Abstract
The “classical” EAU model induced by immunization of mice with the retinal protein IRBP or its peptides has been very useful to study basic mechanisms of ocular inflammation, but is inadequate for some types of studies due to the need for active immunization in the context of strong bacterial adjuvants. We generated transgenic (Tg) mice on the B10.RIII background that express a T cell receptor (TCR) specific for IRBP161–180. Three strains of TCR Tg mice were established. Spontaneous uveitis developed in two of the three strains by 2–3 months of age. Susceptibility correlated with a higher copy number of the transgenic TCR and a higher proportion of TCR Tg T cells in the peripheral repertoire. Even in mice with uveitis, peripheral IRBP-specific CD4+ T cells displayed mostly a naïve phenotype. In contrast, T cells infiltrating uveitic eyes mostly showed an effector/memory phenotype, and included Th1, Th17 as well as T regulatory cells. These mice thus provide a new and distinct model of uveitis from the “classical” EAU, and may represent some types of uveitis more faithfully. Importantly, this new transgenic model of uveitis can serve as a template for therapeutic manipulations, and as a source of naïve retina-specific T cells for a variety of basic and pre-clinical studies. Several examples of such studies will be discussed.
Keywords: Autoimmunity, uveitis, EAU, animal model, Th1, Th17
INTRODUCTION AND BACKGROUND INFORMATION
Human non-infectious uveitis/uveoretinitis is a sight-threatening disease considered to have an autoimmune basis. Uveitis in its various forms is reported to underlie 10–15% of severe visual handicap, or even more, depending on the geographical location [1, 2]. Some types of uveitis are restricted only to the eye, such as idiopathic uveitis, birdshot chorioretinopathy (BC) and sympathetic ophthalmia. Others may be part of a more general, systemic syndrome, such as sarcoidosis, Vogt-Koyanagi-Harada disease (VKH) and Behçet’s disease (BD). The various uveitides may differ in clinical appearance and course, but what they all have in common is that there is no obvious infectious etiology within the eye and patients often exhibit immunological responses to unique retinal proteins (antigens; Ags) localized to the eye, such as retinal arrestin (also known as retinal soluble Ag, or S-Ag) and interphotoreceptor retinoid binding protein (IRBP). Strong MHC associations with some types of uveitis have been reported, e.g., a relative risk of over 200 is reported for HLA-A29-positive individuals to develop BC [3]. Improvement of disease is typically seen with T cell targeting agents, such as CsA, rapamycin and more recently anti-IL-2 receptorantibodies (daclizumab). Other biologics that target immune components have also been reported. Importantly, Th1 and Th17 responses have been reported in association with disease [4, 5].
In the aggregate, these findings support a central role for T cells reactive to eye-specific proteins in the pathogenesis of uveitis. In recent years some of the uveitic diseases considered to be autoimmune were re-classified as “autoinflammatory” and postulated to have an innate immunity basis owing to genetic associations with allelic variants of innate immunity receptors (NLRP3/cryopyrin mutations) and a strong dependence on IL-1 for pathogenesis. One such example of several is BD. As discussed above, however, BD patients also exhibit responses to retinal proteins and IL-17 production by T cells. It is therefore conceivable that, irrespective of the actual etiology, autoimmune responses to retinal Ags released as a result of tissue breakdown may become drivers of disease.
Experimental autoimmune uveitis (EAU) can be induced in mice by immunization with the same retinal Ags that elicit secondary immunological responses in uveitis patients, and serves as a model of the human disease [3]. Some mouse strains, especially “humanized” mice genetically engineered to express HLA molecules, develop strong disease with S-Ag and respond to the same antigenic fragments as uveitis patients [6]. Other mouse strains preferentially develop disease when immunized with IRBP. The most susceptible strain is B10.RIII (H-2r), and its major pathogenic fragment, IRBP residues 161–180, has been identified and can be produced synthetically. Over the years, the EAU model has proved to be an invaluable tool to study basic mechanisms in uveitis. Nevertheless, this model has limitations because mice must be immunized with retinal antigens in complete Freund’s adjuvant (CFA) and some strains also require pertussis toxin as additional adjuvant. Massive immune system stimulation by microbial products can alter immune responses and may not be physiological. Furthermore, by its very nature, the model is not suitable for studying natural triggers of the disease.
