Abstract
Progression through the cell cycle is regulated by CDKs (cyclin-dependent kinases), which associate with activating partners, named cyclins, to efficiently phosphorylate substrates. We previously reported the identification of RINGO, a Xenopus protein that can activate CDK1 and CDK2 despite lack of sequence similarity to cyclins, which plays a role in the regulation of the meiotic cell cycle in oocytes. In the present study we report the characterization of four mammalian RINGO proteins, which are 53–68% identical with Xenopus RINGO in a central core of about 75 residues. We show that all RINGO family members can bind to and activate CDK1 and CDK2, albeit with different efficiencies, but they do not bind to CDK4 or CDK6. The core RINGO sequences are critical for CDK activation. We also identified key residues in CDK2 that are required for RINGO binding. All RINGO proteins can also bind the CDK inhibitor p27Kip1, but with an inverse efficiency of their ability to bind to CDK1. Our results identify a new family of mammalian proteins that can activate CDKs and therefore potentially function as cell cycle regulators. The ability of RINGO proteins to activate CDK1 and CDK2 suggest also cyclin-independent roles for these kinases.
Keywords: cell cycle, cyclin, cyclin-dependent kinase (CDK), RINGO, Speedy, Xenopus oocyte
Abbreviations: CDK, cyclin-dependent kinase; GST, glutathione S-transferase; HA, haemagglutinin; XRINGO, Xenopus RINGO
INTRODUCTION
CDKs (cyclin-dependent kinases) are essential regulators of the eukaryotic cell division cycle. CDKs require association with activating subunits named cyclins, which also provide the CDK with targeting domains responsible for subcellular localization and substrate selection and thus biological specificity [1,2]. The activity of CDKs is also modulated by both inhibitory and activating phosphorylation. In particular, full activity of most CDKs requires the phosphorylation of a conserved threonine located on the activation loop, close to the catalytic cleft of the kinase [3–6].
To date, at least nine mammalian CDKs have been identified, of which only a few appear to play an essential regulatory role in cell division. In the G1 phase of the cell cycle, extracellular signals modulate the activation of cyclin D-bound CDK4 and CDK6. The protein kinase activity of these enzymes results in the expression of genes whose products are involved in G1/S transition and S-phase progression, such as cyclin E and cyclin A. Further cell cycle progression is regulated by CDK1 in association with either cyclin A or cyclin B for the S/G2 and G2/M transitions respectively [7–9].
Contrary to initial belief, activation of CDKs does not necessarily require the presence of cyclins. For example, CDK5 is activated by p35, a protein that shows no sequence similarity to cyclins [10,11]. However, the CDK5–p35 complex does not seem to have a major role in cell cycle regulation and mainly functions in post-mitotic neurons [12].
Surprisingly, CDK1 and CDK2, two key regulators of cell division, can be also activated by a Xenopus protein named RINGO [13] or Speedy [14] that plays an important role in the regulation of the meiotic cell cycle in oocytes. In a manner analogous to p35, RINGO/Speedy exhibits no amino acid sequence homology to cyclins, but can directly activate CDK1 and CDK2 (independently of cyclin binding) and can even bypass the requirement for phosphorylation in the activation loop [15]. Recently, a human homologue of RINGO/Speedy has been reported [16], which can interact with CDK2 and the CDK inhibitor p27Kip1 and has been proposed to play a role in the G1/S transition [17].
In the present study we report the characterization of four mammalian proteins related to XRINGO (Xenopus RINGO). The RINGO proteins are most similar in a region that we refer to as the RINGO core, which is involved in binding to and activation of CDK1 and CDK2. Thus RINGO family members are a family of novel CDK activators that can potentially regulate the cell cycle.
EXPERIMENTAL
DNA cloning and mutagenesis
Ringo1 and Ringo2 cDNAs were obtained as human ESTs (expressed sequence tags) and Ringo4 from the Fantom mouse cDNA collection. Ringo3 was cloned by reverse-transcriptase PCR from mouse testis mRNA and its human orthologue has been reported as Speedy1 [16].
For expression in Xenopus oocytes as N-terminally Myc-tagged proteins, Xenopus CDK1, CDK2 and CDK4 and human CDK5 and CDK6 were amplified by PCR, cloned into PCR2.1 (Invitrogen) and then subcloned into the FTX5 vector as EcoRI–XhoI (CDK1), XhoI (CDK2), BamHI (CDK5), BamHI–XhoI (CDK4) and NcoI–EcoRI (CDK6) fragments. The same approach was used to clone Ringo1 (lacking the first 28 amino acids), Ringo2 and Ringo3 in FTX5 as EcoRI, BamHI–XhoI and EcoRI fragments respectively. The FTX5–XRINGO construct has been described previously [13]. Xenopus cyclin A1 was cloned in FTX5 as an NcoI–EcoRI fragment. For the expression of N-terminally HA (haemagglutinin)-tagged proteins, CDK1, CDK2 and CDK5 were cloned into the FTX4–HA vector as EcoRI–XhoI, XhoI and BamHI fragments respectively, whereas Ringo1, Ringo4 and p27Kip1 were all cloned as EcoRI fragments. For expression in Escherichia coli as GST (glutathione S-transferase)-fusion proteins, mammalian Ringo1–Ringo4 were cloned in pGEX-KG as NcoI–XhoI (Ringo 1 and Ringo3), BamHI (Ringo2) and EcoRI (Ringo4) fragments. The bacterial expression constructs for malE (Escherichia coli maltose-binding protein)–XRINGO and GST–human CDK2 have been described previously [13].
