Abstract
Background/aim
The most commonly prescribed anti-seizures medications (ASMs) for the treatment of epilepsy are currently topiramate, zonisamide, lacosamide, carbamazepine and levetiracetam. The objective of this study was to examine the correlation between preoperative, intraoperative, and postoperative metabolic acidosis and the use of ASMs prior to craniotomy operations.
Materials and methods
This retrospective cross-sectional study evaluated patients who underwent intracranial surgery with craniotomy under general anaesthesia between May 2020 and April 2023 and used ASMs. The patients were classified into four groups based on the pharmacological mechanisms of action of the ASMs administered before intracranial surgery (Group-I, zonisamide or topiramate; Group-II, lacosamide; Group-III, carbamazepine; Group-IV, levetiracetam). Metabolic acidosis severity was defined based on base excess (BE) levels: mild (-3 to -5), moderate (-5 to -10), and severe (below − 10). The study investigated the correlation between ASMs and the severity of metabolic acidosis in preoperative, intraoperative, and postoperative blood gas measurements.
Results
Out of 35 patients, 24 patients underwent intracranial surgery and 11 patients underwent epilepsy surgery. There were statistically significant differences in the severity of metabolic acidosis between preoperative (p < 0.001), intraoperative (p < 0.001) and postoperative (p = 0.01) groups. The preoperative mean BE of group-I was − 4.7, which was statistically lower than that of group-III (p = 0.01) and group-IV (p < 0.001). Intraoperatively and postoperatively, group-I had a mean BE of -7.5 and − 3.2, respectively, which was statistically lower than that of groups II (p = 0.007; p = 0.04), III (p = 0.002; p = 0.03), and IV (p < 0.001; p = 0.009). There was no statistically significant difference in BE between groups II, III and IV at all three time points. Group I had the lowest BE at all three time points. Intraoperative bicarbonate was administered to all patients in group I, whereas no intraoperative bicarbonate was required in the other groups. In group I, 50% of patients required postoperative intensive care.
Conclusion
The use of ASMs in patients undergoing surgery is important in terms of mortality and morbidity. Topirimat and zonisamide are ASMs that can cause preoperative, intraoperative and postoperative metabolic acidosis. Patients receiving topirimat or zonisamide are particularly susceptible to metabolic acidosis. Special care should be taken in the management of anaesthesia in patients receiving these drugs, and monitoring of the perioperative metabolic status is essential.
Keywords: Anti-seizures medications, Antiepileptic drugs, Metabolic acidosis, Craniotomy, Topiramate, Zonisamide, Epilepsy
Background
Epilepsy is a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures [1]. Seizures in epilepsy are associated with increased morbidity. Prior to intracranial surgery, patients with epilepsy are prescribed various anti-seizures medications (ASMs) with different mechanisms of action [2]. These ASMs may cause intraoperative metabolic changes if not appropriately modified before surgery. The most commonly prescribed ASMs for the treatment of epilepsy are currently topiramate, zonisamide, lacosamide, carbamazepine and levetiracetam [3]. It is important to evaluate the postoperative intensive care requirements of patients since the perioperative pharmacological effects of these agents differ, which can affect mortality and morbidity.
Topiramate and zonisamide are sulphonamide derivative compounds used to treat epilepsy. Studies have shown that both drugs are potent carbonic anhydrase (CA) enzyme inhibitors [4, 5]. Recent case reports suggest that both drugs may cause metabolic acidosis by decreasing serum bicarbonate (HCO3) levels in some patients [6–9]. Lacosamide is a new generation ASM. There is currently no direct clinical evidence of metabolic acidosis due to lacosamide reported in the literature [5]. However, it has been reported that the coumarin binding site in CAs may interact with various compounds, such as locosamide [5]. Levetiracetam binds to synaptic vesicle protein 2 A (SV2A) and is presumed to interfere with the release of neurotransmitter stored in the vesicle [10]. The main effect of carbamazepine is to block voltage-sensitive sodium channels, thus stabilising the presynaptic neuron membrane and reducing the release of excitatory neurotransmitters, especially glutamate [10].
Metabolic acidosis is a serious condition that requires immediate treatment during surgery [11]. It is crucial to monitor and manage metabolic acidosis in surgical patients to prevent these complications. It can cause a decrease in cardiac output, electrolyte imbalances, surgical bleeding, and neurological complications, including coma and death [12]. Neurosurgical procedures carry a higher risk of metabolic acidosis due to longer operative times, greater fluid loss, and increased anaesthetic drug requirements [13]. Therefore, it is crucial to monitor the metabolic status of patients undergoing craniotomy surgery.
The objective of this study was to investigate the correlation between preoperative, intraoperative, and postoperative metabolic acidosis and the use of ASMs prior to craniotomy operations. The study highlights the significance of anaesthesia management in this context.
