Abstract
Background
Neurosarcoidosis is an inflammatory granulomatous disease. Up to 25% of occult sarcoidosis affecting the nervous system are only detected by autopsy. In addition, in recent years the suspicion arose that the soluble Interleukin-2 Receptor (sIL-2R) might be useful in differentiating between neurosarcoidosis and neurosarcoidosis-like diseases such as neurotuberculosis, multiple sclerosis, or cerebral lymphoma.
Objectives
Therefore, we aimed to systematically review randomized controlled trials (RCT), observational studies, and case-control studies evaluating sIL-2R levels in neurosarcoidosis patients.
Design
For this systematic review, a comprehensive literature search of electronic databases including EMBASE, The Web Of Science, The Cochrane Library, MEDLINE, and Google Scholar was conducted. The search was limited to the English language and publication date up to January 08th, 2024.
Data Sources and Methods
As part of the search strategy conducted, 6 articles met the inclusion criteria. Two independent reviewers extracted the relevant data from each article. In addition, 2 independent reviewers assessed the quality of each study using the Newcastle-Ottawa Scale (NOS).
Results
We included 6 studies comprising 98 patients suffering from neurosarcoidosis, 525 non-sarcoidosis patients, and 118 healthy controls. Included studies were published between 2010 and 2023. Cerebrospinal fluid (CSF) sIL-2R levels differed significantly between neurosarcoidosis patients and multiple sclerosis, vasculitis, and healthy controls whereas serum sIL-2R levels did not reveal sufficient discriminative power. sIL-2R index was able to discriminate neurosarcoidosis from neurotuberculosis, bacterial/viral meningitis, and healthy controls.
Conclusions
In this systematic review, we found indications that sIL-2R may be a useful biomarker for the diagnosis of neurosarcoidosis. To determine an additional diagnostic value of sIL-2R, large prospective studies are needed that not only examine absolute sIL-2R levels in serum or CSF but also the dynamic changes as well as the implications of renal function on sIL-2R levels.
Keywords: Neurosarcoidosis, soluble IL-2 receptor, diagnosis, biomarker, cerebrospinal fluid
Introduction
Sarcoidosis (Besnier-Boeck-Schaumann disease) is an inflammatory, granulomatous multisystem disease of unknown etiology, which affects the lungs in over 95% and can be associated with involvement of the peripheral or central nervous system or muscles in up to 5–13% of patients.1–3 Among sarcoidosis patients, an incidence of 1% is estimated for isolated neurosarcoidosis, however, there is evidence that occult neurosarcoidosis is identified by autopsy in up to 15–25% of patients with generalized sarcoidosis.4–6 Furthermore, since the therapy of sarcoidosis varies depending on the organ manifestation, it is important not only to differentiate isolated neurosarcoidosis from mimics such as meningitis, multiple sclerosis, seizures, astrocytomas, metastases, or tuberculosis but also to correctly and quickly diagnose neurosarcoidosis in patients with other organ manifestations of sarcoidosis.7–10 For instance, non-steroidal anti-inflammatory drugs such as ibuprofen are primarily used in patients with acute sarcoidosis for symptom control without signs of neurosarcoidosis, as spontaneous remission is very likely (≈85%).7,11 In contrast, medications such as glucocorticoids, azathioprine, mycophenolate mofetil, and methotrexate are used when disease progression is observed.7,11 However, regardless of whether isolated neurosarcoidosis is present, or the nervous system is affected as an additional organ manifestation, glucocorticoids (e.g. prednisone) should be administered from the first day onwards, whereby compared to progressive sarcoidosis other than neurosarcoidosis higher doses are required to overcome the blood-brain barrier (BBB).12–14 The basis for the diagnosis of sarcoidosis is the clinical examination, followed by serological examinations and specific organ-related examinations of the suspected organ systems, such as an X-ray of the lungs and the taking of a biopsy and its histological processing.6,15 In neurosarcoidosis affecting the brain or myelon, cerebrospinal fluid (CSF) analysis and imaging are essential, while confirmation by biopsy is rather secondary, as tissue sampling is often not possible.16–18 In recent years, several attempts have been made to define diagnostic criteria for the diagnosis of neurosarcoidosis. However, as most of the studies examined were retrospective and as neurosarcoidosis can be a chameleon, the diagnostic criteria were categorized as “definite”, “probable” and “possible”.13,19 However, more and more attempts have been made to find a suitable biomarker for the diagnosis of sarcoidosis/neurosarcoidosis.3,20–24 Biomarkers such as angiotensin-converting enzyme (ACE), lysozyme, and neopterin have been shown to correlate with disease progression and are therefore rather predictive of progression than diagnosis, as these biomarkers are both non-sensitive and non-specific.25–31 Another frequently investigated biomarker is the soluble interleukin-2 receptor (sIL-2R), which has been studied in sarcoidosis patients since the 1983s. 32
Interleukin-2 (IL-2) is a key cytokine within the immune system with a critical influence on both protective immunity and the maintenance of immune tolerance achieved through the action of CD4+ regulatory T lymphocytes (Tregs).33–35 IL-2 exerts its effects on cells that possess either high-affinity or low-affinity IL-2 receptors (IL-2R).35,36 The IL-2R complex, consists of IL-2Rα, IL-2Rβ, and IL-2Rγ.37,38 High-affinity IL-2 Rs include IL-2Rα, which enhances the binding of IL-2 to the receptor.35,36 Tregs express IL-2R with high affinity and utilize low physiological IL-2 levels continuously. 35 In contrast, activated CD4+ and CD8+ T cells express IL-2 only transiently. It has been demonstrated that activated T lymphocytes secrete soluble sIL-2R, which is generated by enzymatic cleavage of membrane-bound IL-2Rα, which is a transmembrane glycoprotein receptor.39–41 Soluble IL-2 Rs are regarded as a marker for T cell activation, although they are also produced by other immune cells, including dendritic cells, B lymphocytes, and monocytes.37,39,42,43 In the context of inflammatory processes observed in conditions such as sarcoidosis, Th1 cells are activated, resulting in an increase in the expression of IL-2R on the cell surface. Over time, IL-2 Rs are removed from the cell membrane and released into the bloodstream, where they modulate the immune system.40,41 Elevated levels of sIL-2R in serum as well as CSF have been associated with several pathological conditions, including autoimmune diseases, infections, transplant rejection, and cancers.21,25,40,44,45 In recent years, it has been suggested that sIL-2R could be a useful additional tool for the diagnosis of sarcoidosis. In a retrospective cohort study investigating 189 patients with suspected sarcoidosis sensitivity and specificity of serum sIL-2R for the detection of sarcoidosis were 88% and 85%, respectively, which was found to be superior to ACE (62% and 88%). 24 In addition, a meta-analysis showed a sensitivity of 0.88 (95% confidence interval (CI): 0.75–0.95) and a specificity of 0.87 (95% CI 0.73-0.94) for the differentiation between 592 sarcoidosis patients and 885 non-sarcoidosis patients. 46 However, there has been no systematic review of sIL-2R values in patients with neurosarcoidosis. Therefore, this study aimed to systematically search the literature for evidence of a possible benefit of sIL-2R for the diagnosis of neurosarcoidosis in blood and CSF.