DEVELOPMENT OF A SPONTANEOUS MOUSE MODEL OF UVEITIS AND ITS CHARACTERIZATION
To address these limitations, we sought to generate a spontaneous model of uveitis. Because a higher precursor frequency of T cells that recognize retinal antigens may predispose to uveitis, we chose to generate transgenic (Tg) mice expressing a uveitogenic T cell receptor (TCR) specific to IRBP. Our strategy was to clone the TCR α and β chains from a highly uveitogenic T cell line, specific to the IRBP161–180 sequence, which encodes a major pathogenic epitope for B10.RIII mice, a highly EAU-susceptible strain, on the premise that a known pathogenic TCR would maximize the chances of spontaneous disease development (Fig. 1A, B). The TCR in question, which we called the R161 TCR, turned out to be Vα17/Vβ1. Since this TCR is not detectable by commercially available anti-TCR antibodies, we used an IRBP161–180/MHC class II/IgG1 dimer (p161 dimer) to identify the IRBP-TCR expressing T cells [7] (Fig. 1C). Using the same R161 TCR construct, we generated 3 TCR Tg strains, which differed in the percent of R161 TCR-positive cells in the thymus and periphery detectable by the p161 dimer, and named them according to the proportion and the expression level of IRBP TCR positive CD4+ T cells: R161H (high - around 80% in the thymus), R161M (medium - around 50%) and R161L (low - less than 10%) (Fig. 1D). The frequency of IRBP-specific TCR Tg T cells in the periphery paralleled the efficiency of their positive selection in the thymus, with again the highest frequency of TCR-positive cells in the R161H, and progressively lower frequencies in the R161M and R161L strains ([8] and Fig. 1D).
