Abstract
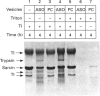
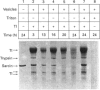
alpha-Sarcin is a cytotoxic protein produced by the mould Aspergillus giganteus. Insertion of alpha-sarcin into asolectin membranes has been demonstrated by protein labelling with photoreactive phospholipids. alpha-Sarcin added externally to tRNA-containing asolectin liposomes degrades the entrapped tRNA. Trypsin-containing asolectin liposomes were also prepared. Encapsulated trypsin degrades alpha-sarcin, even in the presence of a large excess of external hen egg-white trypsin inhibitor to prevent any alpha-sarin degradation outside the vesicles. These processes occur only with acidic phospholipids and were not observed when phosphatidylcholine vesicles were used. These results indicate that alpha-sarcin penetrates the lipid bilayer and becomes exposed to the lumen of negatively charged liposomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Carrasco L., Esteban M. Modification of membrane permeability in vaccinia virus-infected cells. Virology. 1982 Feb;117(1):62–69. doi: 10.1016/0042-6822(82)90507-4. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M. E., Richards F. M. Insertion of apocytochrome c into lipid vesicles. J Biol Chem. 1984 Apr 10;259(7):4147–4156. [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Gasset M., Martinez del Pozo A., Oñaderra M., Gavilanes J. G. Study of the interaction between the antitumour protein alpha-sarcin and phospholipid vesicles. Biochem J. 1989 Mar 1;258(2):569–575. doi: 10.1042/bj2580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Goormaghtigh E., Gavilanes J. G. Acid phospholipid vesicles produce conformational changes on the antitumour protein alpha-sarcin. Biochim Biophys Acta. 1991 Oct 11;1080(1):51–58. doi: 10.1016/0167-4838(91)90111-c. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Martínez del Pozo A., Schiavo G. P., Laynez J., Usobiaga P., Gavilanes J. G. Effect of the antitumour protein alpha-sarcin on the thermotropic behaviour of acid phospholipid vesicles. Biochim Biophys Acta. 1991 Sep 10;1068(1):9–16. doi: 10.1016/0005-2736(91)90055-d. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Thomas P. G., Gavilanes J. G. Fusion of phospholipid vesicles produced by the anti-tumour protein alpha-sarcin. Biochem J. 1990 Feb 1;265(3):815–822. doi: 10.1042/bj2650815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Municio A. M., Oñaderra M. Fluorescence studies on the lipoprotein complex of the fatty acid synthetase from the insect Ceratitis capitata. Biochemistry. 1981 Sep 29;20(20):5689–5694. doi: 10.1021/bi00523a008. [DOI] [PubMed] [Google Scholar]
- Goldin A., Serpick A. A., Mantel N. Experimental screening procedures and clinical predictability value. Cancer Chemother Rep. 1966 May;50(4):173–218. [PubMed] [Google Scholar]
- Hausman S. Z., Burns D. L. Interaction of pertussis toxin with cells and model membranes. J Biol Chem. 1992 Jul 5;267(19):13735–13739. [PubMed] [Google Scholar]
- JENNINGS J. C., OLSON B. H., ROGA V., JUNEK A. J., SCHUURMANS D. M. ALPHA SARCIN, A NEW ANTITUMOR AGENT. II. FERMENTATION AND ANTITUMOR SPECTRUM. Appl Microbiol. 1965 May;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Conformational study of the antitumor protein alpha-sarcin. Biochim Biophys Acta. 1988 Apr 14;953(3):280–288. doi: 10.1016/0167-4838(88)90036-2. [DOI] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Effect of divalent cations on structure-function relationships of the antitumor protein alpha-sarcin. Int J Pept Protein Res. 1989 Nov;34(5):416–422. doi: 10.1111/j.1399-3011.1989.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Arpin M., Hannum C., Margoliash E., Sabatini D. D., Morimoto T. In vitro synthesis and posttranslational uptake of cytochrome c into isolated mitochondria: role of a specific addressing signal in the apocytochrome. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4368–4372. doi: 10.1073/pnas.78.7.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Brunner J., Duflot E., Boquet P., Roa M. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry. 1986 Feb 25;25(4):919–924. doi: 10.1021/bi00352a027. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Castrillo J. L., Carrasco L. Modification of membrane permeability during Semliki Forest virus infection. Virology. 1985 Oct 30;146(2):203–212. doi: 10.1016/0042-6822(85)90004-2. [DOI] [PubMed] [Google Scholar]
- OLSON B. H., GOERNER G. L. ALPHA SARCIN, A NEW ANTITUMOR AGENT. I. ISOLATION, PURIFICATION, CHEMICAL COMPOSITION, AND THE IDENTITY OF A NEW AMINO ACID. Appl Microbiol. 1965 May;13:314–321. doi: 10.1128/am.13.3.314-321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Control of membrane permeability in animal cells by divalent cations. Exp Cell Res. 1987 Apr;169(2):531–542. doi: 10.1016/0014-4827(87)90213-8. [DOI] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Exogenous phospholipase C permeabilizes mammalian cells to proteins. Exp Cell Res. 1988 Jul;177(1):154–161. doi: 10.1016/0014-4827(88)90033-x. [DOI] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Proteins are cointernalized with virion particles during early infection. Virology. 1987 Sep;160(1):75–80. doi: 10.1016/0042-6822(87)90046-8. [DOI] [PubMed] [Google Scholar]
- Oñaderra M., Gasset M., Martínez del Pozo A., Gavilanes J. G. Molecular aspects of alpha-sarcin penetration in phospholipid bilayers. Biochem Soc Trans. 1989 Dec;17(6):999–1000. doi: 10.1042/bst0170999. [DOI] [PubMed] [Google Scholar]
- Papini E., Colonna R., Cusinato F., Montecucco C., Tomasi M., Rappuoli R. Lipid interaction of diphtheria toxin and mutants with altered fragment B. 1. Liposome aggregation and fusion. Eur J Biochem. 1987 Dec 15;169(3):629–635. doi: 10.1111/j.1432-1033.1987.tb13654.x. [DOI] [PubMed] [Google Scholar]
- Papini E., Schiavo G., Tomasi M., Colombatti M., Rappuoli R., Montecucco C. Lipid interaction of diphtheria toxin and mutants with altered fragment B. 2. Hydrophobic photolabelling and cell intoxication. Eur J Biochem. 1987 Dec 15;169(3):637–644. doi: 10.1111/j.1432-1033.1987.tb13655.x. [DOI] [PubMed] [Google Scholar]
- Rietveld A., Jordi W., de Kruijff B. Studies on the lipid dependency and mechanism of the translocation of the mitochondrial precursor protein apocytochrome c across model membranes. J Biol Chem. 1986 Mar 15;261(8):3846–3856. [PubMed] [Google Scholar]
- Rietveld A., de Kruijff B. Is the mitochondrial precursor protein apocytochrome c able to pass a lipid barrier? J Biol Chem. 1984 Jun 10;259(11):6704–6707. [PubMed] [Google Scholar]
- Roga V., Hedeman L. P., Olson B. H. Evaluation of mitogillin (NSC-69529) in the treatment of naturally occurring canine neoplasms. Cancer Chemother Rep. 1971 Apr;55(2):101–113. [PubMed] [Google Scholar]
- Sacco G., Drickamer K., Wool I. G. The primary structure of the cytotoxin alpha-sarcin. J Biol Chem. 1983 May 10;258(9):5811–5818. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi M., Montecucco C. Lipid insertion of cholera toxin after binding to GM1-containing liposomes. J Biol Chem. 1981 Nov 10;256(21):11177–11181. [PubMed] [Google Scholar]