Abstract
Background
The group-I metabotropic glutamate receptor subtype 5 (mGlu5) has been implicated in methamphetamine exposure in animals and in human cognition. Because people with methamphetamine use disorder (MUD) exhibit cognitive deficits, we evaluated mGlu5 in people with MUD and controls and tested its association with cognitive performance.
Methods
Positron emission tomography was performed to measure the total VT of [18F]FPEB, a radiotracer for mGlu5, in brains of participants with MUD (abstinent from methamphetamine for at least 2 weeks, N = 14) and a control group (N = 14). Drug use history questionnaires and tests of verbal learning, spatial working memory, and executive function were administered. Associations of VT with methamphetamine use, tobacco use, and cognitive performance were tested.
Results
MUD participants did not differ from controls in global or regional VT, and measures of methamphetamine use were not correlated with VT. VT was significantly higher globally in nonsmoking vs smoking participants (main effect, P = .0041). MUD participants showed nonsignificant weakness on the Rey Auditory Verbal Learning Task and the Stroop test vs controls (P = .08 and P = .13, respectively) with moderate to large effect sizes, and significantly underperformed controls on the Spatial Capacity Delayed Response Test (P = .015). Across groups, Rey Auditory Verbal Learning Task performance correlated with VT in the dorsolateral prefrontal cortex and superior frontal gyrus.
Conclusion
Abstinent MUD patients show no evidence of mGlu5 downregulation in brain, but association of VT in dorsolateral prefrontal cortex with verbal learning suggests that medications that target mGlu5 may improve cognitive performance.
Keywords: Group-I metabotropic glutamate receptor subtype 5, stimulant use disorder, dorsolateral prefrontal cortex, positron emission tomography, verbal learning
Significance Statement.
We examined mGlu5 in the brains of participants with MUD and controls using PET with [18F]FPEB, a selective radiotracer for mGlu5. Although the total volume of distribution (VT) of [18F]FPEB in brain showed no significant group difference and no relation to indices of methamphetamine use, cigarette smoking was associated with lower VT globally across all participants. VT in the prefrontal cortex correlated with verbal learning, suggesting that mGlu5 may be a useful therapeutic target to improve cognition in MUD.
INTRODUCTION
Methamphetamine use disorder (MUD) is a major public health concern as part of the current overdose and broader public health crisis involving illicit drug use (Rawson et al., 2023). From 1999 to 2021, the methamphetamine mortality rate increased 50-fold among US residents aged 15 to 74 years (Hoopsick and Yockey, 2023). People who use methamphetamine chronically exhibit deficits in several cognitive domains (e.g., Dean et al., 2013; Potvin et al., 2018), including attention; verbal learning; inhibitory control; reward processing; social cognition; and verbal, visual, and working memory. As there is no FDA-approved medication for the treatment of MUD, behavioral approaches are the mainstay of therapy. However, the presence of cognitive impairments can thwart engagement in behavioral treatments and predict poor outcome (Brorson et al., 2013).
While monoamines such as dopamine play a key role in the initiation of and psychomotor effects of psychostimulant use, metabotropic glutamate receptors influence the regulation of dopamine release in these processes (Arai et al., 1996, 1997; Shimazoe et al., 2002). The group-I metabotropic glutamate receptor subtype 5 (mGlu5) is a predominantly postsynaptic receptor that contributes to excitatory neurotransmission (Shigemoto et al., 1993, 1997; Daggett et al., 1995; Gereau and Conn, 1995). Within the brain, its expression is highest in the hippocampus, cerebral cortex, striatum, and thalamus and lowest in the cerebellum (Daggett et al., 1995). Repeated exposure to methamphetamine increases mGlu5 receptor signaling (Szumlinski et al., 2017) and levels in the nucleus accumbens in mice (Wright et al., 2016), and mice selectively bred for high methamphetamine intake have higher methamphetamine-evoked increases in extracellular glutamate, higher levels of mGlu5 in the nucleus accumbens, and higher basal extracellular glutamate levels in the medial prefrontal cortex than mice bred for low intake (Lominac et al., 2016; Szumlinski et al., 2017).
The mGlu5 is implicated in cognition (Homayoun and Moghaddam, 2010; Hullinger et al., 2015), particularly in memory processes (Lu et al., 1997; Bortolotto et al., 2005; Lee et al., 2005; Manahan-Vaughan and Braunewell, 2005; Bikbaev et al., 2008; Neyman and Manahan-Vaughan, 2008). Consistent with evidence of the procognitive effects of positive allosteric modulators of mGlu5 preclinically (Liu et al., 2008; Reichel et al., 2011), several recent studies utilizing positron emission tomography (PET) found positive associations between cognitive performance and mGlu5 availability in brain. Across participants with Alzheimer disease and healthy controls, global cognition scores were positively associated with hippocampal mGlu5 availability measured with [18F]PSS232 (Wang et al., 2024) and [18F]FPEB (Mecca et al., 2020). Higher mGlu5 binding potential in the caudate and left temporal cortex, measured with [11C]ABP688, correlated with faster processing speed in individuals with schizophrenia (Régio Brambilla et al., 2020).
The mGlu5 has not been assessed in people with MUD, but studies using PET with [11C]ABP688 have indicated that cocaine-dependent participants have below-control mGlu5 availability throughout the brain (Martinez et al., 2014; Milella et al., 2014). Here we used PET with [18F]FPEB, a radioligand with high selectivity and specificity for the negative allosteric modulator site on mGlu5 (Wong et al., 2013; Park et al., 2015), to measure the total volume of distribution (VT) of [18F]FPEB in brains of individuals with MUD and matched control participants. In light of the global deficit in mGlu5 observed in cocaine-dependent participants (Martinez et al., 2014; Milella et al., 2014), we hypothesized that VT for [18F]FPEB would be lower in MUD compared with controls. Given prior literature showing positive relationships between cognitive function and mGlu5 binding and the importance of the prefrontal cortex in higher-level cognitive functions, we also expected VT in the prefrontal cortex to be related to cognitive performance across participants. Lastly, prior studies have shown lower mGlu5 availability in the brains of people who smoked cigarettes compared with values in nonsmokers (Akkus et al., 2013, 2017; Hulka et al., 2014; Baldassarri et al., 2023). We therefore tested the interaction between smoking and MUD on VT for [18F]FPEB.