Fig. (1). Cloning of IRBP-specific TCR α and β chains to generate IRBP-specific TCR transgenic (R161) mice.
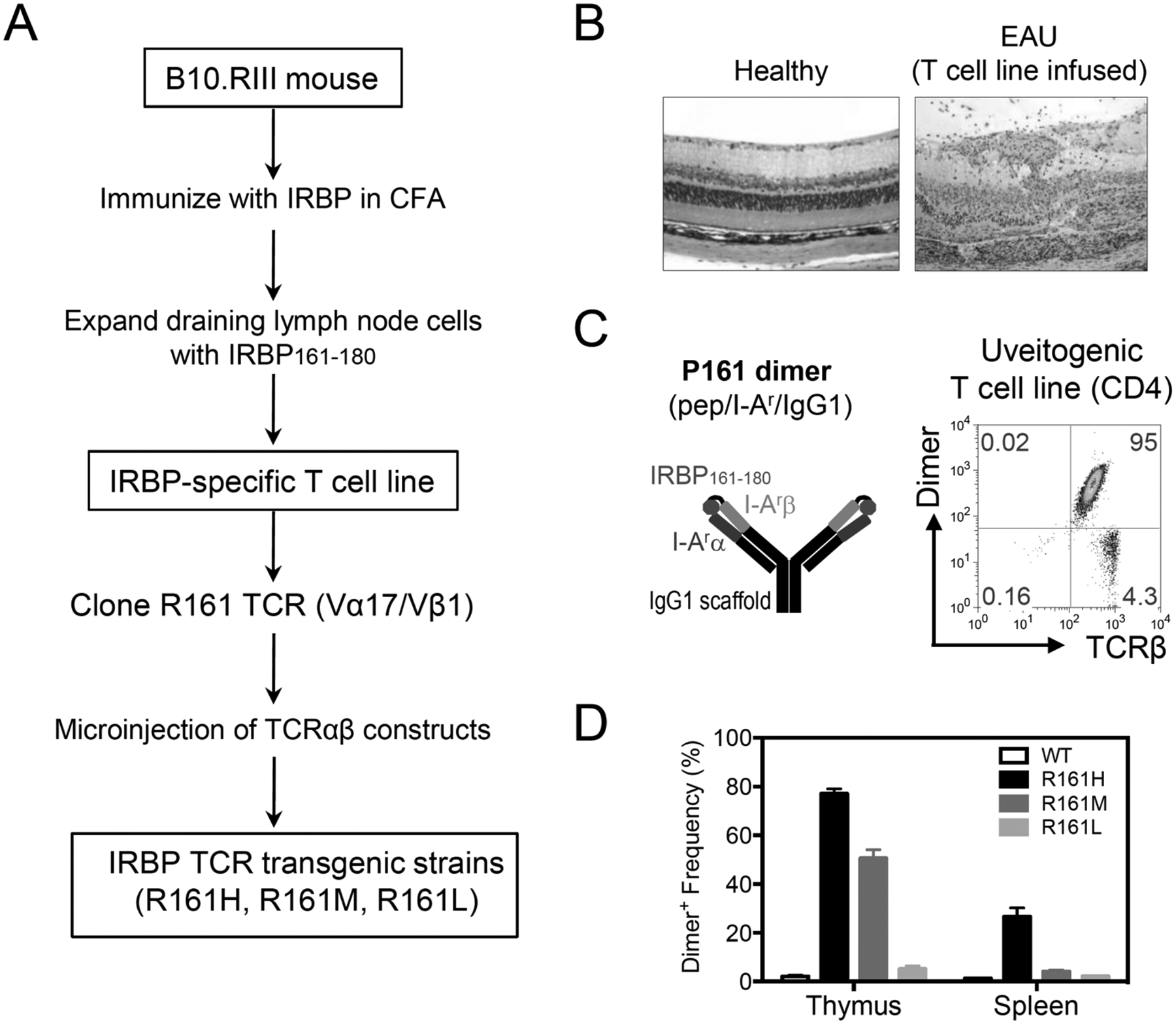
A. Scheme of R161 TCR cloning and transgenic mouse generation. IRBP161–180-specific CD4+ T cell line was established from lymphocytes of B10.RIII WT mice that had been immunized for EAU. R161 TCR was cloned from this line. B. Severe EAU induced in naïve mice by 1×10−6 cells from the uveitogenic T cell line. Compare the histology to healthy retina (original mag. x200). D. Frequency of the p161 dimer cells in thymus and spleen of R161 Tg strains, gated on CD4+ cells.
The high-expressing R161H strain and the medium-expressing R161M strain, but not the low-expressing R161L strain, spontaneously developed uveitis. Ocular inflammation in R161H and R161M strain started to be clinically detectable around 4 weeks of age, and became progressively more severe (Fig. 2A). Around 2–3 months of age for R161H and R161M, respectively, essentially 100% of the mice were positive for disease with a mean clinical score of 1.5 and 1 by fundus examination (which reflects mainly the intensity of inflammation) and with a mean pathology score of 3 and 1.5 by histopathology (which also quantitates damage to the retinal architecture) (Fig. 2B). Thus, disease incidence and severity paralleled frequency of IRBP-specific T cells.
Fig. (2). Spontaneous uveitis in R161 strains of IRBP TCR transgenic mice.

A. Clinical disease scores evaluated by fundus examination. B. Pathology scores evaluated by histology between ages of 8 and 16 wks. Incidence of each strain is shown in the parenthesis.
