect of OA. The effects of naringin on autophagy thus parallel its effects on AMPK phosphorylation (Figure 3), supporting the notion of AMPK as the mediator of AICAR-induced autophagy suppression [7].
Table 1. Antagonistic effect of naringin on autophagy suppression induced by AICAR or OA.
Hepatocytes were incubated for 140 min at 37 °C with or without naringin (100 μM) alone or together with OA (30 nM) or AICAR (100 μM), which were added 20 min after the addition of naringin. Autophagic activity during the last 2 h of incubation was measured and expressed in terms of percentage per hour. Values are the means±S.E.M. for the numbers of experiments given in parentheses.
| Autophagic activity (%/h) | |||
|---|---|---|---|
| Control | AICAR (100 μM) | OA (30 nM) | |
| Control | 2.73±0.13 (4) | 0.69±0.20 (4) | 0.63±0.14 (3) |
| Naringin (100 μM) | 1.63±0.03 (4) | 1.65±0.03 (2) | 1.62±0.18 (3) |
DISCUSSION
Results of the present study show that AMPK is a target for several autophagy-suppressive toxins and suggest that the toxins may use two different AMPK-activating mechanisms: one naringin-sensitive and one naringin-resistant. Such a differential naringin sensitivity was also observed in our previous study of toxin-induced plectin phosphorylation [16], where it was pointed out that naringin sensitivity correlates quite well with the ability of the toxins to inhibit PP2A selectively compared with PP1. Thus, in intact cells, the naringin-antagonized toxins, namely OA and MC, seem to function as selective PP2A inhibitors, whereas the naringin-resistant toxins, namely calyculin A, cantharidin and tautomycin, act as selective inhibitors of PP1 [16].
Although AMPKα can be dephosphorylated at pThr172 by mixtures of PP1 and PP2A in cell extracts [32], the dephosphorylation seems to be performed exclusively by PP2C in intact cells [40]. Therefore, the effects of toxins on AMPK are, probably, indirect, which leaves open whether they, in fact, stimulate phosphorylation or inhibit dephosphorylation at Thr172. Since no alteration in PP2C mobility could be detected in the present study and since the toxins all seemed to induce phosphorylation at several AMPKα sites (detectable as the appearance of low-mobility bands), an indirect (permissive) effect on Thr172 phosphorylation would appear to be most plausible. Although AMPKα1 is phosphorylated inducibly at Thr258 and constitutively at Ser185, these sites do not appear to contribute to AMPK activation [32], suggesting that the AICAR-induced low-mobility AMPK forms reflect phosphorylation at other sites that are more permissive for the activating phosphorylation at Thr172.
AMPK is a heterotrimeric enzyme composed of a catalytic α-subunit and regulatory γ- and β-subunits, all of which can be represented by at least two isoforms. The γ-subunit can bind two molecules of ATP which seem to contact and inhibit the α-subunit, or two molecules of AMP which relieve the inhibition and support α-subunit activity [43]. The γ-subunit is apparently not subject to phosphorylation [32], but the ability of AICAR to stimulate Thr172 phosphorylation of the α-subunit, demonstrated in the present study as well as in previous reports [16,44], indicates that relief from inhibition by ATP and the γ-subunit makes the α-subunit more susceptible to phosphorylation. The β-subunit can, like the α-subunit, be phosphorylated at several sites, one of which (Ser108) is supposed to be an autophosphorylation site of importance for AMPK activity [42]. The fact that this β-subunit site is phosphorylated in parallel with the α-subunit after toxin and AICAR treatment is consistent with secondary phosphorylation by the α-subunit, but a primary, permissive function of the β-subunit phosphorylation cannot be excluded.
The AMPK kinase responsible for phosphorylation at Thr172 was recently identified as LKB1, a tumour-suppressive protein mutated in a cancer-predisposing disease, the Peutz–Jeghers syndrome [30,31]. Although cellular LKB1 can be phosphorylated by a number of protein kinases [45], its ability to phosphorylate AMPK appears to be independent of its phosphorylation state [31]. The unaltered mobility of LKB1 in toxin-treated cells, as demonstrated in the present study, indeed suggests that the toxins (like AICAR) do not stimulate AMPK phosphorylation through LKB1 activation, but rather by increasing the susceptibility of AMPK to phosphorylation by LKB1.