Materials and methods
Study design
This retrospective observational cross-sectional study was started after the ethics committee approval was obtained with the decision of the ethics committee of Trakya University Medical Faculty Ethics Committee (dated 22/05/2023 and numbered TÜTF-GOBAEK 2023/210). This study was registered with the ClinicalTrials.gov international protocol registration and results system under registration number NCT05940935 (21/06/2023). The study complies with the Declaration of Helsinki.
Participants
Between May 2020 and April 2023, a retrospective evaluation was conducted on 74 patients who underwent intracranial surgery with craniotomy under general anaesthesia and were using ASMs. The study included patients aged 18–75 years, with American Society of Anaesthesiologists (ASA) physical status classification I-II-III and undergoing elective surgery. Exclusion criteria for the study were patients with diabetes mellitus, diabetes insipidus, hepatic or renal disease, patients using drugs other than ASMs that may cause metabolic acidosis, patients who received more than two units of blood transfusion intraoperatively, and patients with a duration of surgery longer than 5 h. The study also excluded patients with missing data.
The patients were categorized into different groups based on the pharmacological mechanisms of action of the ASMs used before intracranial surgery. Group-I (n = 1/5) consisted of drugs with strong CA enzyme inhibition, namely zonisamide or topiramate. Group-II (n = 5) included new generation ASMs that are known to inhibit CA enzymes, such as lacosamide. Group-III (n = 4) comprised carbamazepine, while Group-IV (n = 20) consisted of levetiracetam.
Clinical characteristics, medications administered, intraoperative haemodynamic data, blood gas values, and postoperative care unit (PACU) and/or intensive care unit (ICU) follow-up records were obtained from the hospital system and patient files.
Metabolic acidosis severity was determined based on base excess (BE) levels: mild (-3 to -5 BE), moderate (-5 to -10 BE), and severe (BE level below − 10) [9]. The highest metabolic acidosis severity blood gas values from preoperative, intraoperative, and postoperative measurements were analyzed. The study investigated the correlation between this condition and prior use of ASMs.
Interventions
Preoperative management
Long-term use of ASMs may cause dysnatremia, thrombocytopenia, leucopenia, and impaired liver function [14]. Additionally, ASMs blocking the sodium channel may lead to Brugada-type ST changes and J wave abnormalities [14]. The evaluation of patients in the anaesthesia outpatient clinic considered the specified features. Our study recorded consultations for patients with pathological findings in laboratory parameters and electrocardiography. The recommendations of consulted departments were implemented. The seizure types of the patients, including the presence of aura and automatism, were recorded from their files (This information is crucial in evaluating postoperative seizure activity).
Intraoperative management
The fundamental principles of neuroanaesthesia involve maintaining sufficient cerebral perfusion pressure and avoiding increases in intracranial pressure [15]. Therefore, intraoperative patient management is crucial in neuroanaesthesia. Standard general anaesthesia procedures were used to monitor the patients after they were admitted to the operating table. All groups received completely standardised anaesthesia drugs during the intraoperative period. For the induction of anaesthesia, the patient was administered 2–3 mg/kg of propofol, 1 mg/kg of rocuronium, and 1–2 µg/kg of fentanyl. Anaesthesia maintenance was continued with 1 MAC of sevoflurane and a remifentanil infusion (0.25 mg/kg). To avoid resistance to neuromuscular blocking agents associated with prolonged use of ASMs [16], the muscle relaxant recuronium was administered every 30 min. During the operation, ventilation was maintained at a tidal volume of 8 ml/kg, with a fraction of inspired oxygen of 50%, and the ventilatory rate was adjusted to maintain a partial pressure of carbon dioxide (PaCO2) between 30 and 35 mm-Hg. Invasive arterial monitoring was performed by opening a large bore peripheral venous line in each patient. To ensure proper cerebral venous drainage, head positioning and stabilization were carefully maintained, avoiding excessive head rotation. Regular blood gas monitoring was conducted before and during the operation.
Postoperative management
Postoperative patient management was determined based on intraoperative course of operation, haemodynamic data, and blood gas analysis. Patients who were awakened during surgery were monitored in the PACU for at least 60 min after the operation. If their blood gas analysis was normal and they were haemodynamically stable, they were transferred to the ward. Otherwise, they were transferred to the postoperative ICU. Blood gases were taken 30 min after admission to the ICU and analysed as postoperative blood gas.
Outcomes
The primary outcome of the study was to determine the relationship between ASM use before craniotomy operation and the occurrence of preoperative, intraoperative and postoperative metabolic acidosis.
Statistical analysis
Continuous data were presented as mean and standard deviation (SD), while categorical data were presented as number (n) and percentage (%). The normal distribution of all variables was tested using the Shapiro-Wilk test. Numeric continuous variables were analyzed using Welch’s and Fisher’s One-Way ANOVA tests after checking group homogeneity with Levene’s test. The Post-Hoc Tukey Test and Post-Hoc Games-Howell Test were used to analyze differences between groups. Robust ANOVA analysis was used to identify numerical continuous outliers. Categorical data were analyzed using chi-square and Fisher’s Exact tests. Statistical analyses were conducted using IBM SPSS 27.0 (IBM, Armonk, NY). Differences with a P-value < 0.05 were considered statistically significant.
