Abstract
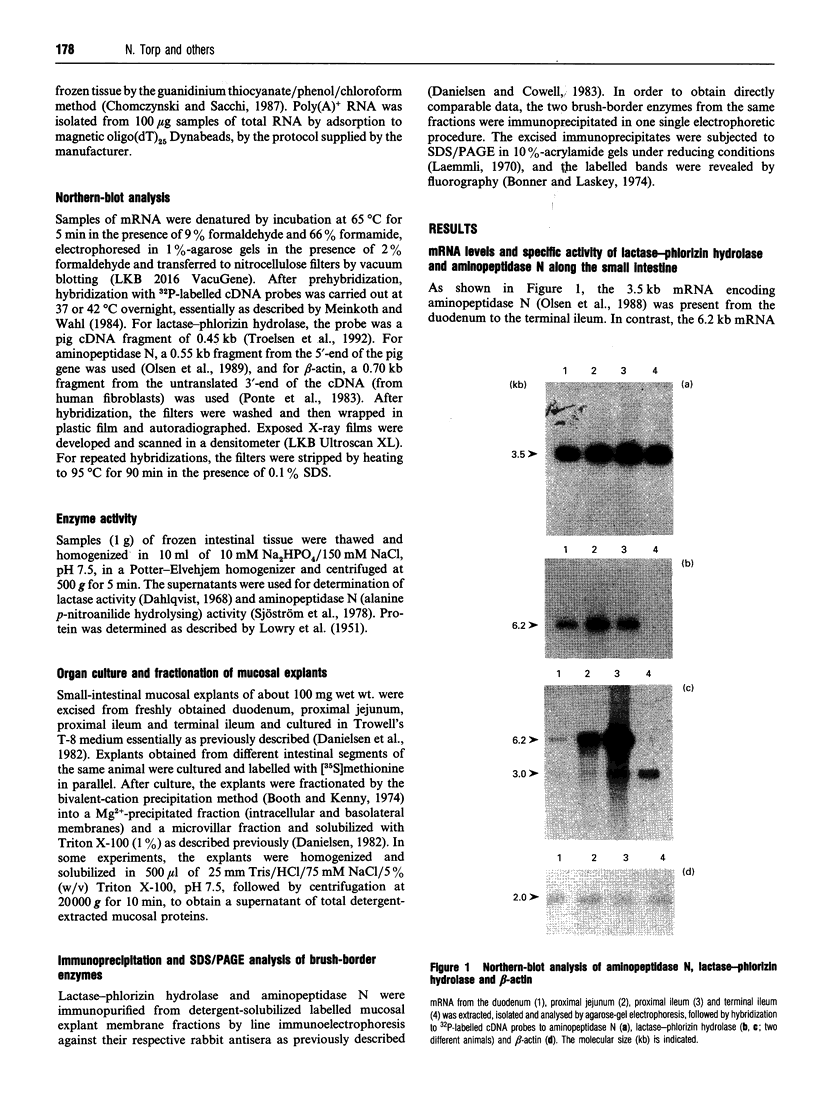
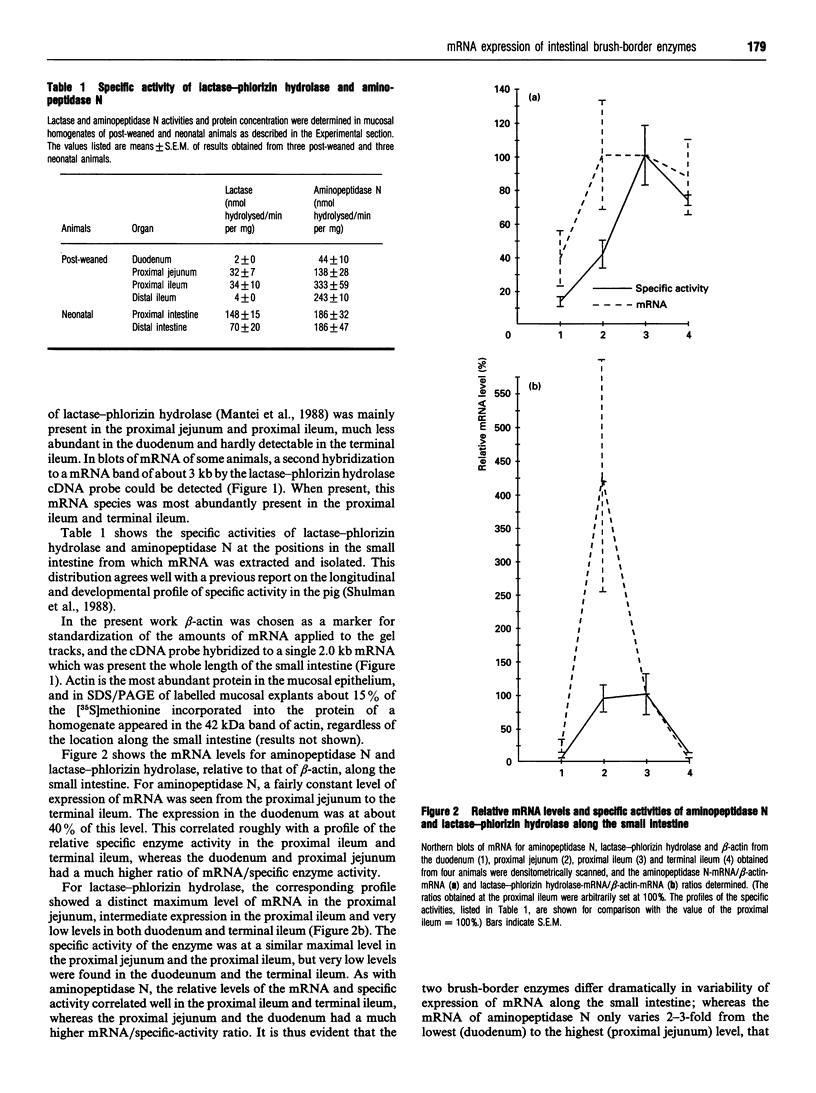
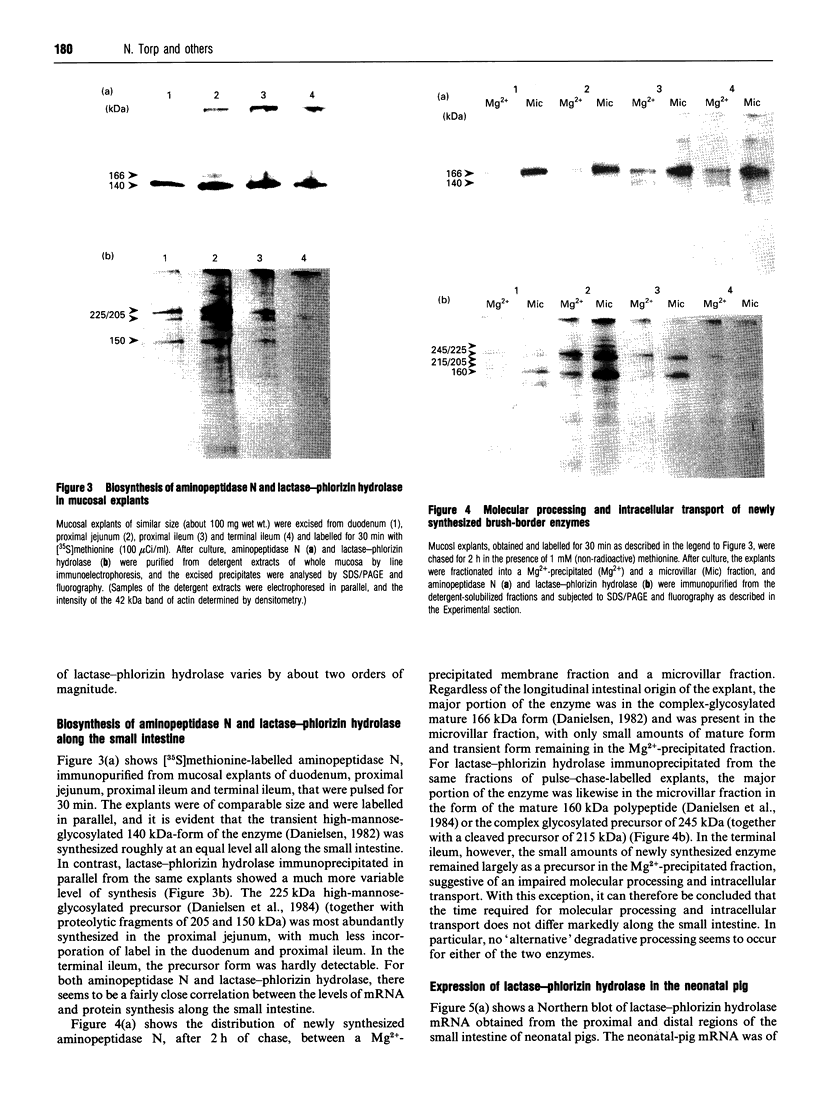
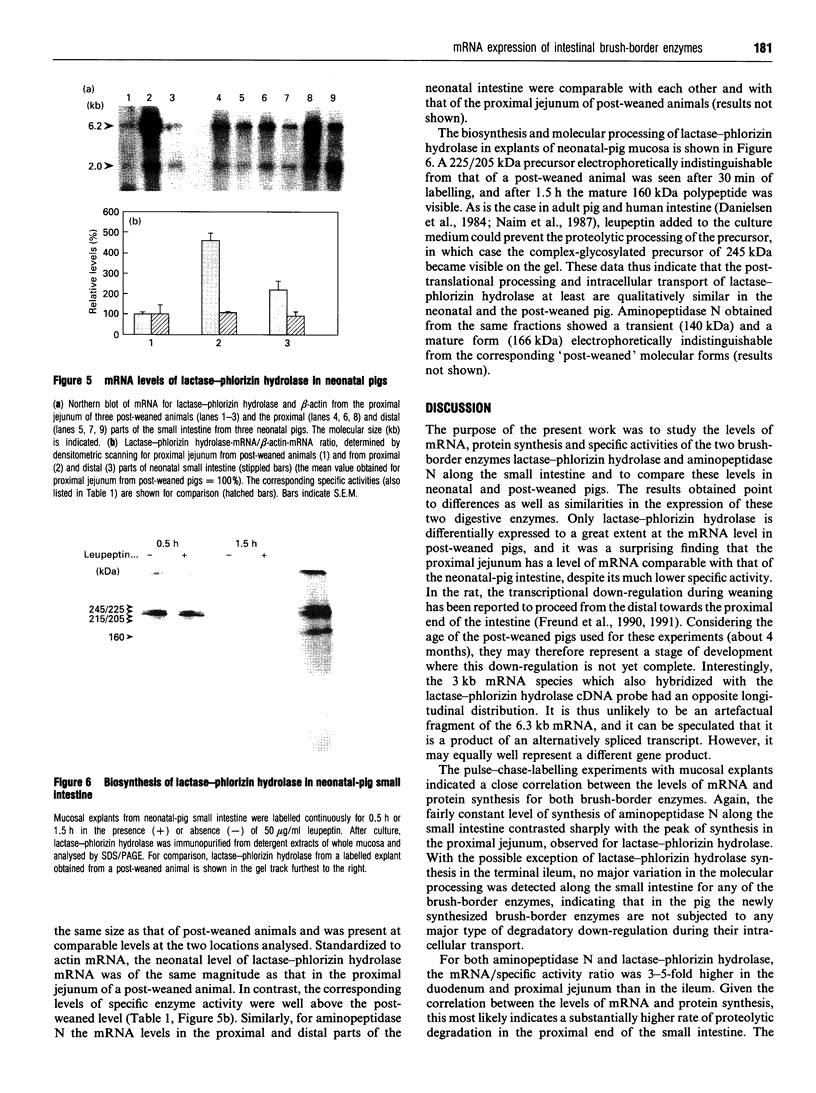
The longitudinal expression of two brush-border enzymes, lactase-phlorizin hydrolase (EC 3.2.1.23/62) and aminopeptidase N (EC 3.4.11.2), was studied in the small intestine of the post-weaned pig. Whereas the level of mRNA, encoding aminopeptidase N (relative to that of beta-actin), only varied moderately from the duodenum to the terminal ileum, the amount of lactase-phlorizin hydrolase mRNA exhibited a sharp maximum in the proximal jejunum. For both enzymes, the level of protein synthesis, studied in cultured mucosal explants, correlated well with the level of mRNA, and no major variation in post-translational processing or intracellular transport was observed along the intestine. The mRNA/specific-activity ratio for both enzymes was markedly (3-5-fold) higher in the duodenum and proximal jejunum, compared with the ileum. This indicates an increased proximal turnover rate, most likely caused by the presence in the gut lumen of pancreatic proteases. In neonatal animals, the level of mRNA for lactase-phlorizin hydrolase in both proximal and distal regions of the intestine was of the same magnitude as in the proximal jejunum of the post-weaned pigs. Our results point to two mechanisms that affect the expression of lactase-phlorizin hydrolase in the pig during development: (1) a primary regulation at the level of mRNA (predominantly in the ileum); (2) an