Abstract
Background
The number and proportion of HIV/AIDS patients among older people are continuously and rapidly increasing in China. We conducted a detailed molecular epidemiological analysis of HIV-1 epidemic strains in a developed city in eastern China and found that elderly people play a crucial role in the transmission of subtypes and high pretreatment drug resistance (PDR).
Methods
A total of 1048 samples were obtained from 1129 (92.8%) newly confirmed HIV-1-positive and treatment-naive patients between 2019 and 2023. The 1316 bp target fragment of the pol gene was amplified by reverse transcription polymerase chain reaction (RT‒PCR) and nested PCR, and Maximum-likelihood (ML) phylogenetic trees and molecular transmission network were constructed to analyse the subtypes and transmission clusters. Molecular transmission network was visualized using Cytoscape with the distance threshold of 0.0075. PDR-associated mutations were determined according to the Stanford University HIV Drug Resistance Database.
Results
A total of 933 pol sequences (89.0%, 933/1048) were successfully obtained, and twelve HIV-1 subtypes were detected. CRF07_BC was the predominant subtype, accounting for 48.1% (449/933) of sequences, followed by CRF01_AE (29.4%, 274/933). A total of 398 individuals (42.7%, 398/933) formed 89 clusters in the network. Multivariable logistic regression analysis revealed that age, nationality, subtype, and PDR were the most significant factors associated with clustering in the transmission network. The prevalence of PDR was 14.6% (136/933).PDR associated with non-nucleoside reverse transcriptase inhibitors (10.0%, 93/933) was much more common than that associated with nucleoside reverse transcriptase inhibitors (1.8%, 17/933) and protease inhibitors (3.2%, 30/933) (χ2 = 77.961, p < 0.001). The most frequent NNRTI mutations were K103N/S/KN/NS (52.2%, 71/136), which led to the highest proportion of high-level resistance to nevirapine and efavirenz (52.2%).
Conclusions
Our study revealed the important influence of elderly people on CRF07_BC transmission and the high prevalence of PDR. The clustering of drug-resistant cases was significant, which suggested the potential for localized widespread transmission of drug-resistant strains. HIV screening and the determination of PDR are recommended for older patients to improve early detection and reduce treatment failure and second-generation transmission.
Keywords: HIV-1, Subtype, Transmission network, Molecular epidemiology, PDR, Pretreatment drug resistance
Background
HIV/AIDS remains one of the most important threats to public health. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS) report in “THE PATH THAT ENDS AIDS”, the number of people living with HIV/AIDS (PLWH) globally by the end of 2022 was 39 million, with 1.3 million new infections and approximately 630,000 people dying from AIDS-related illnesses. According to the infectious disease surveillance data of the China Disease Control and Prevention Information System, by the end of 2022, there were 1.22 million living PLWH in China, among which 107,000 PLWH were newly diagnosed in 2022 [1]. China is facing the challenge of an ageing population. By 2022, individuals aged 50 years and older accounted for 36.8% of the total population [2]. At the same time, the number of PLWH aged 50 years and older continues to increase [3]. The AIDS epidemic in China is also facing challenges related to ageing: the proportion of people with newly reported HIV infections aged older than 50 years increased from 22% in 2011 to 44% in 2020 and reached 48.1% in 2022 [1]. However, there are few studies on HIV transmission among older adults. Men who have sex with men (MSM) are major drivers of HIV transmission in the developed eastern regions of China [4–6]. In contrast, the role of elderly people in HIV and drug resistance transmission might be significantly underestimated.
A molecular transmission network can be used to reveal HIV transmission relationships and patterns among infected individuals via phylogenetic analysis of viral sequences [7] based on the high genetic diversity of HIV-1. In recent years, the application of this network has shown initial results in detecting virus transmission and drug resistance as well as developing precise interventions for targeted populations. Multiple studies have revealed the diversity of HIV-1 prevalence in China, with regional distributions, transmission modes and different prevalences among high-risk groups [8, 9].
This study was designed to reveal the local transmission patterns of HIV though molecular epidemiological analysis, especially focused on the impact of older people on HIV and PDR transmission. This high proportion of sampling could reflect the characteristics of HIV-1 transmission in Shaoxing more comprehensively and reduce deviation and insufficiency. Our study aimed to provide evidence to inform the development of targeted intervention strategies.
Methods
Study population and data collection
From January 1, 2019, to September 30, 2023, 1048 samples were obtained from 1129 (92.8%) newly confirmed HIV-1-positive and treatment-naive patients by the Shaoxing Centers for Disease Control and Prevention (Shaoxing CDC) during the first follow-up visit. The plasma was separated on the day after sampling and stored at − 80 °C. The inclusion criteria were as follows: (1) were aged 16 years and older; (2) were newly diagnosed with HIV-1; and (3) had not received antiretroviral therapy (ART) before enrolment. Sociodemographic information was obtained from the National Center for AIDS/STD Control and Prevention (NCAIDS) information systems.
HIV nucleic acid extraction, amplification and sequencing
HIV RNA was extracted using an automated nucleic acid extraction machine (NP968-C system) and the Tianlong RNA Extraction Kit (Tianlong, Xian, China) according to the manufacturer's standard protocol. If the initial extraction was unsuccessful, another RNA extraction was performed using the High Pure Viral RNA Kit (Roche, Germany). The obtained RNA was amplified using a PrimeScript One Step RT‒PCR Kit (Takara, Dalian, China) for reverse transcription polymerase chain reaction (RT‒PCR) and nested PCR for the pol gene (HXB2: 2253–3306) as previously described [10]. After electrophoretic analysis, the amplified positive products were sent to BioGerm Biotechnology Co., Ltd., for purification and gene sequencing.
HIV subtype analysis
The sequences were assembled and adjusted with Sequencher v5.4.6 software (Genecodes, Ann Arbor, MI). Sequences with ≥ 5% ambiguous bases were excluded from the study. If several sequences from the same patient were available in the database, only the oldest was retained. The assembled sequences were aligned, edited, and analysed with BioEdit 7.2 software using references downloaded from the Los Alamos National Laboratory (LANL) HIV sequence database (https://www.hiv.lanl.gov). Maximum-likelihood (ML) phylogenetic trees for different HIV-1 subtypes were constructed with IQ Tree 2.0.6 under the GTR + G + I nucleotide substitution model. Sequences with bootstrap values ≥ 70% were determined to be the same subtype as the reference sequence. Sequences with possible intersubtype recombination were analysed using Simplot v 3.5.1. Then, the web-based tool iTOL v6.0 (https://itol.embl.de/) was used to visually edit the sequences for further analysis of geographic locations, phylogenetic clusters, and risk groups.
