Abstract
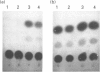
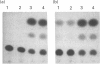
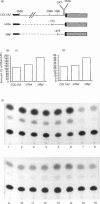
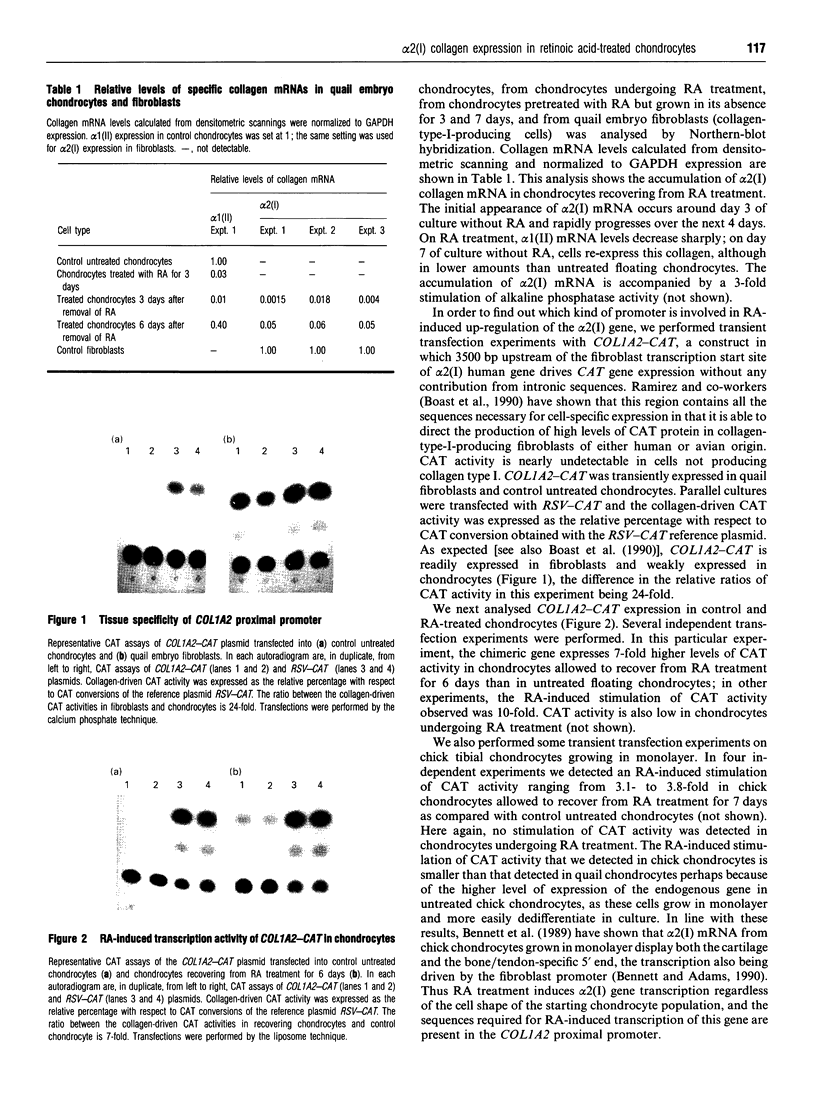
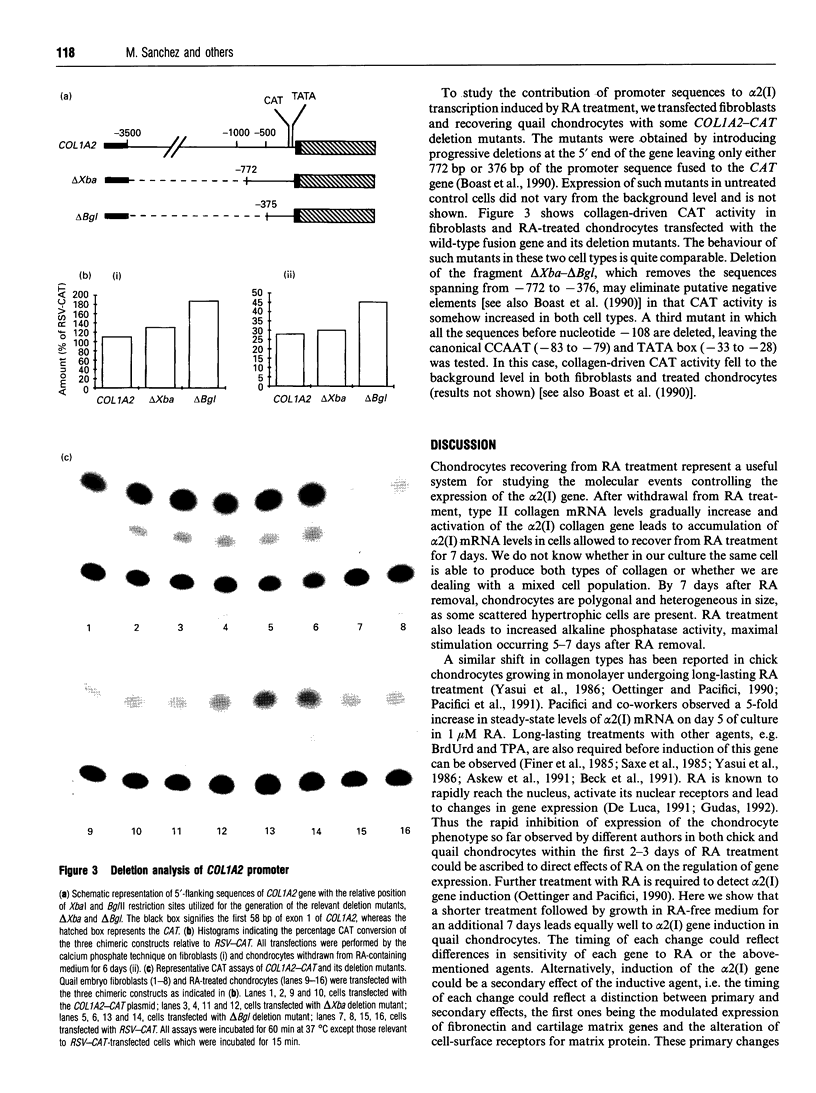
alpha 2(I) collagen gene expression is induced in quail embryo chondrocytes pretreated with retinoic acid (RA). The initial appearance of alpha 2(I) mRNA occurs around day 3 of culture in RA-free medium and rapidly progresses over the next 4 days. In transient transfection assays, expression of COL1A2-CAT, a chimeric gene bearing 3500 bp upstream the bone/tendon transcription start site from the human alpha 2(I) gene fused to the CAT gene, is stimulated severalfold in RA-treated chondrocytes. In contrast, enzyme activity is very low in untreated chondrocytes, suggesting that the sequences required for RA-induced transcription of the alpha 2(I) gene are present in this plasmid. Analysis of alpha 2(I) promoter sequences performed with deletion mutants gives overlapping results in collagen type I-producing fibroblasts and chondrocytes withdrawn from RA treatment. These experiments suggest that RA-induced transcription of the alpha 2(I) collagen gene in chondrocytes is regulated by the binding of transcription factors to the same regulatory sequences that control transcription in fibroblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Boettiger D., Focht R. J., Holtzer H., Pacifici M. Regulation of the synthesis of extracellular matrix components in chondroblasts transformed by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1982 Sep;30(2):373–384. doi: 10.1016/0092-8674(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Allebach E. S., Boettiger D., Pacifici M., Adams S. L. Control of types I and II collagen and fibronectin gene expression in chondrocytes delineated by viral transformation. Mol Cell Biol. 1985 May;5(5):1002–1008. doi: 10.1128/mcb.5.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew G. R., Wang S., Lukens L. N. Different levels of regulation accomplish the switch from type II to type I collagen gene expression in 5-bromo-2'-deoxyuridine-treated chondrocytes. J Biol Chem. 1991 Sep 5;266(25):16834–16841. [PubMed] [Google Scholar]
- Beck K. M., Seekamp A. H., Askew G. R., Mei Z., Farrell C. M., Wang S., Lukens L. N. Association of a change in chromatin structure with a tissue-specific switch in transcription start sites in the alpha 2(I) collagen gene. Nucleic Acids Res. 1991 Sep 25;19(18):4975–4982. doi: 10.1093/nar/19.18.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. D., Adams S. L. Characterization of the translational control mechanism preventing synthesis of alpha 2(I) collagen in chicken vertebral chondroblasts. J Biol Chem. 1987 Oct 25;262(30):14806–14814. [PubMed] [Google Scholar]