All mutants of XRINGO, Ringo1 and CDK2 were prepared using the QuikChange® site-directed mutagenesis kit (Stratagene) and were verified by DNA sequencing. XRINGO and Ringo1 with alanine substitutions were prepared using the FTX5–XRINGO and FTX4–HA–Ringo1 constructs as templates and oligonucleotides designed to replace the indicated residues for alanine. XRINGO C-terminus deletion mutants ΔC233, ΔC207, ΔC194 and ΔC181 were prepared by introducing stop codons at the indicated positions. XRINGO ΔN65 and XRINGO N-terminus ΔN74 mutants were prepared by using oligonucleotides complementary to 20 nt upstream of the first ATG and 20 nt downstream of either position 195 or 222 in FTX5–XRINGO. Ringo1-(Δ263–308) was prepared by using an oligonucleotide complementary to 20 nt upstream of position 787 and 20 nt downstream of position 945 of FTX4–HA–Ringo1.
XRINGO(66–206) was cloned using oligonucleotides designed to introduce an NcoI site at nucleotide 195 and another NcoI site preceded by a stop codon at nucleotide 619. Ringo1(ΔN142) was cloned using oligonucleotides designed to create an Nco1 site at nucleotide 426 and an EcoRI site downstream of the stop codon. PCR fragments were cloned in PCR2.1 vector, digested with the corresponding restriction endonucleases and subcloned in FTX5, pGEX–KG or FTX4–HA vectors.
Immunobloting, immunoprecipitation and kinase assays
Xenopus oocytes were sorted, injected with in vitro-transcribed mRNAs and lysed as described previously [18].
For immunoblotting, Myc and HA monoclonal antibodies from mouse and rat respectively (Roche, Mannheim, Germany) and the Cdc2 (cell division cycle 2) monoclonal antibody 3E1 were used as described previously [13]. The blots were developed using Alexa Fluor 680 (Molecular Probes) or Li-Cor IRDye 800 (Rockland) labelled antibodies with the Odyssey Infrared Imaging System (Li-Cor).
For the immunoprecipitations, 5 μl of anti-Myc (Santa Cruz; sc-40 AC) or anti-HA (Santa Cruz; sc-7392 AC) agarose conjugates were incubated with oocyte lysates for 2 h at 4 °C. The beads were then washed three times in immunoprecipitation buffer [18] and analysed by immunoblotting, or further washed in histone H1 kinase buffer [18] and used for kinase assays.
Recombinant malE- and GST-fusion proteins were expressed in E. coli DE3 and purified using standard protocols [13]. For kinase assays, bacterially produced proteins or immunoprecipitates were incubated in a final volume of 12 μl of histone H1 kinase buffer containing 150 μM unlabelled ATP, 2 μCi of [γ-32P]ATP (3000 Ci/mmol) and 4 μg of histone H1 (Sigma). After 20 min at 30 °C, the reactions were terminated by addition of sample buffer and the gels were analysed by electrophoresis and autoradiography.
Bacterial two-hybrid assays
To be used in the BacterioMatch II two-hybrid system (Stratagene), the XRINGO and Xenopus CDK2 cDNAs were PCR amplified using oligonucleotides that introduced NotI and XhoI restriction sites and subcloned into the pBT and pTRG vectors respectively. CDK2 mutations were generated using pTRG–CDK2 as a template.
BacterioMatch reporter cells (40 μl) were transformed by electroporation with 50 ng of each plasmid pair (XRINGO and CDK2) and processed according to the manfacturer's instructions. Colonies were counted and the percentage of co-transformants on plates with selective versus non-selective medium was calculated for each plasmid pair. Positive XRINGO–CDK2 interactions typically yielded between 7 and 26% of colonies growing on the selective medium that contains 5 mM 3-amino-1,2,4-triazole, whereas this number was always lower than 0.1% for the non-interacting CDK2 mutants.
Computational analysis
Sequence alignments were performed using T-Coffee (http://www.ch.embnet.org/software/TCoffee.html) and secondary structure prediction with JPRED (http://www.compbio.dundee.ac.uk/~www-jpred/). Phylogenetic trees were created using the Neighbour-Joining method implemented in Clustal W (http://www.ebi.ac.uk/clustalw/).
