Abstract
Introduction: Apical periodontitis is one of the common dental diseases. Microorganisms are the main reasons for these lesions; irrigations are used to remove them, but because of limited penetration, the rinsing agents may not always kill the microorganisms. Laser irradiation is effective in canal disinfection. The goal of this study was to compare the effect of calcium hydroxide (Ca(OH)2 ) and diode laser on the improvement of apical periodontitis following root canal retreatment (RCR).
Methods: Twenty-four teeth of 19 patients with periapical lesions which needed RCR were divided into two groups (Ca(OH)2 and Ca(OH)2+laser irradiation [LI]). In the first session, after gutta-percha removal, cleaning, and shaping, Ca(OH)2 was used for 10 days. In the second session, in the Ca(OH)2+LI group, the irradiation utilizing a diode laser (using non-initiated 200-µm fiber, continuous wave (CW), power output of 1W) was done. The periapical radiographic healing was assessed before the retreatment and after 3-month and 6-month follow-ups by periapical index (PAI) Qrstavik. The quantitative data were analyzed (P<0.05).
Results: The initial periapical lesion score was 3.75 and 3.88 in the Ca(OH)2 and Ca(OH)2+LI groups, respectively. In the Ca(OH)2 and Ca(OH)2+LI groups, 3 months after the RCR, the average periapical lesion score was 2.94 and 3.05, respectively. In the Ca(OH)2 and CA(OH)2+LI groups, 6 months after the RCR, the average periapical lesion score was 1.80 and 1.75, respectively. No significant differences were found at the 3 and 6 months between the experimental groups.
Conclusion: The diode laser can reduce the periapical lesion, but there was no significant difference between CA(OH)2+LI and Ca(OH)2 at 3-month and 6-month follow-ups.
Keywords: Diode lasers, Endodontics, Retreatment, Calcium hydroxide
Introduction
Disinfection in root canal retreatment (RCR) is much more difficult because persistent microorganisms have settled in the root canal. The resistant periradicular lesions result from resistant bacteria to irrigations and antimicrobial agents. They can survive for years around the filled root canals.1
The impossibility of proper access of immunity to the root canal can lead to the inadequate removal of the root infection.2 A successful root canal treatment (RCT) is a combination of shaping, disinfection, and filling of the canal. Cleaning and irrigation of root canals have a fundamental role in the success of the treatment.3
Sodium hypochlorite (NaOCl) is the most common irrigating solution in RCT because of its broad antibacterial effect and dissolving organic substances. Without the use of NaOCl with a high concentration, it is not possible to achieve effective cleaning of the canals.1 One of the disadvantages of NaOCl is the cytotoxicity, which causes damage and symptoms in the patient. Also, its strong oxidizing property has a negative effect on the mechanical properties of dentin, including microhardness and elastic coefficient. In general, NaOCl should be used with caution in endodontic treatment to avoid hypochlorite accidents.4
Calcium hydroxide (Ca(OH)2) as an intracanal dressing reduces bacteria inside the root canal. 5 Its antibacterial effect is based on its high pH.6 The conventional root disinfection methods, such as NaOCl, chlorhexidine, and Ca(OH)2, are not always sufficient to reduce the amount of bacteria in the root canal system. 7 For example, NaOCl has a limited ability to penetrate and cannot always affect bacteria that are located in inaccessible areas.1 Also, microorganisms such as Candida albicans and Enterococcus faecalis which are isolated from the root canals of teeth with or without periradicular lesions can resist the antibacterial effect of Ca(OH)2.8,9
In recent years, intracanal laser radiation has been an adjuvant therapy for endodontic treatments due to its disinfection capability in root canal preparation.1 It is easier to reach difficult areas by using lasers.10 The diode laser has recently gained wide acceptance in RCT, and several studies have also shown better disinfection with the help of the diode laser in RCT.11-13 The diode laser causes changes in the dentin surface, such as melting and resolidifying the dentinal walls, which reduces the permeability of dentine, improves the sealing of the root canals, increases the bactericidal effect, and reduces periapical lesions after RCT. In this way, the laser creates a photodynamic effect, reduces inflammation, increases tissue healing, and finally achieves painlessness in the area.1,9-11
To our knowledge, some studies have considered the efficacy of diode laser irradiation and Ca(OH)2 in bone healing of periapical lesions after RCR. The aim of the current study was to evaluate the effect of the diode laser with Ca(OH)2 on the improvement of apical periodontitisin retreatment cases.