increased rate of turnover of the enzyme, mainly in the duodenum and proximal jejunum, and most likely due to an increased secretion into the gut lumen of pancreatic proteases (a mechanism also affecting aminopeptidase N and probably other brush-border enzymes as well).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büller H. A., Grand R. J. Lactose intolerance. Annu Rev Med. 1990;41:141–148. doi: 10.1146/annurev.me.41.020190.001041. [DOI] [PubMed] [Google Scholar]
- Büller H. A., Kothe M. J., Goldman D. A., Grubman S. A., Sasak W. V., Matsudaira P. T., Montgomery R. K., Grand R. J. Coordinate expression of lactase-phlorizin hydrolase mRNA and enzyme levels in rat intestine during development. J Biol Chem. 1990 Apr 25;265(12):6978–6983. [PubMed] [Google Scholar]
- Castillo R. O., Reisenauer A. M., Kwong L. K., Tsuboi K. K., Quan R., Gray G. M. Intestinal lactase in the neonatal rat. Maturational changes in intracellular processing and brush-border degradation. J Biol Chem. 1990 Sep 15;265(26):15889–15893. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on aminopeptidase N and sucrase-isomaltase. Biochem J. 1982 Jun 15;204(3):639–645. doi: 10.1042/bj2040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Microscale purification of proteins by line immunoelectrophoresis: application of the technique in protein biogenesis studies. J Biochem Biophys Methods. 