Abstract
The leukodystrophies are a group of inherited white matter disorders with a heterogeneous genetic background, considerable phenotypic variability and disease onset at all ages. This Review focuses on leukodystrophies with major prevalence or primary onset in adulthood. We summarize 20 leukodystrophies with adult presentations, providing information on the underlying genetic mutations and on biochemical assays that aid diagnosis, where available. Definitions, clinical characteristics, age of onset, MRI findings and treatment options are all described, providing a comprehensive overview of the current knowledge of the various adulthood leukodystrophies. We highlight the distinction between adult-onset leukodystrophies and other inherited disorders with white matter involvement, and we propose a diagnostic pathway for timely recognition of adulthood leukodystrophies in a routine clinical setting. In addition, we provide detailed clinical information on selected adult-onset leukodystrophies, including X-linked adrenoleukodystrophy, metachromatic leukodystrophy, cerebrotendinous xanthomatosis, hereditary diffuse leukoencephalopathy with axonal spheroids, autosomal dominant adult-onset demyelinating leukodystrophy, adult polyglucosan body disease, and leukoencephalopathy with vanishing white matter. Ultimately, this Review aims to provide helpful suggestions to identify treatable adulthood leukodystrophies at an early stage in the disease course.
The leukodystrophies are a class of inherited white matter disorders with diverse genetic underpinnings and substantial phenotypic variability1. Leukodystrophies have become an important differential diagnosis in adulthood white matter diseases2, despite the fact that white matter injury in adulthood is more often caused by acquired leukoencephalopathies with vascular, toxic, degenerative or inflammatory pathology, such as multiple sclerosis (MS), neuromyelitis optica or stroke. Making the differential diagnosis even more challenging, adulthood leukodystrophies must be differentiated from other heritable diseases with prominent white matter involvement that are not leukodystrophies, for example, inherited vasculopathies, which can result in white matter signal abnormalities, or later-onset or slowly progressing inborn errors of metabolism with secondary white matter involvement3.
In this Review, we provide a comprehensive overview of the leukodystrophies that present in adulthood, focusing on those for which treatments are available. We provide information on age of onset, clinical symptoms, genetic mutations and, where available, biochemical assays that assist in diagnosis. For a summary of the leukodystrophies that rarely or never present during adulthood, which are not covered by this Review, see Supplementary information S1 (table). These conditions have also been reviewed elsewhere4.
Classification and definitions
Leukodystrophies are defined as inherited disorders that affect the cerebral white matter. Cells involved in the axon–glia unit, such as oligodendrocytes, astrocytes, ependymal cells and microglia, are specifically affected5. Alterations to these non-neuronal cells lead to myelin sheath — and subsequently axonal — pathology. The underlying pathological mechanisms vary widely, involving inborn errors of metabolism, disrupted protein biosynthesis, oxidative stress and energy failure, among others. In addition to white matter involvement, some of the leukodystrophies include marked axonal pathology, either early in the disease process (though usually to a minor extent) or secondary to progressive myelin disruption in later disease stages. Involvement of the PNS is observed in some but not all leukodystrophies.
In many cases, pathological assessment is not feasible, so clinicians must rely on the molecular aetiology of the disorder and, importantly, on the appearance of the white matter on neuroimaging to define and classify the various disorders6. Leukodystrophies can be broadly subdivided into hypomyelinating leukodystrophies (HLDs), which are characterized by primary deficits in myelin development, and demyelinating leukodystrophies, where myelin develops normally but subsequently undergoes progressive disruption. The two groups can be easily differentiated on MRI, as individuals with HLDs show increased white matter signal on T2-weighted sequences and isointense or hyperintense white matter signal on T1-weighted sequences, whereas demyelination is characterized by increased T2 and substantially decreased T1 signals6. Most adulthood leukodystrophies are demyelinating in nature, and adult-onset HLDs are presumed to be very rare. However, some HLDs, such as Pol-III-related leukodystrophies with childhood or adolescent onset, can progress slowly and might be recognized only in adulthood7. Additional MRI features, such as calcifications, vacuolization or cysts, brainstem or basal ganglia involvement, intramyelinic oedema, or contrast enhancement, can aid MRI pattern recognition of the various leukodystrophies and are of key importance in establishing a diagnosis of an adulthood leukodystrophy6,8.
Clinical presentation
Leukodystrophies can present across the lifespan. It is important to note that leukodystrophies rarely show precise genotype–phenotype correlation, and the same gene defect might cause both childhood and adulthood phenotypes in the same family, as observed in X-linked adrenoleukodystrophy (X-ALD), for example9. Many adulthood leukodystrophies, such as the adrenomyeloneuropathy (AMN) phenotype of X-ALD10, or adult polyglucosan body disease11 (APBD), progress slowly over years or even decades, but rapid deterioration can occur in some diseases (for example, metachromatic leukodystrophy12 (MLD) and cerebral presentation of X-ALD in adulthood). Others, such as megalencephalic leukoencephalopathy with subcortical cysts (MLC), can even improve with age13. TABLE 1 provides a complete overview of the currently known adult-onset leukodystrophies, including clinical and diagnostic signs, age of onset and mutated genes.
Table 1 |.
Leukodystrophies with major prevalence and/or onset in adulthood
| Disease | Age of onset | OMIM entries | Gene(s) | Clinical signs | Biochemical findings |
|---|---|---|---|---|---|
| Hypomyelinating leukodystrophies | |||||
| Hypomyelinating leukodystrophy with atrophy of basal ganglia and cerebellum (HLD6)81 | Infancy to adulthood | 612438 | TUBB4A | • Child: dystonia, nystagmus, mild cognitive deficit, ataxia and spasticity • Adolescent or adult: spastic ataxia or dystonia |
None |
| Pelizaeus-Merzbacher disease (HLD1)82 | Infancy to adolescence | 312080 | PLP1 | • Child: dystonia, nystagmus, ataxia, spasticity, mild cognitive deficit and polyneuropathy in some cases • Adolescent: spastic ataxia |
None |
| Pelizaeus-Merzbacher-like disease (HLD2)83 | Infancy to adolescence | 608804 | GJC2 | • Child: dystonia, nystagmus, ataxia, spasticity and mild cognitive deficit • Adolescent: spastic ataxia |
None |
| Pol-III-related disorders (HLD7 and HLD8)84 | 90%: 2–6 years; 10%: ≥10 years | • 607694 • 614381 |
• POLR3A • POLR3B |
Dystonia, nystagmus, ataxia, spasticity, mild cognitive deficit, hypodontia and delayed or absent puberty | None |
| Demyelinating leukodystrophies | |||||
| Progressive leukodystrophy with ovarian failure (LKENP)85 | Childhood to adulthood | 615889 | AARS2 | Ataxia, spasticity and cognitive decline; all female patients have ovarian failure | None |
| X-Linked adrenoleukodystrophy (X-ALD)10 | • Adrenomyeloneuropathy (AMN): 14–60 years • Adulthood cerebral adrenoleukodystrophy (ACALD): ≥21 years |
300100 | ABCD1 | • AMN: spastic paraparesis, sensory signs, and bladder and sexual dysfunction • ACALD: behavioural changes and psychosis; later: spastic paraparesis, ataxia and dementia |
Elevated saturated very long chain fatty acids in serum |
| Adult polyglucosan body disease (APBD)77 | 50–60 years | 263570 | GBE1 | Spastic paraparesis, distal sensory neuropathy, bladder dysfunction and cerebellar ataxia; Parkinson-like syndrome and amyotrophic lateral sclerosis-like appearance in patients with predominant polyneuropathy; later: cognitive deficits | • Deficient glycogenbranching enzyme • Pathology: polyglucosan accumulation in skin and nerves |
