Abstract
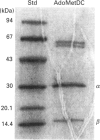
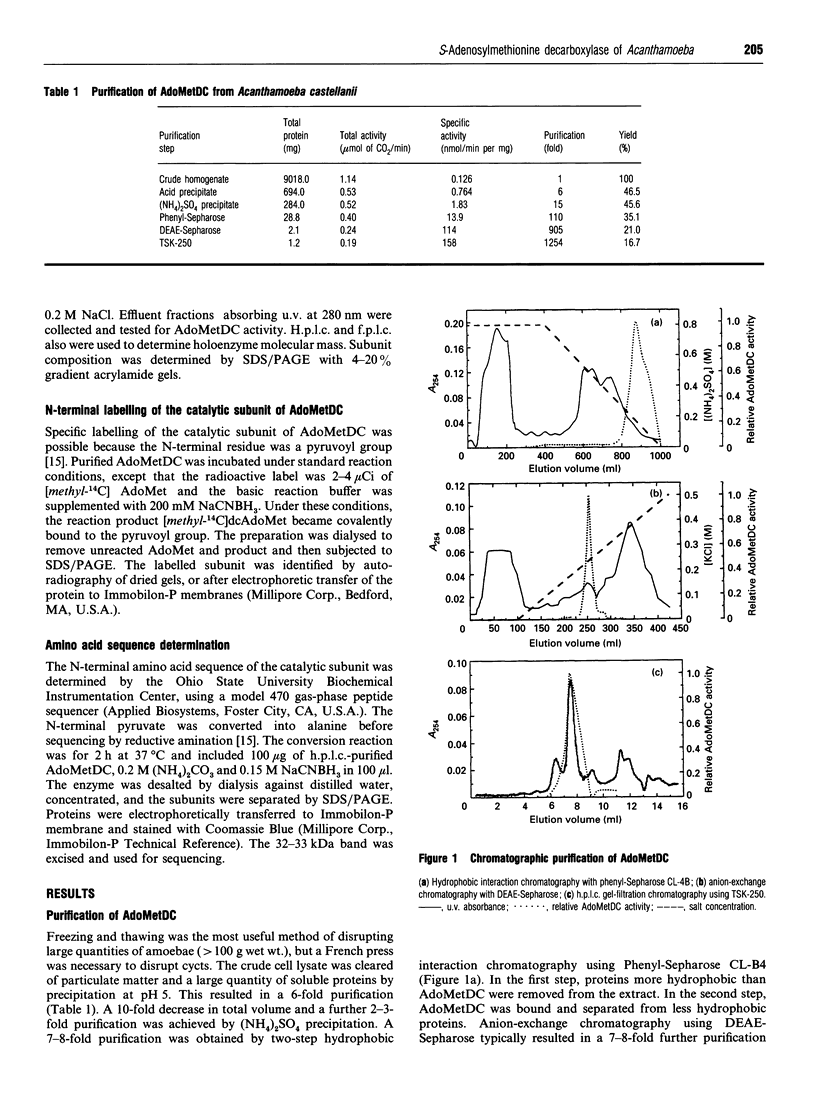
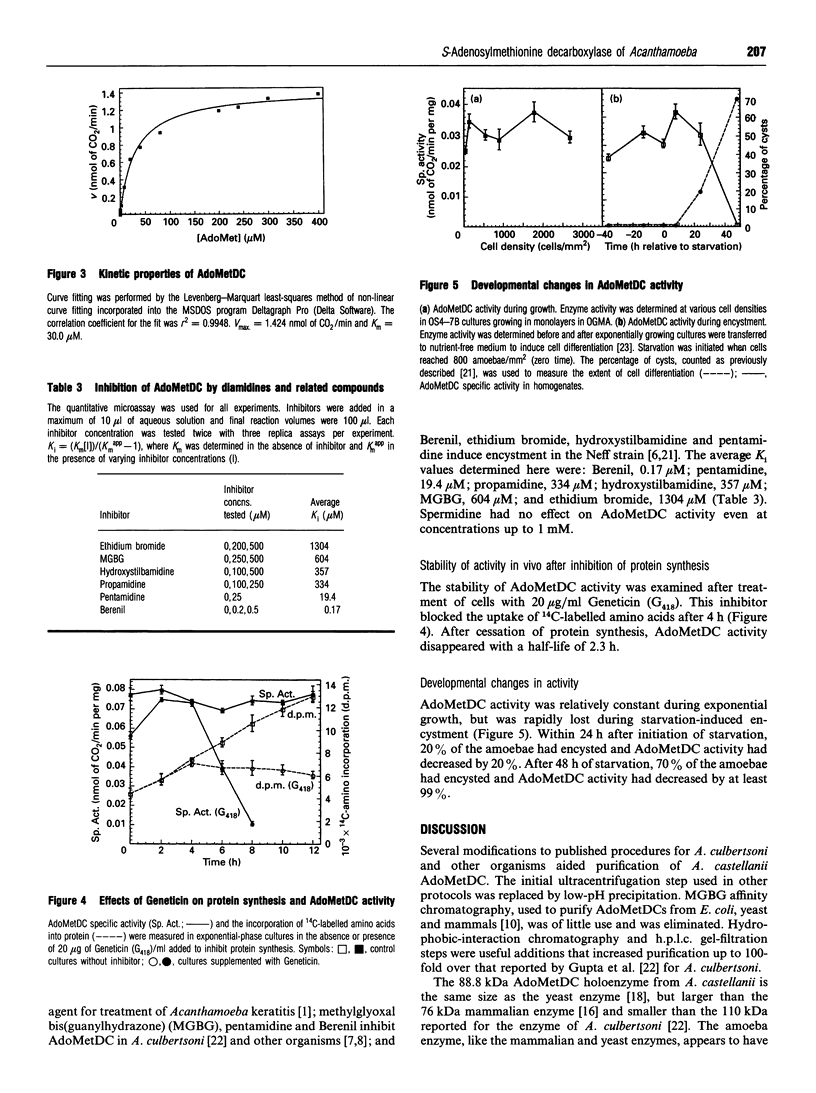
S-Adenosyl-L-methionine decarboxylase (AdoMetDC) has been purified to near homogeneity from the Neff strain of Acanthamoeba castellanii. The holoenzyme molecular mass is 88.8 kDa, including two copies each of a 32.8 kDa alpha-subunit and a 10-15 kDa beta-subunit. The alpha-subunit contains the active site. It has an N-terminal pyruvoyl group, and the first 19 amino acids are 63 and 74% identical with comparable sequences from yeast and mammals, respectively. The apparent Km for S-adenosylmethionine (AdoMet) in the presence of 2 mM putrescine was 30.0 microM. The enzyme was stimulated 2-fold by putrescine, but was unaffected by spermidine. It was inhibited by the following anti-metabolites, listed with their Ki values: Berenil (0.17 microM), pentamidine (19.4 microM), propamidine (334 microM), hydroxystilbamidine (357 microM), methylglyoxal bis(guanylhydrazone) (604 microM) and ethidium bromide (1.3 mM). Activity of the enzyme fell to undetectable levels during cell differentiation (encystment).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Byers T. J. Differentiation promoting factors induced in Acanthamoeba by inhibitors of mitochondrial macromolecule synthesis. Dev Biol. 1980 Jul;78(1):126–140. doi: 10.1016/0012-1606(80)90323-1. [DOI] [PubMed] [Google Scholar]
- Anton D. L., Kutny R. Escherichia coli S-adenosylmethionine decarboxylase. Subunit structure, reductive amination, and NH2-terminal sequences. J Biol Chem. 1987 Feb 25;262(6):2817–2822. [PubMed] [Google Scholar]
- Bacchi C. J. Content, synthesis, and function of polyamines in trypanosomatids: relationship to chemotherapy. J Protozool. 1981 Feb;28(1):20–27. doi: 10.1111/j.1550-7408.1981.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., McCann P. P. Characterization of Trypanosoma brucei brucei S-adenosyl-L-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone). Biochem J. 1986 Aug 1;237(3):685–689. doi: 10.1042/bj2370685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Akins R. A., Maynard B. J., Lefken R. A., Martin S. M. Rapid growth of Acanthamoeba in defined media; induction of encystment by glucose-acetate starvation. J Protozool. 1980 May;27(2):216–219. doi: 10.1111/j.1550-7408.1980.tb04684.x. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Hugo E. R., Stewart V. J. Genes of Acanthamoeba: DNA, RNA and protein sequences (a review). J Protozool. 1990 Jul-Aug;37(4):17S–25S. doi: 10.1111/j.1550-7408.1990.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Kim B. G., King L. E., Hugo E. R. Molecular aspects of the cell cycle and encystment of Acanthamoeba. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 5):S373–S384. doi: 10.1093/clind/13.supplement_5.s373. [DOI] [PubMed] [Google Scholar]