Significance values for multiple comparisons were adjusted using the Bonferroni correction. In the case of comparing four groups, P values below 0.012 were considered statistically significant. The P value was then multiplied by 4 and expressed at a significance level of 0.05.
Post-hoc power
A post-hoc power calculation was conducted based on the hypothesis that there is a relationship between the use of ASM and the development of intraoperative metabolic acidosis. The BE values of ASM that are anticipated to cause metabolic acidosis (Zonisamide, Topiramate, Lacosamide) and those that are not (Carbamazepine, Levetiracetam) were used in the calculation. The BE values were found to be -5.38 ± 3.19 in the drugs with expected metabolic acidosis (n = 11) and − 0.31 ± 2.38 in the drugs without expected metabolic acidosis (n = 24).
The calculation was performed using Version 3.1.9.2 of G*Power software, developed at Heinrich Heine University in Düsseldorf, Germany. The post hoc power of our study, with a two-sided type I error value of 0.05 and an effect size (d) factor of 1.5, was calculated to be 97%.
Results
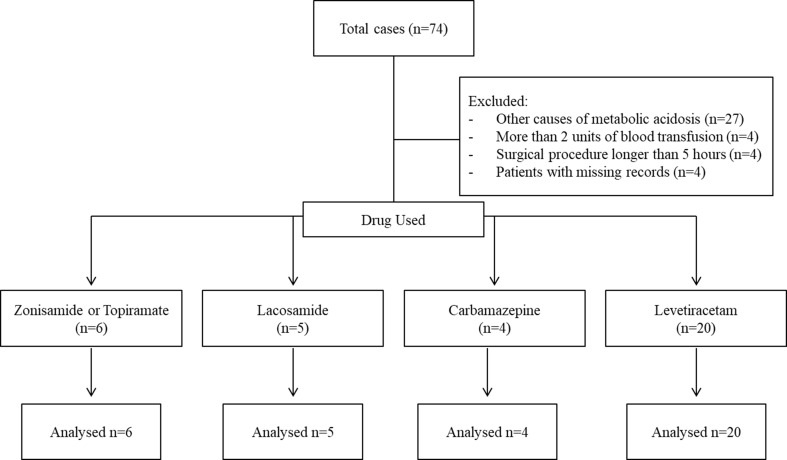
The retrospective analysis included the files of 35 patients who met the inclusion criteria (see Flow chart, Fig. 1). Although there were statistically significant differences between the groups in terms of age and gender (p < 0.001, p = 0.03), there were no significant differences in body mass index (BMI) and duration of surgical procedure (p = 0.29, p = 0.95). Of the 35 patients, 24 underwent intracranial surgery and 11 underwent epilepsy surgery. All patients who received zonisamide, topiramate, and lacosamide underwent epilepsy surgery. Of the patients included in the study, only three were in the ASA III group, while the remaining patients were in the ASA II group. Table 1 summarises the demographic and surgical characteristics of the patients.
Fig. 1.
Flow charts of the patients
Table 1.
Demographic and surgical characteristics of the patients
| Group-I | Group-II | Group-III | Group-IV | P value | ||
|---|---|---|---|---|---|---|
| Anti-seizures medication used |
Zonisamide n = 1 (%2,9) |
Topiramate n = 5 (%14,3) |
Lacosamide n = 5 (%14,3) |
Carbamazepine n = 4 (%11,4) |
Levetiracetam n = 20 (%57,1) |
|
| Age, year, mean ± SD | 20 | 32 ± 6 | 32 ± 4 | 55 ± 6 | 57 ± 10 | < 0.001 |
| Gender, n, female/male | 0 / 1 | 1 / 4 | 5 / 0 | 3 / 1 | 11 / 9 | 0.03 |
| ASA, n, II/III | 1 / 0 | 5 / 0 | 5 / 0 | 4 / 0 | 17 / 3 | |
| BMI, mean ± SD | 23 | 26 ± 2 | 25 ± 2 | 27 ± 1 | 28 ± 3 | 0.29 |
| Surgical pathology, n, ICS/Epilepsy surgery | 0 / 1 | 0 / 5 | 0 / 5 | 4 / 0 | 20 / 0 | |
| Duration of Surgery, min, mean ± SD | 300 | 222 ± 78 | 229 ± 70 | 237 ± 47 | 244 ± 53 | 0.95 |