| Alexander disease (AxD)86 | Childhood to adulthood | 203450 | GFAP | • Child: macrocephaly, dementia or developmental delay, spasticity, seizures and feeding difficulties and/or recurrent vomiting • Adult: bulbar signs, spasticity, cerebellar ataxia, oculomotor signs, palatal myoclonus and autonomic dysfunction |
• Elevated glial fibrillary acidic protein in cerebrospinal fluid • Pathology: Rosenthal fibres |
| Autosomal dominant adult-onset demyelinating leukodystrophy (ADLD)68 | 40–60 years | 169500 | LMNB1 duplication | Autonomic dysfunction, spastic-ataxic gait and cognitive impairment | None |
| Cerebrotendinous xanthomatosis (CTX)61 | Childhood to adulthood | 213700 | CYP27A1 | • Child: Cataracts, chronic diarrhoea and xanthomas • Adult: Cerebellar ataxia, spasticity, seizures, mild cognitive deficits, polyneuropathy and xanthomas |
Elevated serum cholestanol and bile alcohols; reduced mitochondrial sterol 26-hydroxylase activity in leukocytes |
| Leukoencephalopathy with ataxia (LKPAT)87,88 | Childhood to adulthood | 615651 | CLCN2 | Cerebellar ataxia, visual problems, optic neuropathy, cognitive deficits and headaches | None |
| Leukoencephalopathy with vanishing white matter (VWMD)71–75 | Childhood to adulthood | 603896 | EIF2B1–5 | • Child: progressive spastic ataxia • Adult: spastic paraparesis, psychiatric symptoms, ataxia and ovarian failure |
None |
| Fatty acid hydroxylase-associated neurodegeneration89 | 10–20 years | 612319 | FA2H | Spasticity, dystonia and cognitive dysfunction | None |
| Globoid cell leukodystrophy (GLD; also known as Krabbe disease)90 | Childhood to adulthood | 245200 | GALC | • Child: irritability, encephalopathy, polyneuropathy and spastic quadriplegia • Juvenile or adult: spastic tetraparesis, ataxia, polyneuropathy and cognitive impairment; later: blindness and deafness |
Galactocerebrosidase deficiency in leukocytes or fibroblasts |
| Demyelinating leukodystrophies (cont.) | |||||
| Hereditary diffuse leukoencephalopathy with spheroids (HdLS)63 | 40–70 years | 221820 | CSF1R | Cognitive and psychiatric disturbances, spastic-ataxic gait, seizures and bladder dysfunction | None |
| Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL)91 | Childhood to adulthood | 611105 | DARS2 | Child or adult: cerebellar and sensory ataxia, spasticity and dorsal column dysfunction | None |
| Megalencephalic leukoencephalopathy with subcortical cysts (MLC)13 | Childhood to adulthood | • 604004 • 613925 • 613926 |
• MLC1 • HEPACAM • HEPACAM |
• Early-onset macrocephaly, mild cognitive deficits, spasticity and ataxia of variable severity, dysarthria and epilepsy • Mild motor and cognitive delay (40%), clinical improvement possible; early-onset macrocephaly and delayed-onset neurological deterioration, including cerebellar ataxia, spasticity, epilepsy and mild cognitive decline |
None |
| Metachromatic leukodystrophy (MLD) and its biochemical variants12 | Childhood to adulthood | • 250100 • 249900 |
• ARSA • PSAP |
• Child: gait abnormalities, spasticity, ataxia and polyneuropathy • Juvenile or adult: psychosis, cognitive decline and polyneuropathy; later: spastic-ataxic gait and bladder dysfunction |
Decreased arylsulfatase A activity, elevated urinary sulfatides |
| Sjögren-Larsson syndrome (SLS)92 | Birth to 30 months, but it might not be recognized until adulthood | 270200 | ALDH3A2 | Child: spastic paraparesis, mental retardation, macular dystrophy and ichthyosis | Low fatty aldehyde dehydrogenase levels in fibroblasts |
OMIM, Online Mendelian Inheritance in Man.
Neurological and psychiatric symptoms.
The most prominent symptoms in adulthood leukodystrophies tend to be motor impairment and/or varying degrees of cognitive impairment3 (BOX 1). Pyramidal motor symptoms develop in a symmetrical fashion and may start in the lower extremities, mimicking spastic paraparesis. Gait abnormalities can be complicated by gait ataxia, as in hypomyelinating disorders or MLD, or by sensory long-tract signs, as in leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL). Often, bulbar symptoms develop in the later stages, but they might be an early manifestation in certain disorders, including Alexander disease (AxD). Distal symmetric sensory symptoms in the lower extremities and autonomic dysfunctions such as bladder, bowel or sexual impotence, which can mimic transverse myelitis or anterior horn disease, can also be present (for example, in APBD or autosomal dominant adult-onset demyelinating leukodystrophy (ADLD)). Extrapyramidal movement disorders, such as dystonia and/or dyskinesias or seizures, are less frequent but might be a predominant manifestation in certain disorders, including progressive leukoencephalopathy with ovarian failure, various HLDs and AxD. Cognitive symptoms may initially be subtle and, thus, remain undiscovered until other neurological symptoms occur. However, in conditions such as hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) and lysosomal storage disorders, cognitive symptoms are often the presenting manifestations. In contrast to classic dementias such as Alzheimer disease, memory deficits, disorientation and psychosis are less prominent than behavioural changes, mood changes and loss of realistic assessments of daily life experiences14.
Box 1 |. Symptoms of adulthood leukodystrophy.
The leading symptoms of adulthood leukodystrophies are as follows:
Motor symptoms, starting with clumsy gait and diplegia in lower extremities, finally leading to severe quadriplegia, dysarthria and dysphagia
Gait ataxia
Vegetative dysfunction, such as bladder, bowel or sexual dysfunction
Cognitive deficits, which further progress to severe dementia
Consider leukodystrophy in young adults (aged 20–40 years) who exhibit one or more of the following features:
Spastic paraparesis of otherwise unexplained origin (with or without brain MRI changes)
Early onset (<40 years) of dementia
Positive family history
Extraneurological findings.
In the context of adulthood leukodystrophies, extraneurological symptoms can offer important clues to a specific diagnosis (BOX 2). With the exception of Addisonian crisis in X-ALD, such symptoms are usually not life-threatening. However, symptoms such as the lack of pubertal development and cosmetic dental abnormalities in Pol-III-related HLDs can adversely affect functioning and quality of life. These systemic complications are often more amenable to treatment than is the primary neurological disorder, and every effort should be made to manage these complications15.
Box 2 |. Extraneurological symptoms in adulthood leukodystrophies.
Ophthalmological symptoms
Cataracts: cerebrotendinous xanthomatosis (CTX)
Optic nerve atrophy: leukoencephalopathy with vanishing white matter (VWMD), X-linked adrenoleukodystrophy (X-ALD), metachromatic leukodystrophy (MLD), globoid cell leukodystrophy (GLD; also known as Krabbe disease) and hypomyelinating leukodystrophies (HLDs)
Macular dystrophy: Sjögren–Larsson syndrome (SLS)
Nystagmus: Pelizaeus–Merzbacher disease, Pelizaeus–Merzbacher-like disease (HLD2) and other HLDs
Perifoveal glistening white dots: SLS
Cortical blindness: late-stage leukodystrophies, including X-ALD and MLD
Endocrine dysfunction
Adrenal insufficiency: X-ALD (adrenomyeloneuropathy (AMN) phenotype)
Testicular dysfunction: X-ALD (AMN phenotype)
Ovarian dysfunction: VWMD, progressive leukoencephalopathy with ovarian failure
Hypogonadotropic hypogonadism: Pol-III-related HLD
Growth factor deficiency: Pol-III-related HLD
Polyneuropathy
X-ALD (AMN phenotype), adult polyglucosan body disease (APBD), GLD, CTX, MLD and HLD2
Hypodontia
Pol-III-related HLDs
Cutaneous signs
Xanthoma: CTX
Ichthyosis: SLS
Melanoderma: X-ALD (AMN phenotype)
Visceral signs
Chronic diarrhoea: CTX
Gallbladder dysfunction: MLD
Epidemiology
Epidemiological data on the frequency of leukodystrophies are limited; for all leukodystrophies, estimates range from 1 in 50,000 to 1 in 7,500 (REFS 16,17), compared with 1 in 16,800 for all X-ALD phenotypes18. The relative frequencies of specific adulthood leukodystrophies are unclear19.
