Abstract
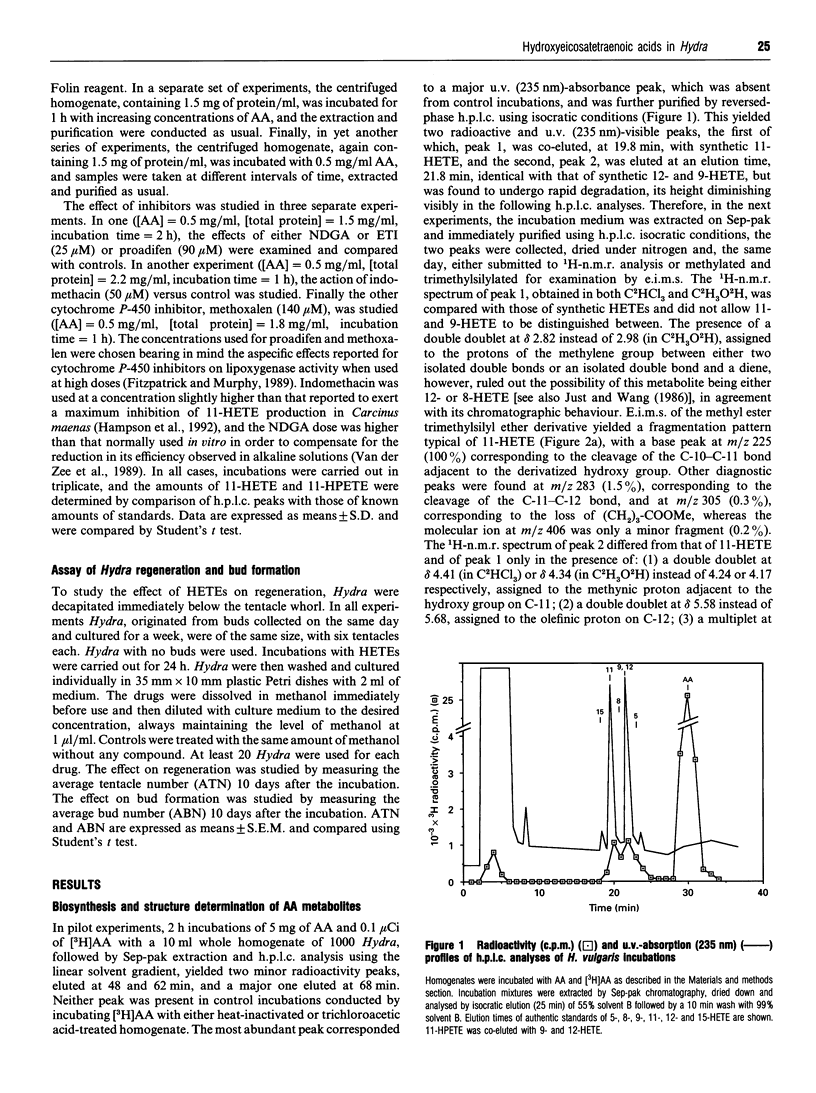
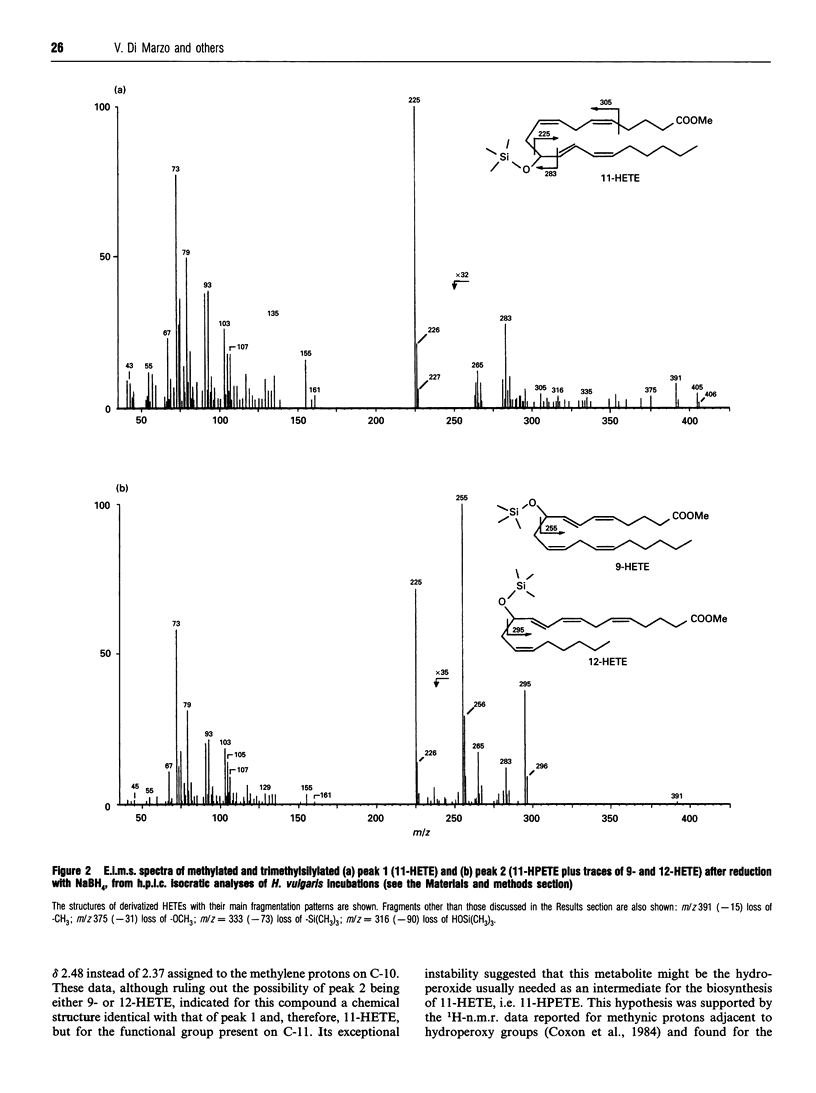
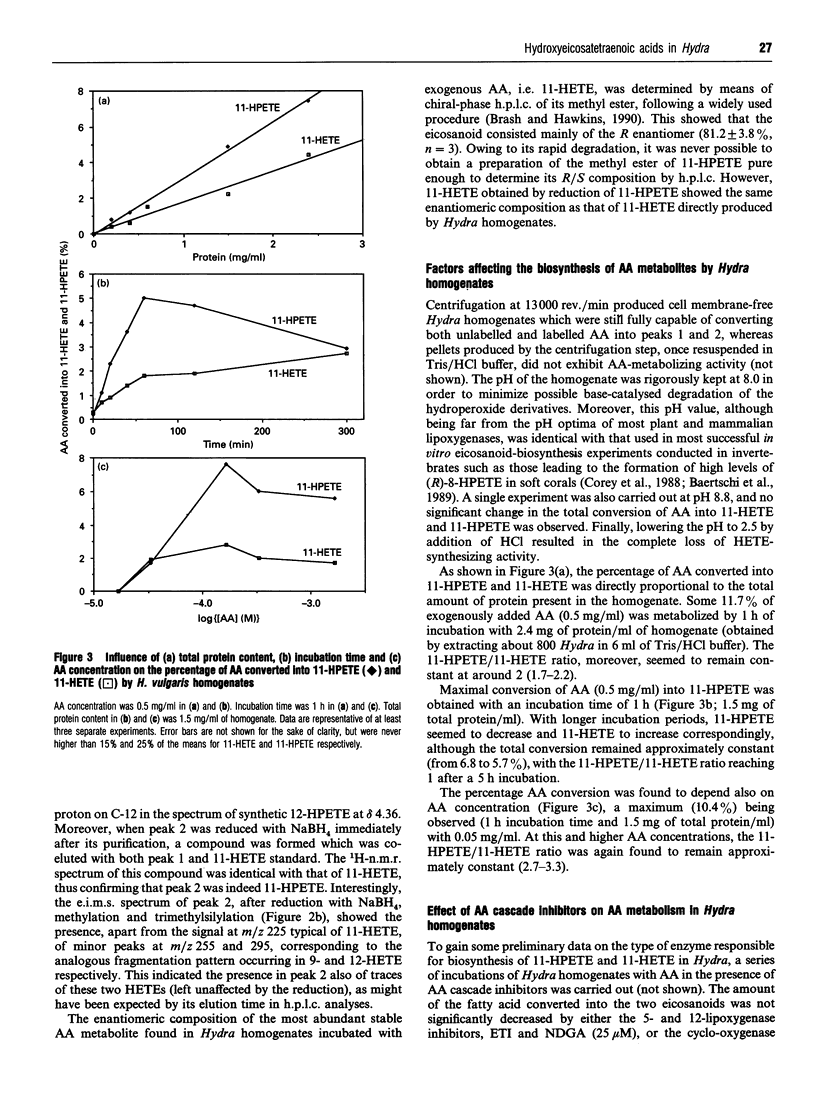
Recent reports have suggested the involvement of arachidonic acid (AA) and its metabolites in the control of body pattern, head and tentacle regeneration and bud formation in Hydra spp. Here we describe for the first time the biosynthesis of hydroxyeicosatetraenoic acids (HETEs) in vitro by hydroid cytosolic extracts. Incubation of both unlabelled and tritiated AA with homogenates of Hydra vulgaris led to the conversion of up to 11% of the exogenous fatty acid into mainly two metabolites. These were characterized as 11-hydroperoxyeicosatetraenoic acid (11-HPETE) and 11-HETE by means of a combination of chromatographic, chemical, 1H-n.m.r. and electron-impact m.s. techniques. Trace amounts of 9-HETE and 12-HETE were also found. Analysis of 11-HETE by chiral-phase h.p.l.c. revealed that this metabolite was composed mainly of the R enantiomer. The production of 11-HPETE and 11-HETE was found to be: (1) associated with the cytosolic fraction of Hydra homogenates; (2) dependent on AA concentration, incubation time and protein amount in the homogenates; (3) unaffected by co-incubation with the 5- and 12-lipoxygenase inhibitors, 5,8,11-eicosatriynoic acid and nordihydroguaiaretic acid, the cyclo-oxygenase inhibitor, indomethacin, or the cytochrome P-450 inhibitors, proadifen and methoxalen. These results strongly suggest the presence of a very active (R)-11-lipoxygenase in H. vulgaris. The activity of both R and S enantiomers of synthetic 9-, 11- and 12-HETE and of 'endogenous' 11-HETE was studied on tentacle regeneration and bud formation in decapitated Hydra. Although almost all compounds tested inhibited budding, only endogenous 11-HETE and synthetic (R)-11-HETE significantly enhanced the average number of tentacles, thus suggesting that this eicosanoid might be one of the cellular regulators of regeneration in H. vulgaris.