Continuous variables are expressed as either the mean ± standard deviation (SD) and categorical variables are expressed as either frequency. Continuous variables were compared with Welch’s and Fisher’s One-Way ANOVA tests or Robust ANOVA test. Categorical variables were compared using Pearson’s chi-square test or fisher exact test. Statistically significant p-values are in bold ASA American Society of Anaesthesiologists, BMI body mass index, ICS intracranial surgery, min: minute, SD standard deviation
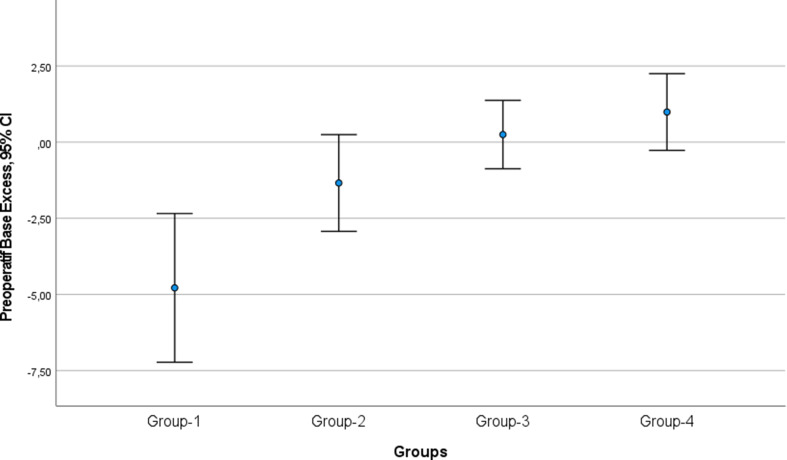
The preoperative blood gas values showed a significant difference in BE between the groups (p < 0.001, Table 2). Group-I had a mean preoperative blood gas BE of -4.7, which was − 5.0 (p = 0.01) lower than Group-III (mean BE 0.2 ± 0.7) and − 5.7 (p < 0.001) lower than Group-IV (mean BE 0.9 ± 2.6) (Fig. 2). However, there was no statistically significant difference between Group-I and Group-II (p = 0.09). Additionally, there was no statistically significant difference in preoperative BE between Groups II, III and IV (Table 2).
Table 2.
The patients’ use of anti-seizures medications, degree of metabolic acidosis, and intensive care requirements
| Group-I | Group-II | Group-III | Group-IV | P value | ||
|---|---|---|---|---|---|---|
| Anti-seizures medication used | Zonisamide n = 1 | Topiramate n = 5 | Lacosamide n = 5 | Carbamazepine n = 4 | Levetiracetam n = 20 | |
|
Concomitant Anti-seizures medication, n Levetiracetam Carbamazepine Carbamazepine + Levetiracetam Carbamazepine + Lacosamide Lamotrigine |
- - - - - |
- - 1 3 - |
1 2 1 - - |
4 - - - - |
- - - - 3 |
|
| Preoperative acidosis, n Mild/Moderate/Severe | 0/ 1 / 0 | 2 / 2 / 0 | 1 / 0 / 0 | 0 / 0 / 0 | 2 / 0 / 0 | < 0.001 |
| Intraoperative acidosis, n Mild/Moderate/Severe | 0 / 1 / 0 | 0 / 4 / 1 | 1 / 1 / 0 | 1 / 0 / 0 | 2 / 0 / 0 | < 0.001 |
| Postoperative acidosis, n Mild/Moderate/Severe | 1 / 0 / 0 | 2 / 0 / 0 | 0 / 0 / 0 | 0 / 0 / 0 | 3 / 0 / 0 | 0.01 |
| Intraoperative bicarbonate, n (%) | 1 (%100) | 5 (%100) | - (%0) | - (%0) | - (%0) | < 0.001 |
| Postoperative ICU, n (%) | 1 (%100) | 2 (%40) | - (%0) | - (%0) | 1 (%5) | 0.03 |
Categorical variables are expressed as either frequency (n) and percentage (%).Categorical variables were compared using Pearson’s chi-square test or fisher exact test. Statistically significant p-values are in bold. ICU Intensive Care Unit
Fig. 2.
Comparison of preoperative base excess of blood gases between groups (CI: confidence interval)
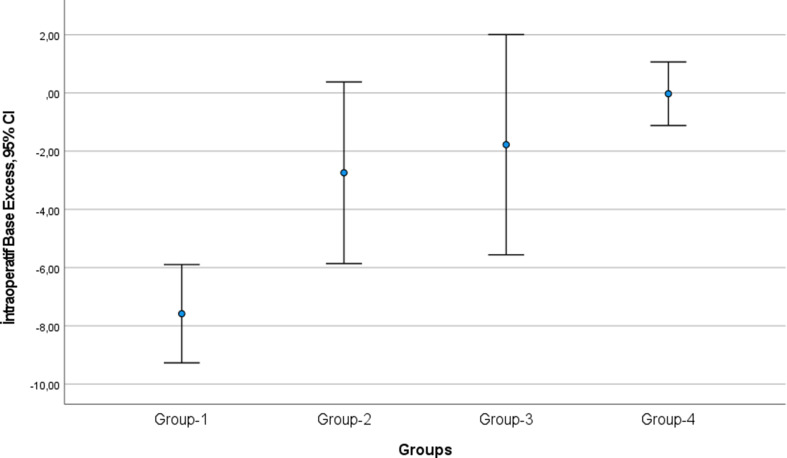
The study found a statistically significant difference in BE values between the groups during intraoperative blood gas analysis (p < 0.001, Table 2). Group-I had a mean intraoperative BE of -7.5, which was − 4.8 (p = 0.007) lower than Group-II (mean BE -2.7 ± 2.5), -5.8 (p = 0.002) lower than Group-III (mean BE -1.7 ± 2.3), and − 7.5 (p < 0.001) lower than Group-IV (mean BE 0 ± 2.3) (Fig. 3). However, there was no statistically significant difference in intraoperative BE between Groups II, III, and IV (Table 2).