Although individual adulthood leukodystrophies are rare, they are increasingly recognized, probably owing to the increased use of MRI. Furthermore, phenotypes in adulthood leukodystrophies are incompletely characterized or remain unsolved in approximately 50% of all cases, resulting in a high estimated number of unreported cases and prolonged diagnostic procedures for patients and families17. The differential diagnosis is further complicated by the fact that other inherited and acquired disorders can present with similar clinical and radiological features20,21.
Diagnosis of an adult-onset leukodystrophy has important implications for family planning and symptom management. In individuals presenting with white matter abnormalities, it is important to obtain a detailed three-generation family history, asking about leukodystrophy-specific symptoms (in adulthood and childhood), early deaths from unclear neurological or psychiatric diseases, and parental consanguinity, to ensure that these disorders are not missed.
Diagnosis
Differential diagnosis.
Before confirming a diagnosis of leukodystrophy, neurologists need rule out more-common causes of white matter abnormality (FIG. 1). Steps such as a family history, history of symptom onset, physical examination, laboratory studies, MRI and lumbar puncture can be helpful. In some cases, laboratory testing might point to risk factors for vascular, inflammatory, degenerative, neoplastic and toxic causes of white matter disease. In other cases, neuroimaging may be helpful to recognize specific disease patterns, for example, the subcortical infarcts in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)22 or specific features in MS23. Careful consideration of treatable aetiologies, including autoimmune and antibody-mediated disorders, vitamin deficiencies and neoplasms, is warranted before determining that the patient is likely to have an inherited disorder. The adulthood leukodystrophies must also be differentiated from other inherited diseases with prominent involvement of the CNS white matter (TABLE 2).
Figure 1 |. Diagnostic pathway for adulthood leukodystrophies.
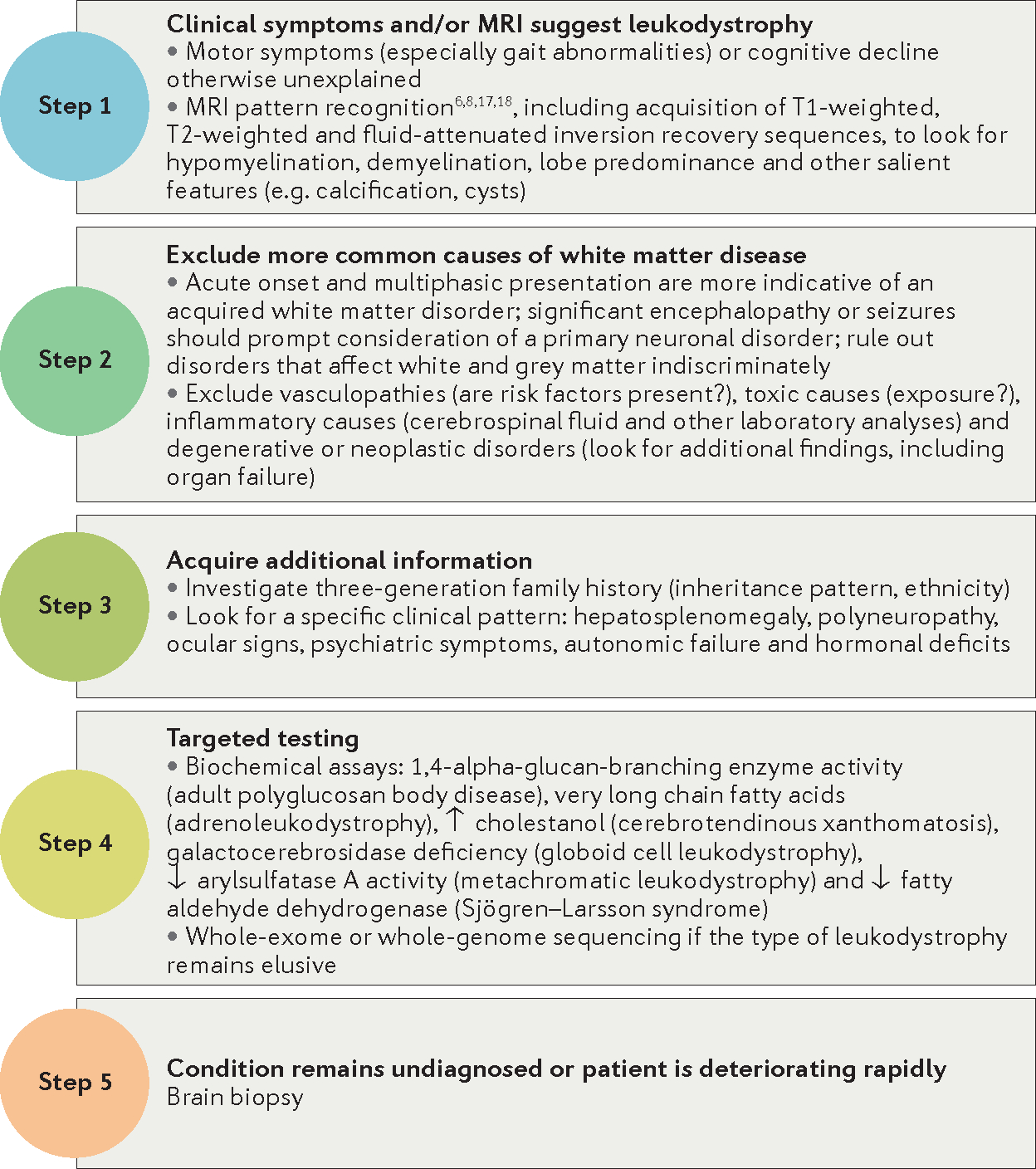
Table 2 |.
Differential diagnoses for adulthood leukodystrophies
| Disease | OMIM entries | Gene(s) |
|---|---|---|
| Inherited vasculopathies with white matter involvement | ||
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)22 | 125310 | NOTCH3 |
| Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL)93 | 600142 | HTRA1 |
| Brain small vessel disease with or without ocular anomalies94 | 607595 | COL4A1 |
| Fabry disease95 | 301500 | GLA |
| Retinal vasculopathy with cerebral leukodystrophy (RVCL)96 | 192315 | TREX1 |
| Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL)97 | 221770 | TREM2, TYROBP |
| Inherited CNS diseases with grey and white matter involvement | ||
| Dentatorubral-pallidoluysian atrophy (DRPLA)98 | 125370 | ATN1 |
| Fragile X tremor/ataxia syndrome (FXTAS) 99 | 300623 | FMR1 |
| Inborn errors of metabolism with white matter involvement | ||
| Aspartylglucosaminuria (AGU)100 | 208400 | AGA |
| Methylmalonic aciduria, isovaleric acidaemia and propionic acidaemia101 | 251000 | MUT |
| 243500 | IVD | |
| 606054 | PCCA, PCCB | |
| Disorders of glycoprotein degradation, including α-mannosidosis (MANSA)102, β-mannosidosis (MANSB)103 and neuraminidase deficiency104 | 248500 | MAN2B1 |
| 248510 | MANBA | |
| 256550 | NEU1 | |
| Galactosaemia105 | 230400 | GALT |
| GM1-gangliosidosis, type III106 | 230650 | GLB1 |
| GM2-gangliosidosis, AB variant107 | 272750 | GM2A |
| Hereditary homocystinurias108, such as methylenetetrahydrofolate reductase deficiency109 or cystathionine β-synthase deficiency110 | 236250 | MTHFR |
| 236200 | CBS | |
| Phenylketonuria (PKU)111 | 261600 | PAH |
| Organic acidurias, such as L-2-hydroxyglutaric aciduria (L2HGA)112 or 3-hydroxy-3-methylglutaryl-CoA lyase deficiency (HMGCLD)113 | 236792 | L2HGDH |
| 246450 | HMGCL | |
| Other diseases with white matter involvement | ||
| Wilson disease114 | 277900 | ATP7B |
The table lists a selection of inherited diseases with involvement of CNS white matter that should be considered in the differential diagnosis of adulthood leukodystrophies. OMIM, Online Mendelian Inheritance in Man.
Diagnostic algorithm.