RESULTS
Identification of mammalian RINGO homologues
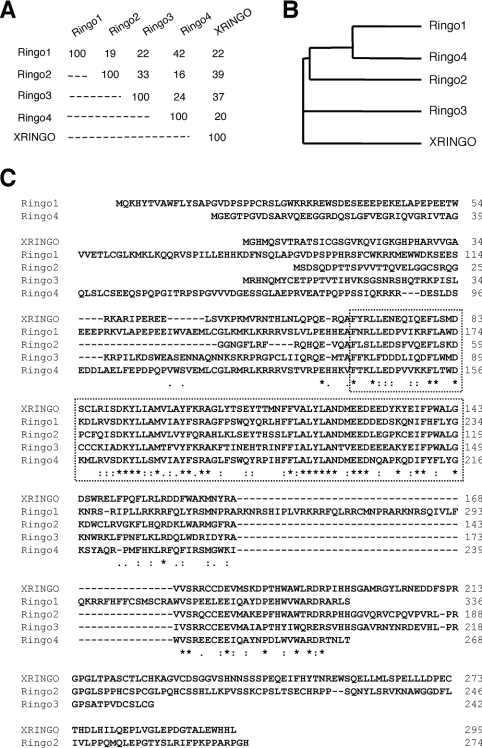
We searched databases for putative homologues of XRINGO and found two human and two mouse clones that share significant homology. We also found RINGO-related sequences in fish (medaka and zebrafish; results not shown). However, no related sequences were found in yeast, worm, flies and plants. We cloned the four mammalian cDNAs related to XRINGO, which were named according to their chronological identification. The mammalian RINGO proteins were 16–42% identical in their amino acid sequences (Figures 1A and 1B). Alignments of the different RINGO protein sequences revealed a central region that was highly conserved in all family members, especially in a stretch of approx. 75 residues, which we named the RINGO core (Figure 1C). Computational analysis using JPRED predicts that the RINGO core may have an α-helical secondary structure.
Figure 1. Sequence alignment and phylogenetic tree of the RINGO protein family.
(A) Percentage of amino acid sequence identity among the different RINGO proteins. (B) Phylogenetic tree of the RINGO proteins. (C) Alignment of the amino acid sequences of XRINGO and the four mammalian RINGO proteins. Asterisks indicate conservation and colons conservative substitutions. The dotted square indicates a highly conserved core region. The GenBank® accession numbers are CAB58366 (XRINGO), NM175064 (Ringo1), BE280100 (Ringo2), AK014910 (Ringo3) and AK015441 (Ringo4).
By Northern blotting, we could detect high levels of expression of the mammalian RINGO proteins only in testis. Using reverse-transcriptase PCR, we also detected low levels of Ringo3 expression in mouse liver, heart and thymus, whereas Ringo2 was found highly expressed in liver (to about the same level as in testis) and at much lower levels in kidney and thymus. However, based on the information available in UniGene (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=unigene), Ringo1 and Ringo2 mRNAs might be also expressed in a variety of human tissues and in some tumours (results not shown).
Mammalian RINGO proteins bind to and activate CDKs
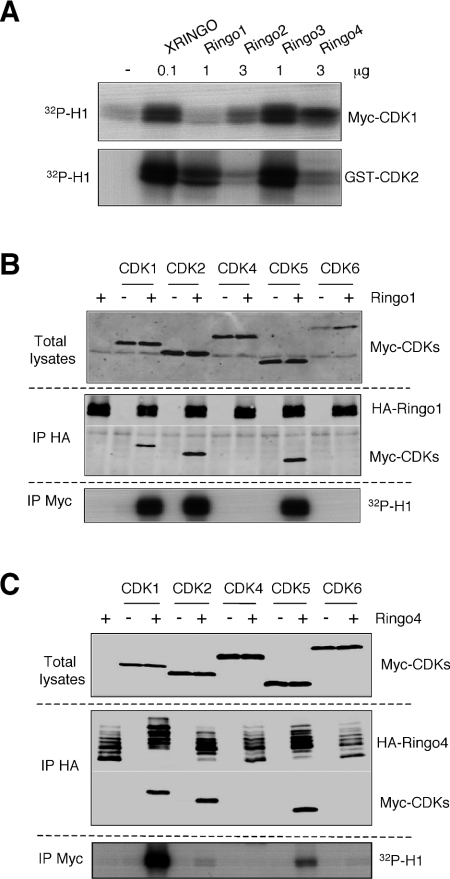
XRINGO has been described to directly activate CDK1 and CDK2 [13–15], we therefore investigated the ability of bacterially produced mammalian RINGO proteins to stimulate the kinase activity of these CDKs using histone H1 as a substrate. For this experiment, we used purified, bacterially produced human CDK2 fused to GST (GST–CDK2). However, bacterially produced GST–CDK1 cannot be activated by XRINGO [13] or cyclins (results not shown), probably due to misfolding, and we used instead Myc-tagged Xenopus CDK1 immunoprecipitated from Xenopus oocytes to assay CDK1 activation. We found that the most efficient activator of both CDKs was XRINGO followed by Ringo3 (note that we used 10-fold less XRINGO to obtain similar levels of CDK1 and CDK2 activity). The other mammalian RINGO proteins were also able to activate CDK1 and/or CDK2, albeit with different efficiencies (Figure 2A). The same results were obtained using either the wild-type or Thr160→Ala mutant GST–CDK2 (results not shown), suggesting that the mammalian RINGO proteins can also activate CDK2 in the absence of activation loop phosphorylation, as reported for XRINGO [15].
