Abstract
Background
Knockdown resistance (kdr) is one of the primary resistance mechanisms present in anopheline species. Although this mutation is largely spread across the Anopheles gambiae s.l. members, its prevalence in other species is still not well documented.
Methods
The present study investigated the distribution and allelic frequencies of kdr in An. gambiae s.l., An. pharoensis, and An. ziemanni samples collected in 2022 and 2023 in nine sites spread across five ecogeographical settings in Cameroon. Members of the An. gambiae complex were identified molecularly by polymerase chain reaction (PCR). kdr L1014F and L1014S alleles were screened by PCR and confirmed by sequencing.
Results
An. gambiae (49.9%), An. coluzzii (36.5%), and An. arabiensis (13%) were identified, and the frequency of the kdr L1014F was high in both An. gambiae and An. coluzzii in all sites. The kdr L1014F allele was detected for the first time in 8 out of 14 An. ziemanni samples examined and in 5 out of 22 An. pharoensis samples examined. The kdr L1014S allele was scarce and found only in the heterozygote “RS” state in An. arabiensis and An. gambiae in Yangah and Santchou.
Conclusions
The present study sheds light on the rapid expansion of the kdr L1014F allele in malaria vectors in Cameroon and stresses the need for surveillance activities also targeting secondary malaria vectors to improve the control of malaria transmission.
Graphical Abstract
Keywords: Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis, Anopheles pharoensis, Anopheles ziemanni, Cameroon, Insecticide resistance, kdr mutation, Malaria
Background
Malaria remains a major public health problem in Cameroon [1]. In 2022, there were over six million malaria cases reported in health care centers across the country. It is estimated that 24% of the 25 million Cameroonians have at least one malaria attack each year [2]. Disease incidence is estimated to vary between 100 and 196 per 1000 according to epidemiological records [1]. Despite the frequent distribution of bed nets across the country, there has not been a significant decline of malaria [1]. Among factors affecting vector control measure performance are the rapid expansion of insecticide resistance and the high diversity of vector populations, which display different feeding, resting, and biting behaviors [3]. Studies characterizing resistance mechanisms in vector populations indicated a rapid increase of insecticide resistance in Anopheles gambiae s.l. and An. funestus with multiple resistance profiles [4–7]. Recent studies also indicated a reduced level of insecticide susceptibility of several other anopheline species, including An. moucheti, An. coluzzii, An. nili, and An. rufipes to dichloro-diphenyl-trichloroethane (DDT) and pyrethroids [8–10]. Apart from An. gambiae s.l. and An. funestus for which resistance mechanisms have been extensively explored, few studies characterizing resistance mechanisms in other anopheline species have been undertaken [9].
Different mechanisms including metabolic, cuticular, and target site mutations [e.g., knockdown resistance (kdr)] drive resistance to insecticides in mosquitoes [11]. kdr mutations, among the most widely spread resistance mechanisms, consist in aminoacidic substitutions in the voltage-gated sodium channel (Vgsc) that reduce the binding and/or action of pyrethroids and DDT and, thus, result in a reduced susceptibility to these insecticides [12, 13]. This resistance mechanism is highly frequent in An. gambiae with two widespread resistance alleles: the L1014F allele widely distributed in West and Central Africa and the L1014S allele more frequent in Eastern Africa [14–16]. However, there are still not enough data on the distribution of these alleles in other Anopheles species. The present study investigated the distribution of these alleles in An. gambiae, An. coluzzii, An. arabiensis, An. pharoensis, and An. ziemanni mosquitoes collected across Cameroon.