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode H., Dunne J., Heimfeld S., Huang L., Javois L., Koizumi O., Westerfield J., Yaross M. Transdifferentiation occurs continuously in adult hydra. Curr Top Dev Biol. 1986;20:257–280. doi: 10.1016/s0070-2153(08)60668-7. [DOI] [PubMed] [Google Scholar]
- Brash A. R., Baertschi S. W., Ingram C. D., Harris T. M. Allene oxides as intermediates in biosynthesis of ketols and cyclopentenones. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:70–73. [PubMed] [Google Scholar]
- Brash A. R., Hawkins D. J. High-performance liquid chromatography for chiral analysis of eicosanoids. Methods Enzymol. 1990;187:187–195. doi: 10.1016/0076-6879(90)87024-w. [DOI] [PubMed] [Google Scholar]
- Buttner N., Siegelbaum S. A., Volterra A. Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature. 1989 Nov 30;342(6249):553–555. doi: 10.1038/342553a0. [DOI] [PubMed] [Google Scholar]
- Cimino G., Crispino A., Di Marzo V., Sodano G., Spinella A., Villani G. A marine mollusc provides the first example of in vivo storage of prostaglandins: prostaglandin-1,15-lactones. Experientia. 1991 Jan 15;47(1):56–60. doi: 10.1007/BF02041252. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L., Di Marzo V., Arcà B., Gavagnin M., Minei R., Cimino G. The effect of diterpenoidic diacylglycerols on tentacle regeneration in Hydra vulgaris. Comp Biochem Physiol C. 1991;100(3):603–607. doi: 10.1016/0742-8413(91)90047-w. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Cimino G., Crispino A., Minardi C., Sodano G., Spinella A. A novel multifunctional metabolic pathway in a marine mollusc leads to unprecedented prostaglandin derivatives (prostaglandin 1,15-lactones). Biochem J. 1991 Feb 1;273(Pt 3):593–600. doi: 10.1042/bj2730593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Murphy R. C. Cytochrome P-450 metabolism of arachidonic acid: formation and biological actions of "epoxygenase"-derived eicosanoids. Pharmacol Rev. 1988 Dec;40(4):229–241. [PubMed] [Google Scholar]