Figure 2. Mammalian RINGO proteins can bind to and activate CDKs.
(A) Lysates from 10 Xenopus oocytes expressing Myc–CDK1 were immunoprecipitated with Myc antibody. Activation of the immunoprecipitated Myc–CDK1 or bacterially produced GST–CDK2 (0.5 μg) with the indicated amounts of recombinant RINGO proteins was analysed by in vitro kinase assays on histone H1 (32P-H1). (B) and (C) Oocytes were injected with mRNAs encoding HA-tagged Ringo1 (B) or Ringo4 (C), incubated overnight and re-injected with mRNAs encoding the indicated Myc-tagged CDKs. After 10 h, lysates from 14 oocytes were immunoprecipitated (IP) with HA antibody and then immunoblotted, together with an aliquot of the total lysates, with anti-Myc (CDKs) and anti-HA (Ringo1 and Ringo4) antibodies. Myc immunoprecipitates prepared from 5 oocytes of the same batch were used for H1 kinase assays.
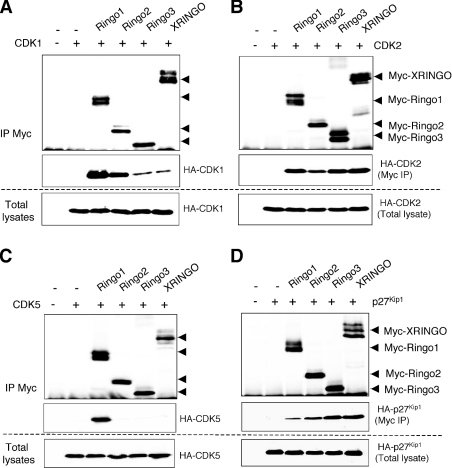
To investigate whether RINGO proteins could bind and activate other CDKs, we co-expressed Ringo1 together with Xenopus CDK1, CDK2 and CDK4 or human CDK5 and CDK6 in Xenopus oocytes. We found that Ringo1 bound to CDK1, CDK2 and CDK5, whereas CDK4 and CDK6 were not detected in the Ringo1 immunoprecipitates (Figure 2B). By direct immunoprecipitation of the CDKs, we found that Ringo1 binding activated CDK1, CDK2 and CDK5 to similar extents. Ringo4 was also able to bind in oocytes to CDK1, CDK2 and CDK5, but not to CDK4 and CDK6. However, in contrast with Ringo1, Ringo4 appeared to be a better activator of CDK1 and CDK5 than of CDK2, at least using histone H1 as a substrate (Figure 2C). We then performed the experiment using different RINGO family members in parallel to compare their abilities to bind to CDKs. All RINGO proteins expressed in oocytes bound to CDK1 and CDK2, albeit with different efficiencies (Figures 3A and 3B). However, only Ringo1 was able to bind to CDK5 in this assay (Figure 3C). All RINGO family members were able to associate with p27Kip1 expressed in Xenopus oocytes (Figure 3D), as was previously reported for human Speedy1 (Ringo3) [17]. We observed an inverse correlation between the ability of RINGO proteins to bind to CDK1 and to p27Kip1, with Ringo3 and XRINGO binding to CDK1 poorly and to p27Kip1 with high efficiency, whereas the opposite was true for Ringo1 and Ringo2 (Figures 3A and 3D).
Figure 3. Binding of mammalian RINGO proteins to different CDKs and p27Kip1.
Xenopus oocytes were injected with mRNAs encoding HA-tagged CDK1 (A), CDK2 (B), CDK5 (C) or p27Kip1 (D) and incubated overnight. The oocytes were then re-injected with mRNAs encoding different Myc-tagged RINGO family members. After 10 h, lysates from 35 oocytes were immunoprecipitated (IP) with Myc antibody and then immunoblotted, together with an aliquot of the total lysates, with Myc (RINGO) and HA (CDK or p27Kip1) antibodies.
RINGO core is essential for RINGO protein function
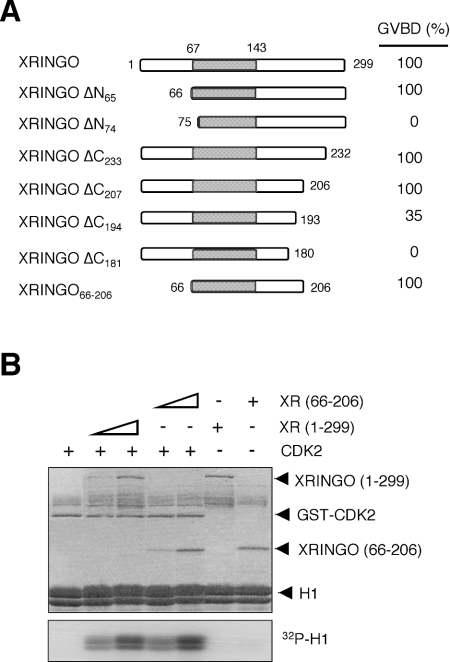
Previous work showed that deletion of residues 5–53 or 218–268 did not impair the ability of XRINGO to induce Xenopus oocyte maturation, whereas internal deletions of 40–50 residue blocks between residues 54 and 218 abolished the activity of XRINGO [16]. To further map the functional boundaries of the RINGO proteins, we generated a number of N-terminal and C-terminal deletions of XRINGO. We found that deletion of the N-terminal 65 residues did not impair the ability XRINGO to induce oocyte maturation, whereas a protein starting at residue 75 was inactive. Likewise, a protein ending at residue 206 was still able to induce oocyte maturation as efficiently as full-length XRINGO, whereas the protein truncated at residue 193 induced oocyte maturation with slower kinetics than full-length XRINGO. To confirm these results, we generated an XRINGO construct comprising only residues 66–206 and found that injecting this truncated protein, which includes the RINGO core, was sufficient to induce oocyte maturation (Figure 4A). This construct was also able to bind to CDK2 (results not shown). Figure 4(B) shows that the central region of XRINGO expressed in bacteria was as efficient as full-length XRINGO at stimulating the protein kinase activity of CDK2 using histone H1 as substrate in vitro.