Fig. 3.
Comparison of intraoperative base excess of blood gases between groups (CI: confidence interval)
A statistically significant difference was observed between the groups in terms of intraoperative bicarbonate administration (p < 0.001, Table 2). Intraoperative bicarbonate was administered to all patients in group-I, while it was not required in the other groups (refer to Table 2).
The postoperative blood gas values showed a significant difference in BE between the groups (P = 0.01, Table 2). Group-I had a mean postoperative BE of -3.2, which was − 1.8 (p = 0.04) lower than Group-II (mean BE -1.4 ± 0.6), -2.7 (p = 0.03) lower than Group-III (mean BE -0.5 ± 1.1) and − 2.7 (p = 0.009) lower than Group-IV (mean BE -0.5 ± 2.6) (Fig. 4). In the postoperative period, no statistically significant difference was found between Groups II, III and IV in terms of BE.
Fig. 4.
Comparison of postoperative base excess of blood gases between groups (CI: confidence interval)
A statistically significant difference was found between the groups regarding postoperative intensive care follow-up (p = 0.03, Table 2). In group-I, 50% of the patients required such follow-up.
None of the patients experienced dysnatremia, thrombocytopenia, leucopenia, impaired liver function tests, or electrocardiography (ECG) findings, which are side effects associated with long-term use of ASMs.
Discussion
In this study, preoperative, intraoperative and postoperative blood gas BE values were found to be statistically lower in the zonisamide and topiramate group, which are ASMs with strong CA enzyme inhibition, compared to patients using other ASMs. Intraoperative bicarbonate was given to all patients using zonisamide or topiramate. In addition, 50% of these patients required postoperative intensive care. The main finding of this study was the risk of metabolic acidosis during craniotomy in patients using ASMs such as zonisamide and topiramate, which have strong CA enzyme inhibition. Monitoring the perioperative metabolic status of this patient group is essential. It is of the utmost importance to exercise caution when administering anaesthesia to this group of patients, and to prioritise intraoperative and postoperative metabolic monitoring.
For patients with epilepsy that is refractory to medical treatment, epilepsy surgery is the best treatment option to improve seizure control [2]. The term ‘epilepsy surgery’ refers to procedures aimed at eliminating epileptogenic foci. Disconnection surgery and neuromodulation procedures are used to reduce the frequency and spread of seizures. Anaesthetic management of these procedures requires a thorough understanding of the underlying disease and a collaborative approach [2, 3]. Craniotomy increases the risk of fluid shifts and therefore requires more anaesthetic for prolonged surgery [11]. These factors may in turn increase pre-existing metabolic acidosis. In our study, a total of 24 patients who had undergone craniotomy for the removal of an intracranial mass and 11 patients who had undergone craniotomy for the treatment of epilepsy were included. The patients had similar operation times and intraoperative hemodynamic data. The effects of topiramate, zonisamide, and other ASMs on metabolic acidosis were compared.
Many different ASMs are used for epilepsy in patients before intracranial surgery. Topiramate and zonisamide have been reported to induce hyperchloremic normal anion gap metabolic acidosis in case reports and small cross-sectional studies [8, 17–19]. Two of these studies were conducted in neurosurgical patients and only topiramate was evaluated. In the study by Türe et al. [13] the effect of topiramate on the incidence and severity of metabolic acidosis was investigated by preoperative blood gas analysis in craniotomy patients, and in the other study [20], the results of blood gas analysis induced by chronic topiramate use were retrospectively evaluated intraoperatively and postoperatively in six neurosurgical patients. Wilner et al. [21] reported that a patient with intractable epilepsy and normal renal function developed a normal anion gap metabolic acidosis that worsened during elective surgery for temporal lobectomy. The authors speculated that the patient’s diarrhoea in the week before surgery exacerbated the clinical picture by increasing alkaline losses. They reported that the metabolic acidosis was caused by an increase in bicarbonate excretion by topiramate, which became clinically significant during surgery. They reported that these findings may be a side effect of topiramate during surgery and may become clinically significant with the use of another CA inhibitor and possibly with a ketogenic diet [21]. Granados et al. [22] reported on the incidence of metabolic acidosis and associated factors in outpatients receiving topiramate as monotherapy or adjunctive therapy for epilepsy. They evaluated 32 adults with epilepsy treated with topiramate as monotherapy or in combination for at least one month in an epilepsy outpatient clinic and found asymptomatic metabolic acidosis in all patients (HCO3 < 22 Eq/L). 9 patients received topiramate monotherapy and 23 patients received at least two ASMs. There was no correlation between bicarbonate levels and dose or duration of treatment. Bicarbonate levels were lower in patients taking more than one ASM. In conclusion, the authors reported that concomitant ASM use increased the known effects of topiramate on serum bicarbonate levels and the presence of metabolic acidosis, and that these effects were independent of the number of ASMs used [22]. In our study, we evaluated the relationship between ASMs and the development of metabolic acidosis in patients using ASMs and undergoing craniotomy. We found that BE levels were statistically significantly lower in the topiramate and zonisamide group preoperatively, intraoperatively and postoperatively, whereas BE levels were higher in the lacosamide, carbamazepine and levetiracetam group. Patients receiving ASMs may require surgery for both epilepsy and other intracranial pathology. Physicians should be aware of the possibility of antiepileptic-induced metabolic acidosis. This condition may be difficult to diagnose unless clinically suspected. Antiepileptic-induced acidosis should not be confused with malignant hyperthermia, hypoventilation or circulatory disturbance [21]. In addition to preoperative blood gas monitoring, it is also crucial to conduct intraoperative and postoperative follow-up assessments.
