Abstract
Objective
To evaluate neurofilament light (NfL) as a biomarker of the presymptomatic phase of amyotrophic lateral sclerosis (ALS).
Methods
The study population includes 84 individuals at risk for developing ALS, 34 controls, 17 ALS patients, and 10 phenoconverters (at-risk individuals observed both before and after the emergence of clinically manifest disease). At-risk individuals are enrolled through Pre-Symptomatic Familial ALS (Pre-fALS), a longitudinal natural history and biomarker study of individuals who are carriers of any ALS-associated gene mutation (in SOD1, C9orf72, TARDBP, FUS, VCP, etc), but who, at the time of enrollment, demonstrated no clinical symptoms or signs (including electromyographic evidence) of manifest disease. NfL in serum and cerebrospinal fluid (CSF) were quantified using an electrochemiluminescence immunoassay.
Results
Serum and CSF NfL are substantially higher in ALS patients compared to controls and at-risk individuals and remain relatively stable over time. Among phenoconverters, however, NfL levels were elevated (ie, above the range observed in controls) as far back as ~12 months preceding the emergence of the earliest clinical symptoms or signs of disease.
Interpretation
Serum (and CSF) NfL are informative biomarkers of presymptomatic ALS, providing a new tool to quantify presymptomatic disease progression and to potentially predict the timing of clinical phenoconversion. As such, quantification of NfL may aid the design and implementation of early therapeutic intervention for affected individuals and/or disease prevention trials for individuals at short-term risk of developing ALS.
Introduction
A major barrier to the development of effective therapies for patients with a range of neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), is the relatively late stage in the course of disease when treatment is initiated.1 Delayed treatment derives, in part, from the long latency from symptom onset to diagnosis and from diagnosis to treatment,2 but also from the fact that the underlying pathological process in neurodegenerative disorders likely begins some time before the emergence of clinical symptoms and signs. For Huntington's,3 Parkinson's,4 and Alzheimer's disease,5 published data suggest that this presymptomatic phase may be decades long, but little is known about the duration of the presymptomatic phase of ALS.6, 7 Given that, by definition, there is no clear clinical evidence of disease during this period, biomarkers are essential for detecting and quantifying presymptomatic disease; and longitudinal changes in biomarkers are critical for differentiating neurodegeneration (which implies a progressive process) from susceptibility (which might explain, at least in part, observed cross-sectional differences between healthy controls and those at higher risk for developing a neurodegenerative disease).8, 9
The study of presymptomatic ALS has been greatly facilitated by the growing number of monogenic causes of disease, which now account for >15% of all cases, with a hexanucleotide repeat expansion (HRE) in C9orf72 and mutations in the CuZn-superoxide dismutase gene (SOD1) being the most frequent in white populations.10 The Pre-Symptomatic Familial ALS (Pre-fALS) study,6 initiated in 2007, uses the presence of pathogenic mutations to define a population of clinically unaffected individuals with greatly increased lifetime risk (ie, beyond the expected 0.1% risk in the general population) for ALS. This unique cohort provides an opportunity to prospectively study the disease process before it becomes clinically manifest, including the identification of biomarkers to detect and quantify the progression of neurodegeneration in the presymptomatic phase and to predict when clinically manifest disease is likely to emerge.
Neurofilaments are three types of intermediate filaments exclusively expressed in neurons and are defined by their molecular weight: neurofilament light (NfL), medium and heavy, which are 68 to 70kDa, 145 to 160kDa, and 200 to 220kDa, respectively.11 They are the most abundant proteins in large myelinated axons. NfL is especially abundant in the large caliber axons that project into deeper brain layers and the spinal cord.12 With the development of a reliable assay for quantification of NfL,13 studies have shown that NfL levels are elevated in the blood and cerebrospinal fluid (CSF) of patients with ALS, but remain largely stable over time, with higher levels predicting poorer survival.13, 14 Hitherto unknown is when, in the natural history of ALS, NfL levels begin to increase. Previous efforts to address this question15 have been limited by a paucity of data from individuals followed longitudinally from the presymptomatic phase, through phenoconversion and the development of clinically manifest disease. Here, we present quantification of NfL in blood and CSF from precisely such a population, and show that levels of NfL increase in the 12-month period preceding the emergence of clinically manifest disease.