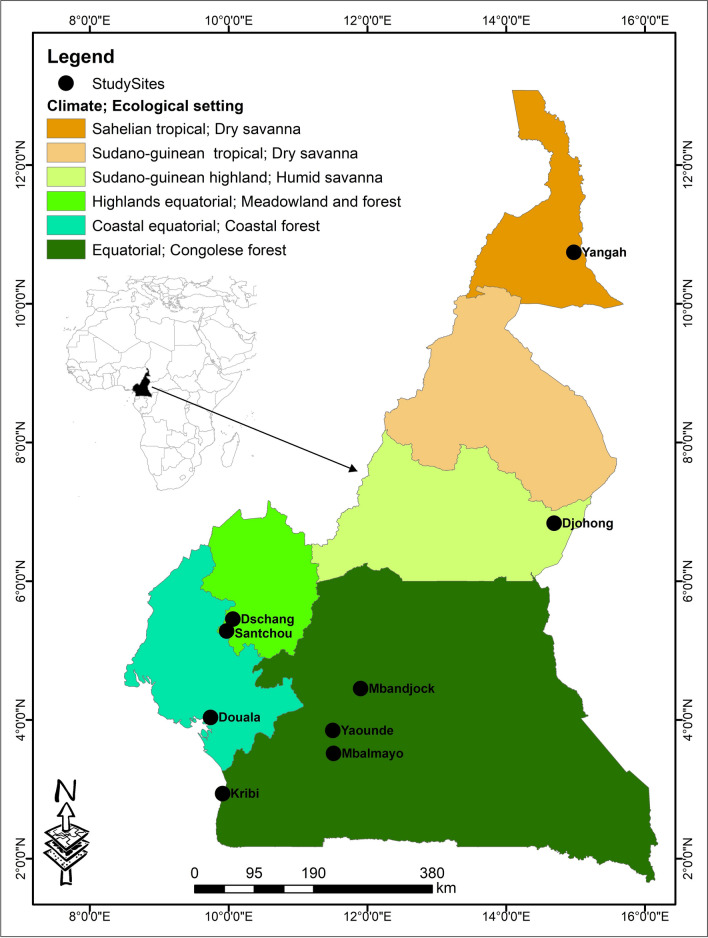
Mosquitoes were collected from nine locations belonging to five different ecogeographical areas in Cameroon (dry savanna, humid savanna, highlands, coastal, and forest) (Fig. 1 and Table 1) during the periods of September to November 2022 and June to August 2023 in the raining season, using different sampling methods, including Centers for Disease Control light traps, human landing catches, and Prokopack aspirators. Adult mosquitoes were identified morphologically using the identification keys of Gillies and Coetzee (1987) [17] and Gillies and De Meillon (1968) [18] and preserved in silica gel for molecular analyses.
Fig. 1.
A map of Cameroon showing the collection sites. The nine collection sites (black dots) are distributed in five of the six ecogeographical areas
Table 1.
Descriptions of the study sites
| Sites | Regions | Ecological settings | Climate | AAT (°C) | Seasons | Crops |
|---|---|---|---|---|---|---|
| Yangah | Far North | Dry savanna | Sahelian | 33 | 4 months rainy/8 months dry | Cotton, rice, millet, sorghum, maize |
| Djohong | Adamawa | Humid savanna | Sudano-Guinean highland | 24 | 7 months rainy/5 months dry | Cotton, coffee millet, sorghum |
| Dschang | West | Meadowland and forest | Highlands equatorial | 21.6 | 8 months rainy/4 months dry | Coffee, Irish potatoes, maize, cabbage, taro |
| Santchou | West | Meadowland and forest | Highlands equatorial | 22.5 | 8 months rainy/4 months dry | Maize, cassava, sweet potato, cocoyam, cocoa, coffee |
| Douala | Littoral | Coastal forest | Coastal equatorial | 27.5 | 9 months rainy/3 months dry | Cocoa, oil palm, rubber, plantain, banana, cassava, yams |
| Yaoundé | Center | Congolese forest | Equatorial | 24 | 9 months rainy/3 months dry | Cocoa, coffee, yams |
| Mbandjock | Center | Congolese forest | Equatorial | 26.5 | 7 months rainy/5 months dry | Sugarcane, oil palm, maize, cassava, yams |
| Mbalmayo | Center | Congolese forest | Equatorial | 25 | 9 months rainy/3 months dry | Cocoa, coffee, cassava, yams, maize |
| Kribi | South | Congolese forest | Equatorial | 26 | 9 months rainy/3 months dry | Cocoa, oil palm, rubber, cassava, plantains, yams |
AAT average annual temperature
DNA extraction was done with the JETFLEX Genomic DNA Purification Kit (Invitrogen by Thermo Fisher Scientific) following the manufacturer’s guidelines. Members of the An. gambiae complex were identified using the rapid high-throughput SYBR green assay described by Chabi et al. (2019) [19] and/or using the protocol of Favia et al. (2001) [20]. Some An. phaorensis and An. ziemanni samples were sequenced at cytochrome c oxidase subunit 1 (COI) loci for species confirmation [21].