Figure 4. Core of XRINGO is sufficient to induce oocyte maturation and CDK activation.
(A) Schematic representation of the XRINGO deletion mutants with the core region shaded in grey. Oocyte maturation (GVBD, germinal vesicle breakdown) was analysed 12 h after the injection of Myc-tagged XRINGO mRNAs. (B) Histone H1 kinase activity of GST–CDK2 (0.5 μg) incubated with bacterially produced XRINGO proteins (0.15 and 0.5 μg). The Coomassie stained gel is shown in the upper panel.
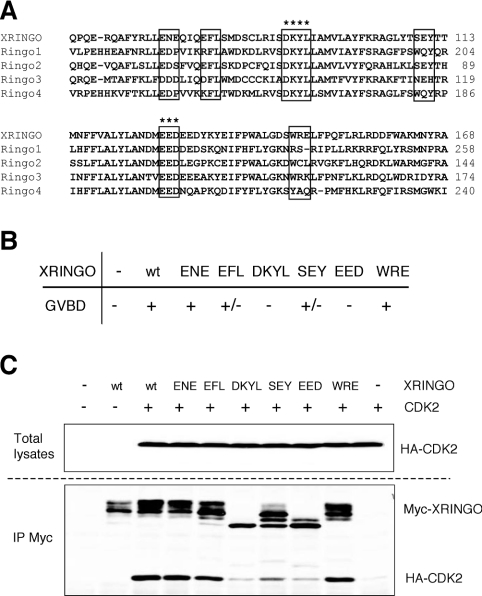
To complement these studies, we generated a number of alanine substitutions in the conserved central region of XRINGO (Figure 5A). We confirmed that mutation of the sequences DKYL and EED, highly conserved in all RINGO proteins (Figure 5A, asterisks), abolished the ability of XRINGO to induce oocyte maturation. In contrast, less conserved regions either had no effect or only partially impaired XRINGO activity in oocytes (Figures 5A and 5B). We also found that the XRINGO mutants that were unable to induce oocyte maturation were also impaired in their ability to bind to CDK2 in oocytes (Figure 5C). Moreover, the inactive XRINGO mutants showed higher electrophoretic mobility, probably indicating their lack of phosphorylation by CDK2.
Figure 5. Core of XRINGO is required to induce oocyte maturation and for CDK binding.
(A) Sequence alignment of the conserved region including the core of the RINGO protein family members. The residues mutated to alanine in XRINGO are boxed. Asterisks indicate two highly conserved sequences. (B) The ability of the mutated proteins described in (A) to induce GVBD (germinal vesicle breakdown) was analysed 20 h after the injection of Myc-tagged XRINGO mRNAs in Xenopus oocytes. + and +/− indicate that more than 70% or less than 30% of the oocytes matured respectively (as an average using different oocyte batches), whereas XRINGO mutants indicated as − were never able to induce oocyte maturation. (C) Oocytes were injected with HA-tagged CDK2 mRNA, incubated overnight and then re-injected with mRNAs encoding the indicated XRINGO mutant proteins. After 8 h, lysates from 25 oocytes were immunoprecipitated (IP) with anti-Myc antibody and then immunoblotted, together with an aliquot of the total lysates, with anti-Myc (XRINGO) and anti-HA (CDK2) antibodies. wt, wild-type.