Patients and Methods
Study Population
Pre-Symptomatic Familial ALS (Pre-fALS) 6 is a longitudinal natural history and biomarker study of individuals recruited from across North America who are carriers of any ALS-causing gene mutation (in SOD1, C9orf72, TARDBP, FUS, VCP, etc), but who, at the time of enrollment, demonstrate no clinical or electromyographic evidence of disease. Asymptomatic carriers of mutations in these genes comprise the only population known to be at significantly greater risk for ALS (compared to the general population), and in whom a study of presymptomatic disease may realistically be considered. Once eligibility has been confirmed and genetic testing performed (along with counseling if results are to be disclosed16), participants travel to the study center at the University of Miami and undergo an in-depth series of assessments that includes: a detailed neuromuscular examination; electromyography (EMG); an array of “dry” biomarker procedures (eg, neuroimaging, electrophysiology, and fine motor function); and collection of biological samples, including urine, blood, and CSF, using established standard operating procedures. These assessments are repeated approximately every 12 to 24 months. Between in-person visits, participants are followed quarterly by telephone to ascertain early symptoms of disease, which, in turn, prompt ad-hoc in-person study visits. Those who develop symptoms or signs suggestive of disease are followed with more frequent in-person visits as needed, depending on the degree of suspicion following the initial ad-hoc assessment and the evolution of symptoms over time. In participants who progress to clinically manifest disease, we communicate this information to them and transition them into a clinical care paradigm. We continue to follow these individuals through in-person visits (whenever possible) and remote assessments between visits (eg, telephone administration of the revised ALS Functional Rating Scale (ALSFRS-R), in-home measurement of forced expiratory volume in 6 seconds (FEV6), remote collection of biological fluids). We thus acquire longitudinal data before the appearance of symptoms, around the time that symptoms begin to emerge, and in the early stages of manifest disease. ALS patients and controls—either healthy individuals with no known family history of ALS or individuals who were found, as part of their Pre-fALS screening, not to harbor the genetic mutation known to cause ALS in their family—are similarly evaluated as done at Pre-fALS in-person visits. Serum and CSF samples included in this experiment were collected at study visits that took place between January 2008 and July 2016. This study was approved by the University of Miami Institutional Review Board, and all participants provided written informed consent. The study is registered on clinicaltrials.gov (NCT00317616).
Sample Collection, Processing, and Storage
For serum analysis, blood was collected in a red top BD vacutainer (BD, Franklin Lakes, NJ) and allowed to clot upright at room temperature for 1 to 2 hours. Following centrifugation (1,750g for 10 minutes at 4°C) serum was aliquoted into cryogenic sterile freestanding conical microtubes (Nalgene [Nalge Nunc International Corporation, Rochester, NY] or Bio Plas Inc. [Vacaville, CA]) and quickly stored at −80°C until use. CSF (free of macroscopic hemoglobin) was collected in polypropylene tubes, centrifuged (1,750g for 10 minutes at 4°C), aliquoted using a sterile pipette into precapped polypropylene cryogenic sterile freestanding conical microtubes, frozen within 15 to 20 minutes of collection, and stored at −80°C until use.
Neurofilament Light
The protocol for quantification of NfL is based on an electrochemiluminescence immunoassay.13, 17 Briefly, 96-well plates (Multi-Array plates; Meso Scale Discovery [MSD], Gaithersburg, MD), including integrated screen-printed carbon ink electrodes on the bottom of the wells, were used. All samples were evenly distributed on each plate and measured in duplicate at the same dilution. All NfL assays were performed blind to group/disease state. Each plate contained calibrators (0–10,000pg/mL) and quality controls; if required, samples were appropriately diluted to fall in the range of the standard curve. Interassay coefficients of variance were below 10%, and mean intra-assay coefficients of variance were below 10%. Linearity of the NfL was established (0–50,000pg/mL) as previously reported.13, 17
Statistical Analysis
NfL concentrations that were below the level of detection were excluded from the summary statistics in Table 2, but included in analyses using an imputed value of 0.37pg/ml, which was the lowest measured value in our data. Because of the highly skewed distribution of NfL concentrations and CSF/serum ratios, and the presence of outliers, analyses were performed using nonparametric tests or natural logarithm-transformed values. For ease of interpretation of log-transformed values, a small constant of 0.63 (=1-0.37) was added to NfL values before taking the natural logarithm, so that the lowest log-transformed value was 0 (rather than a negative value). For CSF NfL, one extreme outlier was excluded. Summary statistics for numerical variables are provided in mean ± standard deviation (SD; or, for variables with a highly skewed distribution, in median and range), and for categorical variables in frequency and percentage. For phenoconverters in Pre-fALS, date of onset was determined based on the earlier of: unequivocal symptoms reported by the participant, or subclinical signs of disease detected through detailed neuromuscular examination or EMG that clearly indicated disease. All 10 phenoconverters described in this article met the former definition. Date of onset of affected individuals was based on participants' recollection and self-report. Spearman rank correlation, Wilcoxon rank-sum test, or Kruskal–Wallace test and regression analyses were used in the analysis of cross-sectional data. Mixed-model analysis (with random intercept, unstructured covariance structure, and Kenward–Roger degrees of freedom) were used in the analysis of longitudinal data. For the Spearman correlation of serum and CSF over follow-up, to correct for the within-person correlation, a bootstrap percentile confidence interval was computed by stratified sampling (with replacement) within the four participant groups. Level of statistical significance was set at 0.05 (two-sided); given the discovery and exploratory nature of this study, however, the focus of our analyses were less on statistical testing, but more on characterizing the NfL concentrations observed. All statistical analyses were performed and graphics produced using SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Table 2.