Genetic transmission network analysis
HIV Trace was used to calculate the genetic distances (GDs) based on the Tamura-Nei 93 (TN93) model. We performed analyses at different GD thresholds (ranging from 0.0025 to 0.015 at intervals of 0.0025) and selected the GD with the maximum number of transmission clusters. Cytoscape v3.7.2 software was used to visualize the HIV molecular transmission network. Each node in the network represents an individual pol sequence or case, and each edge represents the propagation correlation between the nodes. The greater the number of edges is, the greater the inferred communication relationship with more people, and the greater the transmission risk. In this study, according to the aggregation degree of nodes, sequences were divided into large clusters (LCs, ≥ 10 nodes), medium clusters (MCs, 4–9 nodes) and small clusters (SCs, 4–9 nodes). In addition, individuals with ≥ 4 links were identified as high-risk transmission individuals [11].
Drug resistance analysis
The pol sequences were submitted to the Stanford University HIV Resistance Database HIVdb Program (https://hivdb.stanford.edu/hivdb/bysequences/). Drug resistance mutations (DRMs) and their associated resistance levels were determined using the Stanford University HIV Drug Resistance Database as follows: susceptible, potential low-level resistance, low-level resistance, intermediate resistance, and high-level resistance, according to the Stanford penalty.
Statistical analysis
Descriptive statistics were performed on sociodemographic parameters to compare patients in transmission clusters, patients in LCs, and patients with high linkage. Categorical variables were compared using Pearson's chi-square test or Fisher's exact test. Univariate and multivariate logistic regression analyses were performed to identify factors associated with clustering and high-risk individuals. Significant variables identified from the univariate analysis (P < 0.10) were included in a multiple logistic regression model to determine the factors that influence HIV clustering. P values less than 0.05 were considered to indicate statistical significance. Data were analysed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA). P values were two-sided with a significance level of 0.05.
Results
Patient demographic characteristics
A total of 933 (89.0%, 933/1048) qualified sequences were obtained, representing 82.6% (933/1129) newly diagnosed HIV-positive patients in Shaoxing city from 2019 to 2023. As shown in Table 1, the patients were mainly male (82.1%, 766/933) and local residents (84.1%, 785/933); 52.9% (494/933) of patients were married, 33.8% (315/933) had a junior middle school education, 61.8% (577/933) were infected through heterosexual contact, and 48.1% (449/933) were infected with the CRF07_BC subtype. A total of 38.3% (357/933) of the individuals were older than 50 years, and the median age for the entire cohort was 44 years (ranging from 18 to 81 years). There was a significant difference in the composition of subtypes between the ≥ 50-year-old group and the < 50-year-old group (χ2 = 36.929, P < 0.001).
Table 1.
Sociodemographic Characteristics of newly diagnosed HIV-1 patients in Shaoxing from 2019 to 2023
| Categories | Cases(%) (n = 933) | HIV subtypes(%) | χ2 | P | ||
|---|---|---|---|---|---|---|
| CRF07_BC (n = 449) | CRF01_AE (n = 274) | Others (n = 210)a | ||||
| Collection time | 16.236 | 0.039 | ||||
| 2019 | 222(23.8) | 128(28.5) | 61(22.3) | 33(15.7) | ||
| 2020 | 181(19.4) | 77(17.1) | 53(19.3) | 51(24.3) | ||
| 2021 | 210(22.5) | 100(22.3) | 64(23.4) | 46(21.9) | ||
| 2022 | 189(20.3) | 88(19.6) | 56(20.4) | 45(21.4) | ||
| 2023 | 131(14.0) | 56(12.5) | 40(14.6) | 35(16.7) | ||
| Sex | 5.427 | 0.066 | ||||
| Male | 766(82.1) | 366(81.5) | 236(86.1) | 164(78.1) | ||
| Female | 167(17.9) | 83(18.5) | 38(13.9) | 46(21.9) | ||
| Age(years) | 52.198 | < 0.001 | ||||
| ≤ 25 | 124(13.3) | 75(16.7) | 33(12.0) | 16(7.6) | ||
| 26–39 | 266(28.5) | 127(28.3) | 103(37.6) | 36(17.2) | ||
| 40–49 | 186(19.9) | 89(19.8) | 56(20.4) | 41(19.5) | ||
| 50–59 | 223(23.9) | 103(22.9) | 53(19.4) | 67(31.9) | ||
| ≥ 60 | 134(14.4) | 55(12.3) | 29(10.6) | 50(23.8) | ||
| Ethnicity | 0.202 | 0.904 | ||||
| Ethnic Han | 882(94.5) | 426(94.9) | 258(94.2) | 198(94.3) | ||
| Others | 51(5.5) | 23(5.1) | 16(5.8) | 12(5.7) | ||
| Married status | 19.342 | 0.004b | ||||
| Single | 288(30.9) | 149(33.2) | 97(35.4) | 42(20.0) | ||
| Married | 494(52.9) | 235(52.3) | 132(48.2) | 127(60.5) | ||
| Divorced/Widowed | 145(15.5) | 62(13.8) | 42(15.3) | 41(19.5) | ||
| Unknow | 6(0.6) | 3(0.7) | 3(1.1) | 0 | ||
| Education | 36.202 | < 0.001 | ||||
| Junior college or above | 163(17.5) | 68(15.1) | 69(25.2) | 26(12.4) | ||
| High or technical secondary school | 198(21.2) | 113(25.2) | 58(21.2) | 27(12.9) | ||
| Junior middle school | 315(33.8) | 157(35.0) | 79(28.8) | 79(37.6) | ||
| Primary school/illiterate | 257(27.5) | 111(24.7) | 68(24.8) | 78(37.1) | ||
| Household registered | 5.374 | 0.251 | ||||
| Other provinces | 96(10.3) | 41(9.1) | 25(9.1) | 30(14.3) | ||
| Other cities in Zhejiang | 52(5.6) | 23(5.1) | 18(6.6) | 11(5.2) | ||
| Shaoxing city | 785(84.1) | 385(85.8) | 231(84.3) | 169(80.5) | ||
| Sexual contact | 53.657 | < 0.001b | ||||
| Heterosexual | 577(61.8) | 250(55.7) | 157(57.3) | 170(80.9) | ||
| Homosexual | 338(36.2) | 188(41.9) | 115(42.0) | 35(16.7) | ||
| IDUs | 4(0.4) | 4(0.9) | 0 | 0 | ||
| Unknow | 14(1.5) | 7(1.5) | 2(0.7) | 5(2.4) | ||
a: Others were CRF08_BC 105, CRF85_BC 31,CRF55_01B 23,B 13,C 7,CRF87_cpx 3,CRF57_BC 2,02_AG 1, URF(B/C) 1, URF (CRF01_AE/_B) 1 and URF (CRF01_AE/CRF07_BC) 23; b:Fisher’s exact test
HIV subtype distribution
Twelve HIV-1 subtypes and a proportion of unique recombinant forms (URFs) were identified from the 933 pol sequences. CRF07_BC was the predominant subtype, accounting for 48.1% (449/933) of sequences, followed by CRF01_AE (29.4%, 274/933). The other subtypes detected were CRF08_BC (11.3%, 105/933), CRF85_BC (3.3%, 31/933), CRF55_01B (2.5%, 23/933), B (1.4%, 13/933), C (0.8%, 7/933), CRF87_cpx (0.3%, 3/933), CRF57_BC (0.2%, 2/933), 02_AG (0.1%, 1/933), URF (CRF01_AE/CRF07_BC) (2.47%, 23/933), URF (CRF01_AE/B) (0.1%, 1/933), and URF (B/C) (0.1%, 1/933).
