Abstract
How is the essential micronutrient, selenium (Se), transported in the serum and then donated to tissues? In this issue of the Biochemical Journal, Schweizer and colleagues demonstrate, using conditional and total mouse knockout models, that SePP (selenoprotein P) is the major transporter of Se in the serum. Moreover, in the sanctuary area of the brain, SePP was shown to play a hitherto unexpected role as a local Se storage and recycling protein that directly maintains brain Se levels. Considering the function of Se in normal brain metabolism, these results are crucial for our understanding of the role of selenoproteins in redox regulation, antioxidant defences, thyroid hormone metabolism and the development of neurodegenerative conditions.
Keywords: antioxidant defence, neurodegeneration, selenium, selenium transport, selenoprotein, selenoprotein P
Selenium (Se) is an essential micronutrient that is required in a variety of metabolic functions that includes its role in antioxidant defences (for a review see [1]). Se is incorporated into an interesting class of molecules known as selenoproteins that contain the unusual 21st amino acid, selenocysteine [1]. Examples of selenoproteins include those involved in antioxidant defences and redox regulation, such as GPx (glutathione peroxidase), thioredoxin reductases and methionine sulphoxide reductase [1]. Other selenoproteins include the deiodinases that are involved in thyroid hormone metabolism [1]. Interestingly, insufficient Se and increased oxidative stress are associated with neurodegenerative diseases, such as Parkinson's disease [1]. In contrast, like other trace elements, Se over-supply leads to toxicity. Although the human genome contains 25 genes that encode selenoproteins, very little is known concerning many of their basic functions. Indeed, until recently, little was known concerning how Se is transported in the serum and subsequently donated to tissues [1]. Previous studies suggested that SePP (selenoprotein P) was important in serum Se transport, as it may carry 10–17 selenocysteine moieties (depending on the species), and more than 50% of plasma Se exists in the form of SePP [2–5].
In this issue of the Biochemical Journal, Schweizer et al. [6] have investigated the role of SePP as the plasma Se-transporter to tissues. To perform their work, these authors used two SePP knockout models in the mouse, namely a complete SePP knockout [4,5] and a conditional knockout for hepatic SePP only [7]. These studies, consistent with previous suggestions [4,5], definitively demonstrate that SePP in the plasma is almost exclusively hepatically derived and that it is a Se carrier from the liver to the kidney [6]. In the absence of SePP, there is a significant decrease in GPx activity in the kidney, which is a sensitive measure of Se bioavailability [6]. However, surprisingly, brain function was not impaired in the SePP liver-specific conditional knockout mouse, despite an almost complete lack of SePP in the plasma [6]. These results are in clear contrast with those obtained in the complete SePP knockout, where the mice developed neurological deficiencies [5]. Hence, the brain requires local SePP expression for appropriate functioning. Collectively, these findings have three important biological implications. (i) Dietary-derived Se that may be toxic is taken up by the liver and is incorporated into selenocysteine, which is used for the synthesis of SePP. The release of SePP into the plasma provides a relatively inert, but functional, form of Se that is used by distant tissues such as the kidney [6]. (ii) The brain is a privileged organ with respect to Se metabolism, requiring its own local SePP biosynthesis for maintenance of its Se levels. This led the authors to propose the ‘selenoprotein cycle’ in the brain, where SePP may serve as a local Se storage and recycling protein that directly maintains brain Se levels [6]. This was an unexpected finding, because SePP was generally considered as a mere plasma protein. (iii) Brain Se levels are somewhat independent of plasma Se levels, which may, at least in part, explain other intriguing observations, such as the resistance of the brain against dietary Se restriction [8].