Serum and CSF NfL concentration
| Baseline Visit Only | All Available Visits | ||||
|---|---|---|---|---|---|
| Serum NfL | CSF NfL | Serum NfL | CSF NfL | ||
| Control (N=34; total 56 visits) | N=32 d | N=15 f | 53 visits d | 18 visits | |
| Original scale (pg/ml) | Median (Range) |
9.0 (2.2-33.1) |
491 (306-1,573) |
10.7 (0.4-33.5) |
482 (306-2,460) |
| Log-transformed a | Mean ± SD (Range) |
2.4 ± 0.7 (1.0-3.5) |
6.3 ± 0.6 (5.7-7.4) |
2.5 ± 0.7 (0.01-3.5) |
6.4 ± 0.6 (5.7-7.8) |
| At-Risk (N=84; total 213 visits) | N=75 e | N=50 f | 195 visits e | 110 visits | |
| Original scale (pg/ml) | Median (Range) |
12.6 (1.1-62.4) |
641 (301-2,719) |
13.9 (1.1-75.6) |
681 (225-3,629) |
| Log-transformed a | Mean ± SD (Range) |
2.5 ± 0.7 (0.6-4.1) |
6.5 ± 0.5 (5.7-7.9) |
2.7 ± 0.7 (0.6-4.3) |
6.6 ± 0.6 (5.4-8.2) |
| Converter (N=10 b; total 28 visits c) | N=10 | N=3 f | 28 visits | 10 visits g | |
| Original scale (pg/ml) | Median (Range) |
19.1 (6.9-104.3) |
2,558 (772-5,679) |
86.2 (6.9-720.4) |
7,664 (772-16,678) |
| Log-transformed a | Mean ± SD (Range) |
3.1 ± 1.0 (2.0-4.7) |
7.7 ± 1.0 (6.6-8.6) |
4.3 ± 1.3 (2.0-6.6) |
8.6 ± 1.0 (6.6-9.7) |
| Affected (N=17; total 44 visits) | N=17 | N=6 | 44 visits | 16 visits | |
| Original scale (pg/ml) | Median (Range) |
120.5 (20.5-554.7) |
10,655 (2,142-14,964) |
99.0 (20.5-554.7) |
9,413 (2,142-21,658) |
| Log-transformed a | Mean ± SD (Range) |
4.8 ± 0.8 (3.1-6.3) |
9.0 ± 0.8 (7.7-9.6) |
4.7 ± 0.7 (3.1-6.3) |
9.0 ± 0.8 (7.7-10.0) |
Baseline = first visit at which serum sample was available (with or without contemporaneous CSF collection)
N = number of participants. Visits = number of person-visits.
Natural algorithm
All 10 converters were pre-symptomatic at baseline.
The 28 person-visits in the converter group included visits from when participants were pre-symptomatic and after phenoconversion.
In the control group, serum NfL at 3 person-visits (2 baseline, 1 follow-up) were below the level of detection and not included in the summary statistics above.
In the pre-symptomatic group, serum NfL at 18 person-visits (9 baseline, 9 follow-up) were below the level of detection and not included in the summary statistics above.
An additional N=1 control, N=6 at-risk, and N=3 converters had CSF NfL at follow up (but not baseline) visits.
In the converter group, CSF NfL at 1 baseline visit was below the level of detection and not included in summary statistics above.
Results
Study Population
The study population (Tables 1A and 1B) comprises 34 controls; 84 at-risk individuals (who have remained presymptomatic throughout the duration of follow-up to date); 10 converters (who have undergone phenoconversion and followed longitudinally from the presymptomatic to the symptomatic phase); and 17 affected (ie, patients with ALS at the time of initial assessment); the affected group includes 11 ALS patients in whom there is no identified genetic mutation. Median delay from time of onset to diagnosis was 16.4 months (range, 2.6–83.8) among ALS patients, and notably shorter, 1.8 months (range, 0.5–5.5), among the converters. Cross-sectional clinical and NfL data are available from all participants at time of initial sample collection (“baseline”), with longitudinal data available from a subset of 10 controls, 51 at-risk, 8 converters, and 11 affected. Overall, clinical and NfL data are available from 343 person-visits. The at-risk group comprises individuals with an SOD1 mutation (n = 28 A4V, n = 24 non-A4V), a C9orf72 HRE (n = 27) or mutation in TARDBP, FUS, or VCP (n = 5); and all but 1 of the converters were SOD1 mutation carriers. EMG (of both arms and legs, midthoracic paraspinals, and bulbar musculature) was normal in all phenoconverters before phenoconversion, with the exception of the FUS phenoconverter, in whom there were nonspecific changes restricted to L5 innervated muscles 9 months before phenoconversion.
Table 1a.