Zonisamide and topiramate are sulfonamide and sulfamate derivatives, respectively, that inhibit the CA enzyme by binding to its zinc ion [23]. The human kidney’s CA isoforms are crucial for reabsorbing ultrafiltered bicarbonate in the proximal tubule and acidifying urine in the distal tubule [24]. Low plasma potential hydrogen (pH) in patients taking topiramate or zonisamide and low bicarbonate levels in patients taking topiramate have been reported in case reports [7–9, 25] and small cross-sectional studies [26]. In general, a decrease in carbon dioxide in serum usually precedes a change in serum pH, so monitoring of carbon dioxide or bicarbonate is more accurate in detecting small changes in acid-base balance [6, 27]. A recent meta-analysis of studies comparing arterial and venous blood gas analyses has shown that venous blood gas analysis significantly overestimates the partial carbon dioxide value [28]. Therefore, venous plasma pH is not a reliable measure of acid-base status in patients undergoing intracranial surgery. Instead, serum bicarbonate or BE should be used to determine and quantify the presence of metabolic acidosis [6]. In our study, we used the BE value as a measure of metabolic acidosis. Considering that the blood gases obtained may be venous, especially in the postoperative period, we thought that BE evaluation would give more accurate results in order to avoid patient loss and to minimise the secondary margin of error.
Research has demonstrated that a ketogenic diet is as effective as ASM treatment in drug-resistant epilepsy [29]. It is important to note that when used in combination with a ketogenic diet, topiramate may increase the risk of metabolic acidosis [30]. Therefore, physicians and patients should be aware of this potential interaction. Additionally, it is worth noting that water restriction and acidosis, which can accompany a ketogenic diet, may increase the incidence of nephrolithiasis [21]. When topiramate is used in conjunction with a ketogenic diet, renal disease, dialysis, diarrhoea or surgery, there is a risk of severe metabolic acidosis due to a decrease in bicarbonate serum levels. The combination of topiramate with a ketogenic diet or acetazolamide is contraindicated due to the increased risk of metabolic acidosis and the potential for an increased occurrence of nephrolithiasis [27]. Bjurulf et al. [31] found that supplementing with potassium citrate, a mildly alkaline compound, may prevent metabolic acidosis in children with epilepsy treated with a ketogenic diet, without reducing antiepileptic efficacy. Citrate is an organic anion that produces bicarbonate when metabolised in the liver, acting as an alkalising agent [32]. It has been proposed as a substance that may reduce metabolic acidosis due to a ketogenic diet. Potassium citrate is used in renal diseases to prevent metabolic acidosis and improve bone mineral density [32]. It is important to note that this statement is based on current literature and further research is needed to confirm its efficacy. Future studies may determine the use of potassium citrate in reducing perioperative severe metabolic acidosis in patients using topiramate and undergoing craniotomy. This may result in a decrease in the severity of metabolic acidosis and the duration of the patients’ stay in the ICU.
The study has limitations. Firstly, it is a single-centre study with a small number of patients. Prospective studies with larger patient groups are needed. Another limitation is the use of BE value as a measure of metabolic acidosis. Evaluation of carbon dioxide or bicarbonate may provide additional results for the study. As our patient group consisted of those who underwent surgical procedures and received mechanical ventilator support and bicarbonate infusion when necessary, we did not rely on subjective evaluations. Another limitation is that we did not evaluate the ketogenic diet. Extensive studies are needed to evaluate the effectiveness of the ketogenic diet in this patient group. Furthermore, the surgical procedure performed differed between patients. This may have had an impact on the outcomes of the study.