MATERIALS AND METHODS
Participants
Fourteen individuals who met DSM-5 criteria for severe MUD (4 women and 10 men) and 14 control participants (5 women and 9 men) underwent PET scans with [18F]FPEB to measure mGlu5 VT in brain. MUD group participants were recruited from residential (N = 12) and outpatient treatment programs (N = 2) and were abstinent from methamphetamine for at least 14 but fewer than 75 days (mean = 42 days). Control participants were recruited from the community via online and print advertisements. All procedures were approved by the University of California Los Angeles Institutional Review Board and the Greater Los Angeles Veterans Affairs Institutional Review Board and Radiation Safety Committee. All participants were fluent in English and signed informed consent documents after receiving a detailed description of the protocol. Exclusion criteria, determined by a psychiatric evaluation using the Mini International Neuropsychiatric Interview for DSM-5 and a physician-conducted history, physical examination and laboratory tests, included: neurological, cardiovascular, pulmonary, endocrine, autoimmune, untreated renal hepatic, or active infectious disease; psychotic disorders; suicidality; asthma; age outside the range of 18 to 65 years inclusive; MRI contraindications (metallic implants, claustrophobia); pregnancy; head trauma with loss of consciousness >30 minutes; current use of prescribed psychotropic medications with dopaminergic or glutamatergic action; or meeting prisoner status per California Section 3502. Further exclusions for the control group were the use of any psychotropic medications, meeting criteria for a substance use disorder, or more than 5 lifetime uses of substances of abuse other than nicotine, caffeine, or cannabis. Several MUD group participants met criteria for additional substance use disorders: severe opioid use disorder (N = 1), severe alcohol use disorder (N = 4), and severe sedative use disorder (N = 1). Some also met criteria for additional diagnoses, including past depressive episodes (N = 4), current anxiety disorders (N = 2), bulimia nervosa (N = 2), obsessive compulsive disorder (N = 1), panic disorder (N = 1), post-traumatic stress disorder (PTSD; N = 1), and antisocial personality disorder (N = 8). In the control group, some participants met criteria for past depressive episodes (N = 6), anxiety disorder (N = 1), and antisocial personality disorder (N = 2). MUD group participants reported significantly fewer years of education than the control group (Table 1).
Table 1.
Participant Characteristics (Mean ± SE)
| Control | Methamphetamine use disordera | |||
|---|---|---|---|---|
| Smoking N = 6 |
Nonsmoking N = 8 |
Smoking N = 8 |
Nonsmoking N = 6 |
|
| Sex (men/women) | 5/1 | 4/4 | 5/3 | 5/1 |
| Age (y) | 32.0 ± 3.6 | 33.0 ± 2.8 | 36.3 ± 2.0 | 33.5 ± 3.3 |
| Parent’s education (y)b | 15.7 ± 1.1 | 13.8 ± 2.0 | 12.5 ± 0.6 | 14.0 ± 2.0 |
| Education (y)c,d | 15.8 ± 0.8 | 15.9 ± 0.7 | 11.7 ± 0.5 | 13.8 ± 0.7 |
| FSIQ | 107 ± 3.0 | 106 ± 3.4 | 101 ± 3.4 | 103 ± 2.1 |
| Race (ethnicity) | ||||
| Caucasian (non-Hispanic) | 4 | 1 | 1 | 2 |
| Caucasian (Hispanic) | 0 | 3 | 3 | 2 |
| African American | 1 | 2 | 1 | 0 |
| Asian | 0 | 1 | 0 | 0 |
| Hawaiian/Pacific Islander | 1 | 0 | 0 | 0 |
| American Indian/Alaska Native | 0 | 0 | 0 | 0 |
| More than 1 race | 0 | 1 | 3 | 2 |
| Handedness (right-handed) | 6 | 8 | 6 | 6 |
Abbreviations: FSIQ, full scale intelligence quotient; MUD, methamphetamine use disorder.
a Participants met DSM-5 criteria for MUD.
b Missing data: 1 control, 6 MUD.
c Missing data: 1 control, 1 MUD.
d P = .0002.
Although there were no other significant group differences in demographic and drug use variables, the MUD group had more participants who smoked cigarettes than the control group. Among those who smoked, nicotine dependence (Fagerström test scores) and withdrawal on the test day (Shiffman-Jarvik Withdrawal scores) were modest (Table 2). To standardize the timing of nicotine and cannabis exposure across all participants and prevent the effect of nicotine on mGlu5, all participants were instructed to abstain from using tobacco products for at least 12 hours and from cannabis products for at least 48 hours before PET and MRI scans. The average duration of abstinence from tobacco at the time of scanning was nonsignificantly higher in the control group because 1 participant had abstained for 17 days; all other participants in both groups had abstained between 16 and 25 hours before PET. Abstinence from smoking was confirmed by carbon monoxide measurements of <10 ppm or a 50% reduction from baseline intake, determined with a smokerlyzer (Covita Micro+ basic). Nicotine replacement therapies were not used by any participants.
Table 2.
Self-reported Substance Use (Mean ± SE)a
| Control | Methamphetamine use disorder | |||
|---|---|---|---|---|
| Smoking N = 6 |
Non-smoking N = 8 |
Smoking N = 8 |
Non-smoking N = 6 |
|
| Methamphetamine use | ||||
| Duration (y) | 6.52 ± 2.45 | 8.28 ± 3.13 | ||
| Average daily use (g/d) | 2.6 ± 1.7 | 1.3 ± 0.4 | ||
| Days used in last 30 d | 21.6 ± 4.6 | 9.7 ± 3.2 | ||
| Duration of abstinence (d) | 38.9 ± 6.0 | 45.2 ± 5.8 | ||
| Route of administration | ||||
| Smoking, n (%) | 7 (87.5) | 5 (83.3) | ||
| Intravenous injection, n (%) | 0 (0) | 2 (33.3) | ||
| Sniff/snort, n (%) | 3 (37.5) | 5 (83.3) | ||
| Tobacco use | ||||
| Days used in last 30 d | 17.5 ± 4.3 | 26.3 ± 2.1 | ||
| Cigarettes per day | 7.3 ± 2.9 | 7.1 ± 2.0 | ||
| Duration of abstinence (h) | 1063.6 ± 40.7 | 18.8 ± 1.0 | ||
| Nicotine withdrawal (Shiffman- Jarvik average score) |
3.3 ± 0.7 | 3.2 ± 0.5 | ||
| Fagerström (FTND) score | 2.8 ± 1.4 | 2.4 ± 0.8 | ||
| Cannabis use | ||||
| Number of participants, n (%) | 2 (33.3) | 1 (12.5) | 3 (37.5) | 2 (33.3) |
| Days used in last 30 d | 12 ± 8 | 5 | 13.0 ± 8.6 | 1.5 ± 0.5 |
| Alcohol use | ||||
| Number of participants, n (%) | 6 (100) | 4 (50) | 3 (37.5) | 2 (33.3) |
| Days used in last 30 d | 7.7 ± 2.2 | 4.2 ± 2.1 | 10.3 ± 6.6 | 9.5 ± 5.5 |
Abbreviations: FTND, Fagerström test for nicotine dependence.
a No significant differences in tobacco, cannabis, or alcohol use.
MUD group participants were required to maintain abstinence from other substances for at least 4 days before PET and MRI scans, demonstrated by negative urine toxicology (methamphetamine, amphetamine, opiates, cocaine, and benzodiazepines) and breathalyzer (alcohol) tests. Control participants were required to test negative for all substances except cannabis and nicotine. A single participant in the MUD tested positive for cannabis use on scanning and cognitive testing days and had been abstinent from using cannabis products for more than 2 weeks at the time of testing. Tobacco use was not restricted on nonscanning study days, including cognitive testing days.