- Byers T. L., Bush T. L., McCann P. P., Bitonti A. J. Antitrypanosomal effects of polyamine biosynthesis inhibitors correlate with increases in Trypanosoma brucei brucei S-adenosyl-L-methionine. Biochem J. 1991 Mar 1;274(Pt 2):527–533. doi: 10.1042/bj2740527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Morris D. R., Coffino P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase. Microbiol Rev. 1992 Jun;56(2):280–290. doi: 10.1128/mr.56.2.280-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Shukla O. P., Walter R. D. Putrescine-activated S-adenosylmethionine decarboxylase from Acanthamoeba culbertsoni. Mol Biochem Parasitol. 1987 Apr;23(3):247–252. doi: 10.1016/0166-6851(87)90031-4. [DOI] [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Jung G., Korn E. D. Genetic evidence that Acanthamoeba myosin I is a true myosin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4655–4659. doi: 10.1073/pnas.83.13.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Alhonen-Hongisto L., Nikula P., Elo H. S-adenosylmethionine decarboxylase as target of chemotherapy. Adv Enzyme Regul. 1985;24:125–139. doi: 10.1016/0065-2571(85)90073-1. [DOI] [PubMed] [Google Scholar]
- Karvonen E., Kauppinen L., Partanen T., Pösö H. Irreversible inhibition of putrescine-stimulated S-adenosyl-L-methionine decarboxylase by berenil and pentamidine. Biochem J. 1985 Oct 1;231(1):165–169. doi: 10.1042/bj2310165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Taneja S. K., Xie Q. W., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase from Escherichia coli and from Saccharomyces cerevisiae: cloning and overexpression of the genes. Adv Exp Med Biol. 1988;250:73–79. doi: 10.1007/978-1-4684-5637-0_7. [DOI] [PubMed] [Google Scholar]
- Kim B. G., McCann P. P., Byers T. J. Inhibition of multiplication in Acanthamoeba castellanii by specific inhibitors of ornithine decarboxylase. J Protozool. 1987 Aug;34(3):264–266. doi: 10.1111/j.1550-7408.1987.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Kim B. G., Sobota A., Bitonti A. J., McCann P. P., Byers T. J. Polyamine metabolism in Acanthamoeba: polyamine content and synthesis of ornithine, putrescine, and diaminopropane. J Protozool. 1987 Aug;34(3):278–284. doi: 10.1111/j.1550-7408.1987.tb03175.x. [DOI] [PubMed] [Google Scholar]