Conclusion
Metabolic acidosis, whether acute or chronic, can have significant clinical consequences. It has been linked to impaired brain metabolism and cerebral blood flow, which may be of particular concern in neurosurgical patients. Possible side effects of acute metabolic acidosis include hyperventilation, fatigue, anorexia, nausea, delirium, and joint-muscle pain. Severe acute metabolic acidosis can lead to respiratory failure, reduced cardiac output, hypotension, arrhythmias, increased seizure activity, and coma.
Intraoperative and postoperative metabolic acidosis can be caused by topiramate and zonisamide. To stabilize intraoperative hemodynamics, it is important to inquire about the ASMs used in the preoperative evaluation in terms of mortality and morbidity. Prior to intracranial surgery, it is important to evaluate the interchangeability of these drugs with the guidance of an epileptologist and implement a treatment plan. It is also important to consider postoperative intensive care follow-up for patients using zonisamide and topiramate ASMs. It is evident that larger multicentre studies are required to gain further insight into this subject.
Acknowledgements
None.
Abbreviations
- %
Percentage
- ASA
American society of anaesthesiologists
- ASMs
Anti-seizures medication
- BE
Base excess
- BMI
Body mass index
- CA
Carbonic anhydrase
- ECG
Electrocardiography
- HCO3
Bicarbonate
- ICU
Intensive care unit
- n
Number
- PaCO2
Partial pressure of carbon dioxide
- PACU
Postoperative care unit
- pH
Potential hydrogen
- SD
Standard deviation
- SV2A
Synaptic vesicle protein 2 A
Author contributions
Conceptualization, O.K.; methodology, O.K. and S.H.Ş.; validation, O.K.; formal analysis, O.K.; investigation, B.T.; resources, B.T.; data curation, B.T.; writing—review and editing, O.K. and S.H.Ş.; supervision, S.H.Ş.; project administration, S.H.Ş. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval was obtained from the ethics committee at the Trakya University Medical Faculty Ethics Committee (protocol number TÜTF-GOBAEK 2023/210; dated 22/05/2023). Trakya University Medical Faculty Ethics Committee waived the need for informed consent because the current study was retrospective. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration (as revised in 2013) and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr., Forsgren L, French JA, Glynn M, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 2.Rugg-Gunn F, Miserocchi A, McEvoy A. Epilepsy surgery. Pract Neurol. 2020;20(1):4–14. [DOI] [PubMed] [Google Scholar]
- 3.Hakami T. Neuropharmacology of antiseizure drugs. Neuropsychopharmacol Rep. 2021;41(3):336–51. 10.1002/npr2.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winum JY, Poulsen SA, Supuran CT. Therapeutic applications of glycosidic carbonic anhydrase inhibitors. Med Res Rev. 2009;29(3):419–35. 10.1002/med.20141 [DOI] [PubMed] [Google Scholar]
- 5.Temperini C, Innocenti A, Scozzafava A, Parkkila S, Supuran CT. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem. 2010;53(2):850–4. 10.1021/jm901524f [DOI] [PubMed] [Google Scholar]
- 6.Mirza NS, Alfirevic A, Jorgensen A, Marson AG, Pirmohamed M. Metabolic acidosis with topiramate and zonisamide: an assessment of its severity and predictors. Pharmacogenet Genomics. 2011;21(5):297–302. 10.1097/FPC.0b013e3283441b95 [DOI] [PubMed] [Google Scholar]
- 7.Philippi H, Boor R, Reitter B. Topiramate and metabolic acidosis in infants and toddlers. Epilepsia. 2002;43(7):744–7. 10.1046/j.1528-1157.2002.37201.x [DOI] [PubMed] [Google Scholar]
- 8.Ozer Y, Altunkaya H. Topiramate induced metabolic acidosis. Anaesthesia. 2004;59(8):830–830. 10.1111/j.1365-2044.2004.03884.x [DOI] [PubMed] [Google Scholar]
- 9.Burmeister JE, Pereira RR, Hartke EM, Kreuz M. Topiramate and severe metabolic acidosis: case report. Arq Neuropsiquiatr. 2005;63(2B):532–4. 10.1590/S0004-282X2005000300032 [DOI] [PubMed] [Google Scholar]
- 10.Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. 10.1016/j.neuropharm.2020.107966 [DOI] [PubMed] [Google Scholar]
- 11.Waters JH, Miller LR, Clack S, Kim JV. Cause of metabolic acidosis in prolonged surgery. Crit Care Med. 1999;27(10):2142–6. 10.1097/00003246-199910000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Fleisher LA, Roizen MF, Roizen J. Essence of Anesthesia Practice E-Book. Elsevier Health Sciences; 2017.