In individuals in whom clinical symptoms are suggestive of an adulthood leukodystrophy (BOX 1) and MRI demonstrates brain white matter abnormalities, a stepwise diagnostic approach is advisable (FIG. 1). Physical examination or additional historical information, for example, from family history or involvement of extracerebral organs, may aid diagnosis in some cases. Careful review of the MRI scan by a clinician with expertise in pattern recognition for leukodystrophies might provide a specific diagnosis in certain disorders, including X-ALD24, AxD21 and leukoencephalopathy with vanishing white matter (also known as vanishing white matter disease, or VWMD2,6) (BOX 3; FIG. 2). Diagnostic confirmation is further achieved by targeted metabolic or genetic testing if the prior investigations suggest a specific diagnosis; however, in about 50% of all adulthood leukodystrophies, these standard approaches are unlikely to yield a precise diagnosis.
Box 3 |. MRI characteristics in adulthood leukodystrophies.
Hypomyelination
Hypomyelination is characterized by increased white matter signal on T2-weighted sequences and isointense or hyperintense white matter signal on T1-weighted sequences relative to grey matter structures (FIG. 2a,b)
Hypomyelinating leukodystrophy with atrophy of basal ganglia and cerebellum (HLD6)
Pelizaeus–Merzbacher disease (HLD1)
Pelizaeus–Merzbacher-like disease (HLD2)
Pol-III-related disorders (HLD7 and HLD8)
Demyelination
Demyelination is characterized by increased white matter signal on T2-weighted sequences and substantially decreased white matter signal on T1-weighted sequences relative to grey matter structures
Diffuse cerebral (FIG. 2a,c,d):
Leukoencephalopathy with vanishing white matter (VWMD)
Metachromatic leukodystrophy (MLD)
Megalencephalic leukoencephalopathy with subcortical cysts (MLC)
End-stage demyelinating leukodystrophies
Periventricular predominance (FIG. 2d):
MLD
Globoid cell leukodystrophy (GLD; also known as Krabbe disease)
Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL)
Sjögren–Larsson syndrome (SLS)
Adult polyglucosan body disease (APBD)
Fatty acid hydroxylase-associated neurodegeneration
Asymmetric lesions (FIG. 2c,j,k):
APBD
Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS)
VWMD
Cerebellum and/or middle cerebellar peduncles (FIG. 2e,n):
Cerebrotendinous xanthomatosis (dentate nucleus)
X-Linked adrenoleukodystrophy (X-ALD)
Alexander disease (AxD)
Adult-onset demyelinating leukodystrophy (ADLD)
LBSL
Fatty acid hydroxylase-associated neurodegeneration (atrophy)
HLDs (atrophy)
Progressive leukodystrophy with ovarian failure (LKENP)
Brainstem involvement (FIG. 2e,m,n,o):
LBSL
ADLD
APBD
AxD
Frontal predominance (FIG. 2d,f,j):
AxD
MLD
X-ALD (frontal variant)
HDLS
Parieto-occipital predominance (FIG. 2g,h,k):
X-ALD
GLD
APBD
Temporal predominance (FIG. 2l):
APBD
MLC
Multifocal lesions (FIG. 2j,k,m,o):
HDLS
APBD
LBSL
AxD
SLS
Cystic lesions (fluid-attenuated inversion recovery sequences) (FIG. 2l):
VWMD
MLC
Contrast enhancement (FIG. 2 h,n):
AxD
X-ALD
Corpus callosum thinning:
VWMD
ADLD
HDLS
LKENP
Long-tract involvement (FIG. 2i,o,p):
Adrenomyeloneuropathy
GLD
LBSL
Leukoencephalopathy with ataxia (LKPAT)
LKENP
Spinal cord atrophy (FIG. 2m):
Adrenomyeloneuropathy
APBD
AxD
Figure 2 |. Characteristic MRI features of adult leukodystrophies.
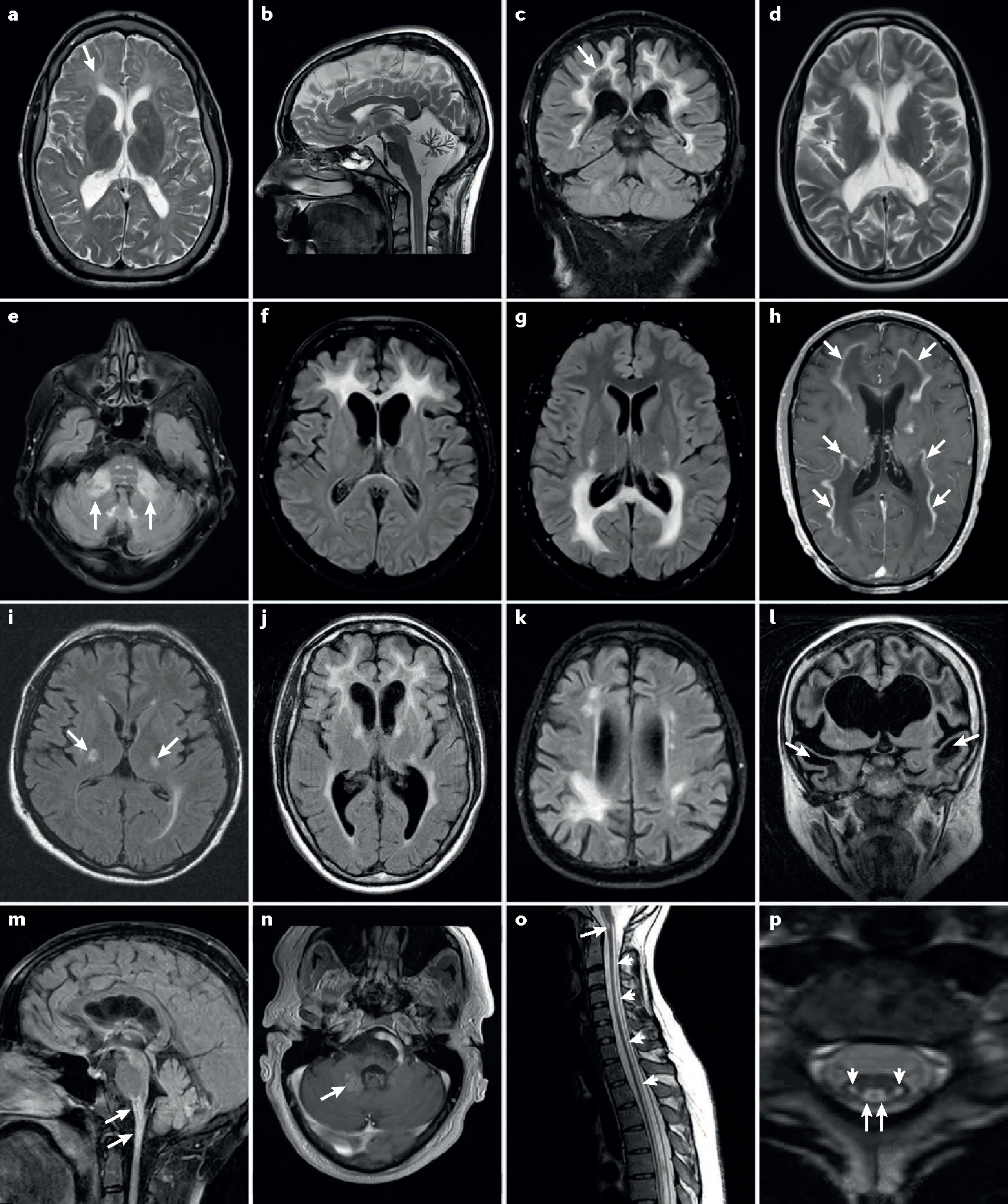
a | Hypomyelination in an adult with a Pol-III-related leukodystrophy. Arrow indicates moderately increased T2-weighted signal in affected white matter. Note relative atrophy, which is common in adults with hypomyelination. b | Severe cerebellar atrophy in a 24-year-old female with Pol-III-related hypomyelinating leukodystrophy. c | Diffuse cerebral white matter changes in a 61-year-old male with vanishing white matter disease. Note rarefaction of affected white matter (arrow) on fluid-attenuated inversion recovery images. d | Periventricular predominance of diffuse white matter changes in a 24-year-old female with metachromatic leukodystrophy. e | Middle cerebellar peduncle involvement (arrows) as well as the characteristic brainstem signal abnormalities in autosomal dominant leukodystrophy with autonomic symptoms. f–h | Cerebral inflammatory demyelinating variants of X-linked adrenoleukodystrophy (X-ALD) with frontal (part f) or parieto-occipital (part g) predominance. Note the contrast enhancement marginal to the demyelinated areas (arrows) (part h). i | Adrenomyeloneuropathy variant of X-ALD with bilateral T2-signal changes in degenerating corticospinal tracts (arrows). j | Large multifocal white matter lesions in a 41-year-old patient with hereditary diffuse leukoencephalopathy with spheroids. Note mild frontal atrophy, which is common in this condition. k | Multifocal lobar white matter changes in a 55-year-old patient with adult polyglucosan body disease. l | Frontotemporal cystic lesions (arrows) in a 33-year-old female with megalencephalic leukoencephalopathy with subcortical cysts. m,n | Alexander disease (AxD) in adulthood. Brainstem and spinal cord atrophy and lesions are shown (arrows in part m). A partially contrast enhancing area is shown (arrow in part n) in a 35-year-old male with AxD. o,p | Long-tract involvement seen in DARS-mutated leukoencephalopathy with brainstem and spinal cord involvement and elevated cerebrospinal fluid lactate. Sagittal images (part o) show T2-signal elevation in the brainstem (arrow) and along spinal tracts (small arrows). Axial sequences (part p) show bilateral involvement of the corticospinal (small arrows) and spinothalamic (arrow) tracts.