Since the permissive effects of toxins on Thr172 phosphorylation appear to be mediated both by naringin-sensitive (PP2A-associated) and naringin-resistant (PP1-associated) mechanisms, several different protein kinases are probably involved. CaMK-II, which has been implicated both in apoptotic and autophagy-suppressive toxin effects [46,47], is naringin-resistant [16] and would thus be a candidate for participation in the PP1-associated mechanism. Similarly, PKA may be involved in a partially naringin-sensitive suppression of hepatocytic autophagy [13] that could reflect the PP2A-associated mechanism. As for the mechanism of action of naringin, further studies will be needed to establish whether the flavonoid acts on AMPK itself (acting, e.g., as an AMP antagonist) or on a protein kinase involved in permissive AMPK phosphorylation.
The good correlation between the effects of toxins, AICAR and naringin on AMPK activation and autophagy suppression is consistent with a role for this kinase in the regulation of hepatocellular autophagy under metabolic and toxic stress. In yeast cells, AMPK/Snf1 stimulates autophagy rather than suppressing it [8], but it is not yet known whether this difference reflects the unique metabolic function of the liver or reflects fundamentally different stress survival strategies in yeast and mammalian cells. Autophagy-regulatory signalling mechanisms downstream from AMPK have not yet been clarified, but some putative elements are beginning to emerge. For example, both AICAR and the PP2A-associated toxins (OA, MC) induce naringin-sensitive phosphorylations of the stress-activated protein kinases SEK1 (stress-activated protein kinase/extracellular-signal-regulated kinase kinase 1) and JNK (c-Jun N-terminal kinase) [16]. Another member of this kinase family, p38, has previously been implicated in the suppression of autophagy induced by osmotic (hypo-osmotic) stress [15,48]. S6 kinase, an enzyme suggested to mediate amino acid-induced autophagy inhibition through phosphorylation of the ribosomal protein S6 [49], is not activated by either toxins or AICAR, but these agents induce, as do the amino acids, regulatory phosphorylations in the tail region of the enzyme [16] compatible with a non-catalytic, autophagy-suppressive function of the protein. Finally, OA and MC have been shown to induce naringin-sensitive phosphorylation of cytoskeletal proteins such as keratin and plectin and to cause naringin-sensitive disruption of the intracellular networks of plectin and keratin intermediate filaments [16,35,36]. These cytoskeletal alterations are probably mediated by AMPK and its downstream effectors and could well play an instrumental role in the suppression of autophagy, e.g. by perturbing the phagophore movements required for the autophagic sequestration of cytoplasm [1].
Acknowledgments
This work was generously supported by the Norwegian Cancer Society and the Research Council of Norway. The skilful technical assistance provided by F. Sætre and S. Finsnes is gratefully acknowledged.
References
- 1.Fengsrud M., Lunde Sneve M., Øverbye A., Seglen P. O. Structural aspects of mammalian autophagy. In: Klionsky D. J., editor. Autophagy, chapter 2. Georgetown, TX: Landes Bioscience; 2004. pp. 11–25. [Google Scholar]
- 2.Klionsky D. J., Cregg J. M., Dunn W. A., Jr, Emr S. D., Sakai Y., Sandoval I. V., Sibirny A., Subramani S., Thumm M., Veenhuis M., Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Dev. Cell. 2003;5:539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- 3.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 4.Fengsrud M., Raiborg C., Berg T. O., Strømhaug P. E., Ueno T., Erichsen E. S., Seglen P. O. Autophagosome-associated variant isoforms of cytosolic enzymes. Biochem. J. 2000;352:773–781. [PMC free article] [PubMed] [Google Scholar]