- 13.Türe H, Keskin Ö, Çakır Ü, Aykut Bingöl C, Türe U. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376–80. 10.1177/0300060516669897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin C, O’Brien D, Maheshwari D. Anaesthesia for Epilepsy surgery. BJA Educ. 2019;19(12):383. 10.1016/j.bjae.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jellish WS, Edelstein S. Neuroanesthesia. Handb Clin Neurol. 2014;121:1623–33. 10.1016/B978-0-7020-4088-7.00106-1 [DOI] [PubMed] [Google Scholar]
- 16.Soriano SG, Martyn J. Antiepileptic-Induced Resistance to Neuromuscular blockers. Clin Pharmacokinet. 2004;43(2):71–81. 10.2165/00003088-200443020-00001 [DOI] [PubMed] [Google Scholar]
- 17.Sacré A, Jouret F, Manicourt D, Devuyst O. Topiramate induces type 3 renal tubular acidosis by inhibiting renal carbonic anhydrase. Nephrol Dialysis Transplantation. 2006;21(10):2995–6. 10.1093/ndt/gfl251 [DOI] [PubMed] [Google Scholar]
- 18.Stowe CD, Bolliger T, James LP, Haley TM, Griebel ML, Farrar HC III. Acute mental status changes and hyperchloremic metabolic acidosis with long-term topiramate therapy. Pharmacotherapy: J Hum Pharmacol Drug Therapy. 2000;20(1):105–9. 10.1592/phco.20.1.105.34662 [DOI] [PubMed] [Google Scholar]
- 19.Izzedine H, Launay-Vacher V, Deray G. Topiramate-induced renal tubular acidosis. Am J Med. 2004;116(4):281–2. 10.1016/j.amjmed.2003.08.021 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez L, Valero R, Fàbregas N. Intraoperative metabolic acidosis induced by chronic topiramate intake in neurosurgical patients. J Neurosurg Anesthesiol. 2008;20(1):67–8. 10.1097/ANA.0b013e31815613ad [DOI] [PubMed] [Google Scholar]
- 21.Wilner A, Raymond K, Pollard R. Topiramate and metabolic acidosis. Epilepsia. 1999;40(6):792–5. 10.1111/j.1528-1157.1999.tb00781.x [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Granados VJ, Márquez-Romero JM. Topiramate in monotherapy or in combination as a cause of metabolic acidosis in adults with epilepsy. Rev Neurol. 2015;60:159–63. [PubMed] [Google Scholar]
- 23.De Simone G, Scozzafava A, Supuran CT. Which carbonic anhydrases are targeted by the antiepileptic sulfonamides and sulfamates? Chem Biol Drug Des. 2009;74(3):317–21. 10.1111/j.1747-0285.2009.00857.x [DOI] [PubMed] [Google Scholar]
- 24.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71(2):103–15. 10.1038/sj.ki.5002020 [DOI] [PubMed] [Google Scholar]
- 25.Inoue T, Kira R, Kaku Y, Ikeda K, Gondo K, Hara T. Renal tubular acidosis associated with zonisamide therapy. Epilepsia. 2000;41(12):1642–4. 10.1111/j.1499-1654.2000.001642.x [DOI] [PubMed] [Google Scholar]
- 26.Garris SS, Oles KS. Impact of topiramate on serum bicarbonate concentrations in adults. Annals Pharmacotherapy. 2005;39(3):424–6. 10.1345/aph.1E437 [DOI] [PubMed] [Google Scholar]
- 27.Montenegro MA, Guerreiro MM, Scotoni AE, Guerreiro CA. Predisposition to metabolic acidosis induced by topiramate. Arq Neuropsiquiatr. 2000;58(4):1021–4. 10.1590/S0004-282X2000000600007 [DOI] [PubMed] [Google Scholar]
- 28.Lim BL, Kelly AM. A meta-analysis on the utility of peripheral venous blood gas analyses in exacerbations of chronic obstructive pulmonary disease in the emergency department. Eur J Emerg Medicine: Official J Eur Soc Emerg Med. 2010;17(5):246–8. 10.1097/MEJ.0b013e328335622a [DOI] [PubMed] [Google Scholar]
- 29.Ruan Y, Chen L, She D, Chung Y, Ge L, Han L. Ketogenic diet for epilepsy: an overview of systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(9):1234–44. 10.1038/s41430-021-01060-8 [DOI] [PubMed] [Google Scholar]
- 30.Heo G, Kim SH, Chang MJ. Effect of ketogenic diet and other dietary therapies on anti-epileptic drug concentrations in patients with epilepsy. J Clin Pharm Ther. 2017;42(6):758–64. 10.1111/jcpt.12578 [DOI] [PubMed] [Google Scholar]
- 31.Bjurulf B, Magnus P, Hallböök T, Strømme P. Potassium citrate and metabolic acidosis in children with epilepsy on the ketogenic diet: a prospective controlled study. Dev Med Child Neurol. 2020;62(1):57–61. 10.1111/dmcn.14393 [DOI] [PubMed] [Google Scholar]
- 32.Starke A, Corsenca A, Kohler T, Knubben J, Kraenzlin M, Uebelhart D, Wüthrich RP, von Rechenberg B, Müller R, Ambühl PM. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clin J Am Soc Nephrology: CJASN. 2012;7(9):1461–72. 10.2215/CJN.01100112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.