A subset of mosquito species collected from each site were used for the screening of kdr alleles 1014L/S. Allele-specific polymerase chain reaction (AS-PCR) was used to detect L1014F (AS-PCR Agd3) and L1014S (AS-PCR Agd5) alleles as described by Verhaeghen et al. (2006) [22]. Some samples of An. gambiae s.l., An. ziemanni, and An. pharoensis were later Sanger sequenced for the confirmation of the presence/absence of the mutation at the Microsynth Company (Germany).
After checking the quality of the chromatograms, we blasted the sequences (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) and aligned them in reverse and forward direction using ClustalW (https://www.genome.jp/tools-bin/clustalw). The kdr L1014F and L1014S mutations were detected studying the picks of the chromatograms corresponding to the mutation sites.
A total of 649 Anopheles mosquitoes (An. gambiae s.l. (N = 507), An. pharoensis (N = 48), and An. ziemanni (N = 94)) were collected and examined. Anopheles gambiae s.l. samples (Djohong N = 48, Douala N = 64, Mbalmayo N = 39, Mbandjock N = 41, Santchou N = 47, Yangah N = 82, Yaoundé N = 66, Kribi N = 58, Dschang N = 62) were screened molecularly to the species level. PCR results revealed three species belonging to the An. gambiae complex: An. gambiae (49.9%), An. coluzzii (36.5%), and An. arabiensis (13%). A few hybrids (An. gambiae/An.coluzzii) were also recorded (0.6%).
Anopheles gambiae was recorded in almost all sites, while An. arabiensis was only found in Yangah together with An. pharoensis and An. ziemanni. In Mbanjock, Djohong and Dschang, only An. gambiae was found, whereas in Kribi, only An. coluzzi was registered. Both An. gambiae and An. coluzzii were found in sympatry in Mbalmayo, Yaoundé, and Douala.
The kdr allele L1014F was found at very high frequency in both An. gambiae (PQ000897) and An. coluzzii (PQ000899) in all sites, while only 2 An. arabiensis out of 59 were found with the allele (PQ000905) (Table 2). The kdr allele L1014S was scarce and detected only at the heterozygote “RS” state in An. arabiensis (PQ000906) and An. gambiae in Yangah and Santchou. One An. gambiae sample (PQ000898) was found with the double mutation L1014F/S (Table 2). It is noteworthy that the kdr allele L1014F was detected for the first time in An. ziemanni and An. pharoensis: out of the 14 An. ziemanni examined, 7 were found to be homozygotes “RR” (PQ000903) and 1 was heterozygote “RS” (PQ000901). Out of the 22 An. pharoensis examined, three were found to be homozygotes “RR” (PQ000907) and 2 were heterozygote “RS” (PQ000909) (Table 2). No kdr 1014S was detected in An. ziemanni and An. pharoensis.
Table 2.