- Hampson A. J., Rowley A. F., Barrow S. E., Steadman R. Biosynthesis of eicosanoids by blood cells of the crab, Carcinus maenas. Biochim Biophys Acta. 1992 Mar 4;1124(2):143–150. doi: 10.1016/0005-2760(92)90090-i. [DOI] [PubMed] [Google Scholar]
- Hawkins D. J., Brash A. R. Eggs of the sea urchin, Strongylocentrotus purpuratus, contain a prominent (11R) and (12R) lipoxygenase activity. J Biol Chem. 1987 Jun 5;262(16):7629–7634. [PubMed] [Google Scholar]
- Meijer L., Brash A. R., Bryant R. W., Ng K., Maclouf J., Sprecher H. Stereospecific induction of starfish oocyte maturation by (8R)-hydroxyeicosatetraenoic acid. J Biol Chem. 1986 Dec 25;261(36):17040–17047. [PubMed] [Google Scholar]
- Müller W. A. Ectopic head and foot formation in Hydra: diacylglycerol-induced increase in positional value and assistance of the head in foot formation. Differentiation. 1990 Feb;42(3):131–143. doi: 10.1111/j.1432-0436.1990.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Asotra S. Biosynthesis, catabolism, and biological properties of HPETEs, hydroperoxide derivatives of arachidonic acid. Free Radic Biol Med. 1989;7(4):409–433. doi: 10.1016/0891-5849(89)90125-1. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Wang J. K., Sihra T. S., Nairn A. C., Czernik A. J., Greengard P. Inhibition of Ca2+/calmodulin-dependent protein kinase II by arachidonic acid and its metabolites. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8550–8554. doi: 10.1073/pnas.86.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. A., Wolf R. A., Pagels W. R., Marnett L. J. A test for the intermediacy of 11-hydroperoxyeicosa-5,8,12,14-tetraenoic acid [11-HPETE] in prostaglandin biosynthesis. Biochem Biophys Res Commun. 1980 Jan 29;92(2):349–355. doi: 10.1016/0006-291x(80)90340-x. [DOI] [PubMed] [Google Scholar]