Materials and methods
Study criteria
For this review, we implemented the PICOS question, and the following eligibility criteria were set. I) Population: age ≥18 years; II) Intervention: patients suffering isolated neurosarcoidosis or neurosarcoidosis as an additional organ manifestation, III) Control: neurosarcoidosis-like syndromes such as multiple sclerosis, bacterial or viral meningitis, neurotuberculosis, vasculitis, healthy controls, Guillain-Barré Syndrome, cerebral lymphoma, non-inflammatory neurological deficits (headache, seizures, polyneuropathy, psychiatric disorders, etc.), patients suffering sarcoidosis without neurological affection, IV) Outcome: clinically confirmed neurosarcoidosis; V) Studies: randomized controlled trial (RCT), observational study, case-control study; VI) English language. VII) Studies reporting on sIL-2R levels of neurosarcoidosis patients without including a control group.
Exclusion criteria were set as follows: I) published whole text language other than English; II) animal studies; and III) case reports, reviews, and letters to the editor IV) studies providing insufficient data about sIL-2R levels.
Data sources and search strategy
This systematic review followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines. 47 The literature search was performed by 2 independent investigators (JR, TL) using EMBASE, The Web Of Science, The Cochrane Library, MEDLINE, and Google Scholar up to January 08th, 2024. Disagreements were resolved by consensus. Used combinations of search strings were “neurosarcoidosis”, “soluble interleukin 2 receptor 2”, “sIL-2R”, “sIL2R”, “sIL-2”, “sIL2R”, “IL-2R”, “IL2R” with the Boolean operators “AND” and “OR”. 48 Reference lists of identified articles were screened for additional sources. Furthermore, relevant meta-analyses and systematic reviews were screened manually to ensure a comprehensive literature review. The complete search strategy can be found in the “Supplemental_Literature_Search_Strategy”. Zotero was used to screen the obtained literature search results and to remove duplicates. 49
Data extraction
The following data was extracted by 2 independent reviewers and entered into an Excel spreadsheet (Microsoft, Redmond, WA): (1) Study characteristics (type of study, study year, number of patients suffering neurosarcoidosis, number of control patients); (2) Country of study investigation (3) Participants’ baseline characteristics (age, gender); (4) Risk factors (hypertension, diabetes mellitus, renal insufficiency, history of pulmonary disorders); (5) sIL-2R measurement method (6) Blood collecting time (7) Blood sIL-2R levels, CSF sIL-2R levels. Disagreements were resolved by consensus. In the systematic review, we focused on the critical discussion of the included studies and refrained from extensive statistical comparisons due to the limited number of studies and persistent heterogeneity. Risk of bias assessment of all included studies, reflected by the “Newcastle-Ottawa Scale” (NOS) was calculated as mean and standard deviation (SD).
Methodological quality assessment - risk of bias
Case-control studies and cohort studies were evaluated according to the NOS to assess the quality of the studies. Studies were rated in the categories “Selection, Comparability, and Exposure/Outcome”. 50 A maximum of 9 points could be achieved. As the literature suggests we classified the studies as follows: ≥ 7 stars were considered as “good-quality”, 2 to 6 stars were considered “fair-quality”, and ≤ 1 star was considered “poor-quality”.51–53 Quality assessment was done by 2 independent authors (AC, JR).
Results
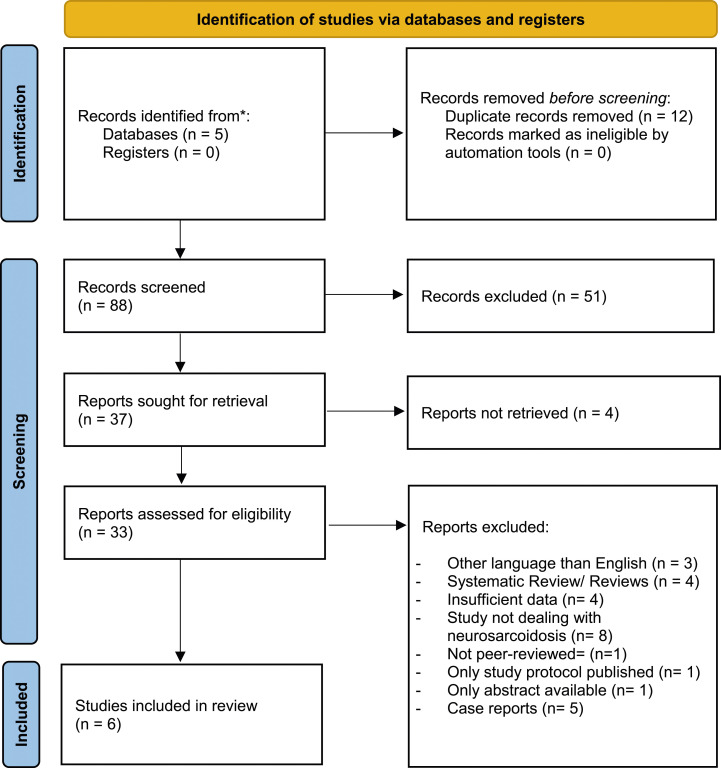
Searching EMBASE, The Web of Science, The Cochrane Library, Medline, and Google Scholar resulted in 100 publications. After removing duplicates, 88 articles were eligible for the first screening phase, where titles and abstracts were searched, resulting in the exclusion of 51 articles by not meeting the inclusion criteria. In the second screening phase, 37 studies were found to be suitable for a full-text search. Of those, 4 studies could not be retrieved, 4 did not provide sufficient data on sIL-2R levels, 3 were written in a language other than English, 4 were reviews/systematic reviews, 8 studies did not deal with neurosarcoidosis, 1 was not peer-reviewed, 1 was only a published protocol, 1 study only provided an abstract and 5 were case reports. (Figure 1).