- Korn E. D. The isolation of the amoeba plasma membrane and the use of latex beads for the isolation of phagocytic vacuole (phagosome) membranes from amoebae including the culture techniques for amoebae. Methods Enzymol. 1974;31:686–698. doi: 10.1016/0076-6879(74)31074-9. [DOI] [PubMed] [Google Scholar]
- Ma P., Visvesvara G. S., Martinez A. J., Theodore F. H., Daggett P. M., Sawyer T. K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990 May-Jun;12(3):490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- Martin S. M., Byers T. J. Acid hydrolase activity during growth and encystment in Acanthamoeba castellanii. J Protozool. 1976 Nov;23(4):608–613. doi: 10.1111/j.1550-7408.1976.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Neff R. J., Neff R. H. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51–81. [PubMed] [Google Scholar]
- Pajunen A., Crozat A., Jänne O. A., Ihalainen R., Laitinen P. H., Stanley B., Madhubala R., Pegg A. E. Structure and regulation of mammalian S-adenosylmethionine decarboxylase. J Biol Chem. 1988 Nov 15;263(32):17040–17049. [PubMed] [Google Scholar]
- Pegg A. E. S-adenosylmethionine decarboxylase: a brief review. Cell Biochem Funct. 1984 Jan;2(1):11–15. doi: 10.1002/cbf.290020105. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Comparison of S-adenosylmethionine decarboxylases from rat liver and muscle. Biochemistry. 1982 Jun 22;21(13):3116–3122. doi: 10.1021/bi00256a013. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Torget R., I J., Freda C. E., Cohen S. S. The binding of polyamines and of ethidium bromide to tRNA. Nucleic Acids Res. 1975 Jul;2(7):1005–1022. doi: 10.1093/nar/2.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Hafner E. W. Convenient method for detecting 14CO2 in multiple samples: application to rapid screening for mutants. J Bacteriol. 1976 Oct;128(1):485–486. doi: 10.1128/jb.128.1.485-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekwani B. L., Stanley B. A., Pegg A. E. Nucleotide sequence of hamster S-adenosylmethionine decarboxylase cDNA. Biochim Biophys Acta. 1992 Mar 24;1130(2):221–223. doi: 10.1016/0167-4781(92)90533-6. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Stehr-Green J. K. Epidemiology of free-living ameba infections. J Protozool. 1990 Jul-Aug;37(4):25S–33S. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Zhu C. M., Cumaraswamy A., Henney H. R., Jr Comparison of polyamine and S-adenosylmethionine contents of growing and encysted Acanthamoeba isolates. Mol Cell Biochem. 1989 Oct 31;90(2):145–153. doi: 10.1007/BF00221214. [DOI] [PubMed] [Google Scholar]
- van Poelje P. D., Snell E. E. Pyruvoyl-dependent enzymes. Annu Rev Biochem. 1990;59:29–59. doi: 10.1146/annurev.bi.59.070190.000333. [DOI] [PubMed] [Google Scholar]