Abstract
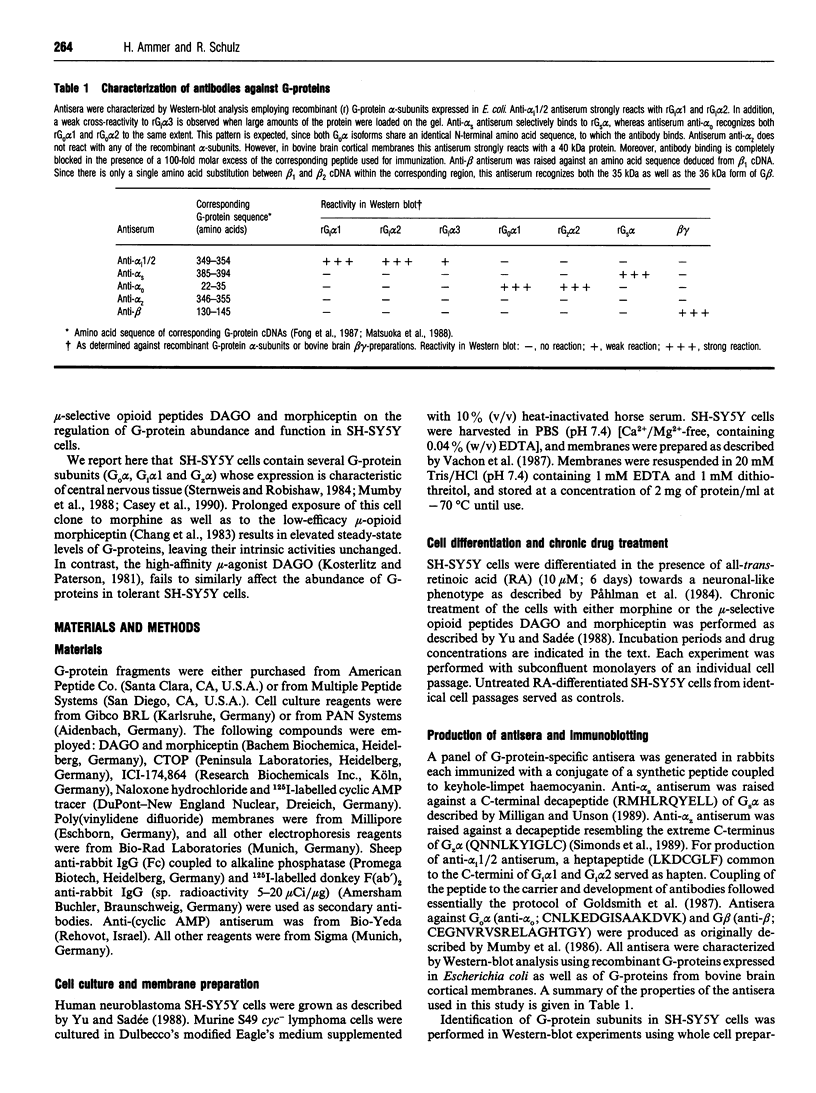
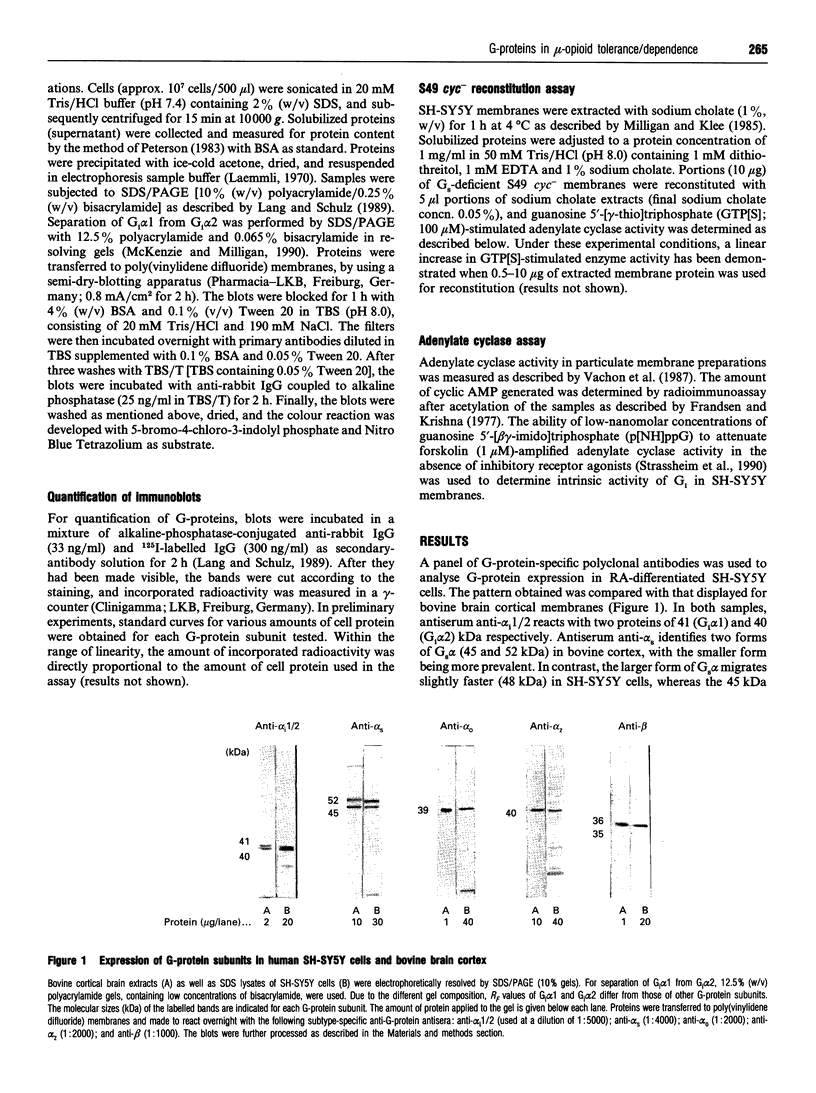
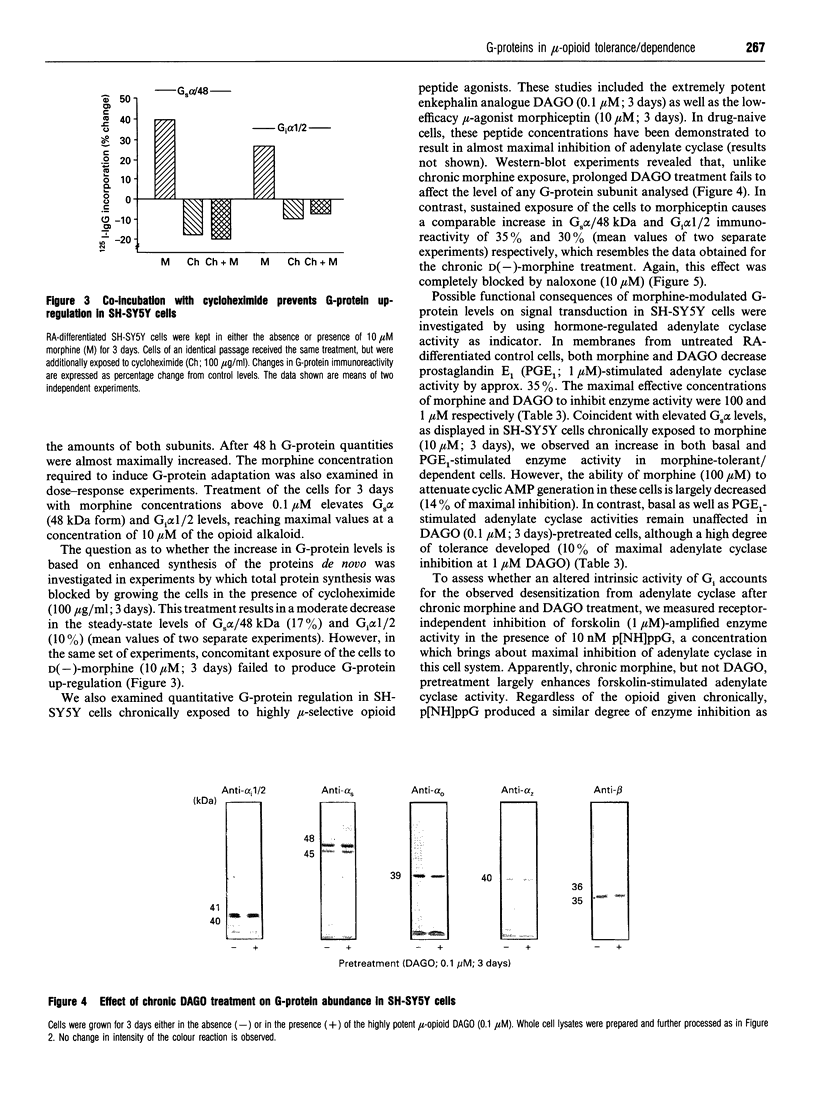
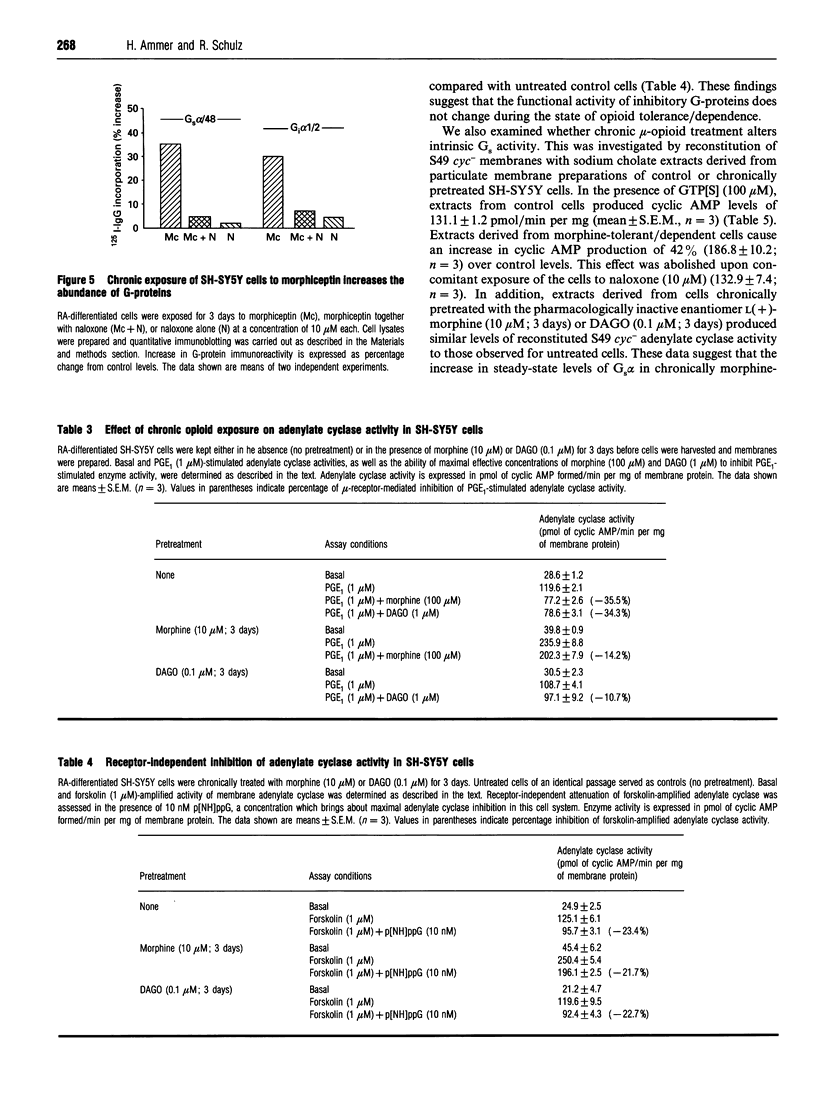
Western-blot analysis of human neuroblastoma SH-SY5Y cells (mu- and delta-receptors) revealed the presence of the following G-protein subunits: Gi alpha 1, Gi alpha 2, Gs alpha, G(o) alpha, Gz alpha, and G beta, a pattern resembling that observed in central nervous tissue. Chronic treatment of differentiated [all-trans-retinoic acid (10 microM; 6 days)] SH-SY5Y cells with D(-)-morphine (10 microM; 3 days) significantly increased the abundance of all G-protein subunits identified. Co-incubation of morphine-exposed cells together with naloxone (10 microM; 3 days) or the mu-selective opioid antagonist CTOP (10 microM; 3 days), but not with the delta-selective antagonist ICI-174,864 (10 microM; 3 days), completely abolished this effect, suggesting that the increase in G-protein abundance is specifically mediated by mu-receptors. Moreover, the biologically inactive enantiomer L(+)-morphine (10 microM; 3 days) failed to produce a similar effect. G-protein up-regulation developed in a time- and dose-dependent manner and is most likely due to enhanced protein synthesis de novo, since concomitant treatment of the cells with cycloheximide (100 micrograms/ml; 3 days) prevented this effect. Chronic treatment with the low-efficacy mu-selective opioid peptide morphiceptin (10 microM; 3 days), but not with the highly potent mu-agonist DAGO (0.1 microM; 3 days) produced a comparable increase in G-protein abundance. Coincident with quantitative effects on G-protein levels in morphine-tolerant/dependent SH-SY5Y cells, we found elevated levels of basal, forskolin (1 microM)- and prostaglandin-E1 (1 microM)-stimulated adenylate cyclase activities. Reconstitution experiments using S49 cyc- lymphoma-cell membranes suggest that this increase is most