Figure 1.
PRISM flow diagram.
All included studies were carried out retrospectively and published between 2010 and 2023. Used measurement methods were Enzyme-linked Immunosorbent Assay (ELISA, 66.7%), semiautomated chemiluminescent immunoassay technology, IMMULITE (16.7%), and no description was provided in 16.7% of all cases. Most of the studies were carried out in Germany (2/6) and Japan (2/6) followed by Netherlands (1/6) and Switzerland (1/6). (Table 1).
Table 1.
Study characteristics.
| Author | Year | Country | Neurosarcoidosis | Controls | Measurement method | Neurosarcoidosis, sIL-2R Levels Mean (SD); Median [IQR] | Controls, sIL-2R Levels Mean (SD); Median [IQR] | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age Mean (SD) Median [IQR] |
N | Age Mean (SD) Median [IQR] |
Serum | CSF | Serum | CSF | |||||
| Sumi et al. | 2003 | Japan | 20 | 64.5 [25-82] | 14 | 62 [48-82] | ELISA | 1004 U/ml [385-2995] | 77 U/ml [25-808] | NA | NA |
MRI, Intraparenchymal enhancement present vs. absent 65.1 U/ml [25-808] vs. 23 U/ml [27.3-489], P = 0.1370 MRI, meningeal enhancement, present vs. absent 77 U/ml [25-348] vs. 316 U/ml [25-808], P = 0.2915 |
| Petereit et al. | 2010 | Germany | 11 | 42 (NA) | NT = 13 BM = 22 VM = 17 VS = 20 MS = 21 HC = 18 |
NT = 57 [NA] BM = 51 [NA] VM = 42[NA] VS = 33 [NA] MS = 37 [NA] HC = NA |
ELISA | 12214 pg/ml (SE: 6445.3) | 2871 pg/ml (SE: 1077.5) | NT = 13918 pg/ml (SE: 4788.7) BM = 11615 pg/ml (SE: 4069.3) VM = 3726 pg/ml (SE: 426.2) VS = 4858 pg/ml (SE: 639.8) MS = 2538pg/ml (SE: 333.3) HC = 2460pg/ml (SE: 161.9) |
NT = 10343 pg/ml (SE: 4593.5) BM = 1578 pg/ml (SE: 450.4) VM = 2129 pg/ml (SE: 658.7) VS = 109 pg/ml (SE: 41.4) MS = 52 pg/ml (SE: 8.7) HC = 34 pg/ml (SE: 0.0) |
Serum sIL-2R NS vs. NT/BM/VM/VS/MS/HC, P > 0.05 CSF sIL-2R NS vs. BM/VM, P > 0.05, NS vs. Neurotuberculosis, P = 0.002 NS vs. VS, P = 0.001; NS vs. MS/HC, P < 0.001 sIL2-R-Index: NS vs. VS/MS, P >0.05; NS vs. HC/VM/BM/NT, P <0.05 |
| Eurelings et al. | 2019 | Netherlands | 6 | NA | HC = 101 | HC = NA | ELISA | 16851 pg/ml [5027-11020] | NA | HC = 1515 pg/ml [1150-1880] | NA | NS vs. SCS, p>0.05 |
| SCS = 101 | SCS = 43 [35-52] | SCS: 6100 pg/ml [4500-9850] | ||||||||||
| Diagnosis other than SCS = 88 | Diagnosis other than SCS = 44 [31-57] | Diagnosis other than SCS = 2600 pg/ml [1925-3300] | ||||||||||
| Otto et al. | 2020 | Germany | 23 | 37 [28-72] | NINDS = 115 MS = 19 NT = 8 BM = 9 VM = 18 GBS = 8 CL = 15 |
NINDS = 61 [2-94] MS = 41 [21-60] NT = 37 [4-73] BM = 53 [38-79] VM = 50 [8-80] GBS = 65 [7-83] CL = 60 [31-78] |
Semiautomated chemiluminescent immunoassay technology (IMMULITE™) | 466 U/ml [201-1034] | 61.1 U/ml [0.9-6970.2] | NIDS = 402 U/ml [155-4334] MS = 313 U/ml [186-775] NT = 750.5 U/ml [420-4964] BM = 924 U/ml [302-4669] VM = 491.5 U/ml [101-1761] GBS = 1057.5 U/ml [465-2093] CL = 582 U/ml [215-1932] |
NINDS = 13 U/ml [8-94] MS = 6.9 U/ml [4.6-23.1] NT = 1605.1 U/ml [46.8-20016] BM = 374.8 U/ml [65.5-2305.9] VM = 57.8 U/ml [18.7-159,3] GBS = 43.3 U/ml [11.1-85.5] CL = 212.3 U/ml [6.4-153.2] |
Serum: NS vs. NINDS (P = 0.076) |
| CSF: NS vs. MS/NINDS, P <0.0001; NINDS vs. GBS/ BM/ VM/ NT, CL (P <0.0001) | ||||||||||||
|
sIL-2R index: NS = 12.4 [0.1-78.6]; NINDS = 3.9 [1.5-12.4]; MS = 4.3 [2-16.9]; NT = 38.8 [4.2-86.4]; BM = 8.2 [1.7-19.4]; VM = 10 [3.2-27]; GBS = 2.0 [0.1-2.6]; CL = 26.6 [1.8-223.8] NINDs vs. NS, CL, VM, NT (P <0.0001); NINDs vs. BM P = 0.19; NINDs vs. MS, P >0.05; NS vs. MS, P = 0.0053* ROC analysis: sIL-2R index: NS vs. MS, AUC: 0.724; NS vs. NINDs, AUC: 0.75 | ||||||||||||
| Fujisawa et al. | 2022 | Japan | 11 | 68 [55.5-74.5] | NA | NA | NA | 1303.28 U/ml (772.55) | 365.4 U/ml (396.51) | NA | NA |
Serum: sIL-2R elevation in 64% (7/11) of NS patients CSF: sIL-2R elevation in 45% (5/11) of NS patients |
| Vlad et al. | 2023 | Switzerland | 27 | 43.5 [40-51.5] | 138 | 32 [28-38] | ELISA | 489.4 pg/ml (303.3) | 183.4 pg/ml (233.9) | 253 pg/ml (83.2) | 11.3 pg/ml (12.8) | Age/Gender: NS vs. MS, P < 0.05 Serum/ CSF: sIL-2R elevation in 43% (9/21) of NS patients Serum: NS vs. MS, P = 0.0016 CSF: NS vs. MS, P = 0.0022 |
N= Number of patients, SD= Standard Deviation, IQR= Interquartile Range, SE= Standard Error, ROC= Receiver Operating Curve, AUC= Area Under Curve, CSF= Cerebrospinal fluid, ELISA= Enzyme-Linked Immunosorbent Assay, NA= Not Applicable, MRI= Magnetic Resonance Imaging, NS= Neurosarcoidosis, NT= Neurotuberculosis, BM= Bacterial Meningitis, VM= Viral Meningitis, VS= Vasculitis, MS= Multiple Sclerosis, HC= Healthy Controls, SCS= Sarcoidosis, NINDS= Non-Inflammatory Neurological Disease, Guillain-Barré syndrome, CL= Cerebral Lymphoma, *Bonferroni correction to a significance level of 0.0016
Overall, the NOS bias assessment showed that all the studies evidenced a fair quality. On average, a value of 4.5 (SD: 0.84) stars was observed (Supplemental Table S1).