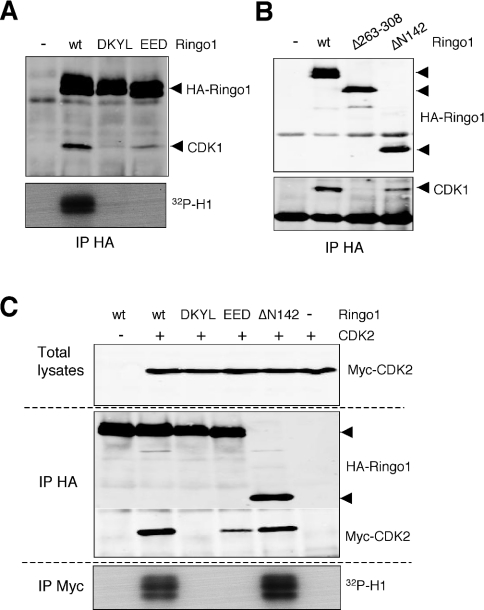
To confirm the functional importance of the RINGO core, we introduced the same alanine mutations that abolished XRINGO activity in Ringo1, a distantly related family member. Mutation of DKYL abolished the ability of Ringo1 to bind to endogenous CDK1 in Xenopus oocytes, whereas Ringo1 mutated at EED was still able to bind to CDK1, albeit with significantly reduced efficiency. However, neither of these Ringo1 mutants were able to trigger histone H1 kinase activation in Xenopus oocytes when compared with wild-type Ringo1 (Figure 6A). Moreover, a Ringo1 protein lacking the N-terminal 142 residues (which retains the RINGO core and a short C-terminal extension) was still able to bind to CDK1, whereas an internal deletion of 45 amino acids, including some very conserved residues in RINGO proteins, abolished CDK1 binding to Ringo1 (Figure 6B). Taken together, the results indicated that the conserved sequences in the core of RINGO proteins are essential for CDK binding and activation.
Figure 6. Mutations in the conserved core impair the ability of Ringo1 to bind to and activate CDKs.
(A) and (B) Lysates from 15 oocytes expressing the indicated Ringo1 proteins were immunoprecipitated (IP) with anti-HA antibody and then immunoblotted with anti-CDK1 and anti-HA (Ringo1) antibodies. HA immunoprecipitates prepared from five oocytes of the same batch were used for H1 kinase assays (32P-H1). DKYL and EED refer to the mutants indicated in Figure 5(A). (C) Oocytes expressing the indicated Ringo1 proteins were re-injected with Myc-tagged CDK2-encoding mRNA. After 10 h, lysates from 15 oocytes were immunoprecipitated with anti-HA antibody and then immunoblotted, together with an aliquot of the total lysates, with anti-Myc (CDK2) and anti-HA (Ringo1) antibodies. Myc immunoprecipitates prepared from 5 oocytes of the same batch were used for H1 kinase assays. wt, wild-type.
We also investigated if the alanine mutations in the RINGO core affected binding of Ringo1 to other CDKs. In agreement with the results mentioned above for CDK1, these mutations rendered the Ringo1 protein unable to activate CDK2 (Figure 6C). However, whereas CDK2 binding was abolished in the DKYL mutant, the EED mutant was still able to bind to CDK2, albeit weakly. The truncated Ringo1 protein corresponding to the core region (ΔN142) was still able to bind to and activate CDK2 (Figure 6C). The DKYL and EED mutations also impaired the ability of Ringo1 to bind to and activate CDK5, whereas the truncated ΔN142 mutant could bind and activate CDK5 as efficiently as the full-length Ringo1 (results not shown).
Identification of CDK2 sequences required for RINGO binding
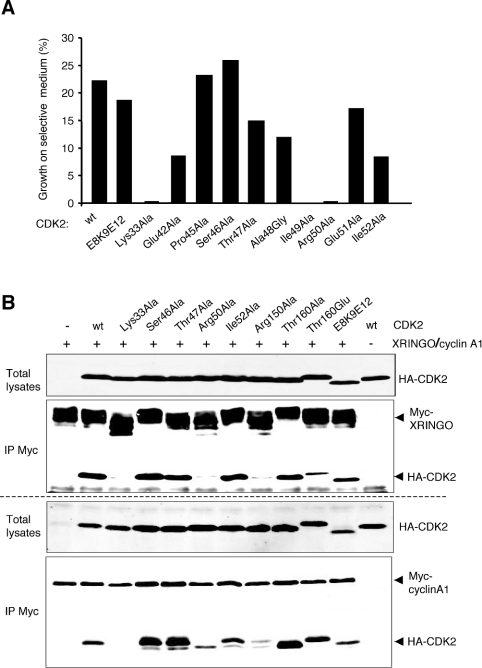
We found that CDK2 and XRINGO were able to interact in the BacterioMatch two-hybrid system, a quick method to study protein–protein interactions. We used this system to investigate the CDK2 sequences that were involved in binding to RINGO proteins (Figure 7A). First, we tested whether the PSTAIRE helix of CDKs, which plays a key role in cyclin binding [19], was also important for RINGO binding. We individually mutated all PSTAIRE amino acids and two adjacent residues in CDK2 and found that only two of these mutations (Ile49→Ala and Arg50→Ala) abolished XRINGO binding in the bacterial two-hybrid system. Additionally, mutation of Lys33 in the ATP-binding region to alanine also abolished CDK2–XRINGO binding. On the other hand, CDK2 containing the cluster mutation E8A/K9A/E12A (single letter amino acid code), which has been proposed to impair binding of 35S-labelled in vitro-translated CDK1 to recombinant cyclin A cross-linked to Sepharose [20], was still able to bind to XRINGO.
Figure 7. Identification of CDK2 sequences required for XRINGO binding.