1983 Aug;8(1):41–47. doi: 10.1016/0165-022x(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Bro B., Dabelsteen E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J. 1982 Mar 15;202(3):647–654. doi: 10.1042/bj2020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Intracellular processing of lactase-phlorizin hydrolase. Biochem Biophys Res Commun. 1984 Jul 18;122(1):82–90. doi: 10.1016/0006-291x(84)90442-x. [DOI] [PubMed] [Google Scholar]
- Escher J. C., de Koning N. D., van Engen C. G., Arora S., Büller H. A., Montgomery R. K., Grand R. J. Molecular basis of lactase levels in adult humans. J Clin Invest. 1992 Feb;89(2):480–483. doi: 10.1172/JCI115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J. N., Duluc I., Raul F. Discrepancy between the intestinal lactase enzymatic activity and mRNA accumulation in sucklings and adults. Effect of starvation and thyroxine treatment. FEBS Lett. 1989 May 8;248(1-2):39–42. doi: 10.1016/0014-5793(89)80427-2. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Duluc I., Raul F. Lactase expression is controlled differently in the jejunum and ileum during development in rats. Gastroenterology. 1991 Feb;100(2):388–394. doi: 10.1016/0016-5085(91)90207-2. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Torp N., Duluc I., Foltzer-Jourdainne C., Danielsen M., Raul F. Comparative expression of the mRNA for three intestinal hydrolases during postnatal development in the rat. Cell Mol Biol. 1990;36(6):729–736. [PubMed] [Google Scholar]