likely due to elevated levels of functionally intact Gs. Chronic treatment with both morphine and DAGO induces high degrees of tolerance in this cell line. However, the intrinsic activity of G1 was unchanged, as assessed in functional studies with low-nanomolar concentrations of guanosine 5'-[beta gamma- imido]triphosphate. Our data demonstrate that chronic treatment of SH-SY5Y cells with low-efficacy mu-opioids increases G-protein abundance, a phenomenon which might contribute to the biochemical mechanisms underlying opioid tolerance/dependence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammer H., Nice L., Lang J., Schulz R. Regulation of G proteins by chronic opiate and clonidine treatment in the guinea pig myenteric plexus. J Pharmacol Exp Ther. 1991 Sep;258(3):790–796. [PubMed] [Google Scholar]
- Ammer H., Schulz R. Coupling of prostaglandin E1 receptors to the stimulatory GTP-binding protein Gs is enhanced in neuroblastoma x glioma (NG108-15) hybrid cells chronically exposed to an opioid. Mol Pharmacol. 1993 Apr;43(4):556–563. [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Boyd R. S., Donnelly L. E., MacDermot J. Opiate-dependent changes in the sensitivity of adenylate cyclase to stimulatory agonists and 5'-guanylylimidodiphosphate are independent of G protein abundance and eukaryotic ADP-ribosyltransferase activity in NG108-15 cells. J Neurochem. 1992 Feb;58(2):688–693. doi: 10.1111/j.1471-4159.1992.tb09772.x. [DOI] [PubMed] [Google Scholar]
- Brabet P., Pantaloni C., Bockaert J., Homburger V. Metabolism of two Go alpha isoforms in neuronal cells during differentiation. J Biol Chem. 1991 Jul 15;266(20):12825–12828. [PubMed] [Google Scholar]
- Casey P. J., Fong H. K., Simon M. I., Gilman A. G. Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem. 1990 Feb 5;265(4):2383–2390. [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979 Apr 25;254(8):2610–2618. [PubMed] [Google Scholar]