Methods
This randomized controlled clinical trial study was registered in the Clinical Trials Registry (IRCT20220713055458N1- https://irct.behdasht.gov.ir/trial/64807) and conducted at the office of an endodontist, between March 2021 and August 2023, in Birjand city. All the patients signed the informed consent form.
Sample Size and Population
Sample size calculation was based on the results of Ahangari and colleagues’ study5 which indicated that 12 samples would be required per group (alpha error = 0.05; power = 0.9)
Twenty-four teeth of 19 patients with periapical lesions which needed RCR were included in the study after they met the inclusion criteria as follows:
Teeth that previously received RCT
Single-rooted tooth
Age from 18 to 50 years without a history of specific medical diseases and using specific drugs
Having the periapical lesion;
The client must agree to participate.
The exclusion criteria were:
Illiterate patient
Pregnant women
Antibiotic consumption within the past month
History of hospitalization
Mobile teeth (mobility more than grade I according to the Miller classification)
Open apex teeth
Teeth with abrupt curvatures
Non-restorable teeth
Teeth that cannot be isolated with a rubber dam
Periodontal pocket depth ≥ 4 mm
Teeth with a history of previous RCT failure due to crack/fracture, resorption, perforation, ledge, overfilling, transportation, and separate files.
Teeth with short root length (less than 16 mm) and long root length (more than 23 mm)
To assess the efficacy of endodontic treatment, radiographs were taken by a radiography machine (Carestream Health, Inc, Rochester, NY, USA) and a phosphor plate sensor (Soredex Digora Optime plate scanner) with size 2 film (STD Plates 900246). The XCP (X-Ray Film holder (kerr) EndoBite) system was used to prevent tube angle change and prepare repeatable radiographs.
The patients were randomly divided into two groups (Ca(OH)2 + LI and Ca(OH)2) by using sealed white envelopes containing the group codes. In this way, only the person who prepared the envelopes knew the contents of the envelopes, and the examiners, patients, and statisticians were blinded.
Treatment Protocol
At the first appointment, the patient’s oral cavity was disinfected with 0.2% chlorhexidine gluconate mouthrinse for one minute. After local anesthesia application and rubber dam isolation, the teeth and rubber dam were disinfected by using 5.25% NaOCl (Chloraxid, Cerkamed, Stalowa Wola, Poland). In both groups, after removing all restorations and caries and access cavity preparation, gutta-percha in the third coronal root canal was removed passively by using Gates Glidden. Afterward, the rest of the canal fillings were removed by M two retreatment files (VDW GmbH, Munich, Germany) and Chloroform solution (Supelco, Merck KGaA, German). Each rotary file was used for the instrumentation of 3 canals. The working length (WL) of the canal was defined by Apex Finder (Dentsply Propex IQ) and confirmed by a digital radiograph. The patency of the teeth included in the study was confirmed by K file #10 (Mani, Japan), and the diameter of the apical foramen was not more than K file #15. Then, the root canal was prepared up to at least three numbers higher than the initial file. After each use of the files, canals were irrigated with 1 ml of 5.25% NaOCl by using a 30-gauge needle (Guangxi Ehall Medical Technology Co., Ltd., Germany) inserted deeply and passively. In the end, creamy Ca(OH)2 (Gholchadent, Golchai, Iran) was applied as an intracanal dressing for 10 days, and the cavity was filled with a temporary material (Coltosol, Coltene).
If there were no signs and symptoms, a second appointment was performed as described below.