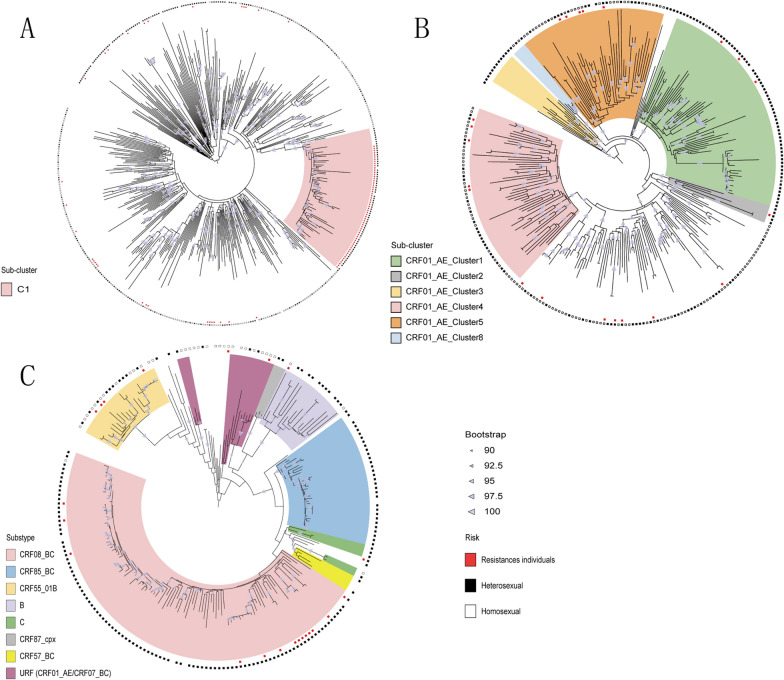
By constructing the ML phylogenetic trees of the different HIV-1 subtypes (Fig. 1), we found that there was a large evolutionary clade in the CRF07_BC subtype named C1. A total of 94.2% (65/69) of the CRF07_BC-C1 individuals were infected through heterosexual contact, 88.4% (61/69) were older than 50 years, and 88.4% (61/69) were drug resistant. CRF01_AE in Shaoxing city was homologous to several CRF01_AE epidemic clusters in China. The major subclusters were CRF01_AE Cluster1 (23.7%, 65/274), CRF01_AE Cluster4 (19.3%, 53/274) and CRF01_AE Cluster5 (8.6%, 24/274). The other detected subclusters were CRF01_AE Cluster8 (1.5%, 4/274), CRF01_AE Cluster2 (1.5%, 4/274), and CRF01_AE Cluster3 (1.8%, 5/274). Individuals in the largest subcluster, CRF01_AE Cluster1, were mainly infected through heterosexual transmission (95.4%, 62/65), and 63.1% (41/65) of the patients were over 50 years old. In addition to the two major HIV-1 subtypes, the other HIV-1 subtypes were concentrated among heterosexual individuals (81.0%, 170/210).
Fig. 1.
Maximum-likelihood phylogenetic trees of patients infected with different HIV-1 subtypes in Shaoxing city from 2019 to 2023. The phylogenetic trees based on the pol region of different HIV-1 subtypes were constructed by an approximate maximumlikehood method with IQ Tree 2.0.6 under the GTR+G+I nucleotide substitution model. Clades of different colours represent different HIV-1 subtypes and subclusters. The inner circle red colour square box represents ressistances individuals; the outer circles shows the risk group, Black colour square box represents heterosexual individuals, White colour square box represents homosexual individuals. A Maximum-likehood phylogenetic tree of CRF07_BC. B Maximum-likehood phylogenetic tree of CRF01_AE.C Maximim-likehood phylogenetic tree of other HIV-1 subtypes.
Genetic transmission network analysis
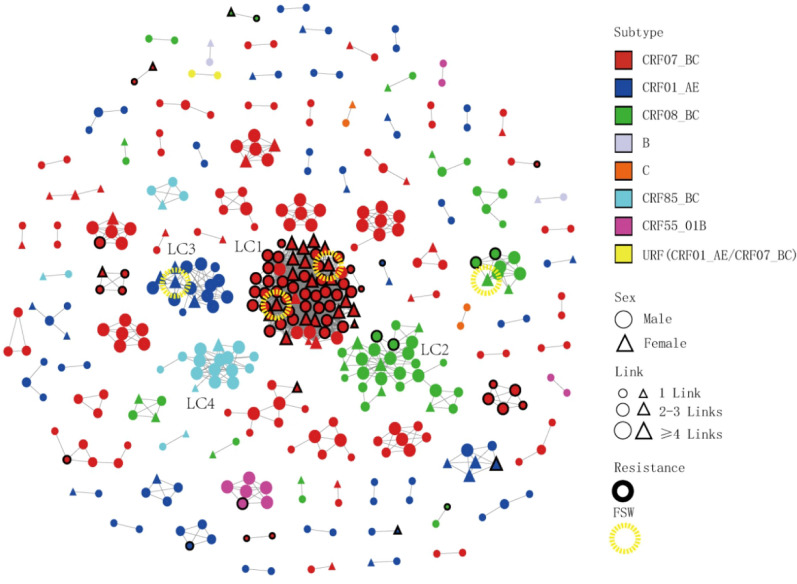
At the optimal GD of 0.0075 substitutions/site, 398 individuals (42.7%, 398/933) formed 98 clusters (mean 4.1, median 2.0, range 2–64, interquartile range 2–3.75) and 1534 edges (mean 7.7, median 3.0, range 1–59, interquartile range 1–5) (Table 2 and Fig. 2). Among the 98 clusters, the subtypes included CRF07_BC (51.3%, 204/398), CRF01_AE (22.4%, 89/398), CRF08_BC (15.3%, 61/398), CRF85_BC (6.0%, 24/398), CRF55_01B (2.5%, 10/398), B (1.0%, 4/398), C (1.0%, 4/398) and URF (CRF01_AE/CRF07_BC) (0.5%, 2/398). There were 4 LCs (4.1%, 4/98), 21 MCs (21.4%, 21/98), and 73 SCs (74.5%, 73/98). Eight MCs clustered patients who were diagnosed after 2021, and 18 clusters exhibited an increasing number of cases ≥ 3 from 2021 to 2023. CRF07_BC formed the most clusters (nodes ranging from 2 to 64) in the network. We also observed that 21.4% (85/398) of the PDR patients were included in the transmission network. A total of 65.9% (56/85) of the PDR patients were concentrated in the LC1 cluster (Table 2 and Fig. 2).