Serum sIL-2R
Five of the 6 studies reported on the likelihood of underlying neurosarcoidosis using the Zajicek criteria.8,20–24 In all of these studies, the majority of patients were classified as having definite or probable neurosarcoidosis. In 82% of cases, neurological deficits were observed as the initial manifestation of sarcoidosis, whereas only 18% presented with uveitis. 3 Highest median serum sIL-2R levels were observed in patients suffering neurological affection such as hearing loss, facial nerve palsy, or epilepsy compared to lung, skin, and ocular affection (16851pg/mL, interquartile range, (IQR): 5027-11020 vs 7300pg/mL, IQR: 4793-11175; 6050pg/mL, IQR: 4975-8750; 6150pg/mL, IQR: 4866-7900, respectively). 24 However, no difference between neurosarcoidosis sIL-2R levels and sarcoidosis other than neurosarcoidosis sIL-2R levels were observed (P > 0.05). 24 There was no relevant increase in sIL-2R levels in some neurosarcoidosis patients.3,21 1 study did not observe differences in serum sIL-2R levels in neurosarcoidosis patients compared to neurotuberculosis, bacterial meningitis diagnosed on average on day 2.5, viral meningitis, vasculitis, and multiple sclerosis as well as healthy controls (P > 0.05). 22 In contrast, another study detected significant differences between neurosarcoidosis and multiple sclerosis in serum sIL-2R levels (253pg/mL, SD: 83.2 vs 11.3pg/mL, SD: 12.8, P = 0.0016). 20 No differences in sIL-2R levels between neurosarcoidosis and noninflammatory neurological diseases (NINDS) such as headache, cranial nerve disorders, encephalopathy, spinal stenosis, polyneuropathy, psychiatric disorders, dementia, encephalopathy, movement disorders, seizures, sleep disorders, motor neuron disease, ataxia, transient global amnesia, cerebrovascular disease and adenocarcinoma of the lung with brain metastasis was observed (466U/ml, IQR: 201-1034 vs 402U/ml, SD: 155-4334, P = 0.076). 21 The sIL-2R levels found in the serum of healthy patients were comparable to those of patients with existing multiple sclerosis (MS). 22
CSF sIL-2R
Five of the 6 studies examined the levels of sIL-2R in CSF. Higher CSF sIL-2R levels in neurosarcoidosis patients compared to healthy controls were observed (2871pg/mL, Standard Error (SE): 1077.5 vs 34pg/mL, SE: 0.0, P < 0.001). 22 Additionally, significantly higher CSF sIL-2R concentrations were observed in neurosarcoidosis patients compared to patients suffering vasculitis or multiple sclerosis.20–22 Interestingly, 66.67% of the multiple sclerosis patients had sIL-2R levels below the detection limit of 34 ppm/ml. 3 CSF sIL-2R did not differ significantly between neurosarcoidosis and infectious meningitis whereas significantly higher sIL-2R levels were detected in neurotuberculosis patients (2871pg/mL, SE: 1077.5 vs 10343pg/mL, SE: 4593.5, P = 0.002). 22 Interestingly, 1 study reported that in more than 50% of samples from patients with neurosarcoidosis CSF, sIL-2R levels were elevated. 21
sIL-2 concentrations in CSF vs blood
Five studies investigated the sIL-2R concentration of neurosarcoidosis patients in serum and CSF, all of which reported higher serum sIL-2R levels.3,20–23 2 studies revealed higher sIL-2R concentrations in the condition of neurotuberculosis in both CSF and serum compared to patients suffering neurosarcoidosis.21,22 2 studies were conducted to examine patients with neurotuberculosis. One study observed higher sIL-2R levels in serum compared to CSF, while contrast results were found in another study.21,22 In patients with acute bacterial meningitis and in patients with bacterial meningitis in whom the sample was taken on a median of day 2.5, higher sIL-2R values were found in the serum compared to the CSF. Similar results were seen in patients suffering from vasculitis, Guillain-Barré syndrome, cerebral lymphoma, or NINDS.21,22
sIL-2R index
In neurological diseases like MS neurotuberculosis, and sarcoidosis, increased sIL-2R levels are found in the CSF, unlike in healthy individuals which is based on the integrity of the BBB.21,22 The blood and the CSF compartments are separated by a barrier that can be regarded as a semi-permeable membrane. 54 This barrier ensures selective permeability of only certain substances such as small lipophilic molecules, ions, or glucose. 54 In physiological conditions, sIL-2R is present in the CSF in only small quantities. Consequently, elevated levels indicate a disruption of the BBB, which can be a consequence of inflammatory processes within the brain. It has been demonstrated that an increase in T-cell activation and immune response can result in an enhanced permeability of the BBB, thereby facilitating the penetration of substances such as cytokines into the brain and spinal cord.55,56 Therefore, the use of the IL-2R index, which represents a ratio between sIL-2R levels in CSF and serum, could provide additional insight into the underlying disease.21,22 The index is calculated according to the following formula: (CSFsIL-2R/SerumsIL-2R)/(CSFalbumin/Serumalbumin).21,22
Different sIL-2R indices between neurosarcoidosis patients and multiple sclerosis, cerebral vasculitis, and healthy controls were observed (P = 0.005; P < 0.001; P = 0.002, respectively). 22 In addition, the sIL-2R index differentiated between neurosarcoidosis and bacterial meningitis diagnosed on day 2.5 in comparison to the absolute serum/CSF sIL-2R values (16.8, SD: 3.14 vs 3.4, SD: 1.02, P < 0.001). 22
Interestingly, the sIL-2R index was higher in active neurosarcoidosis than in patients with clinical remission (32.45, IQR: 15.33-40.94 vs 7.18, IQR: 2.89-17.01, P = 0.0016). Furthermore, sIL-2R indices were more elevated in patients with neurosarcoidosis with diffuse leptomeningeal enhancement than in patients without diffuse leptomeningeal enhancement (37.56, IQR: 30.67-42.04 vs 8.30, IQR: 2.70-15.83, P = 0.022). 21