Behavioral Assessments
All participants completed several interviews and self-report questionnaires to characterize their substance use and health history. These included a neurological history questionnaire used to ascertain study eligibility, and a 147-item assessment of current and past use of addictive substances. In addition, a 26-item questionnaire queried current and past methamphetamine use, and a 10-item questionnaire, adapted from the Cocaine Craving Questionnaire–Brief (Sussner et al., 2006), was used to assess current craving for methamphetamine. Participants who indicated that they smoked cigarettes also were administered the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) during screening and the Shiffman-Jarvik Withdrawal Scale (Shiffman and Jarvik, 1976) immediately before PET scanning.
We administered cognitive tests on a nonscanning day. These included the Rey Auditory Verbal Learning Test (RAVLT; Rey, 1941), which evaluates verbal retention, encoding, and retrieval ability. The variable of interest was immediate memory (age-adjusted Z-score trials A1-A5 total score). The Spatial Capacity Delayed Response Test (SCAP; based on Sternberg 1969; Glahn et al., 2003) was administered to test visual working memory. The primary outcome variable for this test was overall response accuracy, calculated through signal detection procedures (i.e., d-prime). Finally, the Stroop test (Delis et al., 2001) was administered as a test of inhibitory control. The primary outcome variable computed from the 3 subsections was the interference score, calculated as Stroop Color-Word Inhibition test score—(Stroop Color-Naming test score * Stroop Word-Reading test score)/Stroop Color-Naming test score + Stroop Word-Reading test score). The Wechsler test for Adult Reading (Wechsler, 2001) was used to produce an estimated Full-Scale Intelligence Quotient. Participants were allowed to smoke throughout the day as needed.
Image Acquisition and Data Analyses
PET Acquisition
[18F]FPEB was produced via no-carrier-added [18F]fluorination of 3-nitro-5-[2-(pyridinyl)ethynyl]benzonitrile, a precursor for [18F]FPEB, followed by solid phase extraction purification and high-performance liquid chromatography (HPLC) purification (Lim et al., 2014). [18F]FPEB was i.v. administered as a bolus plus continuous infusion with Kbol = 205 minutes to achieve a steady state (Kessler et al., 2014a). The radiotracer dose was 5 mCi ± 10% error (mean = 5.09 ± 0.09) mCi (Supplemental Table 2). Continuous infusion over 140 minutes was ensured via the use of an infusion pump (Graseby 3400 Syringe Pump). In a past study in humans, a Kbol of 205 minutes produced good stability of total uptake at 90 to 130 minutes in high-uptake regions, including the hippocampus (Kessler et al., 2014b).
PET-imaging data were acquired with a Siemens Biograph mCT PET/CT scanner (Hoffman Estates, IL). Dynamic scanning for the acquisition of emission data began 110 minutes after [18F]FPEB bolus administration, lasting for a total of 30 minutes and consisting of three 10-minute frames. The data were acquired within a 2-hour period in the afternoon for all participants to minimize effects of within-day variation on mGlu5 (DeLorenzo et al., 2017). A computed tomography (CT) transmission scan was acquired immediately before dynamic scanning for attenuation correction. Four 8-mL venous samples were collected at 10-minute intervals during the equilibrium period (109, 119, 129, and 139 minutes after the start of [18F]FPEB administration). To measure total plasma radioactivity in the venous samples at equilibrium, the heparinized samples were centrifuged for 10 minutes at 3,000 × g, and activities in 0.2-mL plasma aliquots were determined. Activity was decay-corrected to the start of PET dynamic scanning and was corrected for the fraction of plasma radioactivity represented by unmetabolized parent. To calculate the percent parent [18F]FPEB, 2-mL plasma aliquots from each venous sample were mixed with acetonitrile. The resulting supernatant was analyzed by high-performance liquid chromatography via a Luna 5u C18(2) 100A 250 × 10 mm 5 micron Phenomenex column with a 2-mL loop and a mobile phase of 70/30 methanol/water with 0.1% triethylamine at a flow rate of 4 mL/min. Serial samples were collected every 30 seconds for 12 minutes and the activity counted. The unmetabolized parent fraction was determined as the ratio of the sum of activity in fractions containing the parent compound to the total amount of activity collected in all samples.
MRI Acquisition
Magnetic resonance image (MRI) data were acquired to guide anatomical sampling of the PET data. MR data were acquired with a 3-Tesla PRISMA MRI scanner with a weighted gradient-echo (MPRAGE) sequence, including the following parameters: TE = 2.24 ms, TI = 1060 ms, TR = 2400 ms, FOV = 240 256 mm2, flip angle = 8°, 745 Hz/pixel bandwidth, voxel size = 0.8 mm3, 208 sagittal slices.
PET Image Processing and Quantification
The PET acquisition comprised 3 contiguous frames, each consisting of 10 one-minute scans. The data were reconstructed using TrueX with Time of Flight (5 iterations, 21 subsets) to 30 one-minute volumes (400 × 400 × 109). Measured attenuation correction, relative scatter correction, and 2.0 mm Gaussian smoothing were applied.
Within each frame, the 10 volumes were realigned to the averaged volume for that frame using FSL FLIRT (Jenkinson et al., 2012) with a normalized correlation cost function. The averaged volumes were recomputed, and those for frames 2 and 3 were realigned to that for frame 1. The realigned averaged volumes for all 3 frames underwent co-registration and resampling to the structural MPRAGE image using FSL FLIRT with mutual information as the cost function. Visual inspection confirmed proper alignment and co-registration.
The structural MPRAGE image underwent segmentation via Freesurfer (Fischl et al., 2002; version 6.0.0) and was normalized to MNI space using linear (FSL FLIRT) and nonlinear (FSL FNIRT) registration. Volumes of interest (VOIs) from the FreeSurfer-based Desikan-Killiany Atlas in MNI space transformed to native space. For voxelwise analyses, the aligned co-registered averaged PET volumes were warped from native space to MNI space.
The primary outcome measure of the [18F]FPEB data was the total VT, computed as the ratio of [18F]FPEB activity in tissue (brain) to venous plasma parent activity (Innis et al., 2007). Specifically, VT was calculated as the ratio of activity in brain tissue in each 10-minute frame to the mean activity in venous samples measured immediately before and after the frame. VT was averaged within each VOI in native space. Bilateral VOIs were used for all analyses. The dorsolateral prefrontal cortex VOI was comprised of the rostral middle frontal and caudal middle frontal VOIs. The inferior frontal gyrus VOI was comprised of the pars opercularis, pars triangluaris, and pars orbitalis VOIs. The MNI brain template from FSL was used as a whole-brain VOI. VT also was computed on a voxel-by-voxel basis to create VT maps in MNI space.
VT contains contributions from the number of mGlu5 sites available for specific [18F]FPEB binding and includes contributions from nondisplaceable tissue activity and nonspecific binding. The use of a reference region is precluded here as mGlu5 is expressed throughout the human brain (DeLorenzo et al., 2011, Kågedal et al., 2013), including in the cerebellum (Patel et al., 2007).
Statistical Analyses
Group differences in categorical demographic and substance use variables were tested with Pearson chi-square and Fisher exact tests. Group differences in continuous demographic and substance use variables were assessed with independent t tests. Wilcoxon rank-sum tests were used to assess group differences in non-normally distributed variables (age, education, average daily use of methamphetamine, days used in last 30 days, cigarettes smoked per day, days smoked per month).