In the Ca(OH)2 group, after removing Ca(OH)2 pastes from the root canals with 5 mL NaOCl 5.25% and by using a Hedstrom file corresponding to the master apical file, the canals were obturated with gutta-percha and AH26 sealer (Dentsply, Konstanz, Germany) by using the lateral condensation method after drying with a paper-point. A temporary restorative material was then used to seal the access cavity.
In the Ca(OH)2 + LI group, after final rinsing with distilled water and drying the canal with paper points, a 980 nm laser (Doctor smile) was performed by using a non-initiated 200-μm fiber optic (CW mode and power output of 1 W). In each canal, for each mm of WL, two seconds of irradiation was applied. Each canal was irradiated twice with an interval of 30s between each irradiation. In each canal, irradiation was performed from the apical to the coronal part of the canal in helicoidal movements, 1 mm shorter than the WL. During the laser irradiation, the endodontists, the patient, and the assistants wore protective eyewear. Similar to the Ca(OH)2 group, the canals were obturated. Then, the access cavity was sealed with Coltosol.
In both groups, the smear layer was removed by using 17% EDTA (Cerkamed, Poland) for five minutes and 5.25% NaOCl for one minute. All stages of RCR were performed by an endodontist. The restoration of the dental crown of all patients was performed for a maximum of 2 weeks after the completion of RCR.
Assessment of Radiographic Lesions
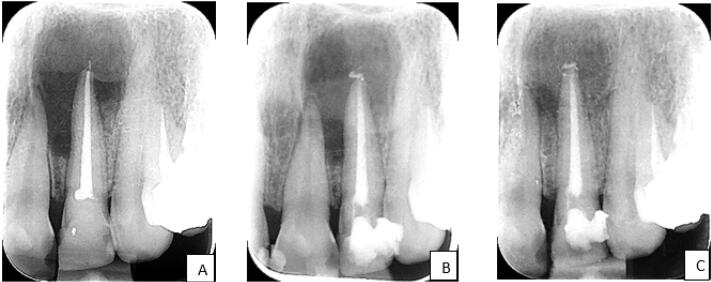
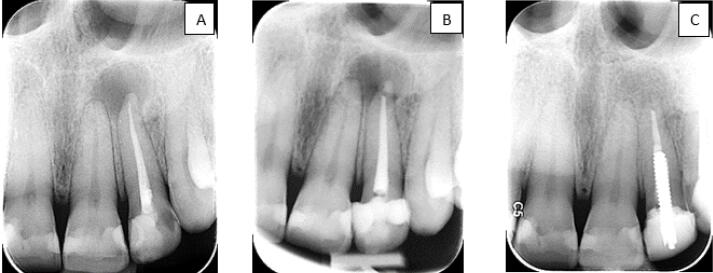
The patients were recalled at intervals of 3 and 6 months after RCR (Figures 1 and 2). A clinical examination was done and the restoration was checked. Follow-up radiographs were prepared as described previously. The healing process of the lesion before the retreatment and at the 3-month and 6-month follow-ups was assessed by Qrstavik’s PAI.14 Two endodontists and one oral, maxillofacial radiologist blinded to the study were calibrated and requested to assess the radiographs according to the images of the Qrstavik PAI pattern. If there was no agreement, the three examiners discussed their findings until they reached a consensus.
Figure 1.
Radiographic Follow-up in the Ca(OH)2 + LI Group: Before Retreatment (A), 3-Month (B) and 6-Month (C) Follow-ups
Figure 2.
Radiographic Follow-up in the Ca(OH)2 Group: Before Treatment (A), 3-Month (B) and 6-Month (C) Follow-ups
Statistical Analysis
The collected data were analyzed and statistically interpreted by chi-square, ANOVA, T-test, and Post Hoc tests. Data analysis was performed by using SPSS Statistics version 22 (IBM Corp., Armonk, USA). We calculated Cohen’s kappa coefficients to assess the inter-observer and intra-observer agreement on the assessment of radiographs (kappa > 0.71).
Results
Demographic Data
RCR was performed on 19 patients (mean age of 34.47, the mean age in the Ca(OH)2 + LI group was 33.5 and in the Ca(OH)2 group was 35.18) and 24 teeth (Table 1).