Table 2.
Molecular network clustering of different HIV-1 subtypes in Shaoxing city from 2019 to 2023
| Subtypes | Cases(n) | Clusters(n) | SCs(2–3 nodes) | MCs(4–9 nodes) | LCs(≥ 10 nodes) | Maximum cluster cases(n) |
|---|---|---|---|---|---|---|
| Total | 398 | 98 | 73 | 21 | 4 | – |
| CRF07_BC | 204 | 43 | 30 | 12 | 1 | 64 |
| CRF01_AE | 89 | 31 | 26 | 4 | 1 | 16 |
| CRF08_BC | 61 | 12 | 8 | 3 | 1 | 28 |
| CRF85_BC | 24 | 4 | 2 | 1 | 1 | 16 |
| CRF55_01B | 10 | 3 | 2 | 1 | 0 | 6 |
| B | 4 | 2 | 2 | 0 | 0 | 2 |
| C | 4 | 2 | 2 | 0 | 0 | 2 |
| URF(CRF01_AE/CRF07_BC) | 2 | 1 | 1 | 0 | 0 | 2 |
Fig. 2.
Molecular transmission network analysis of patients infected with different HIV-1 subtypes in Shaoxing city from 2019 to 2023. All edges represent a genetic distance between nodes of less than 0.0075 substitution/site, and the colour of the node indicates the different HIV-1 subtypes. Different shapes denote sex, and the mode size indicates the number of associated links. Thick border represents resistances individuals.  Represents FSW individuals
Represents FSW individuals
The proportions of PDR in patients with different subtypes were 35.8% (73/204), 4.5% (4/89), and 7.6% (8/105) for CRF07_BC, CRF01_AE, and other subtypes, respectively. The proportion in the CRF07_BC subtype was significantly greater than those of the CRF01_AE and other subtypes (χ2 = 52.148, p < 0.001)(Fig. 2).
Of the four LCs, the CRF07_BC cluster (LC1) had 64 nodes, the CRF08_BC cluster (LC2) had 28 nodes, the CRF01_AE cluster (LC3) had 16 nodes and the CRF85_BC cluster (LC4) had 16 nodes (Fig. 2). All four LCs were defined as elder heterosexual infection clusters. A total of 124 nodes were identified; 93.5% (116/124) were predominantly infected through heterosexual contact, and 80.6% (100/124) were aged 50 years or older. There were 34 females, 52.9% (18/34) of whom were infected by spouses or regular sexual partners. In addition, 4 female sex workers (FSWs) from other provinces were included in these clusters, including two patients in LC1, one patient in LC2 and one patient in LC3. The largest cluster (LC1) contained 64 nodes, a mean of 33 edges (median 39, range 1–59, interquartile range 18–47), and a mean GD of 0.0051 (median 0.0052, interquartile range 0.0042–0.0062). Among the 64 patients, 90.6% (58/64) were local residents, 85.9% (55/64) had a junior middle school education or below, 93.8% (60/64) were infected through heterosexual transmission, 87.5% (56/64) were drug resistant, and 32.8% (21/64) were female; most females (66.7%, 14/21) were detected after their spouses or regular sexual partners were confirmed to be HIV positive. The characteristics of the four LCs are shown in Table 3.
Table 3.
Summary statistics of the four largest Clusters in Shaoxing city from 2019 to 2023
| LC cluster | Cases (n) | subtype | Links Mean [Median, IQR] | Genetic distances Mean[Median, IQR] | Growing case(%) | Age ≥ 50y (%) | Male (%) | Shaoxing household registration(%) | Heterosexual(%) | PDR(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | PI | NRTI | NNRTI | |||||||||
| LC1 | 64 | CRF07_BC | 33 [39,18–47] | 0.0051[0.0052, 0.0042–0.0062] | 24 (37.5) | 10 (15.6) | 15 (23.4) | 13 (20.3) | 2 (3.1) | 44 (68.8) | 42 (65.6) | 58 (90.6) | 60 (93.8) | Q58QE 1(1.6) | 0 | K103N/S/NS Y188F 56(87.5) |
| LC2 | 28 | CRF08_BC | 4.2 [3.5,2.8–5.0] | 0.0049[0.0054, 0.0035–0.0069] | 3 (10.7) | 9 (32.1) | 11 (39.3) | 4 (14.3) | 1 (3.6) | 19 (67.9) | 22 (78.6) | 25 (89.3) | 26 (92.9) | 0 | V75A K65KN 2(7.1) | 0 |
| LC3 | 16 | CRF01_AE | 7.0 [6.0,5.0–9.0] | 0.0049[0.0052, 0.0035–0.0060] | 4 (25.0) | 2 (12.5) | 5 (31.3) | 2 (12.5) | 3 (18.8) | 14 (87.5) | 12 (75.0) | 13 (81.3) | 15 (93.8) | 0 | 0 | 0 |
| LC4 | 16 | CRF85_BC | 6.0 [5.5,3.0–8.3] | 0.0050[0.0054, 0.0042–0.0067] | 2 (12.5) | 5 (31.3) | 4 (25.0) | 2 (12.5) | 3 (18.8) | 13 (81.3) | 14 (87.5) | 14 (87.5) | 14 (87.5) | 0 | 0 | 0 |
Factors associated with clustering and high linkages
Of the individuals included in the clusters, 78.6%(313/398) were male, 50.0%(199/398) were ≥ 50 years old, 59.5%(237/398) were married, 51.3%(204/398) were infected with CRF07_BC, 88.2%(351/398) were local residents, and 67.8%(270/398) were infected through heterosexual transmission (Table 4). Multivariate logistic regression analysis revealed that age, nationality, subtype, and PDR were the most significant factors associated with clustering in the transmission network (Table 4). Patients aged ≥ 50 years were more likely to cluster within the network (adjusted odds ratio [AOR] = 1.868, 95% confidence interval [CI] 1.329–2.625). We also found that individuals with PDR were more likely to cluster than those without PDR (AOR = 2.219, 95% CI 1.491–3.301). Individuals in other ethnic groups (AOR = 0.464, 95% CI 0.232–0.925) and with CRF01_AE (AOR = 0.678, 95% CI 0.487–0.944) had significantly lower odds of clustering than Han individuals and patients with CRF07_BC.
Table 4.