Distribution of the kdr alleles L1014F/S in anopheline species collected in different sites across Cameroon
| L1014F | L1014S | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sites | Species | N | RR (%) | RS (%) | SS (%) | Allele freq | RR (%) | RS (%) | SS (%) | Allele freq |
| Yangah | An. coluzzii | 12 | 7(58.3) | 5(41.7) | 0(0) | 0.79 | 0 | 0 | 12(100) | 0 |
| An. arabiensis | 59 | 0(0) | 2(3.4) | 57(96.6) | 0.02 | 0 | 5(10) | 45(90) | 0.05 | |
| An. pharoensis | 22 | 3(13.6) | 2(9.1) | 17(77.3) | 0.18 | 0 | 0 | 0 | 0 | |
| An. ziemanni | 14 | 7(50) | 1(7.1) | 6(42.9) | 0.54 | 0 | 0 | 0 | 0 | |
| Djohong | An. gambiae | 20 | 20(100) | 0(0) | 0(0) | 1 | 0 | 0 | 20(100) | 0 |
| Santchou | An. gambiae | 20 | 20(100) | 0(0) | 0(0) | 1 | 0 | 1(5) | 19(95) | 0.03 |
| Douala | An. gambiae | 20 | 20(100) | 0(0) | 0(0) | 1 | 0 | 0 | 20(100) | 0 |
| An. coluzzii | 20 | 14(70) | 6(30) | 0(0) | 0.85 | 0 | 0 | 20(100) | 0 | |
| Yaounde | An. gambiae | 20 | 20(100) | 0(0) | 0(0) | 1 | 0 | 0 | 20(100) | 0 |
| An. coluzzii | 20 | 17(85) | 3(15) | 0(0) | 0.92 | 0 | 0 | 20(100) | 0 | |
| Mbandjock | An. gambiae | 20 | 20(100) | 0(0) | 0(0) | 1 | 0 | 0 | 20(100) | 0 |
| Mbalmayo | An. gambiae | 5 | 5(100) | 0(0) | 0(0) | 1 | 0 | 0 | 5(100) | 0 |
| An. coluzzii | 20 | 16(80) | 4(20) | 0(0) | 0.9 | 0 | 0 | 20(100) | 0 | |
| Kribi | An. coluzzii | 58 | 48(82.8) | 10(17.2) | 0(0) | 0.91 | 0 | 0 | 58(100) | 0 |
| Dschang | An. gambiae | 62 | 62(100) | 0(0) | 0(0) | 1 | 0 | 0 | 62(100) | 0 |
N sample size, Allele freq frequency of resistance allele
The present study objective was to investigate the presence of kdr alleles 1014F/S and their frequencies in mosquito samples collected from different parts of Cameroon. The study indicated a high prevalence of the kdr allele L1014F in both An. gambiae and An. coluzzii in all study sites. This result was similar to studies conducted so far in Cameroon reporting a high frequency of the kdr resistance allele in members of the An. gambiae complex [4, 23–25].
Interestingly, the allele L1014F was also detected for the first time in both An. ziemanni and An. pharoensis. These species are considered as secondary malaria vectors in Cameroon owing to their low implication in malaria transmission and their highly zoophilic and exophilic behavior [3, 26]. The presence of this mutation in these species could result from the high selective pressure induced using pesticides in agriculture. Indeed, the site of Yangah where An. ziemanni and An. pharoensis were sampled is a locality where rice, millet, and cotton are cultivated in large surfaces. The production of these crops requires the use of large quantities of pesticides [27, 28]. Although no bioassays were performed in the present study to evaluate the susceptibility of An. ziemanni and An. pharoensis to DDT and pyrethroids, previous studies conducted in the area and surrounding localities indicated a low susceptibility of local anopheline species to these insecticides [8, 10, 24]. It should be important for future studies to explore the presence of other resistance mechanisms also in secondary vector species as a recent study indicated the implication of cuticular resistance in An. pharoensis samples resistant to DDT [10].