Group differences in [18F]FPEB VT were determined using a general linear model while accounting for individual participant variables, including age, sex, and smoking status. GLM analyses were conducted using [18F]FPEB VT in each VOI (whole brain, cortex, thalamus, striatum) as the dependent variable and group (MUD or control), smoking status (smoker or nonsmoker), sex, age, and smoking status and group interaction as independent variables. Main effects were considered significant for α < .05 after Bonferroni correction (α < .05/4). Partial eta squared (η2) values were calculated for each model as a measure of effect size (0.01 = small, 0.06 = medium, 0.14 = large). Post-hoc independent t tests were used to confirm directionality of main effect findings.
Associations of [18F]FPEB VT in the cortex, thalamus, and striatum with measures of methamphetamine use, including average daily use (grams per day), days used per month when actively using, duration of abstinence, and craving on the day of the PET scan, were calculated via partial correlational analyses controlling for age, sex, smoking status, and global proportional scaling (whole brain VT). Spearman rank order correlations were used to assess associations with non-normally distributed variables (average daily use, days used per month when actively using, craving). Correlations were considered significant for α < .05 after Bonferroni correction (α < .05/12).
The correlation between [18F]FPEB VT in the cortex, thalamus, and striatum and measures of tobacco use, including cigarettes smoked per day, days smoked per month, duration of abstinence, Fagerström score, and Shiffman-Jarvik score, were calculated via partial correlational analyses controlling for age, sex, group, and global proportional scaling. Spearman rank order correlations were used to assess associations with non-normally distributed variables (cigarettes smoked per day, days smoked per month, abstinence duration). Correlations were considered significant for α < .05 after Bonferroni correction (α < .05/15).
Associations of [18F]FPEB VT in the dorsolateral prefrontal cortex, inferior frontal gyrus, anterior cingulate cortex, and hippocampus with cognitive performance on the RAVLT, SCAP, and STROOP were calculated via partial correlational analyses, controlling for age, sex, estimated Full-Scale Intelligence Quotient, smoking status, group, and global proportional scaling. Due to the non-normal distribution of SCAP D-prime scores, a Spearman rank order correlation was used to assess its association with VT. Correlations were considered significant for α < .05 after Bonferroni correction (α < .05/12).
An exploratory voxel-wise analysis was conducted using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) to assess the relationship between regional VT and RAVLT performance. Group interactions were not explored due to power limitations. Before analysis, the VT maps were smoothed with a 10-mm FWHM Gaussian filter to reduce noise and improve overlap within individual VT maps. To test for a linear relationship between VT and RAVLT performance independent of group, a multiple regression controlling for sex, age, smoking status, methamphetamine use, and global effects was used. Voxels within any cluster were considered significant at P < .001. The cluster formation threshold was set as the expected voxels per cluster (k > 29) designated by SPM or based on probabilistic threshold-free cluster enhancement (pTFCE Toolbox for SPM12; Spisák et al., 2019). Both threshold options are presented.
Differences in MUD and control performance on cognitive tests were assessed via independent t tests (RAVLT, STROOP) and Wilcoxon rank-sum tests (SCAP). All statistical analyses were conducted in SAS (Version 9.4).
RESULTS
Participants in the MUD group reported significantly fewer years of education than participants in the control group (12.7 vs 15.8, t = −4.34, P = .0002; Table 1). No other significant differences in demographic or substance use variables were found (Table 1; Table 2).
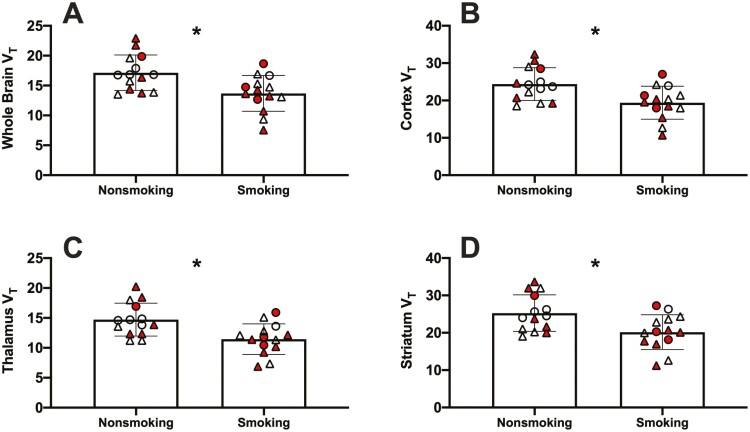
GLM models examining group differences (MUD vs control), smoking status (smoking vs nonsmoking), sex, age, and the interaction of smoking with group on VT found a significant main effect of smoking on VT in the whole brain (F = 10.25, P = .0041, semi-partial η2 = 0.22), cortex (F = 10.35, P = .0040, semi-partial η2 = 0.25), thalamus (F = 11.65, P = .0025, semi-partial η2 = 0.28), and striatum (F = 8.84, P = .0070, semi-partial η2 = 0.22). No significant effect of MUD group, sex, or age was found. No significant smoking and MUD group interaction was found. VT values in participants who smoked were significantly lower than in nonsmokers in the whole brain (t = 3.08, P = .0048), cortex (t = 3.0 P = .0059), thalamus (t = 3.25, P = .0032), and striatum (t = 2.82, P = .0090), independent of MUD group status (Figure 1). VT values for the MUD and control groups, separated by smoking status, are displayed in supplemental Table 1. No significant correlations between measures of methamphetamine or tobacco use and VT were found.
Figure 1.
Significant difference in [18F]FPEB VT between smoking and nonsmoking participants, independent of MUD. Red-colored symbols represent individuals with MUD, and white represent control participants. Circles represent female participants, and triangles represent male participants.
Dorsolateral prefrontal cortical VT showed a significant positive correlation with immediate memory measured by the RAVLT across both groups (r = 0.59, P = .0039). VT in the inferior frontal gyrus trended toward a significant positive correlation with RAVLT performance as well, but the effect did not survive Bonferroni correction (Table 3). Neither SCAP nor STROOP performance was correlated with VT.
Table 3.
Correlation of [18F]FPEB VT in the Prefrontal Cortex With Cognitive Performance
| Dorsolateral prefrontal cortex | Inferior frontal gyrus | Anterior cingulate cortex | Hippocampus | |
|---|---|---|---|---|
| RAVLT Immediate Memory Z-score (r, p) |
0.59, 0.0039a | 0.55, 0.0080 | 0.15, 0.52 | −0.14, 0.52 |
| SCAP D-Prime (rs, p) |
−0.04, 0.88 | 0.05, 0.83 | 0.19, 0.45 | −0.40, 0.09 |
| Stroop Interference (r, p) |
0.16, 0.48 | 0.19, 0.41 | 0.12, 0.61 | −0.03, 0.90 |
Abbreviations: RAVLT, Rey Auditory Verbal Learning Test; SCAP, spatial capacity delayed response test.
a Significant after Bonferroni correction for multiple comparisons and controlling for sex, age, smoking status, participant group, and global effects.