Analysis of the number of copies of the R161 TCR, present in the genome of each of the strains revealed the highest copy number in the R161H strain, and progressively fewer copies in the R161M and L strains. Similarly, the level of expression of the transgenic TCR on the individual T cell (i.e., fluorescence intensity after staining with labeled p161 dimer) also paralleled the number of copies, indicating that fewer molecules of the Tg TCR were expressed per lymphocyte in the different strains. This likely explains the observed differences in TCR expression and in the development of spontaneous disease in these strains.
Extensive phenotypic and immunological characterization done on R161H mice revealed that their eyes contained effector T cells of both the Th1 and Th17 phenotypes. Strikingly, unlike in the “classical” immunization-induced EAU model, double IFN-γ/IL-17 producing cells were rare (Fig. 3). The reason for this is unknown, but may reflect the possibility that due to the chronic disease course in these mice, cell fate decisions may already have occurred previously. T cells isolated from these mice proliferated and produced IL-2 in response to IRBP161–180 peptide in vitro, and transferred severe EAU to naïve WT mice as well as T cell-deficient RAG−/− mice in vivo [8]. Interestingly, however, even when mice were already positive for disease and exhibited a high proportion of effector-phenotype T cells in their eyes, most of the IRBP-specific T cells in the periphery remained naïve. Thus, these mice can serve as donors of naïve retina-specific T cells for basic immunological studies, examples of which will be given ahead.
Fig. (3). Effector cytokine profiles during uveitis in R161H mice vs EAU mice.
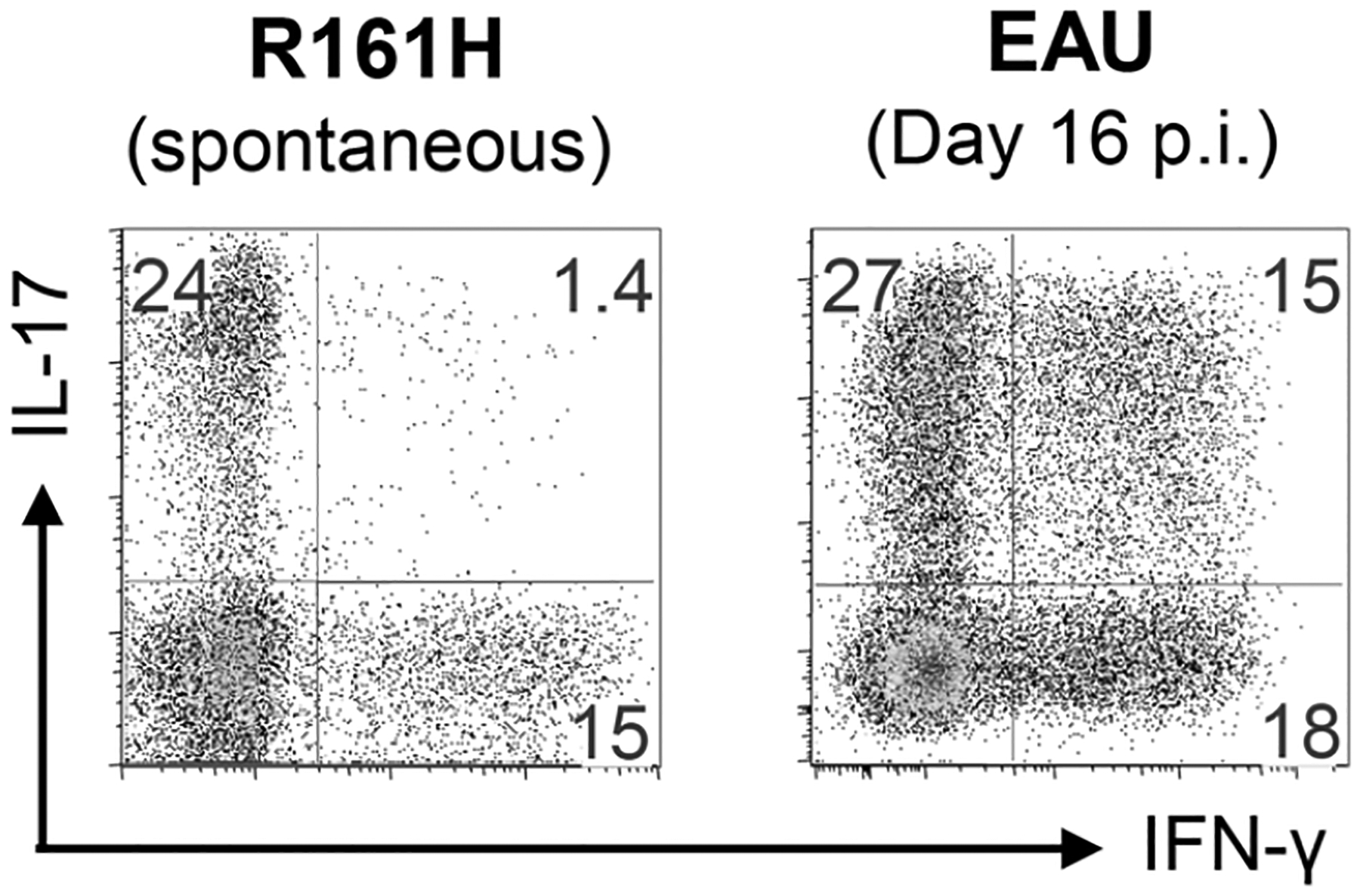
Eye-infiltrating cells of uveitic R161H mice and those of C57BL/6 mice immunized for EAU with IRBP in CFA (+PTX) were compared. Eye-infiltrating cells were stimulated ex vivo with PMA and Ionomycin in the presence of Brefeldin A for 4 h prior to the intracellular staining for IFN-γ and IL-17. Gated on CD4+ T cells. p.i., post immunization.
Examples how R161 TCR Tg Mice and IRBP-Specific T Cells Derived from them Can Be Used to Address Basic Questions in Ocular Immunology