Recent studies using whole-exome sequencing (WES) in leukodystrophies have shown that such next-generation sequencing techniques can increase diagnostic yields to greater than 70%25,26, providing strong support for WES as a first-line diagnostic tool. In cases where an initial review of clinical, imaging and laboratory features does not suggest a specific diagnosis, initiation of next-generation sequencing approaches would be recommended as the next diagnostic step25.
Brain biopsies in cases of suspected leukodystrophy should be considered only in patients with potentially treatable conditions when all other investigations have failed to provide a diagnosis, or in patients who are deteriorating rapidly.
Selected leukodystrophies
In this section, we review the main features of a selection of adulthood leukodystrophies. We have chosen to focus on those conditions for which treatments are currently available.
X-Linked adrenoleukodystrophy.
X-ALD is one of the most frequent leukodystrophies occurring in adulthood. The various phenotypes include a cerebral inflammatory demyelinating form (adult cerebral X-ALD (ACALD)), which is the primary manifestation in ~5% of affected adults, and a more frequent, slowly progressive myelopathic variant (AMN) with spastic paraplegia and bladder and sexual dysfunction10.
X-ALD is caused by mutations in ABCD1, which encodes the peroxisomal membrane protein ATP-binding cassette sub-family D member 1 (ALDP)27. Dysfunction of ALDP results in the accumulation of very long chain fatty acids (VLCFAs) in the nervous system as well as in the adrenal glands, testis and body fluids28. The toxic effects from VLCFAs are thought to provoke oxidative stress phenomena, energy depletion and chronic neurodegeneration. Together, these pathological mechanisms lead to slowly progressive dyingback axonopathy in spinal cord tracts and peripheral nerves29, typically leading to spastic–ataxic gait disturbances starting in the third to fourth decade of life. In addition to neurodegeneration, inflammatory brain demyelination occurs in up to 60% of patients30. The reason for this cerebral transformation remains unknown, but multiple risk factors, including head trauma, autosomal modifier genes, impaired mitochondrial function and vascular and biochemical factors, have been hypothesized.
Allogeneic haematopoietic stem cell transplantation (aHSCT) has been shown to halt cerebral inflammation in childhood cerebral X-ALD (CCALD)31 and ACALD32, and is currently the only available life-saving treatment option in ACALD phenotypes. However, aHSCT carries several risks, including treatment-related mortality, graft failure, graft-versus-host disease (GVHD) and opportunistic infections, due to the immunosuppression used to treat GVHD. Although full chimaerism is achieved in most aHSCT-treated patients, the biochemical abnormality is still present in nervous system cells and ultimately leads to AMN later in life33. Long-term data on aHSCT in adulthood are sparse, but from our own experience, stabilization of inflammatory brain demyelination can be expected in most patients (W.K., unpublished observations). Severe disease (Expanded Disability Status Scale score >6), advanced changes on MRI (Loes score >10) and cognitive changes before transplantation seem to correlate with an unfavourable outcome of aHSCT.
As a proof of principle, an ex vivo lentiviral gene therapy approach in boys with cerebral inflammatory disease has shown long-term disease stabilization34. A multicentre phase II/III study, which was published in October 2017, assessed the efficacy and safety of ex vivo transduction of autologous CD34+ cells with the lentiviral vector elivaldogene tavalentivec (Lenti-D) to treat CCALD. In this study, 15 of 17 boys were alive with no major functional disability 24 months after treatment35. Although the long-term risks and effectiveness are still under investigation, gene therapy shows promise as a treatment option with long-term benefits in patients with CCALD36.
Prevention of neurodegeneration in patients with X-ALD seems to be more challenging37. Studies using high-dose biotin to promote axonal remyelination and enhance energy production38 and studies using peroxisome proliferator-activated receptor-γ agonists to target oxidative stress and enhance mitochondrial function39 are ongoing.
Efforts are underway to implement newborn screening for X-ALD40,41 in an increasing number of countries — an approach that seems to be justified given the substantial progress in therapeutic research.
Metachromatic leukodystrophy.
MLD is a good example of an adulthood leukodystrophy that is frequently misdiagnosed as early-onset dementia and/or a schizophrenic disorder, as the neurological symptoms can occur late in the disease course42,43. MLD is caused by pathogenic mutations in ARSA (which encodes arylsulfatase A (ASA)) or PSAP (which encodes prosaposin), which result in accumulation of toxic metabolites — in particular, sulfatides — within the nervous system and visceral organs, such as the gallbladder12,44–46.
Intravenous enzyme replacement therapy to replace the deficient or missing enzyme with an active recombinant human ASA enzyme was attempted in mice, but the therapeutic enzyme failed to cross the blood–brain barrier47. In a phase I/II study, intrathecal delivery of recombinant ASA is currently being researched as a therapeutic option in children with MLD48.
Haematopoietic stem cell transplantation has been performed in patients with different MLD phenotypes at various ages and disease stages49,50, sometimes with inconclusive or even disappointing results51. Currently, minimally symptomatic patients with juvenile MLD are considered to be potential candidates for aHSCT52–55.
In an ongoing study, patients with MLD are receiving infusions of autologous haematopoietic stem cells transduced with a lentiviral vector encoding ARSA cDNA after myeloablative conditioning with busulfan. The investigators reported preliminary evidence of safety and therapeutic benefit56. The beneficial effects of this new gene therapy approach were primarily seen in patients with early-onset MLD who received treatment in the presymptomatic or very early symptomatic stage of the disease.
Cerebrotendinous xanthomatosis.
In adulthood, cerebrotendinous xanthomatosis (CTX) typically presents with spastic paraparesis and cerebellar ataxia, followed by cognitive decline, dystonia, optic atrophy, polyneuropathy and seizures57,58. Early cataracts, chronic diarrhoea and the typical tendon xanthomas are additional key symptoms that can manifest during childhood. CTX is caused by homozygous or compound heterozygous mutations in the CYP27A1 gene. CYP27A1 encodes the mitochondrial enzyme sterol 26-hydroxylase, which is involved in the synthesis of bile acids from cholesterol59. The enzymatic defect leads to accumulation of large amounts of cholestanol, resulting in premature arteriosclerosis and neurotoxicity. Elevated serum cholestanol levels and the presence of urinary bile alcohols are diagnostic features of CTX60, and the relevant biochemical investigations are highly recommended in patients with unexplained, early-onset cataracts and diarrhoea since childhood.
Treatment of CTX is predominantly preventive, so early diagnosis is crucial. Long-term replacement of bile acid, especially with chenodeoxycholic acid (CDCA; 750 mg daily) is the current best option. CDCA has been shown to improve neurological symptoms, normalize levels of cholestanol and contribute to a better prognosis, especially if initiated early61,62.