Table 1. Sex Distribution of the Patients in the Ca(OH)2 + LI Group and in the Ca(OH)2 Group .
| Sex | Group | |
| Ca(OH)2+LI (n=8) | Ca(OH)2 (n=11) | |
| Male (%) | 2 (25%) | 4 (36%) |
| Female (%) | 6 (75%) | 7 (66%) |
Periapical Radiographic Healing Analysis
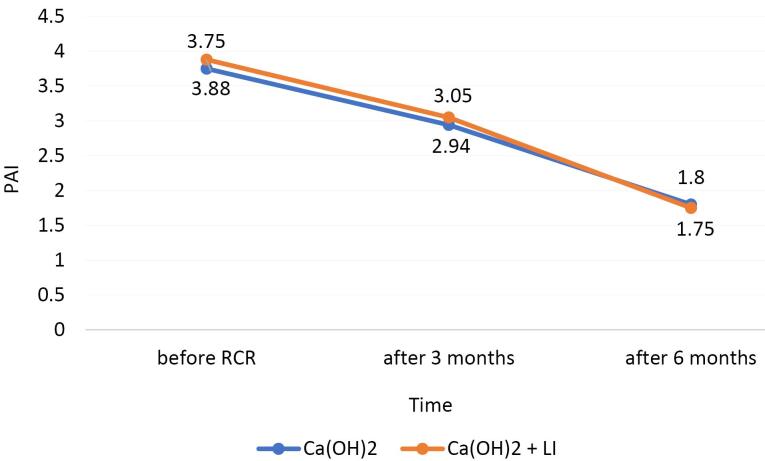
Before starting the RCR, the periapical lesion score was 3.75 and 3.88 in the Ca(OH)2 group and in the Ca(OH)2 + LI group, respectively. In the Ca(OH)2 and Ca(OH)2 + LI groups, 3 months after the RCR, the average periapical lesion score was 2.94 and 3.05, respectively. In the Ca(OH)2 and Ca(OH)2 + LI groups, 6 months after the RCR, the average periapical lesion score was 1.80 and 1.75, respectively (Figure 3).
Figure 3.
Periapical Status of Ca(OH)2 and Ca(OH)2 + LI Groups (Before Retreatment, 3 and 6 Months After RCR)
In both groups, the periapical healing at 6-month recalls improved, compared to 3-month follow-ups. There was no significant difference in the healing of the periapical lesions between the two groups after RCR.
Discussion
Periapical lesions are bacterial infections that result in bone loss and radiolucencies in the radiograph.4 These lesions are more resistant to the treatment and do not heal due to the presence of resistant bacteria.1 This study aimed to investigate the effect of the diode laser as an adjuvant treatment on the healing of periradicular lesions after RCR.
The different anatomy of the root and multiple biofilms make it difficult to disinfect the root canals. Different types of lasers affect the disinfection of root canals due to their antibacterial effect and their ability to penetrate deeper into the dentin, remove soft tissues, and stimulate bone regeneration.5,15-17 The effect of lasers on tissues can be different by changing some parameters such as pulse mode, energy radiation, wavelength, exposure time, optical fiber diameter, and physical characteristics of tissues.4 According to recent studies, the diode laser has been effective in decreasing the number of resistant microorganisms and has significant advantages such as being small and portable, easy to use, affordability, and compactness.4,18 This laser has an antibacterial effect on endodontics bacteria with wavelengths of 810-980 nm.4 Therefore, in our study, the wavelength of 980 nm was used. In addition to the antibacterial effect, this wavelength penetrates the dentinal tubules, where irrigation solutions cannot, and as a result, the sealer penetrates more into them.4 The antibacterial effect of the diode laser occurs due to the photothermal effect related to heat transmission.4 In fact, in ideal parameters, it causes the destruction of the bacterial cell wall, denaturation of proteins, microbial death, and cell lysis.19 Similar to previous studies, in our study, intracanal irradiation was applied by using circular movements to reduce periradicular tissue damage. It was irradiated from the apical direction to the coronal direction so that the temperature of the canal wall was less affected.20
Diode laser radiation modulates processes such as differentiation and proliferation in cell mitochondria, increases the healing of bone structure,21,22,4 reduces the level of inflammatory mediators, and increases vascularization.1,18,23 The process of bone regeneration can change under the influence of systemic or local factors.7 To minimize the systemic effect, we excluded patients with specific systemic diseases from the study, but factors such as malnutrition or differences in the methylation of genes related to the immune response such as FOXP3 or microRNAs could have affected the results of the study.24,25 It is recommended to investigate the methylation of these genes for future studies.