- Keller P., Zwicker E., Mantei N., Semenza G. The levels of lactase and of sucrase-isomaltase along the rabbit small intestine are regulated both at the mRNA level and post-translationally. FEBS Lett. 1992 Nov 30;313(3):265–269. doi: 10.1016/0014-5793(92)81206-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd M., Mevissen G., Fischer M., Olsen W., Goodspeed D., Genini M., Boll W., Semenza G., Mantei N. Regulation of intestinal lactase in adult hypolactasia. J Clin Invest. 1992 Feb;89(2):524–529. doi: 10.1172/JCI115616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantei N., Villa M., Enzler T., Wacker H., Boll W., James P., Hunziker W., Semenza G. Complete primary structure of human and rabbit lactase-phlorizin hydrolase: implications for biosynthesis, membrane anchoring and evolution of the enzyme. EMBO J. 1988 Sep;7(9):2705–2713. doi: 10.1002/j.1460-2075.1988.tb03124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J. 1987 Jan 15;241(2):427–434. doi: 10.1042/bj2410427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988 Oct 10;238(2):307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Olsen J., Sjöström H., Norén O. Cloning of the pig aminopeptidase N gene. Identification of possible regulatory elements and the exon distribution in relation to the membrane-spanning region. FEBS Lett. 1989 Jul 17;251(1-2):275–281. doi: 10.1016/0014-5793(89)81470-x. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R., Santiago N. A., Tsuboi K. K., Gray G. M. Intestinal lactase. Shift in intracellular processing to altered, inactive species in the adult rat. J Biol Chem. 1990 Sep 15;265(26):15882–15888. [PubMed] [Google Scholar]