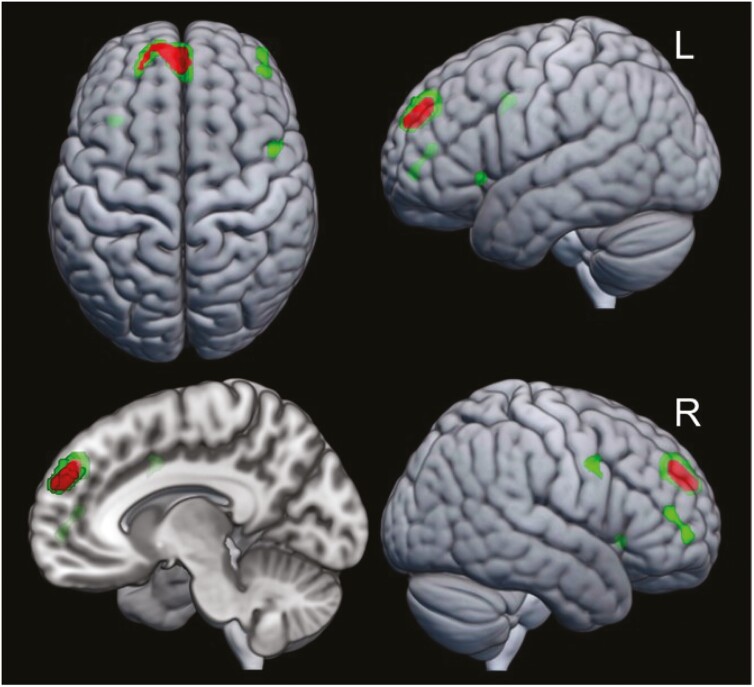
An exploratory voxelwise analysis testing the association between RAVLT and VT identified a significant positive cluster in the dorsolateral prefrontal cortex using the threshold-free cluster enhancement algorithm (Figure 2). Using a minimum cluster size of k > 29, additional positive clusters were found in the superior frontal, rostral middle frontal, caudal middle frontal/precentral gyri, and the insula (Table 4).
Figure 2.
Correlation of [18F]FPEB VT with verbal learning. 3D renderings illustrate statistical parametric mapping analyses that tested the linear relationship between [18F]FPEB VT and immediate memory on the RAVLT (measured in Z-Scores). Green clusters show voxels demonstrating a significant effect at a threshold of P < .001 with a minimum cluster size of k > 29. Significant voxels and clusters, identified using the pTFCE algorithm, are highlighted in red (P < .001). Sex, age, smoking status, participant group, and global effects were controlled.
Table 4.
Correlation of RAVLT Performance With [18F]FPEB VT: Voxelwise Analysis
| Location (Desikan-Killiany) | Hemisphere | MNI coordinates peak | Cluster size | Peak level | ||
|---|---|---|---|---|---|---|
| X | Y | Z | k Voxels | T Values | ||
| Superior frontala | Left | −6 | 54 | 28 | 464 | 6.15 |
| Rostral middle frontalb | Right | 42 | 46 | 12 | 61 | 4.35 |
| Caudal middle frontal/ precentralb |
Right | 48 | 4 | 38 | 31 | 4.27 |
| Insula (anterior)b | Left | −32 | 22 | −4 | 29 | 4.25 |
Abbreviations: RAVLT, Rey Auditory Verbal Learning Test; MNI, Montreal Neurological Institute; TFCE threshold-free cluster enhancement.
a P < .001 TFCE.
bP < .001 (k = 29).
Performance on the SCAP was significantly worse in the MUD group vs the control group (Z = 2.63, P = .0065), with lower D-prime values in the MUD group (Table 5). Though not statistically significant, performance on the RAVLT (d = 0.70) and STROOP (d = 0.61) in the MUD group was also below control values. Effect sizes of the group differences were moderate-to-large for all 3 tests. Cognitive performance did not differ as a function of smoking status.
Table 5.
Cognitive Task Scores (Mean ± SE)
| Control | Methamphetamine use disorder | Group difference (P, Cohen d) |
|||
|---|---|---|---|---|---|
| Smoking N = 6 |
Nonsmoking N = 8 |
Smoking N = 8 |
Nonsmoking N = 6 |
||
| RAVLT Z-Score | 0.65 ± 0.6 | 0.18 ± 0.3 | 0.04 ± 0.4 | −1.05. ± 0.4 | P = .0756, d = 0.70 |
| SCAP D-Primea | 3.3 ± 0.5 | 2.6 ± 0.3 | 2.0 ± 0.4 | 1.7 ± 0.2 | P = .0065, d = 1.03 |
| STROOP interference | 8.3 ± 3.6 | 9.0 ± 4.4 | 2.6 ± 1.8 | 5.6 ± 1.6 | P = .1301, d = 0.61 |
Abbreviations: MUD, methamphatamine use disorder; RAVLT, Rey Auditory Verbal Learning Test; SCAP, spatial capacity delayed response test.
a Significant group difference, control vs MUD (P < .05).
DISCUSSION
In this first assessment, to our knowledge, of mGlu5 in human participants with MUD, we found no significant difference in global or regional mGlu5 in individuals with MUD abstinent from methamphetamine for 19–75 days (average = 42 days) compared with control participants. These findings differ from the below-control mGlu5 receptor availability in individuals with moderate-to-severe cocaine dependence who were actively using the drug when tested (Martinez et al., 2014; Milella et al., 2014). Participants who did not smoke cigarettes exhibited globally higher VT for [18F]FPEB than those who smoked, extending previous findings of lower mGlu5 availability in individuals who smoke (Akkus et al., 2013, 2017; Hulka et al., 2014; Baldassarri et al., 2023). Across participants (but controlling for group status), short-term verbal memory performance evaluated by the RAVLT correlated positively with VT in the dorsolateral prefrontal cortex and superior frontal gyrus, while no association was found between VT and visual working memory performance or inhibitory control.
Lack of an mGlu5 deficit in participants with MUD may reflect the recovery of mGlu5 during abstinence. In the aforementioned studies of cocaine dependence (Martinez et al., 2014; Milella et al., 2014), the maximum duration of abstinence from cocaine was 14 days and duration of abstinence correlated with mGlu5 availability in the striatum, amygdala, and insula (Milella et al., 2014). In our sample of participants with MUD, the association between duration of methamphetamine abstinence and VT in the striatum showed a nonsignificant trend (rs = 0.56, P = .073, uncorrected). The possibility of recovery of mGlu5 with abstinence from methamphetamine is consistent with preclinical findings that mGlu5 expression in the nucleus accumbens is decreased following 2 weeks of cocaine self-administration but not at 60 days after the drug is withdrawn (Ben-Shahar et al., 2009).