Hereditary diffuse leukodystrophy with axonal spheroids.
HDLS primarily manifests in the fourth or fifth decade of life with behavioural changes, depression, gait ataxia and early-onset dementia63. Frontal gait ataxia, rigidity, bradykinesia and resting tremor are frequently observed64. Inheritance is autosomal dominant, although sporadic cases are common. The gene defect affects the tyrosine kinase domain of macrophage colony-stimulating factor 1 receptor, encoded by CSF1R65. An uninformative family history and emergence of clinical symptoms at an advanced age, combined with the multifocal and nonspecific MRI white matter changes that are typical of HDLS, can cause the condition to be misidentified as subcortical arteriosclerotic encephalopathy or as atypical parkinsonism in cerebrovascular disease. Therefore, HDLS is likely to be substantially underdiagnosed in adult neurology. HDLS has a worse prognosis than subcortical arteriosclerotic encephalopathy, with most patients developing severe dementia and dying within 4–6 years of diagnosis66.
No specific treatment options for HDLS are currently available, and supportive management is of crucial importance. However, one patient, who was treated with aHSCT after misdiagnosis of MLD, showed stabilization of disease manifestations67.
Autosomal dominant adult-onset demyelinating leukodystrophy.
ADLD typically presents with autonomic abnormalities, such as bladder dysfunction, impotence, constipation, anhidrosis and postural hypotension, in the fourth to the sixth decade of life. The disease then progresses, and patients have a spastic–ataxic gait and mild dementia in later disease stages68. ADLD is caused by duplications of the LMNB1 gene on chromosome 5q23, which result in overexpression of lamin B1 protein, an intermediate filament protein that is expressed in the nuclear lamina within the nuclear envelope. Overexpression of this protein leads to disruption of myelin homeostasis and slowly progressive, non-inflammatory demyelination, predominantly in deep white matter structures and cerebral peduncles69, which is occasionally mistaken for chronic progressive MS70.
Currently, no disease-specific treatment option is available for ADLD, although supportive care for urinary dysfunction, postural hypotension and gait disturbance can substantially improve quality of life for affected patients. Identification of the gene defect allows prenatal testing and genetic counselling of family members at risk.
Leukoencephalopathy with vanishing white matter.
VWMD, also known as eIF2B-related disorder, is caused by mutations in any one of five genes (EIF2B1–5)71,72. These mutations affect the function of the eukaryotic translation initiation factor eIF2B, which has an essential role in protein synthesis, including its regulation under different stress conditions. Patients with VWMD are highly vulnerable to physiological stress factors such as minor head trauma or fever, presenting with acute motor dysfunction, ataxia or even coma73. In many patients with adult-onset VWMD, a common amino acid substitution (Arg113His) is found in eIF2B subunit ε, causing a milder variant with spastic paraparesis, which sometimes presents primarily with psychiatric manifestations74. After disease onset, symptoms accumulate over the next decade, leading to severe disability, confinement to bed and even death in almost all patients. There are some exceptions to these presentations, for example, vanishing white matter leukodystrophy with ovarian failure (ovarioleukodystrophy), an allelic disorder that is characterized by primary ovarian failure in adult women, and minimal neurological symptoms75.
No disease-specific treatment option for VWMD can be offered to patients, and head trauma, infections and other stress factors should be strictly avoided.
Adult polyglucosan body disease.
APBD typically presents in the fifth to the sixth decade of life with a combination of upper and lower motor neuron impairment resembling amyotrophic lateral sclerosis, along with cerebellar ataxia or Parkinson disease-like symptoms with extrapyramidal movement disorders76. In some patients, polyneuropathic symptoms with hyporeflexia, distal symmetric sensory loss, muscle atrophy and fasciculations can be prominent, with slowed nerve conduction velocity and denervation potentials on electrophysiological testing. Cognitive deficits, reflecting white matter involvement, tend to be very mild11.
The affected gene in APBD, GBE1, encodes a glycogen-branching enzyme (GBE1), dysfunction of which leads to accumulation of polyglucosan bodies in the central and peripheral nerves. The diagnosis is confirmed by detection of reduced GBE1 enzymatic activity in peripheral blood leukocytes or cultured fibroblasts76,77. If assays for GBE1 activity produce equivocal results, sural nerve biopsy or skin biopsy can demonstrate the presence of polyglucosan bodies, which is suggestive of APBD.
No effective treatment is available for APBD, but supportive care for the urological disturbances and gait dysfunction can considerably increase patient safety and quality of life. A randomized controlled trial to study the efficacy of anaplerotic therapy with triheptanoin, a seven-carbon triglyceride, is ongoing78. This approach is based on the hypothesis that decreased glycogen degradation in APBD produces a secondary energy deficit, possibly related to inadequate reserves of normal glycogen for efficient degradation to free glucose. Triheptanoin is thought to provide an efficient substrate to the citric acid cycle to correct this energy deficit. A pilot trial demonstrated arrest of clinical deterioration with limited functional recovery in patients with APBD who received triheptanoin diet therapy79.
General treatment strategies
Despite emerging therapies for the leukodystrophies80, few curative options are available for patients affected by these disorders. An obligation remains, however, to fully treat and manage the many disease-associated complications. To fully serve patients with these conditions, a holistic approach is necessary to improve quality of life and decrease morbidity and mortality from medical complications15. Treatment options in adulthood leukodystrophies should encompass psychological care and psychiatric medications, antiepileptic treatments if needed, early antibiotic treatment for recurrent infections, prevention of secondary complications such as pneumonia and urinary tract infections, attention to general medical care, cognitive and speech therapy, attention to spasticity and functional status, and nutritional support as appropriate for the individual15. This type of approach is best administered at a centre with specialized expertise in these disorders.
Research networks and patient advocacy
Orphan diseases such as leukodystrophies frequently pose severe problems, particularly with respect to early diagnosis and research awareness. Furthermore, development of new drugs and other treatment options for small patient groups may not be attractive for the pharmaceutical industry. Consequently, third-party funding from national and international funds, cooperation with patient groups, and international research collaborations are of major importance (see Further Information box for more information). Close partnerships between clinicians, researchers and patient organizations allow the establishment of large sample sets for genetic and natural history studies and provide high-quality platforms for both basic research and the search for new treatment options.
Conclusions and future perspectives
Genetic leukodystrophies are gaining in importance in the adult neurology field in parallel with the diagnostic and therapeutic advances made in recent years. Diagnosing an adulthood leukodystrophy is still complex and extremely challenging. Targeted genetic testing using next-generation sequencing techniques, paired with comprehensive personal expertise in specialized leukodystrophy centres, will enable the identification of previously undetected or unknown leukodystrophies in adult patients, allowing systematic research for new therapeutic options in hitherto untreatable diseases. Early results from recent treatment studies — of gene therapy approaches, for example — are already starting to fill the therapeutic vacuum, and the hope for new therapeutic options is now becoming a realistic goal for many leukodystrophies.
Supplementary Material
Key points.