(A) Bacterial two-hybrid analysis of the interaction between XRINGO and the indicated CDK2 mutants; E8K9E12 indicate that the 3 residues were mutated to alanine. A representative experiment is shown. CDK2 mutants that interacted with XRINGO yielded more than 7% of co-transformants which grew on plates containing 5 mM 3-amino-1,2,4-triazole, whereas this number was always less than 0.1% for the negative interactors. It should be noted that the technique is not quantitative and higher number of colonies among the interacting mutants does not necessarily imply more efficient binding. (B) Oocytes expressing the indicated HA-tagged CDK2 mutants were re-injected with mRNA encoding either Myc-tagged XRINGO or Xenopus cyclin A1. After 10 h, lysates from 32 oocytes were immunoprecipitated (IP) with anti-Myc antibody and then immunoblotted with anti-Myc (XRINGO and cyclin A1) and anti-HA (CDK2) antibodies. wt, wild-type.
To validate the above results and to further map CDK2 sequences important for XRINGO binding, we performed immunoprecipitation experiments using some of the mutants tested in the bacterial two-hybrid system, as well as additional mutants (Figure 7B, upper panel). Upon co-expression with XRINGO in Xenopus oocytes, the CDK2 Lys33→Ala and Arg50→Ala mutants were both impaired in XRINGO binding, whereas the mutations Ser46→Ala, Thr47→Ala and Ile52→Ala had no effect. Mutation of the activation loop Thr160 to alanine did not affect binding, whereas the Thr160→Glu mutation reduced XRINGO binding, which is in agreement with the previously reported negative effect that Thr160 phosphorylation may have on CDK activation by XRINGO [15]. We also confirmed in Xenopus oocytes that the mutant E8A/K9A/E12A was able to bind to XRINGO, whereas mutation of Arg150, a residue that has been implicated in the CDK2–cyclinA interaction based on structural studies [19], abolished XRINGO binding. For comparison, we performed the same immunoprecipitation experiment using Xenopus cyclin A1. In this assay, cyclin A1 was able to bind all the CDK2 mutants except Lys33→Arg, although binding to the Arg50 and Arg150 mutants was significantly reduced (Figure 7B, lower panel).
DISCUSSION
Here we present the characterization of four mammalian RINGO proteins, which together with XRINGO constitute a family of novel CDK activators. The overall sequence identity of the RINGO proteins is not very high, except for a conserved central core, which is necessary and sufficient for CDK binding and activation. Secondary structure predictions reveal an α-helical structure of the RINGO core, as it has been reported for the cyclin box, the region involved in interaction with the CDKs. This may account for the ability of both types of proteins to activate CDKs, in spite of their lack of sequence homology.
All RINGO family members have similar biochemical properties, including the ability to bind to CDK1, CDK2 and p27Kip1, but there are also clear differences among them. For example, Ringo1 and Ringo2 bind with higher efficiency to CDK1 than to p27Kip1, whereas Ringo3 and XRINGO bind to CDK1 poorly and p27Kip1 with very high efficiency. Moreover, although Ringo1 and Ringo4 can both bind to CDK1, CDK2 and CDK5 with similar efficiencies, they significantly differ in their ability to activate these CDKs. We also found differences in the efficiency of CDK1 and CDK2 activation by RINGO family members. We cannot rule out, however, the possibility that improper folding of the recombinant RINGO proteins might account for some of the differences observed in the in vitro assays. On the other hand, the poorly conserved amino acid sequences outside of the core region might contribute to the differences in partner specificity among RINGO family members.
CDK activation by cyclins involves structural changes, both in the PSTAIRE helix and the activation loop, which are essential for the CDK to refold into a catalytically active conformation [19]. Phosphorylation of a threonine residue on the activation loop further reorganizes the catalytic cleft and substrate binding site, making the CDK more suitable for catalysis [5]. In the absence of the activation loop phosphorylation, XRINGO is a more efficient CDK activator than cyclins [15], suggesting that RINGO binding should mimic, at least partially, the conformational changes that lead to the displacement of the activation loop. This is consistent with the mechanism that has been proposed, on the basis of structural studies, for the activation of CDK5 by p35, another non-cyclin protein [21]. Binding of p35 triggers all conformational changes required for CDK5 activation in the absence of activation loop phosphorylation. The observation that some RINGO proteins can also bind to and activate CDK5, supports the idea that RINGO proteins use the same mechanism for CDK activation as p35. Our results suggest that this phosphorylation-independent mechanism of CDK activation may also be operative in CDK1 and CDK2, as an alternative activation pathway to the well-characterized one that requires their association with specific cyclin subunits, followed by phosphorylation in the activation loop by a CDK-activating kinase.
Our results indicate that RINGO proteins bind to the same part of the CDK molecule as cyclins. XRINGO binding to CDK2 involves residues in the PSTAIRE helix (Arg50) and activation loop (Arg150) that are also important for binding to cyclin A1. This is not surprising, since both cyclin and RINGO would be expected to have similar modes of interaction with the CDK in these two regions in order to induce the conformational changes required for CDK activation. However, the Arg50 and Arg150 mutations have a much stronger inhibitory effect on the binding of RINGO than on cyclin A binding, suggesting that there may be differences between the interaction of the two activators with CDK2.