Study participant characteristics: All participants
| Control (N=34) |
At-Risk (N=84) |
Converter (N=10) |
Affected (N=17) |
||
|---|---|---|---|---|---|
| Baseline age (years) | Mean ± SD (Range) | 46.4 ± 11.4 (24.2-69.2) | 45.1 ± 12.3 (18.9-77.0) | 50.3 ± 11.6 (31.9-74.8) | 59.8 ± 8.2 (45.6-75.8) |
| Male | N (%) | 15 (44%) | 31 (37%) | 4 (40%) | 9 (53%) |
| Genotype | SOD1 A4V | (n/a) | 28 | 8 | 1 |
| SOD1 nonA4V | 24 | 1 | 1 | ||
| C9orf72 HRE | 27 | 0 | 4 | ||
| Other | 5 | 1 | 0 | ||
| Unknown | 0 | 0 | 11 | ||
| Site of onset | Bulbar | (n/a) | 2 | 2 | |
| Limbs | 7 | 14 | |||
| Respiratory | 1 | 0 | |||
| Unknown | 0 | 1 | |||
| Baseline years since onset | Median (Range) | -1.0 (-3.4, -0.1) | 2.0 (0.7, 7.6) | ||
| Baseline years since diagnosis | Median (Range) | -1.3 (-3.7, -0.3) | 0.7 (0.1, 3.4) | ||
| Baseline ALSFRS-R | Mean ± SD (Range) | (n/a) | 35.8 ± 5.6 (27-44) a | ||
| Baseline ΔFRS | Mean ± SD (Range) | (n/a) | 0.50 ± 0.37 (0.15-1.45) a | ||
Baseline = first visit at which serum sample was available (with or without contemporaneous CSF collection)
(n/a) = not applicable.
Baseline ALSFRS-R not available for N=2
ΔFRS = (48 – baseline ALSFRS-R) / months from onset to baseline
Table 1b.
Study participant characteristics: Longitudinal subset only
| Control (N=10) |
At-Risk (N=51) |
Converter (N=8) |
Affected (N=11) |
||
|---|---|---|---|---|---|
| # of collections | Median (Range) | 3 (2-5) | 3 (2-7) | 2.5 (2-5) | 2 (2-6) |
| Follow-up duration (years) | Median (Range) | 1.5 (0.6-5.6) | 3.4 (1.2-7.8) | 1.8 (0.3-4.0) | 1.1 (0.3-1.7) |
| Baseline age (years) | Mean ± SD (Range) | 49.2 ± 13.1 (34.5-69.2) | 47.6 ± 11.1 (18.9-67.6) | 53.3 ± 10.5 (42.8-74.8) | 60.1 ± 7.0 (45.6-67.7) |
| Male | N (%) | 4 (40%) | 17 (33%) | 2 (25%) | 7 (64%) |
| Genotype | SOD1 A4V | (n/a) | 21 | 6 | 1 |
| SOD1 nonA4V | 17 | 1 | 0 | ||
| C9orf72 HRE | 10 | 0 | 2 | ||
| Other | 3 | 1 | 0 | ||
| Unknown | 0 | 0 | 8 | ||
| Site of onset | Bulbar | (n/a) | 1 | 1 | |
| Limbs | 6 | 9 | |||
| Respiratory | 1 | 0 | |||
| Unknown | 0 | 1 | |||
| Baseline years since onset | Median (Range) | −1.2 (−3.4, −0.1) | 2.0 (0.7, 7.6) | ||
| Baseline years since diagnosis | Median (Range) | −1.7 (−3.7, −0.3) | 0.4 (0.1, 3.4) | ||
| Baseline ALSFRS-R | Mean ± SD (Range) | (n/a) | 37.8 ± 4.8 (31.3-44.0) a | ||
| Baseline ΔFRS | Mean ± SD (Range) | (n/a) | 0.42 ± 0.27 (0.15-1.07) a | ||
Baseline = first visit at which serum sample was available (with or without contemporaneous CSF collection)
(n/a) = not applicable.
Baseline ALSFRS-R not available for N=2
ΔFRS = (48 − baseline ALSFRS-R) / months from onset to baseline
The control and at-risk groups are comparable in baseline age (mean ± SD = 46 ± 11 and 45 ± 12 years, respectively), with the converters slightly older (50 ± 12 years) and the affected approximately 10 years older (60 ± 8 years; p < 0.01 when compared to controls or at-risk). As of July 2016, the cut-off date for the NfL experiments reported here, we had conducted a median of two to three (and up to five to seven) longitudinal visits on the N = 80 participants in the longitudinal subset, with varying lengths of follow-up for the different groups (Tables 1A and 1B).
Relationship Between Serum and CSF NfL Levels
Serum and CSF NfL levels were highly correlated: including only cross-sectional data at first CSF collection (total N = 85 participants across all 4 groups), Spearman rank correlation (r = 0.65; p < 0.0001) and including all available data (total 155 person-visits across all 4 groups; r = 0.70; 95% confidence interval [CI], 0.60–0.78). NfL concentrations in CSF were invariably higher than serum by 8- to 286-fold (median, 57-fold). Specifically, CSF/serum ratios of NfL were similar in controls (18 person-visits, mean ± SD = 66 ± 48), at-risk (110 person-visits, 65 ± 52), and converters (10 person-visits, 67 ± 27), and slightly higher in affected (16 person-visits, 77 ± 28). Moreover, CSF/serum ratio remained largely stable over time among the N = 49 participants with longitudinal CSF and serum data (slope estimate on the natural logarithm scale = −0.059; 95% CI, −0.150, 0.032).