Leukodystrophies are a heterogeneous group of inherited disorders with highly variable clinical manifestations and pathogenetic background
Leukodystrophies are characterized by primary glial cell and myelin sheath pathology of variable aetiology; secondary axonal pathology can emerge as the disease progresses
Around 20 distinct disorders are currently defined as adulthood leukodystrophies; additional involvement of grey matter structures or non-cerebral organs distinguishes these conditions from other genetic leukoencephalopathies
Increasing numbers of individual leukodystrophies are being treated using metabolic treatment strategies, enzyme replacement or cell-based options such as allogeneic haematopoietic stem cell transplantation and gene therapy
DATABASES
Online Mendelian Inheritance in Man: http://www.omim.org/
FURTHER INFORMATION
ALD Charity (Switzerland): http://www.ald-charity.ch/
ALD Connect: http://aldconnect.org/
ALD Life (UK): http://www.aldlife.org/
European Leukodystrophy Association: http://ela-asso.com/en/
German Leukodystrophy Network (Leukonet): http://www.leukonet.de/10/?L=1
Global Leukodystrophy Initiative: http://theglia.org/
Leukodystrophy Care Network: http://www.huntershope.org/
LeukoTreat Network: http://www.leukotreat.eu/
StopALD (US): http://www.stopald.org/
The Myelin Project: http://myelin.org/
United Leukodystrophy Foundation: http://ulf.org/
World Leukodystrophy Alliance: http://leukodystrophyalliance.org/
Acknowledgements
The authors thank the patients and their families for their long-lasting confidence and abundance of patience while waiting for relief from the disease burden and new treatment options. The authors also acknowledge many years of constant support and inspiration from family organizations such as the European Leukodystrophy Association, the Myelin Project, the Adrenoleukodystrophy (ALD) Charity, the Stop ALD Foundation and the United Leukodystrophy Foundation. W.K. received funding from the German Ministry of Education and Research as part of the German LEUKONET Network.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Vanderver A et al. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 114, 494–500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikh S et al. A clinical approach to the diagnosis of patients with leukodystrophies and genetic leukoencephelopathies. Mol. Genet. Metab. 114, 501–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed RM et al. A practical approach to diagnosing adult onset leukodystrophies. J. Neurol. Neurosurg. Psychiatry 85, 770–781 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Kohlschutter A & Eichler F Childhood leukodystrophies: a clinical perspective. Expert Rev. Neurother. 11, 1485–1496 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Van der Knaap MS & Buguani M Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 134, 351–182 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffmann R & van der Knaap MS Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72, 750–759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouwels PJ et al. Hypomyelinating leukodystrophies: translational research progress and prospects. Ann. Neurol. 76, 15–19 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Steenweg ME et al. Magnetic resonance imaging pattern recognition in hypomyelinating disorders. Brain 133, 2971–2982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Rocco M, Doria-Lamba L & Caruso U Monozygotic twins with X-linked adrenoleukodystrophy and different phenotypes. Ann. Neurol. 50, 424 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Moser HW Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120, 1485–1508 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Hellmann MA et al. Frequent misdiagnosis of adult polyglucosan body disease. J. Neurol. 262, 2346–2351 (2015). [DOI] [PubMed] [Google Scholar]
- 12.von Figura K, Steckel F, Conary J, Hasilik A & Shaw E Heterogeneity in late-onset metachromatic leukodystrophy. Effect of inhibitors of cysteine proteinases. Am. J. Hum. Genet. 39, 371–382 (1986). [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Hernandez T et al. Mutant GlialCAM causes megalencephalic leukoencephalopathy with subcortical cysts, benign familial macrocephaly, and macrocephaly with retardation and autism. Am. J. Hum. Genet. 88, 422–432 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmahmann JD, Smith EE, Eichler FS & Filley CM Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. NY Acad. Sci. 1142, 266–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adang LA et al. Revised consensus statement on the preventive and symptomatic care of patients with leukodystrophies. Mol. Genet. Metab. 122, 18–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heim P et al. Leukodystrophy incidence in Germany. Am. J. Med. Genet. 71, 475–478 (1997). [PubMed] [Google Scholar]
- 17.Bonkowsky JL et al. The burden of inherited leukodystrophies in children. Neurology 75, 718–725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bezman L et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 49, 512–517 (2001). [PubMed] [Google Scholar]
- 19.Vanderver A, Hussey H, Schmidt JL, Pastor W & Hoffman HJ Relative incidence of inherited white matter disorders in childhood to acquired pediatric demyelinating disorders. Semin. Pediatr. Neurol. 19, 219–223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayrignac X et al. Adult-onset genetic leukoencephalopathies: a MRI pattern-based approach in a comprehensive study of 154 patients. Brain 138, 284–292 (2015). [DOI] [PubMed] [Google Scholar]
- 21.van der Knaap MS, Breiter SN, Naidu S, Hart AA & Valk J Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology 213, 121–133 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E & Bousser MG CADASIL Lancet Neurol. 8, 643–653 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Polman CH et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loes DJ et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am. J. Neuroradiol. 15, 1761–1766 (1994). [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderver A et al. Whole exome sequencing in patients with white matter abnormalities. Ann. Neurol. 79, 1031–1037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kevelam SH et al. Update on leukodystrophies: a historical perspective and adapted definition. Neuropediatrics 47, 349–354 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Mosser J et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Moser HW et al. Adrenoleukodystrophy: elevated C26 fatty acid in cultured skin fibroblasts. Ann. Neurol. 7, 542–549 (1980). [DOI] [PubMed] [Google Scholar]
- 29.Berger J, Forss-Petter S & Eichler FS Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 98, 135–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ & Engelen M Adrenoleukodystrophy — neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 12, 606–615 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Peters C et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 104, 881–888 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Kühl JS et al. Long-term outcomes of allogeneic haematopoietic stem cell transplantation for adult cerebral X-linked adrenoleukodystrophy. Brain 140, 953–966 (2017). [DOI] [PubMed] [Google Scholar]
- 33.van Geel BM et al. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J. Inherit. Metab. Dis. 38, 359–361 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Cartier N et al. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 507, 187–198 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Eichler F et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelen M Optimizing treatment for cerebral adrenoleukodystrophy in the era of gene therapy. N. Engl. J. Med. 377, 1682–1684 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Semmler A, Kohler W, Jung HH, Weller M & Linnebank M Therapy of X-linked adrenoleukodystrophy. Expert Rev. Neurother. 8, 1367–1379 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Sedel F, Bernard D, Mock DM & Tourbah A Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 110, 644–653 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Morato L et al. Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain 136, 2432–2443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbard WC et al. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol. Genet. Metab. 97, 212–220 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Theda C et al. Newborn screening for X-linked adrenoleukodystrophy: further evidence high throughput screening is feasible. Mol. Genet. Metab. 111, 55–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumann N et al. Adult forms of metachromatic leukodystrophy: clinical and biochemical approach. Dev. Neurosci. 13, 211–215 (1991). [DOI] [PubMed] [Google Scholar]
- 43.Betts TA, Smith WT & Kelly RE Adult metachromatic leukodystrophy (sulphatide lipidosis) simulating acute schizophrenia. Report of a case. Neurology 18, 1140–1142 (1968). [DOI] [PubMed] [Google Scholar]
- 44.Gieselmann V, Fluharty AL, Tonnesen T & Von Figura K Mutations in the arylsulfatase A pseudodeficiency allele causing metachromatic leukodystrophy. Am. J. Hum. Genet. 49, 407–413 (1991). [PMC free article] [PubMed] [Google Scholar]
- 45.van Rappard DF et al. Gallbladder and the risk of polyps and carcinoma in metachromatic leukodystrophy. Neurology 87, 103–111 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Cesani M et al. Mutation update of ARSA and PSAP genes causing metachromatic leukodystrophy. Hum. Mutat. 37, 16–27 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Matthes F et al. Efficacy of enzyme replacement therapy in an aggravated mouse model of metachromatic leukodystrophy declines with age. Hum. Mol. Genet. 21, 2599–2609 (2012). [DOI] [PubMed] [Google Scholar]
- 48.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01510028?term=NCT01510028&rank=1 (2017). [DOI] [PubMed]
- 49.Krivit W, Peters C & Shapiro EG Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux–Lamy, and Sly syndromes, and Gaucher disease type III. Curr. Opin. Neurol. 12, 167–176 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Sevin C, Aubourg P & Cartier N Enzyme, cell and gene-based therapies for metachromatic leukodystrophy. J. Inherit. Metab. Dis. 30, 175–183 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Bredius RG et al. Early marrow transplantation in a pre-symptomatic neonate with late infantile metachromatic leukodystrophy does not halt disease progression. Bone Marrow Transplant. 39, 309–310 (2007). [DOI] [PubMed] [Google Scholar]
- 52.van Egmond ME et al. Improvement of white matter changes on neuroimaging modalities after stem cell transplant in metachromatic leukodystrophy. JAMA Neurol. 70, 779–782 (2013). [DOI] [PubMed] [Google Scholar]
- 53.van Rappard DF, Boelens JJ & Wolf NI Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best Pract. Res. Clin. Endocrinol. Metab. 29, 261–273 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Boucher AA et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J. Rare Dis. 10, 94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groeschel S et al. Long-term Outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 73, 1133–1140 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Sessa M et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 388, 476–487 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Dotti MT, Rufa A & Federico A Cerebrotendinous xanthomatosis: heterogeneity of clinical phenotype with evidence of previously undescribed ophthalmological findings. J. Inherit. Metab. Dis. 24, 696–706 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Verrips A et al. Spinal xanthomatosis: a variant of cerebrotendinous xanthomatosis. Brain 122, 1589–1595 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Bjorkhem I et al. Role of the 26-hydroxylase in the biosynthesis of bile acids in the normal state and in cerebrotendinous xanthomatosis. An in vivo study. J. Clin. Invest. 71, 142–148 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koopman BJ et al. Cerebrotendinous xanthomatosis: a review of biochemical findings of the patient population in The Netherlands. J. Inherit. Metab. Dis. 11, 56–75 (1988). [DOI] [PubMed] [Google Scholar]
- 61.Federico A, Dotti MT & Gallus GN Cerebrotendinous xanthomatosis. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1409/ (updated 14 April 2016).