- Chang K. J., Wei E. T., Killian A., Chang J. K. Potent morphiceptin analogs: structure activity relationships and morphine-like activities. J Pharmacol Exp Ther. 1983 Nov;227(2):403–408. [PubMed] [Google Scholar]
- Eriksson P. S., Carlsson B., Isaksson O. G., Hansson E., Rönnbäck L. Altered amounts of G-protein mRNA and cAMP accumulation after long-term opioid receptor stimulation of neurons in primary culture from the rat cerebral cortex. Brain Res Mol Brain Res. 1992 Aug;14(4):317–325. doi: 10.1016/0169-328x(92)90099-w. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Green A., Johnson J. L., Milligan G. Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1990 Mar 25;265(9):5206–5210. [PubMed] [Google Scholar]
- Hadcock J. R., Port J. D., Malbon C. C. Cross-regulation between G-protein-mediated pathways. Activation of the inhibitory pathway of adenylylcylclase increases the expression of beta 2-adrenergic receptors. J Biol Chem. 1991 Jun 25;266(18):11915–11922. [PubMed] [Google Scholar]
- Hsu W. H., Rudolph U., Sanford J., Bertrand P., Olate J., Nelson C., Moss L. G., Boyd A. E., Codina J., Birnbaumer L. Molecular cloning of a novel splice variant of the alpha subunit of the mammalian Go protein. J Biol Chem. 1990 Jul 5;265(19):11220–11226. [PubMed] [Google Scholar]
- Johnson S. M., Fleming W. W. Mechanisms of cellular adaptive sensitivity changes: applications to opioid tolerance and dependence. Pharmacol Rev. 1989 Dec;41(4):435–488. [PubMed] [Google Scholar]
- Kazmi S. M., Mishra R. K. Opioid receptors in human neuroblastoma SH-SY5Y cells: evidence for distinct morphine (mu) and enkephalin (delta) binding sites. Biochem Biophys Res Commun. 1986 Jun 13;137(2):813–820. doi: 10.1016/0006-291x(86)91152-6. [DOI] [PubMed] [Google Scholar]
- Klinz F. J., Yu V. C., Sadée W., Costa T. Differential expression of alpha-subunits of G-proteins in human neuroblastoma-derived cell clones. FEBS Lett. 1987 Nov 16;224(1):43–48. doi: 10.1016/0014-5793(87)80419-2. [DOI] [PubMed] [Google Scholar]