Cross-Sectional NfL Concentration at Baseline
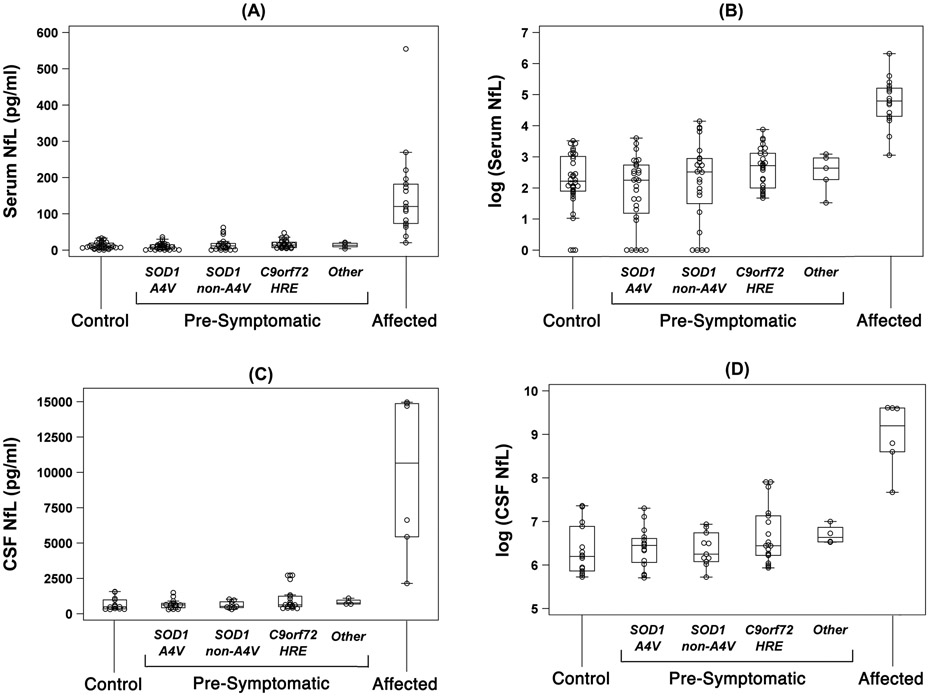
Baseline NfL levels in serum (Table 2) were comparable between controls (median [range] = 9 [2–33] pg/ml) and at-risk individuals (13 [1–62] pg/ml), irrespective of genotype (Fig 1). On the other hand, serum levels were, as expected, higher in patients with ALS (121 [21–555] pg/ml) as compared to controls (Wilcoxon rank-sum test, p < 0.0001) and at-risk (p < 0.0001). Whereas lumbar puncture was only performed at 155 of 343 (45%) person-visits, a similar data pattern was observed in CSF NfL (Table 2; Fig 1).
Figure 1.
Baseline levels of NfL in serum and CSF: (a) serum NfL (pg/ml); (b) log-transformed serum NfL; (c) CSF NfL (pg/ml); and (d) log-transformed CSF NfL. Boxes show median, and 25th and 75th percentiles; whiskers extend to a maximum of 1.5 x interquartile range (IQR), or to the most extreme value if it is less than 1.5 x IQR from the 25th or 75th percentile.
Longitudinal Changes in NfL Concentration
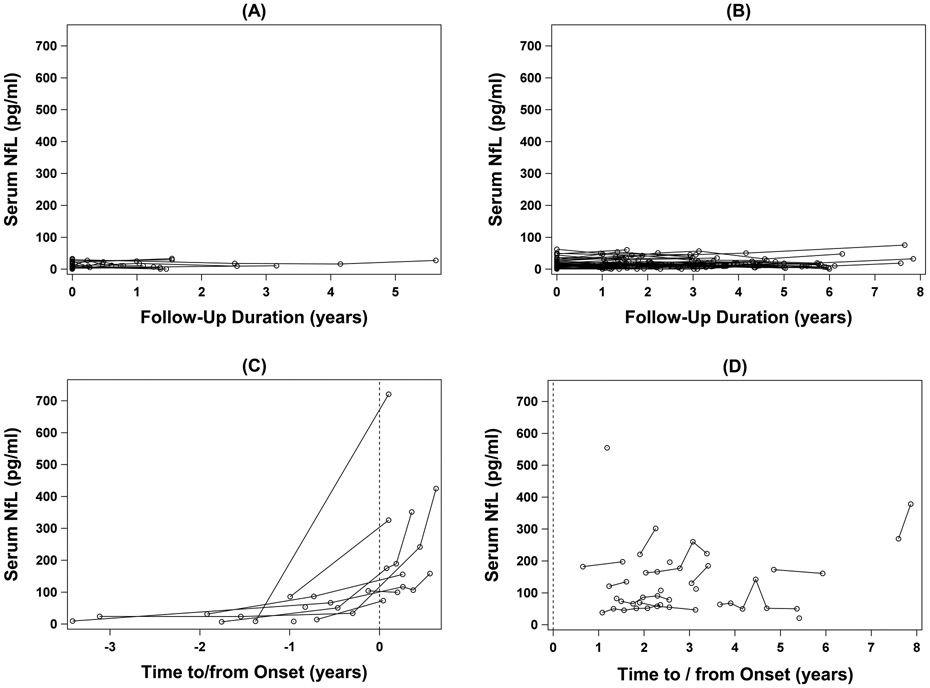
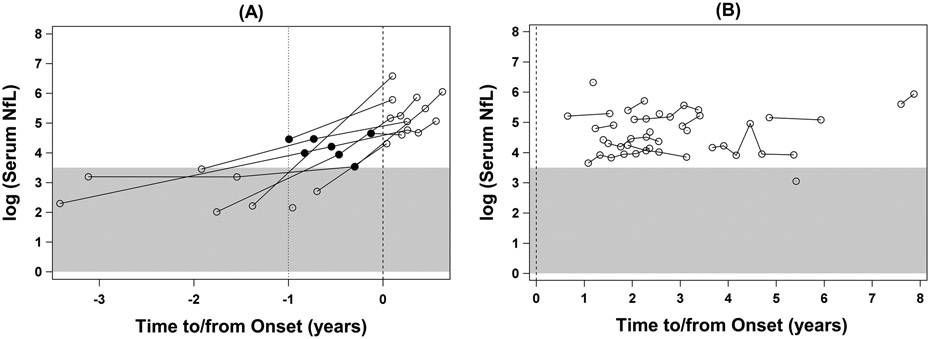
Longitudinally, serum NfL levels were essentially stable in controls and ALS patients (Figs 2 and 3B), although there are as yet insufficient data to reliably estimate the slopes in a mixed-model analysis. By contrast, in at-risk individuals, adjusting for baseline age, serum NfL increased by an average of 2.41pg/ml per 10-year increase in age (p = 0.004). Moreover, among converters, elevated NfL levels (ie, above the highest value observed in controls) were observed as far back as 11.6 months preceding phenoconversion, and their NfL levels continued to increase through at least the first 6 months after symptom onset (Figs 2C and 3A). Although CSF was not available from all participants at every time point, a similar pattern was noted in CSF NfL (data not shown). Noteworthy are two other observations: (1) all available serum NfL measured >1 year before phenoconversion were within the range of controls; and (2) all available serum NfL measured after phenoconversion was well above the range observed among controls.