Our study population was more heterogeneous in drug use and mental health status than participants in prior studies assessing cocaine dependence. Both Martinez et al. and Milella et al. recruited participants who met criteria for cocaine dependence but no other DSM-5 Axis I diagnosis, whereas this study included individuals with polydrug use and histories of depression, anxiety, obsessive compulsive disorder, and bulimia nervosa. More inclusive criteria used here are consistent with capturing changes in drug use behaviors over the past decade, particularly the increase in polydrug use by individuals using stimulants and opioids (Strickland et al., 2021), and consideration of psychiatric co-morbidities. These co-morbidities warrant individual consideration as alcohol use, depression, and anxiety have all been linked to mGlu5 (reviewed in Asch et al., 2023). Notably, in one study (Baldassarri et al., 2023), individuals with co-occurring major depressive disorder and tobacco use did not significantly differ in mGlu5 availability from those with major depressive disorder alone, though individuals with co-occurring PTSD and tobacco use exhibited higher mGlu5 availability in the orbitofrontal, dorsolateral prefrontal, and anterior cingulate cortex than individuals with PTSD alone.
Smoking was associated with lower VT globally in this sample, paralleling the findings of Hulka et al. (2014), in which smoking was associated with lower mGlu5 availability but cocaine use was not. The effect of smoking on mGlu5 availability has been replicated multiple times, with lower receptor availability in individuals who smoked cigarettes (Akkus et al., 2013, 2016, 2017; Hulka et al., 2014; Baldassarri et al., 2023). Smoking has also been associated with lower mGlu5 availability in individuals with cocaine dependence (Martinez et al., 2014) and alcohol use disorder (Hillmer et al., 2021).
Notably, the control group in Milella et al. (2014) contained only 1 current smoker, suggesting that the higher mGlu5 availability in control participants compared with those with cocaine dependence could reflect the lack of participants who smoke. Some studies have also reported associations between mGlu5 availability and smoking-related variables, such as duration of smoking (Akkus et al., 2013) and duration of abstinence (Hulka et al., 2014). However, mGlu5 availability did not correlate with measures of nicotine consumption or severity of nicotine dependence (Akkus et al., 2013; Hulka et al., 2014). We also found no significant associations between the duration of abstinence (even when excluding 1 participant who had abstained for 17 days) or any other smoking-related variable with VT. The generally light-to-moderate levels of smoking in our participants could contribute to the lack of association between smoking variables and VT. Average Fagerström scores in these participants were under 3, representing low nicotine dependence (Table 1), with several participants reporting less than daily smoking. This suggests that even low levels of tobacco use are associated with low mGlu5 availability, replicating the finding that both light and heavy smokers exhibit low mGlu5 availability compared with never-smokers (Hulka et al., 2014) and a main effect of smoking in a study with individuals smoking fewer than 6 cigarettes per day on average (Martinez et al., 2014).
The positive association between prefrontal cortical VT and performance on the RAVLT observed here extends previous findings that link mGlu5 availability to cognitive performance in humans (Mecca et al., 2020; Régio Brambilla et al., 2020; Esterlis et al., 2022; Wang et al., 2024). Global cognition (Mecca et al., 2020; Wang et al., 2024), episodic memory (Mecca et al., 2020), processing speed (Régio Brambilla et al., 2020), and attention (Esterlis et al., 2022) all have been positively associated with mGlu5 availability measured by PET, though the radiotracers and patient populations have differed among studies. However, working memory, executive function, and verbal learning did not associate with mGlu5 VT in a recent study including participants with PTSD, major depressive disorder, and healthy controls (Esterlis et al., 2022). Despite these conflicting findings, the link between cognition and mGlu5 function is well-supported preclinically, with deletion or knockout of the mGlu5 receptor producing impairment of spatial (Jia et al., 2001) and inhibitory learning (Xu et al., 2009) in rodents.
Pharmacological studies have also linked mGlu5 to cognition. In rodents, MPEP (2-Methyl-6-(phenylethynyl)pyridine), an mGlu5 antagonist, impaired working and spatial memory (Simonyi et al., 2010; Clifton et al., 2013), whereas positive allosteric modulators of mGlu5 have produced improvements in spatial memory (Balschun et al., 2006) and reaction time (Liu et al., 2008; Uslaner et al., 2009). Taken together, these findings suggest that development of pharmacotherapies targeting mGlu5 to ameliorate cognitive impairment, which interferes with behavioral treatment for MUD, may be of particular interest (Petzold et al, 2021).
There are some limitations in this study, of which sample size is one, that may have affected our ability to detect sex-specific effects. Whereas preclinical data have suggested [11C]ABP688 binding potential (Smart et al., 2019) and effects of methamphetamine exposure on mGlu5 expression (Denning et al., 2024) may be sex dependent, no effect of sex was observed in this study.
Another limitation is the inclusion of participants with polydrug use, which may have affected the findings. Additionally, the inclusion of only treatment-seeking participants may add a selection bias, since only participants already willing to abstain enrolled in this study. Recruitment methods did not require enrollment in a treatment program, but all potential participants who were not receiving treatment failed to progress past screening procedures. Efforts were made to recruit individuals who smoked cigarettes to the control group to match smoking in the MUD group. The percentages of individuals who smoked in the 2 groups did not significantly differ, but the control group had fewer smokers than the MUD group. Additionally, several “nonsmokers” in the MUD group and 1 nonsmoker in the control group had formerly smoked. Although mGlu5 availability recovers after 6 months of abstinence in former smokers (Akkus et al., 2016), several of the participants had abstained from cigarettes for a shorter period.
The state of smoking abstinence may be a consideration. While all scan procedures were performed in a state of abstinence from nicotine and other substances of abuse to ensure measurement of accurate mGlu5 VT, participants were not abstinent from nicotine during cognitive testing. Nicotine has positive effects on cognitive function, particularly memory and attention (reviewed in Rezvani and Levin, 2001; Heishman, Kleykamp and Singleton, 2010), and withdrawal produced deficits (reviewed in Heishman, Kleykamp and Singleton, 2010; Ashare, Falcone and Lerman, 2014). Participants in this study who smoked primarily reported light-to-moderate cigarette use, with average Fagerström scores under 3.3 indicating low dependence (Table 2). However, a small percentage of smokers had higher scores (5–8). The degree of nicotine dependence influences the effect of smoking and withdrawal on cognitive performance (Tait et al., 2000; Nesic et al., 2011). Heavy smokers perform worse on a short-term memory task during withdrawal than light smokers, with smoking ameliorating this difference (Tait et al., 2000). Cognitive testing was performed in a non-abstinent state to avoid any impact of withdrawal on cognitive performance.
Supplementary Material
Acknowledgments
The authors thank the staff and clients at the Cri-Help Inc. rehabilitation center for their participation and support. We also thank the Veterans Administration of Greater Los Angeles nuclear medicine technologists, particularly Ralph Mendoza and Josephine Ribe, for their assistance with collection of radiotracer metabolite data.
This work was supported by grants from the National Institute on Drug Abuse at the National Institutes of Health: grant number R33 DA031441 (to R.M.K.), grant number R01 DA045162 (to E.D.L.), grant number R21 DA042347 (to E.D.L.), and grant number F32 DA047111 (to M.N.M.).
Contributor Information
Megan N McClintick, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA; Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Robert M Kessler, Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Mark A Mandelkern, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA; Department of Physics, University of California Irvine, Irvine, California, USA.
Tarannom Mahmoudie, Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Hilary Lachoff, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA; Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Jean-Baptiste F Pochon, Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Dara G Ghahremani, Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Judah B Farahi, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA.
Edwin Partiai, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA.