Discriminative value of sIL-2 R for diagnosis of neurosarcoidosis
Two of the studies reviewed included information on the ability of sIL-2R levels to differentiate between neurosarcoidosis and other neurological conditions (MS, bacterial meningitis, viral meningitis, neurotuberculosis, vasculitis).20,22 1 study revealed a sensitivity of 61% and a specificity of 93% in detecting neurosarcoidosis when applying a cut-off of 150pg/mL for CSF compared to patients suffering chronic inflammatory disorders other than neurosarcoidosis (vasculitis, multiple sclerosis) or healthy controls (overall accuracy = 0.83). 22 Comparable results were obtained in another study, which showed a sensitivity of 40.0 % (95% CI, 20.8–59.2%) and a specificity of 95.0% (95% CI, 85.4–100.0%) in the serum, while the sensitivity of discrimination by using the CSF was 38.1 % (95% CI, 17.3–58.9%) and the specificity 100%. 20 A calculated area under curve of 0.724 by using the sIL-2R index for differentiation neurosarcoidosis and multiple sclerosis was observed. 21 However, sIL-2R levels in neurosarcoidosis and infectious meningitis did not reveal a good discriminative power (sensitivity 61%, specificity 13%, overall accuracy = 0.3). 22
Discussion
Sarcoidosis is a systemic inflammatory disease that affects the lungs in ≈95% of cases.1,2 Central nervous system involvement has been shown to occur in up to 13% of patients, although it has also been noted that 25% of patients with sarcoidosis were not diagnosed with central involvement until autopsy.15,57,58 It is important to diagnose neurosarcoidosis quickly, as appropriate treatment and patient outcome depends on it. 59 Biomarkers such as ACE, neopterin, lysozyme, chitinase-like protein YKL40, sCD163, C-C motive chemokine ligand 18 (CCL18), serum amyloid A and chitotriosidase did not show an additional benefit in the diagnosis of sarcoidosis. However, several recently published studies suggested a benefit of sIL-2R in the diagnosis of sarcoidosis; therefore, we systematically summarized the current literature on sIL-2R in neurosarcoidosis.31,60–64
We identified 6 studies investigating sIL-2R in patients suffering neurosarcoidosis showing elevated sIL-2R levels in neurosarcoidosis patients as well as in several other neuroinflammatory diseases (multiple sclerosis, neurotuberculosis, bacterial meningitis, viral meningitis, Guillain-Barré-Syndrome, cerebral lymphoma) but also in patients suffering noninflammatory neurological deficits (headache, cranial nerve disorders, encephalopathy, spinal stenosis, polyneuropathy, psychiatric disorders etc.).3,20–24 As part of immune activation, T-lymphocytes cleave sIL-2R from membrane-bound IL-2R, thus elevated sIL-2R levels in serum can be detected in various diseases.33–35,65 For instance, diseases such as malignancies, transplant rejection, and autoimmune diseases can show elevated sIL-2R levels, indicating that sIL-2R does not appear to be specific enough for the diagnosis of neurosarcoidosis.66–68 However, the combined use of a comprehensive medical history, in combination with sIL-2R could be useful in the discrimination of certain differential diagnoses. For example, in all of our included studies patients with neurosarcoidosis showed higher CSF sIL-2R values than multiple sclerosis patients.20–22 Furthermore, the highest sIL-2R measurements were found in sarcoidosis patients with neurological involvement (hearing loss, facial nerve paralysis, epilepsy, etc.), which could represent an important additional benefit for the diagnostics. 24 Nevertheless, some neurosarcoidosis patients did not show elevated sIL-2R levels. 3 Notably, it has been shown not only in some patients with neurosarcoidosis but also in isolated cardiac sarcoidosis, patients can have normal sIL-2R levels.3,69 Possible explanations are, firstly, that the specific role of sIL-2R in the immune response has not yet been fully described, so there may be differences in the release of sIL-2R in different organ manifestations. Furthermore, it has been described that certain underlying genetic variations in human leukocyte antigen alleles may influence sIL-2R levels.24,70,71 Secondly, the sIL-2R level is dependent on renal elimination, so increased levels could be conceivable in the context of impaired elimination in the presence of renal insufficiency. 72 A significant difference between sIL-2R and an estimated glomerular filtration rate (eGFR) of <60 compared to a high eGFR of >90 mL/min−1 per 1.73 m2 was detected. 73 Therefore, the assessment of renal function is essential. However, none of the included studies reported on kidney function, which could have led to a distortion of sIL-2R values. Irrespectively, several studies investigated CSF sIL-2R. Interestingly, CSF sIL-2R concentrations of neurosarcoidosis patients were significantly lower than in serum, indicating that a possible influence of renal function as well as a crossing of sIL-2R from the blood into the CSF due to a disturbed blood-brain barrier seemed unlikely.20–22 To further address this hypothesis, 2 of the included studies investigated the sIL-2R index of neurosarcoidosis patients as well as non-neurosarcoidosis patients. In both studies, suspicion arose that CSF sIL-2R in neurosarcoidosis is mainly formed in the brain. Therefore, 1 hypothesis might be that sIL-2R levels in CSF may be more reliable than sIL-2R levels in serum, as CSF sIL-2R is mostly eliminated by the arachnoid cells and therefore kidney-independent.74,75 However, it must be considered that arachnoid cell dysfunction may be present in neurosarcoidosis