Figure 2.
Longitudinal changes in serum NfL concentration (pg/ml): (a) controls; (b) at-risk individuals who remain pre-symptomatic throughout follow-up; (c) phenoconverters; and (d) ALS patients. The x-axis in (a) and (b) shows years since baseline. The x-axis in (c) and (d) shows years to or since the onset of symptoms or signs, which is marked by the vertical dashed line at year=0.
Figure 3.
Longitudinal changes in serum NfL concentration, natural log-transformed: (a) phenoconverters; and (b) ALS patients. The x-axis shows years to or since the onset of symptoms or signs, which is marked by the vertical dashed line at year=0. The gray area covers the range of serum NfL values observed in the control group. The closed circles mark the elevated levels of NfL (i.e. above the highest value seen in controls) that were measured in serum collected before the onset of symptoms or signs.
Effects of Age, Sex, and Gene Mutation on NfL Concentration
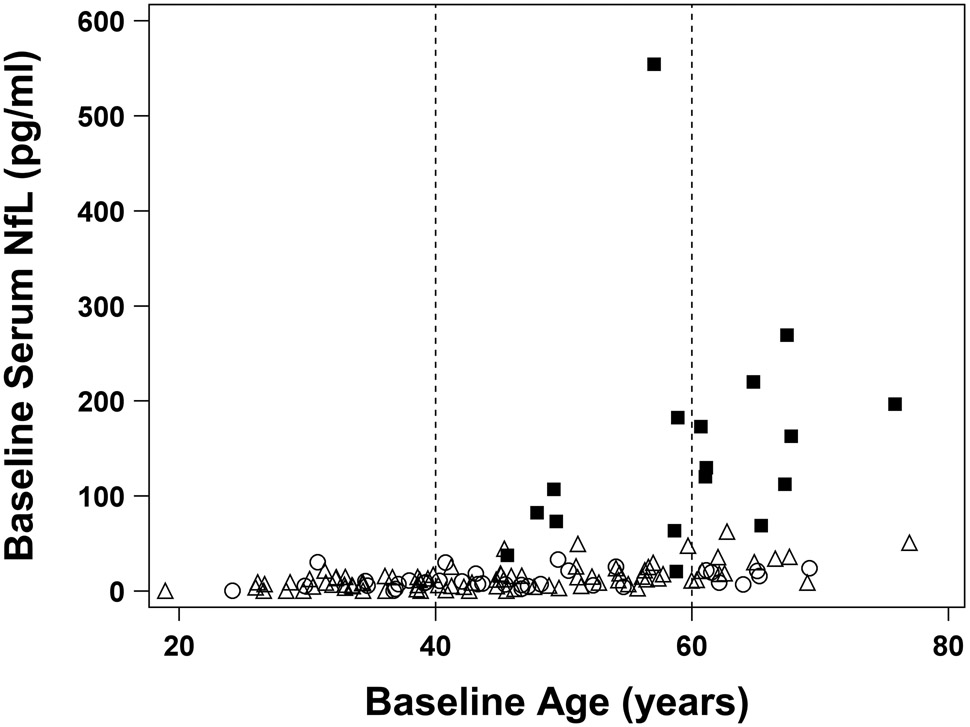
In cross-sectional analysis among n = 34 controls, there is a modest correlation between age and baseline serum NfL (Spearman rank correlation, r = 0.32; p = 0.065), with an average increment of 1.39pg/ml for every decade increase in age. Although we had, thus far, too few control participants with sufficiently long follow-up duration to reliably estimate the annual increase over time, the cross-sectional data suggest that the magnitude of the age-related increase in serum NfL was negligible considering the levels observed in the affected group (Figs 2-4) and converter group (Figs 2 and 3). Furthermore, baseline serum and CSF NfL levels did not differ by sex (p > 0.16 across the four groups; Table S1) or by gene mutation (at-risk group only, SOD1 A4V versus SOD1 non-A4V versus C9orf72 versus other gene mutations, p = 0.12 for serum and 0.52 for CSF; Tables S2 and S3).