What are the natural triggers of uveitis?
The R161L strain, which does not develop appreciable spontaneous uveitis throughout their life span, is an interesting strain in itself. Because it does have autoreactive T cells that respond to IRBP, but yet remains healthy under normal animal facility rearing conditions, this strain has potential to serve for the study of environmental triggers and endogenous regulatory mechanisms of uveitis. As an example, injecting these mice with “blank” CFA, containing only oil and a suspension of heat-killed mycobacteria, triggered a transient mild uveitis in these mice, which subsequently resolved spontaneously [8]. This demonstrates that a nonspecific perturbation of the immune system can cause a temporary lowering of the susceptibility threshold, which in a genetically predisposed individual can be sufficient to precipitate disease. Notably, incomplete Freund’s adjuvant, containing only oil but no mycobacteria, did not have this effect. Therefore, these findings specifically point to microbial stimuli, here represented by the mycobacteria incorporated into CFA, as potentially able to elicit disease. Inspired by this finding, we are currently using the R161H mice to study the role of endogenous commensal flora in the development of spontaneous uveítis [9].
The apparent contradiction of uveitis and ocular immune privilege:
The eye is an immunologically privileged organ. This means that normally it is devoid of migrating lymphocytes, which are largely excluded from the eye due to presence of the blood-retinal barrier (BRB). Furthermore, if a lymphocyte crosses the BRB and enters the eye, it encounters a profoundly immunosuppressive ocular environment, created by soluble inhibitory factors in ocular fluids and on the surface of ocular cells [10]. Ocular immune privilege is exploited clinically, and accounts for the extraordinary success of (tissue unmatched) corneal transplants, 90% of which are still in place at the end of one year [11]. Therefore, a question that has perplexed immunologists and vision researchers is, why does autoimmune uveitis develop despite ocular immune privilege. Retina-specific R161 TCR Tg T cells can be used to address this.