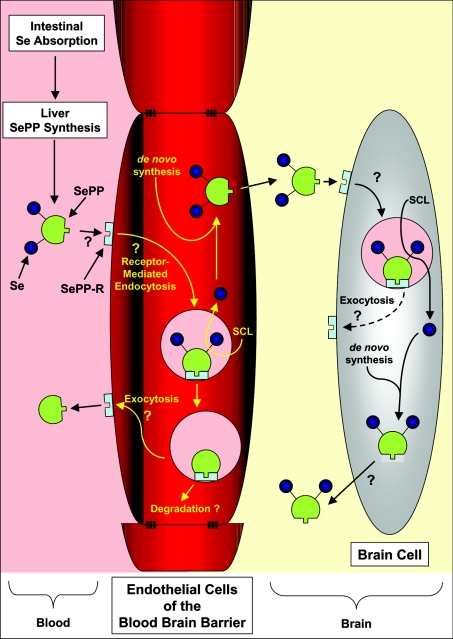
Considering their results, Schweizer et al. [6] propose a model of Se metabolism that entails the intestinal absorption of Se, hepatic SePP biosynthesis and secretion, Se transport by SePP and uptake of the protein by tissues [6] (Figure 1). At present, the mechanism by which SePP donates Se to cells remains unclear, although kinetic studies of brain Se uptake [3] and evidence from ligand-binding experiments [9] suggest a specific receptor-mediated mechanism. Uptake of Se by the brain under physiological conditions may involve plasma SePP donating Se to the brain via endothelial cells of the blood–brain barrier, followed by its incorporation into brain SePP (Figure 1). Cells of the brain may secrete SePP as a potential extracellular storage form of Se [6]. When cellular Se concentrations are low, extracellular Se as SePP may then be taken up by brain cells to maintain Se levels (Figure 1). The results of Schweizer et al. [6] clearly demonstrate that, in the absence of SePP synthesis, Se levels cannot be maintained in the brain at concentrations necessary to ensure normal functioning. In fact, while hyper-supplementation of Se can prevent the generation of neurological deficits in the complete SePP knockout mouse, it is of interest that, when supplementation ceases, the animals lose Se from the brain and develop neurological problems [6].
Figure 1. Hypothetical model of Se transport via SePP and its uptake mechanism by the brain and other tissues.
Dietary-derived Se is taken up by the liver, and is used for the synthesis of SePP. The release of SePP into the plasma provides Se that is used by distant tissues. SePP may donate Se to cells via its binding to a postulated SePP receptor (SePP-R) [3,9]. Such a delivery mechanism is necessary for targeting Se to tissues [4]. This is not unreasonable to suggest, as it is pre-empted by the mechanism by which Tf donates Fe to cells and also transfers Fe across the blood–brain barrier [10]. Delivery of Se from SePP to the brain may involve uptake of the protein via the SePP-R on the blood–brain barrier endothelial cells, its subsequent internalization and the release of Se, perhaps via the recently identified selenocysteine lyase (SCL) [6]. Whether, after Se release, the SePP and SePP-R can be recycled to the cell surface for re-utilization, like Tf and its receptor [10], or is degraded remains unknown, but testable. Se may then be re-incorporated into de novo synthesized SePP that is then released from the abluminal membrane of the endothelial cell into the interstitial space of the brain. Uptake of SePP via SePP-R on brain cells and the release of Se via SCL could provide the cell with Se for metabolism. De novo synthesis of brain SePP would result in local ‘organification of Se’, while release of some of this newly synthesized SePP would provide an extracellular ‘store of Se’ that can be utilized by brain cells [6]. This mechanism would explain why the brain does not lose Se during times of Se restriction, as it is trapped within the ‘selenoprotein cycle’ [6].
The functional dichotomy between plasma and brain SePP transport is reminiscent of the iron (Fe) transport protein Tf (transferrin) [10]. This latter protein is expressed in the liver as the primary source of plasma Tf, but is also expressed in sanctuary sites, such as the brain (for a review see [10]). The Tf synthesized in the liver and secreted into the serum transports its bound Fe to endothelial cells of the blood–brain barrier, where Tf then subsequently binds to the transferrin receptor 1 (TfR1) on their luminal membrane [10]. The Tf–TfR1 complex is then internalized by endothelial cells via receptor-mediated endocytosis, and the Fe is removed and is transported into the cell [10]. This Fe is released from the abluminal membrane by an unknown mechanism and is then bound by Tf in the interstitial space of the brain [10]. Similarly, under physiological conditions, it can be speculated that plasma SePP also may be involved in donating Se to endothelial cells, perhaps via a receptor-mediated mechanism (Figure 1). However, clearly, this remains to be validated experimentally, and the hypothetical model in Figure 1 poses questions to test in further studies. Consistent with the suggestion of a receptor-mediated process is the fact that the plasma half-life of Se is only 3–4 h, suggesting that Se cycles through SePP at a high rate [3]. As clearly demonstrated by Schweizer et al. [6], brain SePP may then be involved in the sequestration of Se and its transport and utilization within this organ. This type of mechanism is not only observed for micronutrients, but is also reminiscent of the important role that transthyretin performs for plasma and brain thyroid hormone transport (for a review see [11]). Of relevance, transthyretin is also thought to be taken up by a receptor-mediated process [11].