Figure 4.
Scatterplot of baseline serum NfL levels vs. baseline age shows that, although NfL levels are slightly higher among older individuals in the control (open circles) and at-risk groups (open triangles), the magnitude of this increase is negligible in comparison to the levels of NfL observed among ALS (closed squares) patients (i.e. older age does not explain the increase in serum NfL among ALS patients). Open circles represent controls; open triangles represent at-risk individuals who remain pre-symptomatic throughout follow-up; and filled squares represent ALS patients. Vertical dashed lines demarcate age groups (< 40, 40-60 and > 60 years of age).
Discussion
Consistent with previously published cross-sectional results, we found that serum and CSF NfL concentrations are significantly higher in ALS patients compared to controls; are largely stable over time in both groups18; and do not differ between controls and presymptomatic gene mutation carriers at risk for ALS.15 In addition, we found no difference in serum NfL content between presymptomatic individuals with various SOD1 mutations (AV4 vs all others), or between those with an SOD1 mutation compared to those with a C9orf72 HRE. Our data also confirm the previous observations19-21 that, whereas NfL concentrations do not differ by sex, NfL concentrations correlate with age—though the magnitude of the annual increase, as shown in our control data, is clinically negligible.
Until now, the timing of the increase in NfL levels in ALS has been unclear. This is, in large part, because very few individuals have ever been followed longitudinally from the presymptomatic phase through manifest disease, and because the early symptomatic phase of disease is rarely observed given that diagnosis is typically delayed, with an average of ~11 months from symptom onset to diagnosis.22, 23 Past efforts to study neurofilament levels during the presymptomatic phase of disease (ALS and frontotemporal dementia) have been largely cross-sectional, with the temporal course of NfL levels constructed by measuring NfL in presymptomatic gene mutation carriers and estimating their age at onset—and thereby the number of years they were before symptom onset—based on that of family members.15, 21 These studies both concluded that the elevation in NfL level is a characteristic of early symptomatic (but not presymptomatic) disease.
Here, we show, for the first time, that NfL levels, in samples collected from almost all phenoconverters up to almost 1 year before the initial symptoms, are elevated above the highest value observed in controls and, in fact, are typical of those observed in ALS patients. We recognize that most of these phenoconverters have the SOD1 A4V mutation, which is an especially aggressive form of the disease; 2 phenoconverters, however, had other mutations (SOD1 I113T and FUS) that are associated with less aggressive disease. Although the generalizability of our findings will require further observations in individuals with a broader array of genotypes, these data provide a first glimpse into the duration of the presymptomatic phase of ALS and evidence that the neurodegenerative process begins at least 6 to 12 months preceding the first emergence of overt clinical evidence of disease. These data also suggest that an increase in serum NfL level may be a useful predictor of the emergence of clinically manifest disease and perhaps could be used as an eligibility criterion for a disease prevention trial, though further validation is needed before we can draw this conclusion. For example, it is unclear to what extent the observed gradual, but small, increase over time in presymptomatic individuals at genetic risk for ALS represents an even earlier onset of the neurodegenerative process; and whether or not those individuals with the highest levels are approaching the time of phenoconversion will only become apparent through their continued follow-up.
These observations were possible because of the longitudinal design of the Pre-fALS study, in which presymptomatic gene mutation carriers are studied regularly over an extended period. As such, Pre-fALS overcomes the limitations of previous studies that developed quasi-longitudinal constructs of disease based on cross-sectional data, relying, for example, upon parental or other family members' age of onset to estimate when a presymptomatic person is likely to develop ALS (an approach that is not supported by our experience24). In addition, the longitudinal nature of Pre-fALS enables us to differentiate neurodegeneration (which entails change, such as progressive axonal loss, attributed to degeneration over time) from the susceptibility to neurodegeneration (which, aside from the effects of aging, is likely to be a largely stable state).
Notwithstanding its strengths, this study does have limitations. Longitudinal CSF was available from only a relatively small number of participants, especially from converters before and after the development of manifest disease. Although CSF and serum NfL are highly correlated, the small number of longitudinal CSF samples limits our ability to comment on whether CSF levels might rise in advance of serum levels as presymptomatic individuals approach phenoconversion. The ongoing Pre-fALS study, which now includes ~150 individuals with follow-up duration of as long as 10 years, will yield a larger number of phenoconverters over time; and given that lumbar puncture is performed at ~50% of visits, it is highly likely that additional pre- and post-phenoconversion CSF samples will be available in the future. These efforts notwithstanding, the ease of serum (as opposed to CSF) collection makes the use of serum NfL a pragmatically more appealing biomarker.