In summary, we describe a new family of mammalian RINGO proteins that can bind to and directly activate CDK1 and CDK2, two kinases that play key roles in cell cycle progression. Since the activation of CDKs by RINGO and cyclins has different requirements, it is reasonable to postulate that RINGO-activated CDKs could have different functions. One obvious possibility is that RINGO proteins may direct the CDKs to different set of substrates, since cyclins are thought to contribute, at least in part, to the substrate specificity of the CDK–cyclin complex [1,2,22]. The ability of some RINGO family members to activate CDK5 may also reflect additional functions for these proteins beyond cell cycle regulation.
Acknowledgments
We thank Randy Poon (Hong Kong University of Science and Technology) and Gordon Peters (Cancer Research UK London Institute) for the CDK5 and CDK6 cDNAs respectively, Filippo del Bene for initial attempts to clone mammalian RINGO cDNAs, Philippe Beaufils for help in setting up the BacterioMatch system and Gustavo Gutierrez for comments on the manuscript. A. D. acknowledges an EMBL pre-doctoral fellowship.
References
- 1.Schulman B. A., Lindstrom D. L., Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams P. D., Li X., Sellers W. R., Baker K. B., Leng X., Harper J. W., Taya Y., Kaelin W. G. J. Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin–cdk complexes. Mol. Cell. Biol. 1999;19:1068–1080. doi: 10.1128/mcb.19.2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol. Cell. Biol. 1992;3:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Y., Rosenblatt J., Morgan D. O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A. A., Jeffrey P. D., Pavletich N. P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat. Struct. Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 6.Brown N. R., Noble M., Lawrie A. M., Morris M. C., Tunnah P., Divita G., Johnson L. N., Endicott J. A. Effects of phosphorylation of threonine 160 on cyclin-dependent kinase 2 structure and activity. J. Biol. Chem. 1999;274:8746–8756. doi: 10.1074/jbc.274.13.8746. [DOI] [PubMed] [Google Scholar]
- 7.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem. J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 9.Morgan D. O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 10.Lew J., Huang Q. Q., Qi Z., Winkfein R. J., Aebersold R., Hunt T., Wang J. H. A brain-specific activator of cyclin-dependent kinase 5. Nature (London) 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 11.Tsai L. H., Delalle I., Jr, Chae C. V. S., Harlow T. E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature (London) 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 12.Dhavan R., Tsai L. H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 13.Ferby I., Blazquez M., Palmer A., Eritja R., Nebreda A. R. A novel p34cdc2-binding and activating protein that is necessary and sufficient to trigger G2/M progression in Xenopus oocytes. Genes Dev. 1999;13:2177–2189. doi: 10.1101/gad.13.16.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenormand J. L., Dellinger R. W., Knudsen K. E., Subramani S., Donoghue D. J. Speedy: a novel cell cycle regulator of the G2/M transition. EMBO J. 1999;18:1869–1877. doi: 10.1093/emboj/18.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaiskou A., Perez L. H., Ferby I., Ozon R., Jessus C., Nebreda A. R. Differential regulation of Cdc2 and Cdk2 by RINGO and cyclins. J. Biol. Chem. 2001;276:36028–36034. doi: 10.1074/jbc.M104722200. [DOI] [PubMed] [Google Scholar]
- 16.Porter L. A., Dellinger R. W., Tynan J. A., Barnes E. A., Kong M., Lenormand J. L., Donoghue D. J. Human Speedy: a novel cell cycle regulator that enhances proliferation through activation of Cdk2. J. Cell Biol. 2002;157:357–366. doi: 10.1083/jcb.200109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter L. A., Kong-Beltran M., Donoghue D. J. Spy1 interacts with p27Kip1 to allow G1/S progression. Mol. Cell. Biol. 2003;14:3664–3674. doi: 10.1091/mbc.E02-12-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perdiguero E., Nebreda A. R. Use of Xenopus oocytes and early embryos to study MAP kinase signalling. Methods Mol. Biol. 2004;250:299–314. doi: 10.1385/1-59259-671-1:299. [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey P. D., Russo A. A., Polyak K., Gibbs E., Hurwitz J., Massague J., Pavletich N. P. Mechanism of CDK activation revealed by the structure of a cyclinA–CDK2 complex. Nature (London) 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 20.Marcote M. J., Knighton D. R., Basi G., Sowadski J. M., Brambilla P., Draetta G., Taylor S. S. A three-dimensional model of the Cdc2 protein kinase: localization of cyclin- and Suc1-binding regions and phosphorylation sites. Mol. Cell. Biol. 1993;13:5122–5131. doi: 10.1128/mcb.13.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarricone C., Dhavan R., Peng J., Areces L. B., Tsai L. H., Musacchio A. Structure and regulation of the CDK5–p25nck5a complex. Mol. Cell. 2001;8:657–669. doi: 10.1016/s1097-2765(01)00343-4. [DOI] [PubMed] [Google Scholar]
- 22.Brown N. R., Noble M. E., Endicott J. A., Johnson L. N. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1999;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]