The Orstavik PAI, which has high reliability and reproducibility among the periapical indexes of two-dimensional radiography, is used to evaluate the healing process and the condition of the periapical lesions.26 The process of recovery or failure of treatment of periapical lesions is interpreted by this index.26 Considering the possibility of observer bias in radiographic evaluation,26 three observers scored separately and then we averaged the scores. The lack of determination of the relationship between the periapical lesion and the surrounding anatomical structures and the high possibility of false negative results are other disadvantages of this index.26 To solve these problems, we recommend using cone beam computed tomography (CBCT) indexes in doubtful cases, considering its high cost and radiation dose,26 although CBCT artifacts, especially gutta-percha, metal post, and endodontic cement, make the diagnosis more difficult.27,28
The healing of apical periodontitisis most obvious from 3 months to 2 years after treatment.29 Different studies consider different follow-up sessions. Huumonen and Ørstavik recommended follow-up treatment from 3 months to 2 years, but the European Association of Endodontists recommends evaluating periapical lesions up to 4 years after treatment.29,30 Although a long-term follow-up is better to evaluate healing in endodontic treatment, two stages of improvement of the PAI can clinically be considered as an indicator of recovery.31 Thus, follow-ups of more than 6 months should be considered. Whether the laser has a faster healing effect or not, we considered the 3-month and 6-month follow-up as an indicator for the short-term healing of lesions due to the effect of LI.
Pelozo et al showed that the diode laser had a positive effect on periapical lesion healing during treatment follow-ups.32 In their study, the effectiveness of the laser was investigated in terms of microbiology and radiology. Radiographic follow-ups of their study included 3, 6, 9, and 12 months. The radiographic images were classified based on complete recovery (healthy PDL), violation (no change in size or reduction in size without achieving a healthy PDL), and treatment failure (enlargement of lesion size). Irradiation was done with a diode laser (980 nm, 350-μm optical fiber, power of 1.5 W, pulsed mode,100 Hz for 20 seconds). In this study, a 980 nm diode laser (continuous wave, power of 1 W) was applied. The discrepancy between the result of our study with that study may be because of the design of the research, the characteristics of the laser used, and the larger number of samples.
Some researchers found no positive relationship between the size of periradicular lesions and treatment success,33,34 but in Bytyqi and colleagues’ study, the size of the lesion had a considerable effect on the prognosis of the treatment.35 The negative impact of bigger lesions on treatment success is because of a greater variety of microorganisms and their correlation with long-term infections because bacteria have penetrated deeply. In addition, these lesions may be cysts.36 Moreover, some larger lesions have a slower response to ecological changes due to the treatment protocol.13 Bytyqi et al have found that the use of a laser in small lesions ( ≤ 6 mm) can be effective, but in larger lesions ( ≥ 10 mm), it can have little effect on Enterococcus faecalis or Streptococcus mitis.35 Consequently, we recommend considering lesions with similar sizes for further studies or using prolonged heating by pulsed mode for larger lesions.35
Meire et al, in a systematic review, concluded that there was not any additional effect of adjunctive therapy like LI over conventional endodontic treatment in the improvement of periradicular lesions.37 Our findings are similar to their results. The little evidence on the efficacy of the LI in the healing of periapical lesions does not necessarily invalidate the effect of laser irradiation, but it does emphasize the need for further studies to establish the impacts of laser irradiation on RCR results.