- 5.Abeliovich H., Zhang C., Dunn W. A., Jr, Shokat K. M., Klionsky D. J. Chemical genetic analysis of Apg1 reveals a non-kinase role in the induction of autophagy. Mol. Biol. Cell. 2003;14:477–490. doi: 10.1091/mbc.E02-07-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller M. T. N., Samari H. R., Fengsrud M., Strømhaug P. E., Østvold A. C., Seglen P. O. Okadaic acid-induced, naringin-sensitive phosphorylation of glycine N-methyltransferase in isolated rat hepatocytes. Biochem. J. 2003;373:505–513. doi: 10.1042/BJ20030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samari H. R., Seglen P. O. Inhibition of hepatocytic autophagy by adenosine, AICAR and N6-mercaptopurine riboside: evidence for involvement of AMP-activated protein kinase. J. Biol. Chem. 1998;273:23758–23763. doi: 10.1074/jbc.273.37.23758. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Wilson W. A., Fujino M. A., Roach P. J. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 2001;21:5742–5752. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codogno P., Meijer A. J. Signaling pathways in mammalian autophagy. In: Klionsky D. J., editor. Autophagy, chapter 3. Georgetown, TX: Landes Bioscience; 2004. pp. 26–47. [Google Scholar]
- 10.Noda T., Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 11.Gordon P. B., Tolleshaug H., Seglen P. O. Use of digitonin extraction to distinguish between autophagic-lysosomal sequestration and mitochondrial uptake of [14C]sucrose in hepatocytes. Biochem. J. 1985;232:773–780. doi: 10.1042/bj2320773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada Y., Funakoshi T., Shintani T., Nagano K., Ohsumi M., Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holen I., Gordon P. B., Strømhaug P. E., Seglen P. O. Role of cAMP in the regulation of hepatocytic autophagy. Eur. J. Biochem. 1996;236:163–170. doi: 10.1111/j.1432-1033.1996.00163.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogier-Denis E., Pattingre S., El Benna J., Codogno P. Erk1/2-dependent phosphorylation of Gα-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J. Biol. Chem. 2000;275:39090–39095. doi: 10.1074/jbc.M006198200. [DOI] [PubMed] [Google Scholar]
- 15.Häussinger D., Schliess F., Dombrowski F., Vom Dahl S. Involvement of p38MAPK in the regulation of proteolysis by liver cell hydration. Gastroenterology. 1999;116:921–935. doi: 10.1016/s0016-5085(99)70076-4. [DOI] [PubMed] [Google Scholar]
- 16.Ruud Larsen A.-K., Møller M. T. N., Blankson H., Samari H. R., Holden L., Seglen P. O. Naringin-sensitive phosphorylation of plectin, a cytoskeletal crosslinking protein, in isolated rat hepatocytes. J. Biol. Chem. 2002;277:34826–34835. doi: 10.1074/jbc.M205028200. [DOI] [PubMed] [Google Scholar]
- 17.Arico S., Petiot A., Bauvy C., Dubbelhuis P. F., Meijer A. J., Codogno P., Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 18.Strømhaug P. E., Fengsrud M., Berg T. O., Møller M. T. N., Grotterød E. M., Samari H. R., Blankson H., Halvorsen K., Holen I., Kovács A. L., et al. Regulation of autophagy by protein phosphorylation. In: Hopsu-Havu V. K., Järvinen M., Kirschke H., editors. Proteolysis in Cell Functions. Amsterdam: IOS Press; 1997. pp. 357–366. [Google Scholar]
- 19.Inbal B., Bialik S., Sabanay I., Shani G., Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holen I., Strømhaug P. E., Gordon P. B., Fengsrud M., Berg T. O., Seglen P. O. Inhibition of autophagy and multiple steps in asialoglycoprotein endocytosis by inhibitors of tyrosine protein kinases (tyrphostins) J. Biol. Chem. 1995;270:12823–12831. doi: 10.1074/jbc.270.21.12823. [DOI] [PubMed] [Google Scholar]
- 21.Tallóczy Z., Jiang W. X., Virgin H. W., Leib D. A., Scheuner D., Kaufman R. J., Eskelinen E. L., Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holen I., Gordon P. B., Seglen P. O. Inhibition of hepatocytic autophagy by okadaic acid and other protein phosphatase inhibitors. Eur. J. Biochem. 1993;215:113–122. doi: 10.1111/j.1432-1033.1993.tb18013.x. [DOI] [PubMed] [Google Scholar]
- 23.Gordon P. B., Holen I., Seglen P. O. Protection, by naringin and some other flavonoids, of hepatocytic autophagy and endocytosis against inhibition by okadaic acid. J. Biol. Chem. 1995;270:5830–5838. doi: 10.1074/jbc.270.11.5830. [DOI] [PubMed] [Google Scholar]