- Bennett V. D., Adams S. L. Identification of a cartilage-specific promoter within intron 2 of the chick alpha 2(I) collagen gene. J Biol Chem. 1990 Feb 5;265(4):2223–2230. [PubMed] [Google Scholar]
- Benya P. D., Brown P. D., Padilla S. R. Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol. 1988 Jan;106(1):161–170. doi: 10.1083/jcb.106.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R. Modulation of the rabbit chondrocyte phenotype by retinoic acid terminates type II collagen synthesis without inducing type I collagen: the modulated phenotype differs from that produced by subculture. Dev Biol. 1986 Nov;118(1):296–305. doi: 10.1016/0012-1606(86)90096-5. [DOI] [PubMed] [Google Scholar]
- Boast S., Su M. W., Ramirez F., Sanchez M., Avvedimento E. V. Functional analysis of cis-acting DNA sequences controlling transcription of the human type I collagen genes. J Biol Chem. 1990 Aug 5;265(22):13351–13356. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J Biol Chem. 1985 Feb 25;260(4):2315–2320. [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Finer M. H., Gerstenfeld L. C., Young D., Doty P., Boedtker H. Collagen expression in embryonic chicken chondrocytes treated with phorbol myristate acetate. Mol Cell Biol. 1985 Jun;5(6):1415–1424. doi: 10.1128/mcb.5.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht R. J., Adams S. L. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984 Sep;4(9):1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionti E., Capasso O., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. Transformation by rous sarcoma virus induces a switch in the collagen type synthesis and enhances fibronectin expression. J Biol Chem. 1983 Jun 10;258(11):7190–7194. [PubMed] [Google Scholar]
- Gionti E., Jullien P., Pontarelli G., Sanchez M. A continuous line of chicken embryo cells derived from a chondrocyte culture infected with RSV. Cell Differ Dev. 1989 Sep;27(3):215–223. doi: 10.1016/0922-3371(89)90701-6. [DOI] [PubMed] [Google Scholar]
- Gionti E., Pontarelli G., Cancedda R. Avian myelocytomatosis virus immortalizes differentiated quail chondrocytes. Proc Natl Acad Sci U S A. 1985 May;82(9):2756–2760. doi: 10.1073/pnas.82.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gudas L. J. Retinoids, retinoid-responsive genes, cell differentiation, and cancer. Cell Growth Differ. 1992 Sep;3(9):655–662. [PubMed] [Google Scholar]