Conclusion
Bearing research limitations in mind, there is no significant difference between Ca(OH)2 + 980 nm LI and Ca(OH)2 RCR at 3-month and 6-month follow-ups. However, more studies are required to assess the effect of LI over Ca(OH)2 in terms of apical periodontitis healing.
Acknowledgments
Dr Sediqe Ebrahimipour provided help with the scoring of radiographs, and Dr Freshteh Osmani helped us with data analysis.
Authors’ Contribution
Conceptualization: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Data curation: Maryam Noferesti.
Funding acquisition: Soheila Darmiani.
Investigation: Maryam Nofersti, Soheila Darmiani.
Methodology: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Project administration: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Resources: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Software: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Supervision: Soheila Darmiani, Homa Rastegar.
Validation: Maryam Noferesti, Soheila Darmiani, Homa Rastegar.
Visualization: Maryam Noferesti.
Writing-original draft: Maryam Noferesti.
Writing-review & editing: Maryam Noferesti, Soheila Darmiani.
Competing Interests
The authors deny any conflicts of interest.
Ethical Approval
This study was approved by the Research Ethics Committee of Birjand University of Medical Sciences (IR.BUMS.REC.1401.036).
Funding
None.
Please cite this article as follows: Noferesti M, Darmiani S, Rastegar H. A 980 nm diode laser as an adjunctive therapy on the healing of apical periodontitis following endodontic retreatment: a randomized controlled clinical trial study. J Lasers Med Sci. 2024;15:e36. doi:10.34172/jlms.2024.36.
References
- 1.Genc Sen O, Kaya M. Effect of root canal disinfection with a diode laser on postoperative pain after endodontic retreatment. Photobiomodul Photomed Laser Surg. 2019;37(2):85–90. doi: 10.1089/photob.2018.4539. [DOI] [PubMed] [Google Scholar]
- 2.Tibúrcio-Machado CS, Michelon C, Zanatta FB, Gomes MS, Marin JA, Bier CA. The global prevalence of apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2021;54(5):712–35. doi: 10.1111/iej.13467. [DOI] [PubMed] [Google Scholar]
- 3.Do QL, Gaudin A. The efficiency of the Er:YAG laser and photoninduced photoacoustic streaming (PIPS) as an activation method in endodontic irrigation: a literature review. J Lasers Med Sci. 2020;11(3):316–34. doi: 10.34172/jlms.2020.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susila AV, Sai S, Sharma N, Balasubramaniam A, Veronica AK, Nivedhitha S. Can natural irrigants replace sodium hypochlorite? A systematic review. Clin Oral Investig. 2023;27(5):1831–49. doi: 10.1007/s00784-023-04913-7. [DOI] [PubMed] [Google Scholar]
- 5.Ahangari Z, Mojtahed Bidabadi M, Asnaashari M, Rahmati A, Tabatabaei FS. Comparison of the antimicrobial efficacy of calcium hydroxide and photodynamic therapy against Enterococcus faecalis and Candida albicans in teeth with periapical lesions; an in vivo study. J Lasers Med Sci. 2017;8(2):72–8. doi: 10.15171/jlms.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berman LH, Hargreaves KM. Cohen’s Pathways of the Pulp: Cohen’s Pathways of the Pulp-E-Book. Elsevier Health Sciences; 2020.