patients. Further prospective studies may help address these questions. Interestingly, high sIL-2R concentrations in CSF and a low sIL-2R index have been observed in acute bacterial meningitis, as well as those diagnosed on day 2.5, suggesting that they originate from the serum and pass the severely impaired blood-brain barrier.21,22 The sIL-2R index for distinguishing between neurosarcoidosis and inflammatory/non-inflammatory neurological diseases demonstrated an association indicating potential utility in cases of neurotuberculosis and infectious meningitis in the studies we included. However, due to the limited number of included patients, the interpretation of the potential utility of the sIL-2R index should be approached with caution. One potential explanation for the observed differences is that the blood-brain barrier is less damaged in patients with mild neurosarcoidosis than in patients with a more severe course of the disease. However, this hypothesis requires further investigation in future studies. 22
However, another approach for an additional potential benefit of sIL-2R for the differentiation of neurosarcoidosis and other diseases could be the repeated measurement of sIL-2R levels, since sIL-2R is increasingly released as the inflammatory process progresses.28,76 Therefore, dynamic changes in sIL-2R levels could provide an additional benefit. In addition, a possible influence on kidney function could be minimized, since not the total amount of sIL-2R concentration is solely taken into account for interpretation, but its development over time.28,77–79
All in all, the findings of this systematic review should be interpreted with caution for several reasons. Firstly, all the included studies were retrospective, and thus biases such as selection bias or information bias are likely. Secondly, none of the included studies reported on the time of the blood draw which might had a great impact on measured sIL-2R levels. Thirdly, there is only limited data on neurosarcoidosis which is reflected by the limited number of studies included in this systematic review. Given the paucity of available literature, we have included a study that did not report a measurement method for determining sIL-2R which should be considered when interpreting the results of this review. Fourthly, not only renal function might had an influence on sIL-2R concentrations but also gender and age which were not accounted for. 80 To the best of the author’s knowledge, this is the first analysis summarizing the existing literature on sIL-2R levels in patients suffering neurosarcoidosis. Our findings indicate that sIL-2R concentrations in CSF may be useful in differentiating between Neurosarcoidosis and multiple sclerosis, though this distinction may not be as clinically significant given the ability to clinically differentiate between these conditions in most cases. Nevertheless, the insights gained could potentially inform further hypotheses and subsequent investigations aimed at elucidating the underlying immunopathological mechanisms. Especially, 1 study showed that implementing clinical features in combination with the consideration of sIL-2R levels and the underlying disease could increase the accuracy of the correct diagnosis. 21 Therefore, the construction of a panel composed of clinical characteristics could be an approach for future studies.
Conclusion
In summary, sIL-2R is present in diseases that are associated with immune activation, such as multiple sclerosis, meningitis, or autoimmune diseases, and therefore a reliable differentiation from neurosarcoidosis by using total serum sIL-2R concentration is not possible. However, CSF sIL-2R levels may be useful in differentiating neurosarcoidosis from multiple sclerosis. Further prospective studies should be conducted to investigate the kinetics of sIL-2R, the influence of renal function on sIL-2R levels, as well as the production/elimination of sIL-2R in CSF compared to serum to test the potential utility of sIL-2R in the diagnosis of neurosarcoidosis.
Supplemental Material
Supplemental Material for Diagnostic value of soluble Interleukin-2 receptor in patients suffering neurosarcoidosis: A systematic review by Aditya Chanpura, Rajesh K. Gupta, Shitiz K. Sriwastava, Jan Rahmig in Journal of Central Nervous System Disease
Abbreviations
- ACE
Angiotensin-Converting Enzyme
- BBB
Blood-brain barrier
- CI
Confidence Interval
- CCL18
C-C Motive Chemokine Ligand 18
- CSF
Cerebrospinal Fluid
- eGFR
Estimated Glomerular Filtration Rate
- ELISA
Enzyme-linked Immunosorbent Assay
- IQR
Interquartile Range
- NINDS
Noninflammatory Neurological Diseases
- NOS
Newcastle-Ottawa Scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized Controlled Trials
- SD
Standard Deviation
- SE
Standard Error
- sIL-2R
Soluble Interleukin-2 Receptor
Acknowledgements
We acknowledge support by the German Research Foundation, the Medical Faculty Carl Gustav Carus, the SLUB as well as the Open Access Publication Funds of the TU Dresden. The funding bodies had no impact on any aspect of the study and the content of the manuscript.
Author contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Aditya Chanpura: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing - review & editing, Rajesh Kumar Gupta: Conceptualization, Methodology, Validation, Writing - review & editing, Shitiz Kumar Sriwastava: Conceptualization, Methodology, Validation, Writing - review & editing, Jan Rahmig: Conceptualization, Data curation, Formal analysis, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing - original draft.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Sächsische Landesbibliothek - Staats-und Universitätsbibliothek Dresden (VAT-ID: DE 190021717).