It is of interest that Se is still incorporated into the brain in the conditional SePP knockout mouse that does not express this protein in the liver [6]. As noted by others [4], this suggests that other forms of Se can donate this micronutrient to the brain in the absence of the physiological transporter, SePP. At present, these forms of Se remain unknown. However, it is clear that approx. 5% of Se exists in the serum in an unknown chemical form that could potentially donate Se to the brain. Schweizer et al. [6] suggest that selenite or selenomethionine could potentially fulfil this function, although other selenoproteins cannot be completely ruled out [4]. However, the relevance of small-molecular-mass species to brain Se uptake under physiological conditions may be minimal, as most Se would be present as SePP. Indeed, it is known that, in the absence of SePP, Se administered as inorganic selenite enters the brain and rescues the SePP knockout phenotype [6]. Again, a parallel can be drawn between Tf and SePP: when Tf is virtually absent, such as in the mutant hypotransferrinaemic mouse, non-Tf-bound Fe in the serum is present which may supply Fe to the brain [10]. However, this is not a physiological mechanism, as, under normal circumstances, there is very little non-Tf-bound Fe in the serum, with most Fe being bound to Tf [10].
The investigation of Schweizer et al. [6] demonstrates that brain Se levels are largely independent of plasma Se levels. This observation might explain the failure to correlate plasma Se levels with neurodegenerative diseases, such as dementia, Alzheimer's disease or Parkinson's disease, despite the pivotal role of Se as part of antioxidative enzymes [1]. Considering this, the evaluation of SePP in the cerebrospinal fluid rather than the serum may be a better indicator of brain Se status [6].
In summary, knowledge obtained from the work of Schweizer et al. [6] provides new insights into the metabolism and function of Se in the brain, including redox-control processes and thyroid hormone metabolism. Moreover, the study invites new research directions that will markedly expand knowledge of Se biology.
Acknowledgments
I thank Dr Ralph Watts (Iron Metabolism and Chelation Program; IMC) for his tremendous help with creating Figure 1 and assessing the manuscript. Critical evaluation of the paper by Dr Neil Davies is also kindly appreciated. This work was supported by a fellowship and project grant from the National Health and Medical Research Council of Australia and grants from the Australian Research Council, Muscular Dystrophy Association USA and Friedreich's Ataxia Research Alliance USA. Children's Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children's Hospital.
References
- 1.Schweizer U., Brauer A. U., Köhrle J., Nitsch R., Savaskan N. E. Selenium and brain function: a poorly recognized liaison. Brain Res. Rev. 2004;45:164–178. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Motsenbocker M. A., Tappel A. L. A selenocysteine-containing selenium-transport protein in rat plasma. Biochim. Biophys. Acta. 1982;719:147–153. doi: 10.1016/0304-4165(82)90318-x. [DOI] [PubMed] [Google Scholar]
- 3.Burk R. F., Hill K. E., Read R., Bellew T. Response of rat selenoprotein P to selenium administration and fate of its selenium. Am. J. Physiol. 1991;261:E26–E30. doi: 10.1152/ajpendo.1991.261.1.E26. [DOI] [PubMed] [Google Scholar]
- 4.Hill K. E., Zhou J., McMahan W. J., Motley A. K., Atkins J. F., Gesteland R. F., Burk R. F. Deletion of selenoprotein P alters distribution of selenium in the mouse. J. Biol. Chem. 2003;278:13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 5.Schomburg L., Schweizer U., Holtmann B., Flohé L., Sendtner M., Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem. J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweizer U., Streckfuss F., Pelt P., Carlson B. A., Hatfield D. L., Köhrle J., Schomburg L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem. J. 2005;386:221–226. doi: 10.1042/BJ20041973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson B. A., Novoselov S. V., Kumaraswamy E., Lee B. J., Anver M. R., Gladyshev V. N., Hatfield D. L. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J. Biol. Chem. 2004;279:8011–8017. doi: 10.1074/jbc.M310470200. [DOI] [PubMed] [Google Scholar]
- 8.Savaskan N. E., Brauer A. U., Kuhbacher M., Eyupoglu I. Y., Kyriakopoulos A., Ninnemann O., Behne D., Nitsch R. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J. 2003;17:112–114. doi: 10.1096/fj.02-0067fje. [DOI] [PubMed] [Google Scholar]
- 9.Gomez B., Jr, Tappel A. L. Selenoprotein P receptor from rat. Biochim. Biophys. Acta. 1989;979:20–26. doi: 10.1016/0005-2736(89)90518-x. [DOI] [PubMed] [Google Scholar]
- 10.Ponka P., Beaumont C., Richardson D. R. Function and regulation of transferrin and ferritin. Semin. Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 11.Schussler G. C. The thyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]