- 7.Holland R, Gomes JE, Cintra LT, de Azevedo Queiroz ÍO, Estrela C. Factors affecting the periapical healing process of endodontically treated teeth. J Appl Oral Sci. 2017;25(5):465–76. doi: 10.1590/1678-7757-2016-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiniforush N, Pourhajibagher M, Shahabi S, Kosarieh E, Bahador A. Can antimicrobial photodynamic therapy (aPDT) enhance the endodontic treatment? J Lasers Med Sci. 2016;7(2):76–85. doi: 10.15171/jlms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xhevdet A, Stubljar D, Kriznar I, Jukic T, Skvarc M, Veranic P, et al. The disinfecting efficacy of root canals with laser photodynamic therapy. J Lasers Med Sci. 2014;5(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Preethee T, Kandaswamy D, Arathi G, Hannah R. Bactericidal effect of the 908 nm diode laser on Enterococcus faecalis in infected root canals. J Conserv Dent. 2012;15(1):46–50. doi: 10.4103/0972-0707.92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asnaashari M, Ebad LT, Shojaeian S. Comparison of antibacterial effects of 810 and 980- nanometer diode lasers on Enterococcus faecalis in the root canal system - an in vitro study. Laser Ther. 2016;25(3):209–14. doi: 10.5978/islsm.16-OR-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragana R, Jelena M, Jovan M, Biljana N, Dejan M. Antibacterial efficiency of adjuvant photodynamic therapy and high-power diode laser in the treatment of young permanent teeth with chronic periapical periodontitis A prospective clinical study. Photodiagnosis Photodyn Ther. 2023;41:103129. doi: 10.1016/j.pdpdt.2022.103129. [DOI] [PubMed] [Google Scholar]
- 13.Dalaei Moghadam M, Saberi EA, Farhad Molashahi N, Shahraki Ebrahimi H. Comparative efficacy of depotphoresis and diode laser for reduction of microbial load and postoperative pain, and healing of periapical lesions: a randomized clinical trial. G Ital Endod. 2021;35(2):75–87. [Google Scholar]
- 14.Orstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol. 1986;2(1):20–34. doi: 10.1111/j.1600-9657.1986.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 15.Bahrololoomi Z, Fekrazad R, Zamaninejad S. Antibacterial effect of diode laser in pulpectomy of primary teeth. J Lasers Med Sci. 2017;8(4):197–200. doi: 10.15171/jlms.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romeo U, Palaia G, Nardo A, Tenore G, Telesca V, Kornblit R, et al. Effectiveness of KTP laser versus 980 nm diode laser to kill Enterococcus faecalis in biofilms developed in experimentally infected root canals. Aust Endod J. 2015;41(1):17–23. doi: 10.1111/aej.12057. [DOI] [PubMed] [Google Scholar]
- 17.Zaccara IM, Mestieri LB, Pilar EFS, Moreira MS, Grecca FS, Martins MD, et al. Photobiomodulation therapy improves human dental pulp stem cell viability and migration in vitro associated to upregulation of histone acetylation. Lasers Med Sci. 2020;35(3):741–9. doi: 10.1007/s10103-019-02931-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan T, Sezgin GP, Sönmez Kaplan S. Effect of a 980-nm diode laser on post-operative pain after endodontic treatment in teeth with apical periodontitis: a randomized clinical trial. BMC Oral Health. 2021;21(1):41. doi: 10.1186/s12903-021-01401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Mobadder M, Nammour S, Namour M, Namour A, Grzech-Leśniak K. Disinfection potential of 980 nm diode laser and hydrogen peroxide (3%) in “critical probing depths” periodontal pockets: retrospective study. Life (Basel) 2022;12(3):370. doi: 10.3390/life12030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutknecht N. Lasers in endodontics. J Laser Health Acad. 2008;4:1–4. [Google Scholar]
- 21.Sasaki H, Hirai K, Martins CM, Furusho H, Battaglino R, Hashimoto K. Interrelationship between periapical lesion and systemic metabolic disorders. Curr Pharm Des. 2016;22(15):2204–15. doi: 10.2174/1381612822666160216145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva LA, de Souza Lopes ZM, de Sá RC, Novaes Júnior AB, Romualdo PC, Lucisano MP, et al. Comparison of apical periodontitis repair in endodontic treatment with calcium hydroxide-dressing and aPDT. Braz Oral Res. 2019;33:e092. doi: 10.1590/1807-3107bor-2019.vol33.0092. [DOI] [PubMed] [Google Scholar]