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Jan Rahmig https://orcid.org/0000-0002-7061-8995
References
- 1.sti . Die Sarkoidose ist ein Chamäleon. MMW - Fortschritte Med. 2014;156:22. [PubMed] [Google Scholar]
- 2.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155-1167. [DOI] [PubMed] [Google Scholar]
- 3.Fujisawa M, Koga M, Sato R, Oishi M, Takeshita Y, Kanda T. Spinal cord sarcoidosis in Japan: utility of cerebrospinal fluid examination and nerve conduction study for diagnosis and prognosis prediction. J Neurol. 2022;269:4783-4790. [DOI] [PubMed] [Google Scholar]
- 4.Tetikkurt C. Neurosarcoidosis. J Neurol Neuromed. 2018;3:108-112. [Google Scholar]
- 5.Joubert B, Chapelon-Abric C, Biard L, et al. Association of prognostic factors and immunosuppressive treatment with long-term outcomes in neurosarcoidosis. JAMA Neurol. 2017;74:1336-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tana C, Wegener S, Borys E, et al. Challenges in the diagnosis and treatment of neurosarcoidosis. Ann Med. 2015;47:576-591. [DOI] [PubMed] [Google Scholar]
- 7.Lexa FJ, Grossman RI. MR of sarcoidosis in the head and spine: spectrum of manifestations and radiographic response to steroid therapy. AJNR Am J Neuroradiol. 1994;15:973-982. [PMC free article] [PubMed] [Google Scholar]
- 8.Zajicek JP. Neurosarcoidosis. Curr Opin Neurol. 2000;13:323-325. https://journals.lww.com/co-neurology/fulltext/2000/06000/neurosarcoidosis.16.aspx [DOI] [PubMed] [Google Scholar]
- 9.Berntsson SG, Elmgren A, Gudjonsson O, Grabowska A, Landtblom AM, Moraes-Fontes MF. A comprehensive diagnostic approach in suspected neurosarcoidosis. Sci Rep. 2023;13:6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubart FC, Chazal T, Lhote R, et al. Chapter 10 - neurosarcoidosis. In: Baughman RP, Valeyre D, eds. Sarcoidosis. Philadelphia: Elsevier; 2019:115-125. [Google Scholar]
- 11.Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58:2004079. [DOI] [PubMed] [Google Scholar]
- 12.Judson MA. The treatment of pulmonary sarcoidosis. Respir Med. 2012;106:1351-1361. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw MJ, Pawate S, Koth LL, Cho TA, Gelfand JM. Neurosarcoidosis: pathophysiology, diagnosis, and treatment. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt KA, Sandoval KE. Steroids and the blood-brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361-390. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki K, Judson MA. Neurosarcoidosis: clinical manifestations, diagnosis and treatment. Presse Med. 2012;41:e331-348. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Frohman EM. Spinal neurosarcoidosis mimicking an idiopathic inflammatory demyelinating syndrome. Arch Neurol. 2004;61:586-589. [DOI] [PubMed] [Google Scholar]
- 17.Feizi P, Tandon M, Khan E, et al. Overcoming the elusiveness of neurosarcoidosis: learning from five complex cases. Neurol Int. 2021;13:130-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanoff RB, Ruberg RL. Intramedullary sarcoidosis of the spinal cord: report of a case. J Am Osteopath Assoc. 1978;77:868-875. [PubMed] [Google Scholar]
- 19.Stern BJ, Royal W, Gelfand JM, et al. Definition and consensus diagnostic criteria for neurosarcoidosis: from the neurosarcoidosis consortium consensus group. JAMA Neurol. 2018;75:1546-1553. [DOI] [PubMed] [Google Scholar]
- 20.Vlad B, Neidhart S, Hilty M, Ziegler M, Jelcic I. Differentiating neurosarcoidosis from multiple sclerosis using combined analysis of basic CSF parameters and MRZ reaction. Front Neurol. 2023;14:1135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto C, Wengert O, Unterwalder N, Meisel C, Ruprecht K. Analysis of soluble interleukin-2 receptor as CSF biomarker for neurosarcoidosis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petereit H-F, Reske D, Tumani H, et al. Soluble CSF interleukin 2 receptor as indicator of neurosarcoidosis. J Neurol. 2010;257:1855-1863. [DOI] [PubMed] [Google Scholar]
- 23.Sumi K, Masuda T, Kimura N, Akiyoshi Y, Obayashi K, Matsubara E. Cerebrospinal fluid B-cell activating factor levels as a novel biomarker in patients with neurosarcoidosis. J Neurol Sci. 2023;449:120668. doi: 10.1016/j.jns.2023.120668. [DOI] [PubMed] [Google Scholar]
- 24.Eurelings LEM, Miedema JR, Dalm VASH, et al. Sensitivity and specificity of serum soluble interleukin-2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS One. 2019;14:e0223897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petereit HF, Reske D, Tumani H, et al. Soluble CSF interleukin 2 receptor as indicator of neurosarcoidosis. J Neurol. 2010;257:1855-1863. [DOI] [PubMed] [Google Scholar]
- 26.Ungprasert P, Carmona EM, Crowson CS, Matteson EL. Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: a population-based study. Lung. 2016;194:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasowski MD, Savage J, Ehlers A, et al. Ordering of the serum angiotensin-converting enzyme test in patients receiving angiotensin-converting enzyme inhibitor therapy: an avoidable but common error. Chest. 2015;148:1447-1453. [DOI] [PubMed] [Google Scholar]
- 28.Vorselaars ADM, van Moorsel CHM, Zanen P, et al. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. 2015;109:279-285. [DOI] [PubMed] [Google Scholar]
- 29.Tomita H, Sato S, Matsuda R, et al. Serum lysozyme levels and clinical features of sarcoidosis. Lung. 1999;177:161-167. [DOI] [PubMed] [Google Scholar]
- 30.Bergantini L, Bianchi F, Cameli P, et al. Prognostic biomarkers of sarcoidosis: a comparative study of serum chitotriosidase, ACE, lysozyme, and KL-6. Dis Markers. 2019;2019:8565423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann G, Rieder J, Smolny M, et al. Neopterin-induced expression of intercellular adhesion molecule-1 (ICAM-1) in type II-like alveolar epithelial cells. Clin Exp Immunol. 1999;118:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunninghake GW, Bedell GN, Zavala DC, Monick M, Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983;128:634-638. [DOI] [PubMed] [Google Scholar]
- 33.Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18:648-659. [DOI] [PubMed] [Google Scholar]
- 34.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180-190. [DOI] [PubMed] [Google Scholar]
- 36.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924-1927. [DOI] [PubMed] [Google Scholar]
- 37.von Bergwelt-Baildon MS, Popov A, Saric T, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228-237. [DOI] [PubMed] [Google Scholar]
- 38.Leonard WJ, Lin JX. Cytokine receptor signaling pathways. J Allergy Clin Immunol. 2000;105:877-888. [DOI] [PubMed] [Google Scholar]
- 39.Rubin LA, Kurman CC, Fritz ME, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172-3177. [PubMed] [Google Scholar]
- 40.Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008;13:1-26. [DOI] [PubMed] [Google Scholar]
- 41.Kirschke S, Ogunsulire I, Selvakumar B, et al. The metalloprotease ADAM10 generates soluble interleukin-2 receptor alpha (sCD25) in vivo. J Biol Chem. 2022;298:101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediat Inflamm. 2005;2005:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson DL, Rubin LA, Kurman CC, Fritz ME, Boutin B. An analysis of the cellular requirements for the production of soluble interleukin-2 receptors in vitro. J Clin Immunol. 1986;6:114-120. [DOI] [PubMed] [Google Scholar]