Robert A Casillas, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA.
Larissa J Mooney, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA; Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Andy C Dean, Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Edythe D London, Veterans Administration of Greater Los Angeles System, Los Angeles, California, USA; Semel Institute and Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California, USA.
Interest Statement
L.J.M. has received research support from Aelis Farma.
Data Availability
All self-report, cognitive test, and summary PET data discussed in this manuscript are publicly available from the Open Science Framework web site under project title, “Brain mGlu5 is linked to cognition and cigarette smoking, but does not differ among those abstinent from chronic methamphetamine use and controls.”
Author Contributions
Megan McClintick (Data curation [Lead], Formal analysis [Lead], Investigation [Equal], Methodology [Equal], Project administration [Equal], Supervision [Supporting], Validation [Equal], Writing—original draft [Lead], Writing—review and editing [Equal]), Robert Kessler (Conceptualization [Lead], Formal analysis [Supporting], Funding acquisition [Lead], Investigation [Equal], Methodology [Equal], Resources [Supporting], Supervision [Supporting], Validation [Supporting], Writing—review and editing [Equal]), Mark Mandelkern (Data curation [Supporting], Formal analysis [Equal], Investigation [Supporting], Methodology [Equal], Supervision [Supporting], Validation [Supporting], Writing—review and editing [Equal]), Tarannom Mahmoudie (Data curation [Supporting], Investigation [Supporting], Writing—review and editing [Supporting]), Daicia Allen (Data curation [Supporting], Investigation [Supporting], Writing—review and editing [Supporting]), Hilary Lachoff (Investigation [Supporting], Supervision [Supporting], Writing—review and editing [Supporting]), Jean-Baptiste Pochon (Formal analysis [Equal], Visualization [Supporting], Writing—original draft [Supporting], Writing—review and editing [Supporting]), Dara Ghahremani (Data curation [Supporting], Formal analysis [Supporting], Investigation [Supporting], Writing—review and editing [Equal]), Judah Farahi (Investigation [Supporting], Methodology [Supporting], Supervision [Supporting], Writing—review and editing [Supporting]), Edwin Partiai (Investigation [Supporting], Methodology [Supporting], Writing—review and editing [Supporting]), Robert Casillas (Investigation [Supporting], Methodology [Supporting], Writing—review and editing [Supporting]), Larissa Mooney (Investigation [Supporting], Project administration [Supporting], Supervision [Equal], Writing—review and editing [Supporting]), Andy Dean (Data curation [Supporting], Formal analysis [Supporting], Writing—review and editing [Equal]), and Edythe London (Formal analysis [Equal], Funding acquisition [Supporting], Investigation [Equal], Methodology [Lead], Project administration [Lead], Resources [Lead], Supervision [Lead], Writing—review and editing [Lead]).
References
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, Hasler G (2013) Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A 110:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, Buck A, Hasler G (2016) Association of long-term nicotine abstinence with normal metabotropic glutamate receptor-5 binding. Biol Psychiatry 79:474–480. [DOI] [PubMed] [Google Scholar]
- Akkus F, Treyer V, Ametamey SM, Johayem A, Buck A, Hasler G (2017) Metabotropic glutamate receptor 5 neuroimaging in schizophrenia. Schizophr Res 183:95–101. [DOI] [PubMed] [Google Scholar]
- Arai I, Shimazoe T, Shibata S, Inoue H, Yoshimatsu A, Watanabe S (1996) Enhancement of dopamine release from the striatum through metabotropic glutamate receptor activation in methamphetamine sensitized rats. Brain Res 729:277–280. [PubMed] [Google Scholar]
- Arai I, Shimazoe T, Shibata S, Inoue H, Yoshimatsu A, Watanabe S (1997) Methamphetamine-induced sensitization of dopamine release via a metabotropic glutamate receptor mediated pathway in rat striatal slices. Jpn J Pharmacol 73:243–246. [DOI] [PubMed] [Google Scholar]
- Asch RH, Hillmer AT, Baldassarri SR, Esterlis I (2023) The metabotropic glutamate receptor 5 as a biomarker for psychiatric disorders. Int Rev Neurobiol 168:265–310. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C (2014) Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology 76 Pt B:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarri SR, Asch RH, Hillmer AT, Pietrzak RH, DellaGioia N, Esterlis I, Davis MT (2023) Nicotine use and metabotropic glutamate receptor 5 in individuals with major depressive and posttraumatic stress disorders. Chronic Stress (Thousand Oaks) 7:24705470231154842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W (2006) Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience 142:691–702. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK (2009) Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 63:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn PJ, Nicoletti F, Manahan-Vaughan D (2008) MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS One. 3:e2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL (2005) The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology 49:13–25. [DOI] [PubMed] [Google Scholar]
- Brorson HH, Arnevik EA, Rand-Hendriksen K, Ducket F (2013) Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev 33:1010–1024. [DOI] [PubMed] [Google Scholar]
- Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F (2013) Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology (Berl) 225:579–594. [DOI] [PubMed] [Google Scholar]
- Daggett LP, Sacaan AI, Akong M, Rao SP, Hess SD, Liaw C, Urrutia A, Jachec C, Ellis SB, Dreessen J, Knöpfel T, Landwehrmeyer GB, Testa CM, Young AB, Varney M, Johnson EC, Veliçelebi G (1995) Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology 34:871–886. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED (2013) An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001) Delis–Kaplan executive function system (D-KEFS): examiner’s manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- DeLorenzo C, Kumar JS, Mann JJ, Parsey RV (2011) In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab 31:2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Gallezot JD, Gardus J, Yang J, Planeta B, Nabulsi N, Ogden RT, Labaree DC, Huang YH, Mann JJ, Gasparini F, Lin X, Javitch JA, Parsey RV, Carson RE, Esterlis I (2017) In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB. J Cereb Blood Flow Metab 37:2716–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning CJE, Madory LE, Herbert JN, Cabrera RA, Szumlinski KK (2024) Neuropharmacological evidence implicating drug-induced glutamate receptor dysfunction in affective and cognitive sequelae of subchronic methamphetamine self-administration in mice. Int J Mol Sci 25:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, DeBonee S, Cool R, Holmes S, Baldassari SR, Maruff P, Pietrzak RH, Davis MT (2022) Differential role of mGluR5 in cognitive processes in posttraumatic stress disorder and major depression. Chronic Stress (Thousand Oaks) 6:24705470221105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Gereau RW, Conn PJ (1995) Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci 15:6879–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD (2003) Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry 53:624–626. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 210:453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Angarita GA, Esterlis I, Anderson JM, Nabulsi N, Lim K, Ropchan J, Carson RE, Krystal JH, Malley SSO, Cosgrove KP (2021) Longitudinal imaging of metabotropic glutamate 5 receptors during early and extended alcohol abstinence. Neuropsychopharmacology 46:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B (2010) Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol 639:33–39. [DOI] [PubMed] [Google Scholar]
- Hoopsick RA, Andrew Yockey R (2023) Methamphetamine-related mortality in the United States: co-involvement of Heroin and Fentanyl, 1999-2021. Am J Public Health 113:416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Treyer V, Scheidegger M, Preller KH, Vonmoos M, Baumgartner MR, Johayem A, Ametamey SM, Buck A, Seifritz E, Quednow BB (2014) Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Mol Psychiatry 19:625–632. [DOI] [PubMed] [Google Scholar]