Factor associated with clustering and high linkages of newly diagnosed HIV-1 patients in Shaoxing from 2019 to 2023
| Categories | Cluster cases(%) (n = 398) | Univariate analysis | Multivariate analysis | High-risk cases(%) (n = 157) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | AOR (95% CI) | P | OR (95% CI) | P | AOR (95% CI) | P | |||
| Age(years) | ||||||||||
| < 50 | 199(50.0) | 1.000 | 1.000 | 44(28.0) | 1.000 | 1.000 | ||||
| ≥ 50 | 199(50.0) | 2.386(1.821–3.127) | 0.000 | 1.868(1.329–2.625) | 0.000 | 113(72.0) | 4.629(2.991–7.164) | 0.000 | 5.149(2.689–9.859) | 0.000 |
| Sex | ||||||||||
| Male | 313(78.6) | 1.000 | 1.000 | 123(78.3) | 1.000 | |||||
| Female | 85(21.4) | 1.500(1.072–2.099) | 0.018 | 1.282(0.867–1.896) | 0.213 | 34(21.7) | 1.030(0.631–1.680) | 0.906 | ||
| Ethnicity | ||||||||||
| Ethnic Han | 398(96.7) | 1.000 | 1.000 | 154(98.1) | 1.000 | |||||
| Others | 13(3.3) | 0.442(0.232–0.841) | 0.013 | 0.464(0.232–0.925) | 0.029 | 3(1.9) | 0.450(0.122–1.661) | 0.231 | ||
| Married status | ||||||||||
| Single | 100(25.1) | 1.000 | 1.000 | 25(15.9) | 1.000 | 1.000 | ||||
| Married | 237(59.5) | 1.734(1.284–2.340) | 0.000 | 1.083(0.750–1.563) | 0.671 | 101(64.3) | 2.228(1.324–3.750) | 0.003 | 0.573(0.264–1.245) | 0.160 |
| Divorced/Widowed | 58(14.6) | 1.253(0.831–1.891) | 0.282 | 0.803(0.507–1.273) | 0.351 | 30(19.1) | 3.214(1.619–6.381) | 0.001 | 1.533(0.646–3.639) | 0.333 |
| Unknow | 3(0.8) | 1.880(0.373–9.486) | 0.445 | 1.095(0.176–6.830) | 0.922 | 1(0.6) | 1.500(0.130–17.257) | 0.745 | 0.076(0.004–1.288) | 0.074 |
| Education | ||||||||||
| Junior college or above | 51(12.8) | 1.000 | 1.000 | 11(7.0) | 1.000 | 1.000 | ||||
| High or technical secondary school | 82(20.6) | 0.152(1.004–2.399) | 0.048 | 1.439(0.914–2.265) | 0.117 | 24(15.3) | 1.505(0.663–3.415) | 0.328 | 1.407(0.547–3.617) | 0.478 |
| Junior middle school | 138(34.7) | 1.712(1.149–2.552) | 0.008 | 1.281(0.829–1.981) | 0.265 | 57(36.3) | 2.559(1.211–5.408) | 0.014 | 1.510(0.611–3.733) | 0.372 |
| Primary school/illiterate | 127(31.9) | 2.145(1.422–3.238) | 0.000 | 1.331(0.819–2.164) | 0.249 | 65(41.4) | 3.812(1.796–8.091) | 0.000 | 1.528(0.604–3.866) | 0.371 |
| Household registered | ||||||||||
| Other provinces | 32(8.0) | 1.000 | 1.000 | 15(9.6) | 1.000 | 0.231 | ||||
| Other cities in Zhejiang | 15(3.8) | 0.811(0.389–1.691) | 0.576 | 0.702(0.325–1.517) | 0.368 | 3(1.9) | 0.283(0.067–1.200) | 0.087 | ||
| Shaoxing city | 351(88.2) | 1.618(1.034–2.529) | 0.035 | 1.481(0.923–2.376) | 0.103 | 139(88.5) | 0.746(0.359–1.537) | 0.423 | ||
| Subtype | ||||||||||
| CRF07_BC | 204(51.3) | 1.000 | 1.000 | 101(64.3) | 1.000 | 1.000 | ||||
| CRF01_AE | 89(22.4) | 0.578(0.422–0.791) | 0.001 | 0.678(0.487–0.944) | 0.021 | 18(11.5) | 0.259(0.144–0.464) | 0.000 | 0.303(0.148–0.622) | 0.001 |
| Other | 105(26.4) | 1.201(0.865–1.667) | 0.274 | 1.185(0.829–1.683) | 0.353 | 38(24.2) | 0.578(0.357–0.938) | 0.026 | 0.410(0.214–0.786) | 0.007 |
| Sexual contact | ||||||||||
| Heterosexual | 270(67.8) | 1.000 | 1.000 | 125(79.6) | 1.000 | 1.000 | ||||
| Homosexual | 121(30.4) | 0.634(0.481–0.836) | 0.001 | 1.053(0.744–1.489) | 0.771 | 25(15.9) | 0.302(0.183–0.498) | 0.000 | 0.494(0.244–0.999) | 0.050 |
| Unknow | 7(1.8) | 0.724(0.277–1.893) | 0.510 | 0.632(0.222–1.799) | 0.390 | 7(4.5) | / | |||
| PDR | ||||||||||
| No | 313(78.6) | 1.000 | 1.000 | 95(60.5) | 1.000 | 1.000 | ||||
| Yes | 85(21.4) | 2.577(1.771–3.750) | 0.000 | 2.219(1.491–3.301) | 0.000 | 62(39.5) | 6.186(3.620–10.570) | 0.000 | 3.899(1.971–7.711) | 0.000 |
| Current place of residence | ||||||||||
| Yuecheng | 127(31.9) | 1.000 | 67(42.7) | 1.000 | 1.000 | |||||
| Keqiao | 113(28.4) | 1.521(0.767–3.018) | 0.230 | 48(30.6) | 0.661(0.397–1.102) | 0.112 | 1.232(0.654–2.320) | 0.519 | ||
| Zhuji | 84(21.1) | 1.169(0.589–2.317) | 0.656 | 25(15.9) | 0.379(0.212–0.680) | 0.001 | 0.822(0.401–1.686) | 0.593 | ||
| Shangyu | 22(5.5) | 0.833(0.417–1.664) | 0.604 | 3(1.9) | 0.141(0.040–0.502) | 0.002 | 0.469(0.112–1.967) | 0.301 | ||
| Shengzhou | 36(9.0) | 0.518(0.232–1.157) | 0.109 | 13(8.3) | 0.506(0.236–1.087) | 0.081 | 1.528(0.599–3.900) | 0.375 | ||
| Xingchang | 16(4.0) | 1.101(0.509–2.382) | 0.807 | 1(0.6) | 0.060(0.008–0.466) | 0.007 | 0.121(0.014–1.073) | 0.058 | ||
Others genotypes were CRF08_BC, CRF85_BC,CRF55_01B,B,C,CRF87_cpx,CRF57_BC,02_AG、URF(B/C)、URF(CRF01_AE/_B) and URF(CRF01_AE/CRF07_BC); PDR: pretreatment drug resistance; OR: odds ratio;AOR: adjusted odds ratio; red data represent P ≤ 0.05
We defined nodes with ≥ 4 links as high-risk individuals. Multivariate logistic regression analysis also revealed that age, subtype, and PDR were significant factors associated with high-risk individuals (Table 4). The results showed that the effect of age ≥ 50 years was 5.149 times greater than that of age < 50 years (95% CI = 2.689–9.859), that of individuals with PDR was 3.899 times greater than that of individuals without PDR (95% CI = 1.971–7.711), and that of CRF01_AE and other subtypes were 0.303 times (95% CI = 0.148–0.622) and 0.410 times (95% CI = 0.214–0.786) greater than that of CRF07_BC.