- 62.Nie S, Chen G, Cao X & Zhang Y Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 9, 179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman SH et al. Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol. 19, 39–47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundal C et al. Parkinsonian features in hereditary diffuse leukoencephalopathy with spheroids (HDLS) and CSF1R mutations. Parkinsonism Relat. Disord. 19, 869–877 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rademakers R et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 44, 200–205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sundal C et al. Hereditary diffuse leukoencephalopathy with spheroids with phenotype of primary progressive multiple sclerosis. Eur. J. Neurol. 22, 328–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eichler FS et al. CSF1R mosaicism in a family with hereditary diffuse leukoencephalopathy with spheroids. Brain 139, 1666–1672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padiath QS et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet. 38, 1114–1123 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Schuster J et al. Genomic duplications mediate overexpression of lamin B1 in adult-onset autosomal dominant leukodystrophy (ADLD) with autonomic symptoms. Neurogenetics 12, 65–72 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Eldridge R et al. Hereditary adult-onset leukodystrophy simulating chronic progressive multiple sclerosis. N. Engl. J. Med. 311, 948–953 (1984). [DOI] [PubMed] [Google Scholar]
- 71.van der Knaap MS et al. A new leukoencephalopathy with vanishing white matter. Neurology 48, 845–855 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Schiffmann R et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann. Neurol. 35, 331–340 (1994). [DOI] [PubMed] [Google Scholar]
- 73.van der Knaap MS, Pronk JC & Scheper GC Vanishing white matter disease. Lancet Neurol. 5, 413–423 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Labauge P et al. Natural history of adult-onset eIF2B-related disorders: a multi-centric survey of 16 cases. Brain 132, 2161–2169 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Fogli A et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am. J. Hum. Genet. 72, 1544–1550 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mochel F et al. Adult polyglucosan body disease: natural history and key magnetic resonance imaging findings. Ann. Neurol. 72, 433–441 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein CJ Adult polyglucosan body disease. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK5300/ (updated 2 April 2009).
- 78.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00947960?term=NCT00947960&rank=1 (2016). [DOI] [PubMed]
- 79.Roe CR, Bottiglieri T, Wallace M, Arning E & Martin A Adult polyglucosan body disease (APBD): anaplerotic diet therapy (triheptanoin) and demonstration of defective methylation pathways. Mol. Genet. Metab. 101, 246–252 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Helman G et al. Disease specific therapies in leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 114, 527–536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Knaap MS et al. New syndrome characterized by hypomyelination with atrophy of the basal ganglia and cerebellum. AJNR Am. J. Neuroradiol. 23, 1466–1474 (2002). [PMC free article] [PubMed] [Google Scholar]
- 82.Garbern JY et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125, 551–561 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Uhlenberg B et al. Mutations in the gene encoding gap junction protein α12 (connexin 46.6) cause Pelizaeus–Merzbacher-like disease. Am. J. Hum. Genet. 75, 251–260 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolf NI et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology 83, 1898–1905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dallabona C et al. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology 82, 2063–2071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balbi P et al. The clinical spectrum of late-onset Alexander disease: a systematic literature review. J. Neurol. 257, 1955–1962 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Jeworutzki E et al. GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl− channel auxiliary subunit. Neuron 73, 951–961 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Depienne C et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study. Lancet Neurol. 12, 659–668 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Edvardson S et al. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 83, 643–648 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graziano AC & Cardile V History, genetic, and recent advances on Krabbe disease. Gene 555, 2–13 (2015). [DOI] [PubMed] [Google Scholar]
- 91.van Berge L et al. Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain 137, 1019–1029 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Lossos A et al. Phenotypic variability among adult siblings with Sjogren–Larsson syndrome. Arch. Neurol. 63, 278–280 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Onodera O, Nozaki H & Fukutake T CARASIL. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK32533/ (updated 11 September 2014).
- 94.Choi JC Genetics of cerebral small vessel disease. J. Stroke 17, 7–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bottcher T et al. Fabry disease — underestimated in the differential diagnosis of multiple sclerosis? PLoS ONE 8, e71894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kolar GR et al. Neuropathology and genetics of cerebroretinal vasculopathies. Brain Pathol. 24, 510–518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paloneva J, Autti T, Hakola P & Haltia MJ Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL). GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1197/ (updated 12 March 2015).
- 98.Girard JM, Turnbull J, Ramachandran N & Minassian BA Progressive myoclonus epilepsy. Handb. Clin. Neurol. 113, 1731–1736 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Hagerman PJ & Hagerman RJ Fragile X-associated tremor/ataxia syndrome. Ann. NY Acad. Sci. 1338, 58–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aronson NN Jr. Aspartylglycosaminuria: biochemistry and molecular biology. Biochim. Biophys. Acta 1455, 139–154 (1999). [DOI] [PubMed] [Google Scholar]
- 101.Baumgartner MR et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 9, 130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borgwardt L et al. Alpha-mannosidosis: correlation between phenotype, genotype and mutant MAN2B1 subcellular localisation. Orphanet J. Rare Dis. 10, 70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sedel F et al. Psychiatric manifestations revealing inborn errors of metabolism in adolescents and adults. J. Inherit. Metab. Dis. 30, 631–641 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Verheijen FW et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat. Genet. 23, 462–465 (1999). [DOI] [PubMed] [Google Scholar]
- 105.Rubio-Agusti I et al. Movement disorders in adult patients with classical galactosemia. Mov. Disord. 28, 804–810 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Yoshida K et al. GM1 gangliosidosis in adults: clinical and molecular analysis of 16 Japanese patients. Ann. Neurol. 31, 328–332 (1992). [DOI] [PubMed] [Google Scholar]
- 107.Argov Z & Navon R Clinical and genetic variations in the syndrome of adult GM2 gangliosidosis resulting from hexosaminidase A deficiency. Ann. Neurol. 16, 14–20 (1984). [DOI] [PubMed] [Google Scholar]
- 108.Wilcken B Leukoencephalopathies associated with disorders of cobalamin and folate metabolism. Semin. Neurol. 32, 68–74 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Lossos A et al. Severe methylenetetrahydrofolate reductase deficiency: clinical clues to a potentially treatable cause of adult-onset hereditary spastic paraplegia. JAMA Neurol. 71, 901–904 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Skovby F, Gaustadnes M & Mudd SH A revisit to the natural history of homocystinuria due to cystathionine beta-synthase deficiency. Mol. Genet. Metab. 99, 1–3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding XQ et al. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J. Magn. Reson. Imag. 27, 998–1004 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Marcel C et al. L-2-hydroxyglutaric aciduria diagnosed in a young adult with progressive cerebellar ataxia and facial dyskinesia. Rev. Neurol. (Paris) 168, 187–191 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Reimao S et al. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: initial presentation in a young adult. J. Inherit. Metab. Dis. 32 (Suppl. 1), 49–52 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Bandmann O, Weiss KH & Kaler SG Wilson’s disease and other neurological copper disorders. Lancet Neurol. 14, 103–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