- Schaller H. C., Bodenmüller H. Isolation and amino acid sequence of a morphogenetic peptide from hydra. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7000–7004. doi: 10.1073/pnas.78.11.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty B. N., Stuart M. J., Walenga R. W. Formation of 11-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid in human umbilical arteries is catalyzed by cyclooxygenase. Biochim Biophys Acta. 1985 Mar 6;833(3):484–494. doi: 10.1016/0005-2760(85)90106-7. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Gordon J. A., Moore S. A. Hydroxyeicosatetraenoic acids (HETEs). Prog Lipid Res. 1988;27(4):271–323. doi: 10.1016/0163-7827(88)90009-4. [DOI] [PubMed] [Google Scholar]
- Stanley-Samuelson D. W. Comparative eicosanoid physiology in invertebrate animals. Am J Physiol. 1991 May;260(5 Pt 2):R849–R853. doi: 10.1152/ajpregu.1991.260.5.R849. [DOI] [PubMed] [Google Scholar]
- Van der Zee J., Eling T. E., Mason R. P. Formation of free radical metabolites in the reaction between soybean lipoxygenase and its inhibitors. An ESR study. Biochemistry. 1989 Oct 17;28(21):8363–8367. doi: 10.1021/bi00447a015. [DOI] [PubMed] [Google Scholar]
- Weinheimer A. J., Spraggins R. L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla chemistry of coelenterates. XV. Tetrahedron Lett. 1969 Dec;(59):5185–5188. doi: 10.1016/s0040-4039(01)88918-8. [DOI] [PubMed] [Google Scholar]
- Wollard P. M., Cunnigham F. M., Murphy G. M., Camp R. D., Derm F. F., Greaves M. W. A comparison of the proinflammatory effects of 12(R)- and 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid in human skin. Prostaglandins. 1989 Oct;38(4):465–471. doi: 10.1016/0090-6980(89)90129-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto S. "Enzymatic" lipid peroxidation: reactions of mammalian lipoxygenases. Free Radic Biol Med. 1991;10(2):149–159. doi: 10.1016/0891-5849(91)90008-q. [DOI] [PubMed] [Google Scholar]