- Hullinger R, O’Riordan K, Burger C (2015) Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem 125:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, et al. (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu YM, Agopyan N, Roder J (2001) Gene targeting reveals a role for the glutamate receptors mGluR5 and GluR2 in learning and memory. Physiol Behav 73:793–802. [DOI] [PubMed] [Google Scholar]
- Kågedal M, Cselényi Z, Nyberg S, Raboisson P, Ståhle L, Stenkrona P, Varnäs K, Halldin C, Hooker AC, Karlsson MO (2013) A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066 - estimating occupancy in the absence of a reference region. Neuroimage 82:160–169. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Roth T, Kim H, Ansari M, Zald D, Wong D, Cowan R (2014a) Bolus/infusion studies for PET [18F]FPEB studies of cerebral mGluR5 receptors. J Nucl Med 55:318. [Google Scholar]
- Kessler RM, Seibyl J, Cowan RL, Zald D, Young JS, Ansari MS, Stabin MG (2014b) Radiation dosimetry of (18)F-FPEB in humans. J Nucl Med 55:1119–1121. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A (2005) NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci 8:1657–1659. [DOI] [PubMed] [Google Scholar]
- Lim K, Labaree D, Li S, Huang Y (2014) Preparation of the metabotropic glutamate receptor 5 (mGluR5) PET tracer [(18)F]FPEB for human use: an automated radiosynthesis and a novel one-pot synthesis of its radiolabeling precursor. Appl Radiat Isot 94:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, et al. (2008) ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1, 2, 4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK (2016) Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur J Neurosci 43:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC (1997) Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 17:5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH (2005) The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex 15:1703–1713. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NB, Perez A, Liu F, Lin SF, Ropchan J, Mao X, Kegeles LS, Shungu DC, Carson RE, Huang Y (2014) Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry 75:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AP, McDonald JW, Michalak HR, Godek TA, Harris JE, Pugh EA, Kemp EC, Chen MK, Salardini A, Nabulsi NB, Lim K, Huang Y, Carson RE, Strittmatter SM, van Dyck CH (2020) PET imaging of mGluR5 in Alzheimer’s disease. Alzheimers Res Ther 12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, Benkelfat C, Leyton M (2014) Limbic system mGluR5 availability in cocaine dependent subjects: a high-resolution PET [(11)C]ABP688 study. Neuroimage 98:195–202. [DOI] [PubMed] [Google Scholar]
- Nesic J, Rusted J, Duka T, Jackson A (2011) Degree of dependence influences the effect of smoking on cognitive flexibility. Pharmacol Biochem Behav 98:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D (2008) Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci 27:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Sullivan JM, Planeta B, Gallezot JD, Lim K, Lin SF, Ropchan J, McCarthy TJ, Ding YS, Morris ED, Williams WA, Huang Y, Carson RE (2015) Test-retest reproducibility of the metabotropic glutamate receptor 5 ligand [¹⁸F]FPEB with bolus plus constant infusion in humans. Eur J Nucl Med Mol Imaging 42:1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE (2007) Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18 F] F-PEB. Nucl Med Biol 34:1009–1017. [DOI] [PubMed] [Google Scholar]
- Petzold J, Szumlinski KK, London ED (2021) Targeting mGlu5 for methamphetamine use disorder. Pharmacol Ther 224:107831. [DOI] [PubMed] [Google Scholar]
- Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T (2018) Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav 80:154–160. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Erath TG, Clark HW (2023) The fourth wave of the overdose crisis: examining the prominent role of psychomotor stimulants with and without fentanyl. Prev Med 176:107625. [DOI] [PubMed] [Google Scholar]
- Régio Brambilla C, et al. (2020) mGluR5 receptor availability is associated with lower levels of negative symptoms and better cognition in male patients with chronic schizophrenia. Hum Brain Mapp 41:2762–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Foster Olive M, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacol 36:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1941) L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie 28:21. [Google Scholar]
- Rezvani AH, Levin ED (2001) Cognitive effects of nicotine. Biol Psychiatry 49:258–267. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 50:35–39. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17:7503–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazoe T, Doi Y, Arai I, Yoshimatsu A, Fukumoto T, Watanabe S (2002) Both metabotropic glutamate I and II receptors mediate augmentation of dopamine release from the striatum in methamphetamine-sensitized rats. Jpn J Pharmacol 89:85–88. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR (2010) Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. Eur J Pharmacol 639:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart K, Cox SML, Scala SG, Tippler M, Jaworska N, Boivin M, Séguin JR, Benkelfat C, Leyton M (2019) Sex differences in [11C]ABP688 binding: a positron emission tomography study of mGlu5 receptors. Eur J Nucl Med Mol Imaging 46:1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spisák T, Spisák Z, Zunhammer M, Bingel U, Smith S, Nichols T, Kincses T (2019) Probabilistic TFCE: a generalized combination of cluster size and voxel intensity to increase statistical power. Neuroimage 185:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S (1969) The discovery of processing stages: extensions of Donder’s method. Acta Psychol (Amst) 30:276–315. [Google Scholar]
- Strickland JC, Stoops WW, Dunn KE, Smith KE, Havens JR (2021) The continued rise of methamphetamine use among people who use heroin in the United States. Drug Alcohol Depend 225:108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D (2006) The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend 83:233–237. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugmann M, Phillips TJ, Kippin TE (2017) Methamphetamine addiction vulnerability: the glutamate, the bad, and the ugly. Biol Psychiatry 81:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R, Martin-Iverson M, Michie PT, Dusci L (2000) The effects of cigarette consumption on the Stenberg visual memory search paradigm. Addiction 95:437–446. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH (2009) Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538. [DOI] [PubMed] [Google Scholar]
- Wang J, He Y, Chen X, Huang L, Li J, You Z, Huang Q, Ren S, He K, Schibli R, Mu L, Guan Y, Guo Q, Zhao J, Xie F (2024) Metabotropic glutamate receptor 5 (mGluR5) is associated with neurodegeneration and amyloid deposition in Alzheimer’s disease: a [18F]PSS232 PET/MRI study. Alzheimers Res Ther 16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2001) Wechsler test of adult reading. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wong DF, Waterhouse R, Kuwabara H, Kim J, Brašić JR, Chamroonrat W, Stabins M, Holt DP, Dannals RF, Hamill TG, Mozley PD (2013) 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: a first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J Nucl Med 54:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SR, Zanos P, Georgiou P, Yoo JH, Ledent C, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A (2016) A critical role of striatal A2A R-mGlu5 R interactions in modulating the psychomotor and drug-seeking effects of methamphetamine. Addict Biol 21:811–825. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF (2009) mGluR5 has a critical role in inhibitory learning. J Neurosci 29:3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All self-report, cognitive test, and summary PET data discussed in this manuscript are publicly available from the Open Science Framework web site under project title, “Brain mGlu5 is linked to cognition and cigarette smoking, but does not differ among those abstinent from chronic methamphetamine use and controls.”