- 44.Chopra A, Kalkanis A, Judson MA. Biomarkers in sarcoidosis. Expet Rev Clin Immunol. 2016;12:1191-1208. [DOI] [PubMed] [Google Scholar]
- 45.Murakami K, Koh J, Taruya J, Ito H. Neurosarcoidosis mimicking the recurrence of malignant lymphoma. Case Rep Neurol. 2021;13:605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S, Parmar M, Padappayil RP, Bansal A, Daouk S. Role of serum soluble interleukin-2 receptor level in the diagnosis of sarcoidosis: a systematic review and meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis. 2023;40:e2023005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011. https://handbook-5-1.cochrane.org/chapter_6/6_4_7_boolean_operators_and_or_and_not.htm (accessed 17 August 2023). [Google Scholar]
- 49.Ivey C, Crum J. Choosing the right citation management tool: endnote, mendeley, refworks, or zotero. J Med Libr Assoc. 2018;106:399-403. [Google Scholar]
- 50.Wells G, Shea B, O’Connell D, et al. The newcastle–ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 21 April 2024). [Google Scholar]
- 51.Desyibelew HD, Dadi AF. Burden and determinants of malnutrition among pregnant women in Africa: a systematic review and meta-analysis. PLoS One. 2019;14:e0221712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fekadu Dadi A, Miller ER, Mwanri L. Antenatal depression and its association with adverse birth outcomes in low and middle-income countries: a systematic review and meta-analysis. PLoS One. 2020;15:e0227323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mengist B, Desta M, Tura AK, Habtewold TD, Abajobir A. Maternal near miss in Ethiopia: protective role of antenatal care and disparity in socioeconomic inequities: a systematic review and meta-analysis. Int J Afr Nurs Sci. 2021;15:100332. [Google Scholar]
- 54.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harbor Perspect Biol. 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelhardt B. T cell migration into the central nervous system during health and disease: different molecular keys allow access to different central nervous system compartments. Clin Exp Neuroimmunol. 2010;1:79-93. [Google Scholar]
- 56.Angelini G, Bani A, Constantin G, Rossi B. The interplay between T helper cells and brain barriers in the pathogenesis of multiple sclerosis. Front Cell Neurosci. 2023;17:1101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol. 2004;182:289-295. [DOI] [PubMed] [Google Scholar]
- 58.Allen RKA, Sellars RE, Sandstrom PA. A prospective study of 32 patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:118-125. [PubMed] [Google Scholar]
- 59.Vorselaars ADM, Culver DA. Hit-hard and early versus step-up treatment in severe sarcoidosis. Curr Opin Pulm Med. 2022;28:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Mark SC, Bajnath VWS, Veltkamp M. Biomarkers in sarcoidosis: beginning of a new era? Clin Chest Med. XXX;45:33-43. [DOI] [PubMed] [Google Scholar]
- 61.Schimmelpennink MC, Quanjel M, Vorselaars A, et al. Value of serum soluble interleukin-2 receptor as a diagnostic and predictive biomarker in sarcoidosis. Expet Rev Respir Med. 2020;14:749-756. [DOI] [PubMed] [Google Scholar]
- 62.Bennett D, Cameli P, Lanzarone N, et al. Chitotriosidase: a biomarker of activity and severity in patients with sarcoidosis. Respir Res. 2020;21:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uysal P, Durmus S, Sozer V, et al. YKL-40, soluble IL-2 receptor, angiotensin converting enzyme and C-reactive protein: comparison of markers of sarcoidosis activity. Biomolecules. 2018;8. doi: 10.3390/biom8030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraaijvanger R, Janssen Bonás M, Vorselaars AD, Veltkamp M. Biomarkers in the diagnosis and prognosis of sarcoidosis: current use and future prospects. Front Immunol. 2020;11:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dik WA, Heron M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J Med. 2020;78:220-231. [PubMed] [Google Scholar]
- 66.Ali H, Mohiuddin A, Sharma A, et al. Implication of interleukin-2 receptor antibody induction therapy in standard risk renal transplant in the tacrolimus era: a meta-analysis. Clin Kidney J. 2019;12:592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKeage K, McCormack PL. Basiliximab: a review of its use as induction therapy in renal transplantation. BioDrugs. 2010;24:55-76. [DOI] [PubMed] [Google Scholar]
- 68.Shaik I, Carmody IC, Chen PW. Chapter 93 - treatment of acute and chronic rejection. In: Busuttil RW, Klintmalm GBG, eds. Transplantation of the Liver. 3rd ed.. Philadelphia: W.B. Saunders; 2015:1317-1328. [Google Scholar]
- 69.Kiko T, Yoshihisa A, Kanno Y, et al. A multiple biomarker approach in patients with cardiac sarcoidosis. Int Heart J. 2018;59:996-1001. [DOI] [PubMed] [Google Scholar]
- 70.Sato H, Woodhead FA, Ahmad T, et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet. 2010;19:4100-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keindl M, Fedotkina O, du Plessis E, et al. Increased plasma soluble interleukin-2 receptor alpha levels in patients with long-term type 1 diabetes with vascular complications associated with IL2RA and PTPN2 gene polymorphisms. Front Endocrinol. 2020;11:575469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Junghans RP, Waldmann TA. Metabolism of Tac (IL2Ralpha): physiology of cell surface shedding and renal catabolism, and suppression of catabolism by antibody binding. J Exp Med. 1996;183:1587-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verwoerd A, Vorselaars ADM, van Moorsel CHM, Bos WJW, van Velzen-Blad H, Grutters JC. Discrepant elevation of sIL-2R levels in sarcoidosis patients with renal insufficiency. Eur Respir J. 2015;46:277-280. [DOI] [PubMed] [Google Scholar]
- 74.Kaur J, Fahmy LM, Davoodi-Bojd E, et al. Waste clearance in the brain. Front Neuroanat. 2021;15:665803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hladky SB, Barrand MA. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood–brain barrier. Fluids Barriers CNS. 2018;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi Y, Sato T, Nagai T, et al. Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Fail. 2021;8:5282-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Yu F, Feng X, et al. Immunity and inflammation predictors for short-term outcome of stroke in young adults. Int J Neurosci. 2018;128:634-639. [DOI] [PubMed] [Google Scholar]
- 78.Grutters JC. Establishing a diagnosis of pulmonary sarcoidosis. J Clin Med. 2023;12:6898. doi: 10.3390/jcm12216898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogata-Suetsugu S, Hamada N, Takayama K, Tsubouchi K, Arimura-Omori M, Nakanishi Y. The clinical value of serum soluble interleukin-2 receptor in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alende-Castro V, Alonso-Sampedro M, Fernández-Merino C, et al. Factors influencing serum concentrations of soluble interleukin-2 receptor: a general adult population study. Life. 2023;16:2169958. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Diagnostic value of soluble Interleukin-2 receptor in patients suffering neurosarcoidosis: A systematic review by Aditya Chanpura, Rajesh K. Gupta, Shitiz K. Sriwastava, Jan Rahmig in Journal of Central Nervous System Disease