Infusion of naïve R161H cells into WT recipients does not induce uveitis, but if these cells have been stimulated in vitro with antigen before transfer, the recipients develop severe disease [8]. Together with other data not cited here, this has led to the conclusion that stimulated, i.e., antigen-experienced, T cells can actively enter the healthy eye and cause uveitis, whereas naïve T cells are excluded. However, naïve T cells do enter the eye following retinal capillary damage during a microtrauma or vascular abnormality, probably more often than is realized. As well, even if uveitogenic antigen-experienced T cells can cross the BRB, why are they not inhibited by the immunosuppressive ocular environment? To address these questions, we injected directly into the eye either naïve or antigen-experienced R161H T cells, purified from naïve or IRBP-immunized R161H donors, respectively. The donor T cells could be distinguished from the recipient’s own cells by the allotypic marker CD90.2. The results of a series of experiments showed that injected naïve R161H T cells were efficiently converted into FoxP3+ T-regulatory cells (Tregs) rather than to T-effector cells within the healthy eye, and did not cause ocular inflammation. In contrast, however, the antigen-experienced R161H T cells did not convert into Tregs and induced severe uveitis [12].
These data point to the nature and the limitations of ocular immune privilege. They demonstrate that immune privilege is effective in restraining naïve retina-specific T cells that have accidentally made their way into the eye due to a microtrauma or a vascular abnormality. However, immune privilege cannot control activated retina-specific T cells that have acquired effector function outside the eye and are able to actively penetrate the BRB. These findings help to explain why uveitis can occur despite immune privilege and provide new insights into the biology of uveitic disease.
Unraveling the effects of immunotherapy on uveitogenic Th1 and Th17 effector cells:
Our previous data demonstrated that EAU can be mediated either by Th1 or Th17 effector responses, and that Th1 or Th17 cells are each independently able to cause uveitis upon adoptive transfer. In all models of uveitis both Th1 and Th17 cells are detectable, although one or the other can predominate [8, 13, 14]. We now know that T cell responses have inherent plasticity. At the single cell level, Th17 cells can convert to Th1. At the population level, a shift in either direction is possible, because these effectors draw from a common pool of naïve precursors. It is therefore conceivable that suppressing one effector pathway may drive the response towards the other pathway, making treatment ineffective. Clearly, the ideal approach would inhibit both Th1 and Th17.
One such approach is using IL-27p28 treatment for concurrent inhibition of both Th1 and Th17 responses by antagonizing the gp130 receptor, whose signaling is involved in promoting both these effector responses [15]. Mice immunized for EAU and treated with IL-27p28 develop strongly attenuated disease and reduced Th1 and Th17 responses (Fig. 4A). However, to mimic the clinical situation it was important to ask whether this treatment is effective in controlling already differentiated Th1 and Th17 effector T cells. To address this, we again used R161H mice as donors of antigen specific T cells, that were polarized in vitro to Th1 or Th17 and infused into naïve WT recipients. Untreated recipients developed severe uveitis, but in recipients treated with or genetically overexpressing IL-27p28, disease was attenuated (Fig. 4B, C). To examine mechanistically what happened to the Th1 and Th17 cells as a result of in vivo exposure to IL-27p28, we injected the in vitro polarized effector cells into CD90.1 congenic recipients, so that they could be retrieved several days later and analyzed for changes in gene expression (Fig. 4B, C). The data showed that after 4 days of in vivo exposure to IL-27p28 expression of numerous lineage-specific genes in these cells was downregulated, leading to the conclusion that IL-27p28 reduces lineage stability of Th1 and Th17 effector T cells, which may help to explain its therapeutic effect.
Fig. (4). IL-27p28 attenuates EAU by suppressing Th1 and Th17 lineages.