- Kurose H., Katada T., Amano T., Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lameh J., Eiger S., Sadée W. Interaction among mu-opioid receptors and alpha 2-adrenoceptors on SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 1992 Sep 1;227(1):19–24. doi: 10.1016/0922-4106(92)90137-k. [DOI] [PubMed] [Google Scholar]
- Lang J., Costa T. Chronic exposure of NG 108-15 cells to opiate agonists does not alter the amount of the guanine nucleotide-binding proteins Gi and Go. J Neurochem. 1989 Nov;53(5):1500–1506. doi: 10.1111/j.1471-4159.1989.tb08544.x. [DOI] [PubMed] [Google Scholar]
- Lang J., Schulz R. Chronic opiate receptor activation in vivo alters the level of G-protein subunits in guinea-pig myenteric plexus. Neuroscience. 1989;32(2):503–510. doi: 10.1016/0306-4522(89)90097-3. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Loss of opiate receptor activity in neuroblastoma X glioma NG108-15 hybrid cells after chronic opiate treatment. A multiple-step process. Mol Pharmacol. 1982 Jul;22(1):1–4. [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Opiate receptor down-regulation and desensitization in neuroblastoma X glioma NG108-15 hybrid cells are two separate cellular adaptation processes. Mol Pharmacol. 1983 Nov;24(3):413–424. [PubMed] [Google Scholar]
- Lux B., Schulz R. Effect of cholera toxin and pertussis toxin on opioid tolerance and dependence in the guinea-pig myenteric plexus. J Pharmacol Exp Ther. 1986 Jun;237(3):995–1000. [PubMed] [Google Scholar]
- Matsuoka M., Itoh H., Kozasa T., Kaziro Y. Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein alpha subunit. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. Biochem J. 1990 Apr 15;267(2):391–398. doi: 10.1042/bj2670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Milligan G., Unson C. G. Persistent activation of the alpha subunit of Gs promotes its removal from the plasma membrane. Biochem J. 1989 Jun 15;260(3):837–841. doi: 10.1042/bj2600837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Williamson A. R. Structural mutations in a mouse immunoglobulin light chain resulting in failure to be secreted. Cell. 1980 Jun;20(2):283–292. doi: 10.1016/0092-8674(80)90614-5. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S., Pang I. H., Gilman A. G., Sternweis P. C. Chromatographic resolution and immunologic identification of the alpha 40 and alpha 41 subunits of guanine nucleotide-binding regulatory proteins from bovine brain. J Biol Chem. 1988 Feb 5;263(4):2020–2026. [PubMed] [Google Scholar]
- Nestler E. J., Erdos J. J., Terwilliger R., Duman R. S., Tallman J. F. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989 Jan 9;476(2):230–239. doi: 10.1016/0006-8993(89)91243-2. [DOI] [PubMed] [Google Scholar]
- Nishino K., Su Y. F., Wong C. S., Watkins W. D., Chang K. J. Dissociation of mu opioid tolerance from receptor down-regulation in rat spinal cord. J Pharmacol Exp Ther. 1990 Apr;253(1):67–72. [PubMed] [Google Scholar]
- Pelton J. T., Kazmierski W., Gulya K., Yamamura H. I., Hruby V. J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986 Nov;29(11):2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Port J. D., Hadcock J. R., Malbon C. C. Cross-regulation between G-protein-mediated pathways. Acute activation of the inhibitory pathway of adenylylcyclase reduces beta 2-adrenergic receptor phosphorylation and increases beta-adrenergic responsiveness. J Biol Chem. 1992 Apr 25;267(12):8468–8472. [PubMed] [Google Scholar]
- Påhlman S., Ruusala A. I., Abrahamsson L., Mattsson M. E., Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984 Jun;14(2):135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Strassheim D., Milligan G., Houslay M. D. Diabetes abolishes the GTP-dependent, but not the receptor-dependent