- Horton W. E., Yamada Y., Hassell J. R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev Biol. 1987 Oct;123(2):508–516. doi: 10.1016/0012-1606(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Horton W., Hassell J. R. Independence of cell shape and loss of cartilage matrix production during retinoic acid treatment of cultured chondrocytes. Dev Biol. 1986 Jun;115(2):392–397. doi: 10.1016/0012-1606(86)90258-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger H. F., Pacifici M. Type X collagen gene expression is transiently up-regulated by retinoic acid treatment in chick chondrocyte cultures. Exp Cell Res. 1990 Dec;191(2):292–298. doi: 10.1016/0014-4827(90)90017-5. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Golden E. B., Iwamoto M., Adams S. L. Retinoic acid treatment induces type X collagen gene expression in cultured chick chondrocytes. Exp Cell Res. 1991 Jul;195(1):38–46. doi: 10.1016/0014-4827(91)90497-i. [DOI] [PubMed] [Google Scholar]
- Pawlowski P. J., Brierley G. T., Lukens L. N. Changes in the type II and type I collagen messenger RNA population during growth of chondrocytes in 5-bromo-2-deoxyuridine. J Biol Chem. 1981 Aug 10;256(15):7695–7698. [PubMed] [Google Scholar]
- Ramirez F., Di Liberto M. Complex and diversified regulatory programs control the expression of vertebrate collagen genes. FASEB J. 1990 Apr 1;4(6):1616–1623. doi: 10.1096/fasebj.4.6.2180769. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Gionti E., Pontarelli G., Arcella A., De Lorenzo F. Expression of type X collagen is transiently stimulated in redifferentiating chondrocytes pretreated with retinoic acid. Biochem J. 1991 May 15;276(Pt 1):183–187. doi: 10.1042/bj2760183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe S. A., Lukens L. N., Pawlowski P. J. Changes in the nuclear and cytoplasmic levels of type I and type II collagen RNAs during growth of chondrocytes in 5-bromo-2'-deoxyuridine. J Biol Chem. 1985 Mar 25;260(6):3812–3819. [PubMed] [Google Scholar]
- Vuorio E., de Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- Yasui N., Benya P. D., Nimni M. E. Coordinate regulation of type IX and type II collagen synthesis during growth of chick chondrocytes in retinoic acid or 5-bromo-2'-deoxyuridine. J Biol Chem. 1986 Jun 15;261(17):7997–8001. [PubMed] [Google Scholar]
- von der Mark K. Immunological studies on collagen type transition in chondrogenesis. Curr Top Dev Biol. 1980;14(Pt 2):199–225. doi: 10.1016/s0070-2153(08)60195-7. [DOI] [PubMed] [Google Scholar]