(A) Naïve WT mice were immunized with IRBP/CFA for EAU and treated with IL-27p28 expression plasmid (pmIL27p28) or recombinant IL-27p28 (p28). (B, C) Antigen specific T cells were obtained from R161H mice, polarized under (B) Th1 or (C) Th17 conditions, and transferred to naïve WT recipient mice, which were then treated with pmIL27p28 or p28. After the treatment, the donors cells were retrieved to investigate their Th1 and Th17 lineage specific genes expression by real time PCR. (Modified from Chong et al., 2014) [15].
SUMMARY
We have generated a new model of uveitis in IRBP-specific TCR Tg (R161) mice. Spontaneous uveitis develops in 2 of 3 Tg strains, whereas the third is largely disease-free, but has a low threshold of susceptibility. R161 mice thus provide a new model of spontaneous uveitis targeting a native retinal Ag that is distinct from the “classical” immunization-induced EAU model, and may represent some human uveitic diseases more faithfully than the “classical” model of uveitis. These mice can serve as a source of retina-specific autoreactive T cells to study basic mechanisms involved in ocular homeostasis, activation and function of pathogenic effector T cells. Finally, R161 TCR Tg mice can serve as a platform for development of novel therapeutic approaches, that complements and extends the immunization-induced EAU model and may be more appropriate as a clinical surrogate for some types of human uveitis.
ACKNOWLEDGEMENTS
The authors thank the NEI Genetic Engineering Core for assistance in generating the R161 TCR mouse strains. This study was supported by NIH/NEI Intramural funding, Project # EY000184.
ABBREVIATIONS
- BC
Birdshot Chorioretinopathy
- BD
Behçet’s Disease
- BRB
Blood-Retinal Barrier
- CFA
Complete Freund’s Adjuvant
- EAU
Experimental Autoimmune Uveitis
- IRBP
Interphotoreceptor Retinoid Binding Protein
- S-Ag
Retinal Soluble Antigen (retinal arrestin)
- TCR
T cell receptor
- Tg
Transgenic
- VKH
Vogt-Koyanagi-Harada’s disease
Biography
R.R. Caspi

Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica 2004; 218(4): 223–36. [DOI] [PubMed] [Google Scholar]
- [2].Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004; 111(3): 491–500; discussion. [DOI] [PubMed] [Google Scholar]
- [3].Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest 2010; 120(9): 3073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang Y, Xiao X, Li F, Du L, Kijlstra A, Yang P. Increased IL-7 expression in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci 2012; 53(2): 1012–7. [DOI] [PubMed] [Google Scholar]
- [5].Chi W, Zhu X, Yang P, et al. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest Ophthalmol Vis Sci 2008; 49(7): 3058–64. [DOI] [PubMed] [Google Scholar]
- [6].Mattapallil MJ, Silver PB, Mattapallil JJ, et al. Uveitis-associated epitopes of retinal antigens are pathogenic in the humanized mouse model of uveitis and identify autoaggressive T cells. J Immunol 2011; 187(4): 1977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karabekian Z, Lytton SD, Silver PB, Sergeev YV, Schneck JP, Caspi RR. Antigen/MHC class II/Ig dimers for study of uveitogenic T cells: IRBP p161–180 presented by both IA and IE molecules. Invest Ophthalmol Vis Sci 2005; 46(10): 3769–76. [DOI] [PubMed] [Google Scholar]
- [8].Horai R, Silver PB, Chen J, et al. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun 2013; 44: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity 2015; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol 2006; 7(4): 354–9. [DOI] [PubMed] [Google Scholar]
- [11].Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Influence of donor and histocompatibility factors on corneal graft outcome. Transplantation 1994; 58(11): 1210–6. [PubMed] [Google Scholar]
- [12].Zhou R, Horai R, Silver PB, et al. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J Immunol 2012; 188(4): 1742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med 2008; 205(4): 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol 2007; 178(9): 5578–87. [DOI] [PubMed] [Google Scholar]
- [15].Chong WP, Horai R, Mattapallil MJ, et al. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J Autoimmun 2014; 50: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