inhibitory function of the inhibitory guanine-nucleotide-binding regulatory protein (Gi) on adipocyte adenylate cyclase activity. Biochem J. 1990 Mar 1;266(2):521–526. doi: 10.1042/bj2660521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger R. Z., Beitner-Johnson D., Sevarino K. A., Crain S. M., Nestler E. J. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991 May 10;548(1-2):100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Toll L. Mu-opioid receptor binding in intact SH-SY5Y neuroblastoma cells. Eur J Pharmacol. 1990 Feb 6;176(2):213–217. doi: 10.1016/0014-2999(90)90530-j. [DOI] [PubMed] [Google Scholar]
- Ueda H., Harada H., Nozaki M., Katada T., Ui M., Satoh M., Takagi H. Reconstitution of rat brain mu opioid receptors with purified guanine nucleotide-binding regulatory proteins, Gi and Go. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7013–7017. doi: 10.1073/pnas.85.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon L., Costa T., Herz A. GTPase and adenylate cyclase desensitize at different rates in NG108-15 cells. Mol Pharmacol. 1987 Feb;31(2):159–168. [PubMed] [Google Scholar]
- Van Vliet B. J., Wardeh G., Mulder A. H., Schoffelmeer A. N. Reciprocal effects of chronic morphine administration on stimulatory and inhibitory G-protein alpha subunits in primary cultures of rat striatal neurons. Eur J Pharmacol. 1991 Dec 12;208(4):341–342. doi: 10.1016/0922-4106(91)90082-s. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Eiger S., Duan D. S., Lameh J., Sadée W. Regulation of cyclic AMP by the mu-opioid receptor in human neuroblastoma SH-SY5Y cells. J Neurochem. 1990 Oct;55(4):1390–1396. doi: 10.1111/j.1471-4159.1990.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Sadée W. Efficacy and tolerance of narcotic analgesics at the mu opioid receptor in differentiated human neuroblastoma cells. J Pharmacol Exp Ther. 1988 Apr;245(1):350–355. [PubMed] [Google Scholar]