Drug resistance mutation analysis
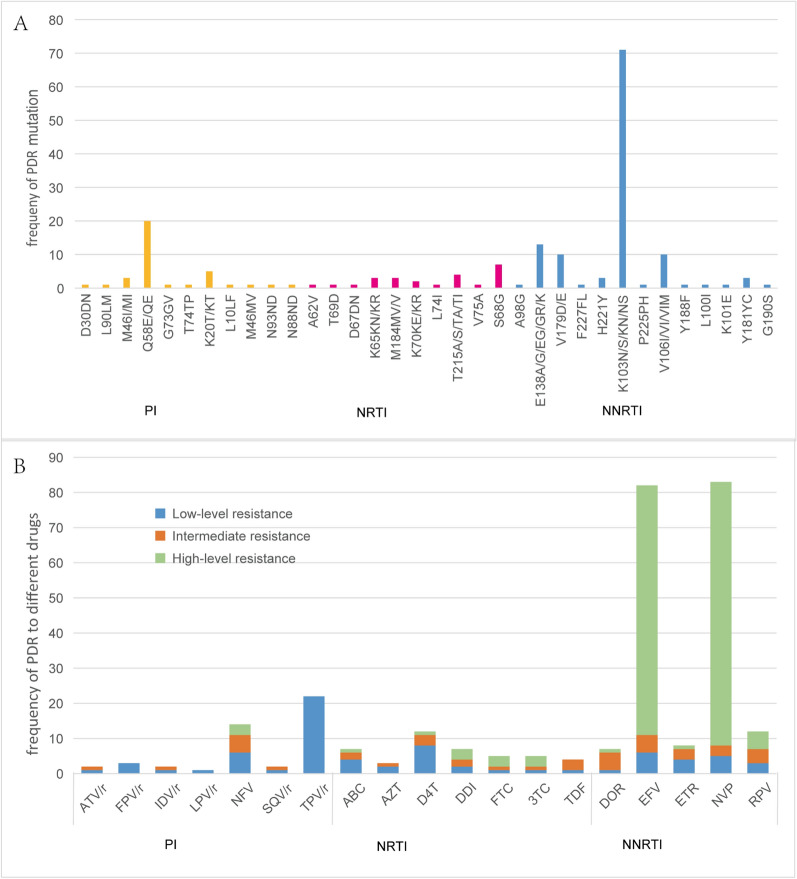
The prevalence of PDR among newly diagnosed patients was 14.6% (136/933). Eleven protease inhibitor (PI) resistance-associated mutation patterns, 10 nucleoside reverse transcriptase inhibitor (NRTI) mutation patterns, and 13 non-nucleoside reverse transcriptase inhibitor (NNRTI) mutation patterns were identified. The overall prevalence of HIV-1 PDR to NNRTIs (10.0%, 93/933) was much greater than that to NRTIs (1.8%, 17/933) and PIs (3.2%, 30/933) (χ2 = 77.961, p < 0.001). In individuals with NNRTI resistance, the most frequent mutations were K103N/S/KN/NS (52.2%, 71/136), which were responsible for the greatest proportion of high-level resistance to nevirapine (NVP) and efavirenz (EFV) (52.2%). In individuals with PI resistance, resistance to tipranavir (TPV/r) was the most common (16.2%, 22/136), which was mainly related to the Q58E/QE mutation (14.7, 20/136)(Fig. 3). Four (2.9%, 4/136) strains harboured two classes of drug resistance mutations. No HIV-1 strains with PDR mutations to three classes of drugs were found in this study.
Fig. 3.
A: Frequency of HIV-1 pretreatment drug resistance (PDR) mutations to PIs, NRTIs, and NNRTIs. B: Level of HIV-1 PDR associated mutations to different ART drug among 136 newly diagnosed HIV/AIDS patients. PI, Protease inhibitor; NRTI, Nucleoside reverse transcriptase inhibitor; NNRTI, Non-nucleoside reverse transcriptase inhibitor; ATV/r:Atazanavir/ritonavir; FPV/R:Fosamprenavir/ritonavir; IDV/r:Indinavir/ritonavir; LPV/r:Lopinavir/ritonavir; NFV:Nelfinavir; SQV/r:Saquinavir/ritonavir; TPV/r: Ritonavir/ritonavir; ABC: Abacavir; AZT: Zidovudine; D4T: Stavudine; DDI: Didanosine; FTC: Emtricitabine; 3TC, Lamivudine; TDF, Tenofovir; DOR, Doravirine; EFV, Efavirenz; ETR: Etravirine; NVP: Nevirapine; RPV: Rilpivirine
Discussion
A genetic transmission network based on the HIV-1 pol gene is widely used in HIV-targeted precision intervention [4, 12–14]. A high coverage rate of sample collection (≥ 60%) is conducive to avoiding bias caused by insufficient sampling [15]. Via consecutive cross-sectional sampling of a high proportion of newly diagnosed HIV-1 patients between 2019 and 2023 and a detailed molecular epidemiological approach, we constructed a molecular network for Shaoxing, a developed city in eastern China. We detected a high rate of infection, clustering in the transmission network, and a high number of linkages in elderly people (≥ 50 years) in Shaoxing. In addition, four large molecular clusters were identified in people over 50 years old, and the largest molecular cluster had a high proportion of patients with PDR (87.5%, 56/64), which has never been observed before, suggesting that people over 50 years of age are the main drivers of HIV transmission in Shaoxing. The spread of drug-resistant molecular clusters resulted in a high rate of PDR in Shaoxing, a typical epitome of the HIV epidemic in ageing people in China. Our study provides a basis for the assessment of epidemic development and the formulation of targeted prevention and intervention strategies.
The CRF07_BC subtype formed the largest number of clusters in the network (Table 2) and was present in significantly greater proportions in different years, male patients, and elderly people over 50 years old compared with the other subtypes (Table 1). These findings indicated that CRF07_BC spreads rapidly through people over 50 years old, which may be related to the transmission characteristics of CRF07_BC [16]. Its pathogenicity is less pronounced than that of the CRF01_AE and B subtypes [17], but it has greater transmission efficiency and slower disease progression in group of men who have sex with men. Therefore, CRF07_BC represents a new form of rapid expansion in China [16, 18, 19] and has become the most commonly detected subtype in many areas.