In conclusion, NfL—which is easily measured using well-established laboratory assays—appears to be a promising biomarker of presymptomatic ALS in the ~12 months before the emergence of clinically overt disease. Notwithstanding a relatively small number of converters and the variable timing of sample collection vis-à-vis phenoconversion, our data provide the first biochemical evidence of presymptomatic ALS. The significance of these findings may be best understood in the context of efforts to initiate (experimental) therapeutics in the early symptomatic or even presymptomatic phase of disease, with the goal of delaying or preventing the onset of clinically manifest disease in the population at (genetic) risk for developing ALS. The rise in serum (and CSF) NfL levels before the appearance of clinically manifest disease may serve as a presymptomatic biomarker of disease progression; and potentially as a pharmacodynamic biomarker if an experimental therapeutic were to show normalization of NfL levels. Moreover, the presymptomatic rise in NfL levels may serve as a prognostic biomarker insofar as it may predict the timing of phenoconversion to clinically manifest disease. As such, the rise in NfL levels, if replicated in future studies, might be used as an eligibility criterion for enrollment in a disease prevention trial with the appearance of manifest disease as the primary outcome measure. In light of these potential clinical and clinical trial applications, it is significant that, compared to CSF, serum may be equally good (or even better) as a biological fluid for quantification of this informative biomarker.
Supplementary Material
Acknowledgment
The study was sponsored by the Muscular Dystrophy Association (Grant #4365 and #172123), the ALS Association (Grant #2015), the ALS Recovery Fund, the Kimmelman Estate, the Knut and Alice Wallenberg Foundation, the Swedish Research Council, and the Swedish Brain Research Foundation.
We extend thanks to our research staff at the University of Miami for participant recruitment and evaluation (Eliana Reyes, Danielle Dauphin, Maria Catalina Fernandez, Danielle Sheldon, Sumaira Hussain, Anne Cooley, Jessica Medina, and Ashley Manso) and data management (Christine Clayman); and Christine Stanislaw for providing genetic counseling. Most important, we are grateful to all the individuals who participated in this study for their altruism and contribution to advancing ALS therapy development efforts.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Benatar M. ALS therapy development: challenges and opportunities. J Neuromuscul Dis 2016;3:S10. [Google Scholar]
- 2.Benatar M, Wuu J. The challenge of early therapeutic intervention in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:5. [Google Scholar]
- 3.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry 2008;79:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postuma RB, Gagnon JF, Montplaisir J. Clinical prediction of Parkinson’s disease: planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry 2010;81:1008–1013. [DOI] [PubMed] [Google Scholar]
- 5.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benatar M, Wuu J. Pre-symptomatic studies in ALS: rationale, challenges and approach. Neurology 2012;79:1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen A, Kiernan M, Mitsumoto H, Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry 2014;85:1232–1238. [DOI] [PubMed] [Google Scholar]
- 8.Carew JD, Nair G, Andersen PM, et al. Presymptomatic spinal cord neurometabolic findings in SOD1-positive people at risk for familial ALS. Neurology 2011;77:1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng MC, Ho JT, Ho SL, et al. Abnormal diffusion tensor in nonsymptomatic familial amyotrophic lateral sclerosis with a causative superoxide dismutase 1 mutation. J Magn Reson Imaging 2008;27:8–13. [DOI] [PubMed] [Google Scholar]
- 10.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 2011;7:603–615. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Xie F, Siedlak SL, et al. Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci 2004;61:3057–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec 1970;167:379–387. [DOI] [PubMed] [Google Scholar]
- 13.Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 2016;79:152–158. [DOI] [PubMed] [Google Scholar]
- 16.Benatar M, Stanislaw C, Reyes E, et al. Presymptomatic ALS genetic counseling and testing: experience and recommendations. Neurology 2016;86:2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain in blood is a prognostic, and a potential pharmacodynamic biomarker for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:32. [Google Scholar]
- 19.Steinacker P, Feneberg E, Weishaupt J, et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 2016;87:12–20. [DOI] [PubMed] [Google Scholar]
- 20.Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM. Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur J Neurol 2007;14: 1329–1333. [DOI] [PubMed] [Google Scholar]
- 21.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 2016;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell JD, Callagher P, Gardham J, et al. Timelines in the diagnostic evaluation of people with suspected amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)—a 20-year review: can we do better? Amyotroph Lateral Scler 2010;11:537–541. [DOI] [PubMed] [Google Scholar]
- 23.Paganoni S, Macklin EA, Lee A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener 2014;15:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benatar M, Polak M, Feng R. Predicting the risk of disease in familial amyotrophic lateral sclerosis. In: American Neurological Association 133rd Annual Meeting, Salt Lake City, UT, September 21–24, 2008. [Google Scholar]
- 25.Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–1661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.