Conclusions
The spread of kdr alleles in other anopheline species is problematic for the use of pyrethroids in public health. Even though, during the last distribution campaign new-generation bed nets, pyrethroid-piperonyl butoxide (PBO) (Olyset Plus®), and interceptor® G2 (IG2), combining pyrethroids with other active ingredients were distributed to combat resistant vector populations [1], the impact of this new control strategy is still awaited. The rapid expansion of resistance in vector populations must therefore continue to be the subject of particular attention, as it could compromise the control efforts implemented in the field.
Acknowledgements
We are grateful to the administrative and traditional authorities and the population from the different collection sites for their assistance. We also thank Mrs. Sonhafouo-Chiana Nadège, Mrs. Kaminsi Héléna, Mr. Ngangue Nasser, and Mr. Talipouo Abdou for their assistance during field work and Mr. Juluis Foyet for the conception of the map.
Author contributions
GF conceived the work. M.P.A.M., C.D., A.C., B.D., L.D.D., and M.I.K.N. were responsible for methodology; M.P.A.M., C.A.N., R.B., C.D., A.C., and G.F. were involved in the data curation and validation; M.P.A.M. and G.F. were responsible for conceptualization, project administration, and writing of the paper. C.A.N., C.D., V.P., I.R., and G.F. were involved in the writing—review and editing. G.F. supervised and provided resources. All authors reviewed the manuscript.
Funding
The research was funded by the Italian Ministry of University and Research (MUR) with the grant P20225TJWB to G.F. Marie Paul Audrey Mayi was supported by a long-term fellowship from the European Molecular Biology Organization (EMBO) (EMBO ALTF 369–2022). The funding bodies did not have any role in the experimental design, collection of data, analysis, interpretation of data, or writing of the manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
The study was conducted under ethical clearance no. 2020/04/1209/CE/CNERSH/SP delivered by the Cameroon National Ethics (CNE) Committee for Research on Human Health.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Institute of Statistics (NIS), National Malaria Control Program (NMCP), and DHS. 2023. Cameroon Malaria Indicator Survey 2022. Yaoundé, Cameroon, and Rockville, Maryland, USA: NIS, NMCP, and DHS. https://www.dhsprogram.com/publications/publication-MIS42-MIS-Final-Reports.cfm. Accessed 08 Jul 2023.
- 2.World malaria report 2022 Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 08 Jul 2023.
- 3.Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12:501. 10.1186/s13071-019-3753-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, Doumbe-Belisse P, Talipouo A, Djamouko-Djonkam L, et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit Vectors. 2017;10:472. 10.1186/s13071-017-2417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menze BD, Riveron JM, Ibrahim SS, Irving H, Antonio-Nkondjio C, Awono-Ambene PH, et al. Multiple insecticide resistance in the malaria vector Anopheles funestus from Northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS ONE. 2016;11:e0163261. 10.1371/journal.pone.0163261. 10.1371/journal.pone.0163261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamou R, Sonhafouo-Chiana N, Mavridis K, Tchuinkam T, Wondji CS, Vontas J, et al. Status of insecticide resistance and its mechanisms in Anopheles gambiae and Anopheles coluzzii populations from forest settings in South Cameroon. Genes. 2019;10:741. 10.3390/genes10100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonhafouo-Chiana N, Nkahe LD, Kopya E, Awono-Ambene PH, Wanji S, Wondji CS, et al. Rapid evolution of insecticide resistance and patterns of pesticides usage in agriculture in the city of Yaoundé Cameroon. Parasit Vectors. 2022;15:186. 10.1186/s13071-022-05321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamou R, Kopya E, Nkahe LD, Menze BD, Awono-Ambene P, Tchuinkam T, et al. Increased prevalence of insecticide resistance in Anopheles coluzzii populations in the city of Yaoundé, Cameroon and influence on pyrethroid-only treated bed net efficacy. Parasite. 