We found that older people (aged ≥ 50 years) were more likely to form clusters in the transmission network (50%, 199/398), and high-risk linkages (72.0%, 113/157) and individuals who carried the resistance mutation, i.e.,K103N (87.5%, 56/64), were part of the largest CRF07_BC cluster (LC1)(Tables 4 and 2). Most male (94.4%, 85/90) individuals in the four LCs self-reported nonmarital heterosexual sexual contact, which reflected elderly men, especially younger ones, still have psychological and physical needs [20]. Rich economic conditions, easier communication, convenient transportation and more open minds allow these individuals to experience nonmarital heterosexual sex through commercial or noncommercial sources for thrill seeking. All of these factors maintain the growth of commercial sex [21]. Most of the patients in clusters (67.8%, 204/398) and in high-risk groups (79.6%, 125/157) were infected through heterosexual transmission. Moreover, poor knowledge of AIDS prevention has resulted in decreased condom use, increasing the likelihood of HIV transmission between older male clients and FSWs [22] and of transmission from males to their spouses or regular sexual partners [23]. Our study conclusively confirmed this finding; we detected 4 FSWs from other provinces within the 4 LCs (Fig. 2), and 52.9% (18/34) of the female individuals in the four LCs were infected by their spouses or permanent partners (Table 3). All of these findings indicated that patients aged older than 50 years play an important role in HIV transmission in Shaoxing and should be monitored.
Since China had offered a free ART program for AIDS patients for more than 20 years, the rapid growth of PDR in recent years may have been underestimated due to a lack of resistance monitoring and testing before ART. Our study showed that PDR was significantly greater in Shaoxing (14.6%) than in Yunnan [24], Guangxi [25] and other severe HIV epidemic areas [26, 27]. According to the WHO definition of the prevalence of HIV drug resistance, our study revealed moderate (5–15%) rates of PDR in the Shaoxing region, which is extremely close to cut-off for a high rate of resistance (> 15%). Some studies have suggested that PDR is primarily driven by resistance to NNRTIs [28], which is consistent with our study results. The overall prevalence of HIV-1 PDR to NNRTIs reached 10.0% (93/933), which significantly differed from that of PI and NRTI (χ2 = 77.961, P < 0.001) in Shaoxing. Our study revealed a specific regional characteristic: 78.9% (56/71) of patients with K103N/S/KN/NS mutations in Shaoxing were older than 50 years, and 77.5% (55/71) were concentrated in the largest molecular cluster (LC1). The difference was significant (χ2 = 10.000, P = 0.001). These findings suggested that K103N/S/KN/NS mutations spread rapidly through the large molecular transmission cluster LC1 in patients over 50 years old, resulting in a significantly high proportion of PDR in Shaoxing. In addition to LC1, two drug resistance genes in CRF07_BC contained 4 and 5 nodes. The aggregation of drug-resistant strains is a target for intervention priority. Effective monitoring and intervention of the LC1 cluster could significantly reduce PDR in Shaoxing. Due to the lack of pretreatment resistance testing, patients who are experiencing drug resistance before treatment may be more prone to antiviral failure. Specifically, elderly people need more safe and complex treatment because of poor health.
Our study provided some insights and information to explore the transmission characteristics and epidemic patterns of HIV in ageing individuals. This study also inevitably has several limitations. First, our results represent the Shaoxing epidemic alone. Due to the limited activities of elderly people, it is believed that they are infected mainly by heterosexual contact in local places. Our sample had little influence from MSM or injection drug use. Although the epidemic among elderly people continues to grow across China, there are still significant differences between different regions. Therefore, future research should focus on a larger geographic range of older individuals with infections. Second, all of the patients were newly diagnosed, but we do not know exactly when they were infected. Elderly people have a higher rate of late diagnosis [29]. Thus, the network constructed may not reflect the true prevalence status. Finally, given limited time and funds, subtypes were identified based only on the pol gene sequence, which may not represent the exact subtype.
Conclusion
In summary, our study revealed the important influence of elderly people on the transmission of the CRF07_BC subtype and the high prevalence of PDR. Four clusters confirmed the transmission relationship between elderly men and FSWs and between elderly men and their spouses. The clustering of drug-resistant cases is significant, leading to the potential for localized widespread transmission of drug-resistant strains. Finally, a high prevalence of PDR could compromise the efficacy of ART and impact the overall management of the epidemic. Therefore, it is essential to conduct PDR testing for HIV patients and strengthen the dissemination of AIDS knowledge to older people.
Acknowledgements
We are thankful to the participants, primary clinicians and nurses for sample collection.
Abbreviations
- HIV
Human immunodeficiency virus
- AIDS
Acquired immunodeficiency syndrome
- PLWHA
People living with HIV/AIDS
- ART
Antiretroviral therapy
- WHO
World Health Organization
- PDR
Pretreatment drug resistance
- PCR
Polymerase chain reaction
- PI
Protease inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitor
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- ATV/r
Atazanavir/ritonavir
- FPV/r
Fosamprenavir /ritonavir
- IDV/r
Indinavir/ritonavir
- LPV/r
Lopinavir/ritonavir
- NFV
Nelfinavir
- SQV/r
Saquinavir/ritonavir
- TPV/r
Ritonavir/ritonavir
- ABC
Abacavir
- AZT
Zidovudine
- D4T
Stavudine
- DDI
Didanosine
- FTC
Emtricitabine
- 3TC
Lamivudine
- TDF
Tenofovir
- DOR
Doravirine
- EFV
Efavirenz
- ETR
Etravirine
- NVP
Nevirapine
- RPV
Rilpivirine
Author contributions
DC is responsible for sample detection,study design, analysis and writing article. HX, YF, JZ, and TH are responsible for guiding the whole study and revising the article. JL, JC, and JZ are responsible for performed experiments, statistics and figures, translation. All authors read and approved the final version of the paper.
Funding
This work was supported by Projects of Science Technology Bureau of Shaoxing (Grant number: 2018C30051, 2022A14007), and the 2022 Zhejiang Province Higher Education Curriculum Ideological and Political Education Research Project (Project Number: ZJSJ2022406).
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request, and with permission from Shaoxing Center for Disease Control and Prevention.
Declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Review Board of the Shaoxing CDC in China (SXCDC2018-002 and SXCDC2021-001). Participants provided written informed consent to use sequencing data both for HIV genetic transmission network and drug resistance studies. The study was conducted in accordance with the «Helsinki Declaration».
Consent for publication
Not applicable.
Competing interests
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui Xing, Email: xingh@chinaaids.cn.
Yi Feng, Email: fengyi@chinaaids.cn.
Tingting He, Email: winterdq616@163.com.
Jiafeng Zhang, Email: jfzhang@cdc.zj.cn.
References
- 1.Han MJ. Analysis of the epidemic situation and prospects of HIV/AIDS prevention and control in China. Chin J AIDS STD. 2023;29(3):247–50. [Google Scholar]
- 2.China NBoSo. China statistical yearbook; 2023.