- 24.Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 25.Gjessing R., Seglen P. O. Adsorption, simple binding and complex binding of rat hepatocytes to various in vitro substrata. Exp. Cell Res. 1980;129:239–249. doi: 10.1016/0014-4827(80)90347-x. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Kopitz J., Kisen G. Ø., Gordon P. B., Bohley P., Seglen P. O. Non-selective autophagy of cytosolic enzymes in isolated rat hepatocytes. J. Cell Biol. 1990;111:941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovács A. L., Gordon P. B., Grotterød E. M., Seglen P. O. Inhibition of hepatocytic autophagy by adenosine, adenosine analogues and AMP. Biol. Chem. 1998;379:1341–1347. doi: 10.1515/bchm.1998.379.11.1341. [DOI] [PubMed] [Google Scholar]
- 29.Hardie D. G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell. Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 30.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Woods A., Vertommen D., Neumann D., Turk R., Bayliss J., Schlattner U., Wallimann T., Carling D., Rider M. H. Identification of phosphorylation sites in AMP-activated protein kinase for upstream AMP-activated protein kinase kinases and study of their roles by site-directed mutagenesis. J. Biol. Chem. 2003;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 33.Kovács A. L., Grinde B., Seglen P. O. Inhibition of autophagic vacuole formation and protein degradation by amino acids in isolated hepatocytes. Exp. Cell Res. 1981;133:431–436. doi: 10.1016/0014-4827(81)90336-0. [DOI] [PubMed] [Google Scholar]
- 34.Hardie D. G., Carling D., Sim A. T. R. The AMP-activated protein kinase: a multisubstrate regulator of lipid metabolism. Trends Biochem. Sci. 1989;14:20–23. [Google Scholar]
- 35.Blankson H., Grotterød E. M., Seglen P. O. Prevention of toxin-induced cytoskeletal disruption and apoptotic liver cell death by the grapefruit flavonoid, naringin. Cell Death Differ. 2000;7:739–746. doi: 10.1038/sj.cdd.4400705. [DOI] [PubMed] [Google Scholar]
- 36.Blankson H., Holen I., Seglen P. O. Disruption of the cytokeratin cytoskeleton and inhibition of hepatocytic autophagy by okadaic acid. Exp. Cell Res. 1995;218:522–530. doi: 10.1006/excr.1995.1187. [DOI] [PubMed] [Google Scholar]
- 37.Jinsart W., Ternai B., Polya G. M. Inhibition of rat liver cyclic AMP-dependent protein kinase by flavonoids. Hoppe-Seyler's Z. Physiol. Chem. 1992;373:205–211. doi: 10.1515/bchm3.1992.373.1.205. [DOI] [PubMed] [Google Scholar]
- 38.Ferriola P. C., Cody V., Middleton E. Protein kinase C inhibition by plant flavonoids. Biochem. Pharmacol. 1989;38:1617–1624. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 40.Davies S. P., Helps N. R., Cohen P. T. W., Hardie D. G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2Ac. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 41.Moore F., Weekes J., Hardie D. G. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur. J. Biochem. 1991;199:691–697. doi: 10.1111/j.1432-1033.1991.tb16172.x. [DOI] [PubMed] [Google Scholar]
- 42.Warden S. M., Richardson C., O'Donnell J., Jr, Stapleton D., Kemp B. E., Witters L. A. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem. J. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubbelhuis P. F., Meijer A. J. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett. 2002;521:39–42. doi: 10.1016/s0014-5793(02)02815-6. [DOI] [PubMed] [Google Scholar]
- 45.Boudeau J., Sapkota G., Alessi D. R. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546:159–165. doi: 10.1016/s0014-5793(03)00642-2. [DOI] [PubMed] [Google Scholar]
- 46.Fladmark K. E., Brustugun O. T., Mellgren G., Krakstad C., Bøe R., Vintermyr O. K., Schulman H., Døskeland S. O. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- 47.Holen I., Gordon P. B., Seglen P. O. Protein kinase-dependent effects of okadaic acid on hepatocytic autophagy and cytoskeletal integrity. Biochem. J. 1992;284:633–636. doi: 10.1042/bj2840633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vom Dahl S., Dombrowski F., Schmitt M., Schliess F., Pfeifer U., Häussinger D. Cell hydration controls autophagosome formation in rat liver in a microtubule-dependent way downstream from p38MAPK activation. Biochem. J. 2001;354:31–36. doi: 10.1042/0264-6021:3540031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blommaart E. F. C., Luiken J. J. F. P., Blommaart P. J. E., Van Woerkom G. M., Meijer A. J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J. Biol. Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]