2021;28:8. 10.1051/parasite/2021003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awono-Ambene PH, Etang J, Antonio-Nkondjio C, Ndo C, Eyisap WE, Piameu MC, et al. The bionomics of the malaria vector Anopheles rufipes Gough, 1910 and its susceptibility to deltamethrin insecticide in North Cameroon. Parasit Vectors. 2018;11:253. 10.1186/s13071-018-2809-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kala-Chouakeu NA, Ndjeunia-Mbiakop P, Ngangue-Siewe IN, Mavridis K, Balabanidou V, Bamou R, et al. Pyrethroid resistance situation across different eco-epidemiological settings in Cameroon. Molecules. 2022;27:6343. 10.3390/molecules27196343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–65. 10.1016/j.ibmb.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84. 10.1046/j.1365-2583.1998.72062.x [DOI] [PubMed] [Google Scholar]
- 13.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7. 10.1046/j.1365-2583.2000.00209.x [DOI] [PubMed] [Google Scholar]
- 14.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A, et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7:74. 10.1186/1475-2875-7-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AP, Santos JM, Martins AJ. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids—a review. Parasit Vectors. 2014;7:450. 10.1186/1756-3305-7-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson CS, Miles A, Harding NJ, O’Reilly AO, Weetman D, Kwiatkowski D, et al. The genetic architecture of target-site resistance to pyrethroid insecticides in the African malaria vectors Anopheles gambiae and Anopheles coluzzii. Mol Ecol. 2021;30:5303–17. 10.1111/mec.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillies MT, Coetzee M. Supplement to the Anophelinae of Africa south of the Sahara (afrotropical region). South Afr Inst Med Res. 1987;1:55–143. [Google Scholar]
- 18.Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara. South African Institute for Medical Research.1968;54–343.
- 19.Chabi J, Vant Hof A, Ndri LK, Datsomor A, Okyere D, Njoroge H, et al. Rapid high throughput SYBR green assay for identifying the malaria vectors Anopheles arabiensis, Anopheles coluzzii and Anopheles gambiae s.s. Giles PLoS One. 2019;14:e0215669. 10.1371/journal.pone.0215669. 10.1371/journal.pone.0215669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. 10.1046/j.1365-2583.2001.00236.x [DOI] [PubMed] [Google Scholar]
- 21.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–9. [PubMed] [Google Scholar]
- 22.Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16. 10.1186/1475-2875-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nwane P, Etang J, Chouaїbou M, Toto JC, Mimpfoundi R, Simard F. Kdr-based insecticide resistance in Anopheles gambiae s.s. populations in Cameroon: spread of the L1014F and L1014S mutations. BMC Res Notes. 2011;4:463. 10.1186/1756-0500-4-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etang J, Mandeng SE, Nwane P, Awono-Ambene HP, Bigoga JD, Ekoko WE, et al. Patterns of Kdr-L995F allele emergence alongside detoxifying enzymes associated with deltamethrin resistance in Anopheles gambiae s.l. from North Cameroon. Pathogens. 2022;11:253. 10.3390/pathogens11020253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piameu M, Nwane P, Toussile W, Mavridis K, Wipf NC, Kouadio PF, et al. Pyrethroid and etofenprox resistance in Anopheles gambiae and Anopheles coluzzii from vegetable farms in Yaoundé, Cameroon: dynamics, intensity and molecular sasis. Molecules. 2021;26:5543. 10.3390/molecules26185543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–21. 10.1093/jmedent/43.6.1215 [DOI] [PubMed] [Google Scholar]
- 27.Chouaïbou M, Etang J, Brévault T, Nwane P, Hinzoumbé CK, Mimpfoundi R, et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in Northern Cameroon. Trop Med Int Health. 2008;13:476–86. 10.1111/j.1365-3156.2008.02025.x [DOI] [PubMed] [Google Scholar]
- 28.Müller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008. 10.1371/journal.pgen.1000286. 10.1371/journal.pgen.1000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.