- 3.Yue Q, Liu YF, Li H, Zhao Y. Characteristics and the first CD4(+)T lymphocytes test of newly-reported HIV/ AIDS cases aged 50 years and above in the third round of China comprehensive AIDS response program. Zhonghua Liu Xing Bing Xue Za Zhi. 2021;42(10):1823–8. [DOI] [PubMed] [Google Scholar]
- 4.Qin F, Zhang JF, Luo MY, Feng Y, Ge R, Yan Y, et al. Molecular genetics and epidemiological characteristics of HIV-1 epidemic strains in various sexual risk behaviour groups in developed Eastern China, 2017–2020. Emerg Microbes Infect. 2022;11(1):2326–39. 10.1080/22221751.2022.2119167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Guo Z, Pan X, Zhang WJ, Yang JZ, Ding XB, et al. Highlighting the crucial role of Hangzhou in HIV-1 transmission among men who have sex with men in Zhejiang, China. Sci Rep. 2017;7(1):13892. 10.1038/s41598-017-14108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZY, Zhang M, Zhang RF, Liu L, Shen YZ, Wang JR, et al. Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China. BMC Infect Dis. 2019;19(1):313. 10.1186/s12879-019-3927-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan AS, Pybus OG, Sanders EJ, Albert J, Esbjörnsson J. DefiningHIV-1 transmission clusters based on sequence data. AIDS. 2017;31(9):1211–22. 10.1097/QAD.0000000000001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong P. Progress in research and practice of molecular epidemiology of HIV-1. Electron J Emerg Infect Diseases. 2019;4(3):137–44. [Google Scholar]
- 9.Vrancken B, Zhao B, Li X, Han X, Liu H, Zhao J, et al. Comparative circulation dynamics of the five main HIV types in China. J Virol. 2020;94(23):e00683-e720. 10.1128/JVI.00683-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Guo Z, Yang J, Pan X, Jiang J, Ding X, et al. Genetic diversity of HIV-1 and transmitted drug resistance among newly diagnosed individuals with HIV infection in Hangzhou. China J Med Virol. 2015;87(10):1668–76. 10.1002/jmv.24223 [DOI] [PubMed] [Google Scholar]
- 11.Oster AM, Wertheim JO, Hernandez AL, Ocfemia MC, Saduvala N, Hall HI. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. JAIDS J Acquir Immune Defic Syndr. 2015;70(4):444–51. 10.1097/QAI.0000000000000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JF, Xu K, Jiang J, Qin F, Ding XB, Zhong P, et al. Combining molecular network analysis and field epidemiology to quantify local HIV transmission and highlight ongoing epidemics. Int J Infect Dis. 2023;128:187–93. 10.1016/j.ijid.2022.12.033 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Lu J, Zhang Z, Sun Q, Xu XQ, Hu HY. Characteristics of the different HIV-1 risk populations based on the genetic transmission network of the newly diagnosed HIV cases in Jiangsu, Eastern China. Heliyon. 2023;9(12): e22927. 10.1016/j.heliyon.2023.e22927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Yang Y, Liang N, Liang HY, Chen YZ, Lin ZS, et al. Transmission network and phylogenetic analysis reveal older male-centered transmission of CRF01_AE and CRF07_BC in Guangxi, China. Emerg Microbes Infect. 2023;12(1):2147023. 10.1080/22221751.2022.2147023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevention detecting and responding to HIV transmission clusters: A guide for health department. 2018.
- 16.Ye JR, Chen J, Wang J, Wang YC, Xing H, Yu FT, et al. CRF07_Bc is associated with slow hiv disease progression in Chinese patients. Sci Rep. 2022;12(1):3773. 10.1038/s41598-022-07518-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Z, Yan H, Li Q, Ablan SD, Kleinpeter A, Freed EO, et al. Enhanced transmissibility and decreased virulence of HIV-1 CRF07_BC may explain its rapid expansion in China. Microbiol Spectr. 2022;10(4): e0014622. 10.1128/spectrum.00146-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin PH, Lai CC, Yang JL, Huang HL, Huang MS, Tsai MS, et al. Slow immunological progression in hiv-1 Crf07_Bc-infected injecting drug users. Emerg Microbes Infect. 2013;2(12): e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge ZW, Feng Y, Li K, Lv B, Zaongo SD, Sun J, et al. CRF01_AE and CRF01_AE cluster 4 are associated with poor immune recovery in Chinese patients under combination antiretroviral therapy. Clin Infect Dis. 2021;72(10):1799–809. 10.1093/cid/ciaa380 [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Feng C, Feng L, Xiao TC, Zhao XR, Liu H, et al. An exploratory transmission mode of HIV/AIDS among older people based on data from multiple sources in China. Sci Rep. 2022;12(1):16077. 10.1038/s41598-022-20146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odimegwu CO, Mutanda N. Covariates of high-risk sexual behaviour of men aged 50 years and above in sub-Saharan Africa. SAHARA J. 2017;14(1):162–70. 10.1080/17290376.2017.1392340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Qin CW, Chen RF, Huang YX. Epidemiological profile and molecular genetic characterization of HIV-1 among female sex workers and elderly male clients in Guangxi. China Emerg Microbes Infect. 2021;10(1):384–95. 10.1080/22221751.2021.1888659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Hu D, Yin YQ, Zhu ZB, Wang N, Wang B. HIV prevalence and correlated factors among male clients of female sex workers in a border region of China. PLoS ONE. 2019;14(11): e0225072. 10.1371/journal.pone.0225072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Zhu QM, Xing H, Chen HC, Jin XM, Dong LJ, et al. The characteristics of pretreatment HIV-1 drug resistance in western Yunnan, China. Epidemiol Infect. 2020;8(148): e102. 10.1017/S095026882000093X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu XS, Luo LH, Song C, Li JJ, Chen HH, Zhu QY, et al. Survey of pretreatment HIV drug resistance and the genetic transmission networks among HIV-positive individuals in southwestern China, 2014–2020. BMC Infect Dis. 2021;21(1):1153. 10.1186/s12879-021-06847-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Dong AB, Liao LJ, Feng Y, Shao YM, Shu L, et al. Survey of pretreatment HIV drug resistance and genetic transmission network analysis among HIV patients in a high drug-use area of Southwest China. Curr HIV Res. 2019;17:441–51. 10.2174/1570162X17666191128101426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang RH, Liang SJ, Ma YL, Liang S, Xiao L, Zhang XH, et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect Dis Poverty. 2020;9(1):54. 10.1186/s40249-020-00668-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo LL, Liu K, Liu HL, Hu YH, Zhang ZJ, Qin JR, et al. Trend of HIV-1 drug resistance in China: a systematic review and meta-analysis of data accumulated over 17 years (2001–2017). EClinicalMedicine. 2020;5(18): 100238. 10.1016/j.eclinm.2019.100238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun CQ, Li JJ, Liu XY, Zhang Z, Qiu T, Hu HY, et al. HIV/AIDS late presentation and its associated factors in China from 2010 to 2020: a systematic review and meta-analysis. AIDS Res Ther. 2021;18(1):96. 10.1186/s12981-021-00415-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request, and with permission from Shaoxing Center for Disease Control and Prevention.