- 23.Morsy DA, Negm M, Diab A, Ahmed G. Postoperative pain and antibacterial effect of 980 nm diode laser versus conventional endodontic treatment in necrotic teeth with chronic periapical lesions: a randomized control trial. F1000Res. 2018;7:1795. doi: 10.12688/f1000research.16794.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos K, Franscisconi CF, Okehie V, de Souza LC, Trombone AP, Letra A, et al. FOXP3 DNA methylation levels as a potential biomarker in the development of periapical lesions. J Endod. 2015;41(2):212–8. doi: 10.1016/j.joen.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhong S, Zhang S, Bair E, Nares S, Khan AA. Differential expression of microRNAs in normal and inflamed human pulps. J Endod. 2012;38(6):746–52. doi: 10.1016/j.joen.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekhar R, Soman S, Sebastian VM, Muliyar S, Cherian NM. Indexes for periapical health evaluation: a review. Int Dent Res. 2022;12(2):97–106. doi: 10.5577/intdentres.2022.vol12.no2.8. [DOI] [Google Scholar]
- 27.Pope O, Sathorn C, Parashos P. A comparative investigation of cone-beam computed tomography and periapical radiography in the diagnosis of a healthy periapex. J Endod. 2014;40(3):360–5. doi: 10.1016/j.joen.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Kruse C, Spin-Neto R, Reibel J, Wenzel A, Kirkevang LL. Diagnostic validity of periapical radiography and CBCT for assessing periapical lesions that persist after endodontic surgery. Dentomaxillofac Radiol. 2017;46(7):20170210. doi: 10.1259/dmfr.20170210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huumonen S, Ørstavik D. Radiographic follow-up of periapical status after endodontic treatment of teeth with and without apical periodontitis. Clin Oral Investig. 2013;17(9):2099–104. doi: 10.1007/s00784-013-0926-2. [DOI] [PubMed] [Google Scholar]
- 30.Paljevic E, Brekalo Prso I, Hrstic JV, Pezelj-Ribaric S, Persic Bukmir R. Impact of smoking on the healing of apical periodontitis after nonsurgical endodontic treatment. Eur J Dent. 2024;18(1):124–30. doi: 10.1055/s-0043-1761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurado Patrón OD, Vargas López A, Vega Lizama EM, Alvarado Cárdenas G, López Villanueva ME, Ramírez Salomón M. Radiographic characteristics in the periapical healing post endodontic treatment in patients of the Autonomous University of Yucatan, School of Dentistry. Nova Scientia. 2018;10(21):379–90. doi: 10.21640/ns.v10i21.1592. [DOI] [Google Scholar]
- 32.Pelozo LL, Silva-Neto RD, Salvador SL, Sousa-Neto MD, Souza-Gabriel AE. Adjuvant therapy with a 980-nm diode laser in root canal retreatment: randomized clinical trial with 1-year follow-up. Lasers Med Sci. 2023;38(1):77. doi: 10.1007/s10103-022-03659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aksoy F. Outcomes of nonsurgical endodontic treatment in teeth with large periapical lesion. Ann Med Res. 2019;26(11):2642. doi: 10.5455/annalsmedres.2019.08.444. [DOI] [Google Scholar]
- 34.Moazami F, Sahebi S, Sobhnamayan F, Alipour A. Success rate of nonsurgical endodontic treatment of nonvital teeth with variable periradicular lesions. Iran Endod J. 2011;6(3):119–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Bytyqi A, Aliu X, Barani M, Stubljar D, Jukic T, Starc A, et al. Disinfection of infected artificial dental periapical lesions with diode laser: an in vitro study. Med Sci Monit Basic Res. 2021;27:e932492. doi: 10.12659/msmbr.932492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karamifar K, Tondari A, Saghiri MA. Endodontic periapical lesion: an overview on the etiology, diagnosis and current treatment modalities. Eur Endod J. 2020;5(2):54–67. doi: 10.14744/eej.2020.42714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meire MA, Bronzato JD, Bomfim RA, Gomes B. Effectiveness of adjunct therapy for the treatment of apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2023;56 Suppl 3:455–74. doi: 10.1111/iej.13838. [DOI] [PubMed] [Google Scholar]