- Rossi M., Maiuri L., Russomanno C., Auricchio S. In vitro biosynthesis of lactase in preweaning and adult rabbit. FEBS Lett. 1992 Nov 30;313(3):260–264. doi: 10.1016/0014-5793(92)81205-z. [DOI] [PubMed] [Google Scholar]
- Sebastio G., Villa M., Sartorio R., Guzzetta V., Poggi V., Auricchio S., Boll W., Mantei N., Semenza G. Control of lactase in human adult-type hypolactasia and in weaning rabbits and rats. Am J Hum Genet. 1989 Oct;45(4):489–497. [PMC free article] [PubMed] [Google Scholar]
- Shulman R. J., Henning S. J., Nichols B. L. The miniature pig as an animal model for the study of intestinal enzyme development. Pediatr Res. 1988 Mar;23(3):311–315. doi: 10.1203/00006450-198803000-00016. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Mills P. R., Fransen J. A., Hauri H. P., Lentze M. J., Naim H. Y., Ginsel L., Bond J. Biogenesis of intestinal lactase-phlorizin hydrolase in adults with lactose intolerance. Evidence for reduced biosynthesis and slowed-down maturation in enterocytes. J Clin Invest. 1990 Oct;86(4):1329–1337. doi: 10.1172/JCI114842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelsen J. T., Olsen J., Norén O., Sjöström H. A novel intestinal trans-factor (NF-LPH1) interacts with the lactase-phlorizin hydrolase promoter and co-varies with the enzymatic activity. J Biol Chem. 1992 Oct 5;267(28):20407–20411. [PubMed] [Google Scholar]
- Witte J., Lloyd M., Lorenzsonn V., Korsmo H., Olsen W. The biosynthetic basis of adult lactase deficiency. J Clin Invest. 1990 Oct;86(4):1338–1342. doi: 10.1172/JCI114843. [DOI] [PMC free article] [PubMed] [Google Scholar]