Abstract
Background
It is well known that physical exercise can trigger asthma symptoms and can induce bronchial obstruction in people without clinical asthma. International guidelines on asthma management recommend the use of beta2‐agonists at any stage of the disease. At present, however, no consensus has been reached about the efficacy and safety of beta2‐agonists in the pretreatment of exercise‐induced asthma and exercise‐induced bronchoconstriction. For the purpose of the present review, both of these conditions are referred to by the acronymous EIA, independently from the presence of an underlying chronic clinical disease.
Objectives
To assess the effects of inhaled short‐ and long‐acting beta2‐agonists, compared with placebo, in the pretreatment of children and adults with exercise‐induced asthma (or exercise‐induced bronchoconstriction).
Search methods
Trials were identified by electronic searching of the Cochrane Airways Group Specialised Register of Trials and by handsearching of respiratory journals and meetings. Searches are current as of August 2013.
Selection criteria
We included randomised, double‐blind, placebo‐controlled trials of any study design, published in full text, that assessed the effects of inhaled beta2‐agonists on EIA in adults and children. We excluded studies that did not clearly state diagnostic criteria for EIA.
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration.
Main results
We included 53 trials consisting of 1139 participants. Forty‐eight studies used a cross‐over design, and five were performed in accordance with a parallel‐group design. Forty‐five studies addressed the effect of a single beta2‐agonist administration, and eight focused on long‐term treatment. We addressed these two different intervention regimens as different comparisons.
Among primary outcomes for short‐term administration, data on maximum fall in forced expiratory volume in 1 second (FEV1) showed a significant protective effect for both short‐acting beta‐agonists (SABA) and long‐acting beta‐agonists (LABA) compared with placebo, with a mean difference of ‐17.67% (95% confidence interval (CI) ‐19.51% to ‐15.84%, P = 0.00001, 799 participants from 72 studies). The subgroup analysis of studies performed in adults compared with those performed in children showed high heterogeneity confined to children, despite the comparable mean bronchoprotective effect.
Secondary outcomes on other pulmonary function parameters confirmed a more positive and protective effect of beta2‐agonists on EIA compared with placebo. Occurrence of side effects was not significantly different between beta2‐agonists and placebo.
Overall evaluation of the included long‐term studies suggests a beta2‐agonist bronchoprotective effect for the first dose of treatment. However, long‐term use of both SABA and LABA induced the onset of tolerance and decreased the duration of drug effect, even after a short treatment period.
Authors' conclusions
Evidence of low to moderate quality shows that beta2‐agonists, both SABA and LABA, when administered in a single dose, are effective and safe in preventing EIA.
Long‐term regular administration of inhaled beta2‐agonists induces tolerance and lacks sufficient safety data. This finding appears to be of particular clinical relevance in view of the potential for prolonged regular use of beta2‐agonists as monotherapy in the pretreatment of EIA, despite the warnings of drug agencies (FDA, EMA) regarding LABA.
Keywords: Adult; Child; Humans; Administration, Inhalation; Adrenergic beta‐2 Receptor Antagonists; Adrenergic beta‐2 Receptor Antagonists/administration & dosage; Asthma, Exercise‐Induced; Asthma, Exercise‐Induced/drug therapy; Bronchoconstriction; Bronchodilator Agents; Bronchodilator Agents/administration & dosage; Forced Expiratory Volume; Forced Expiratory Volume/drug effects; Randomized Controlled Trials as Topic
Plain language summary
Asthma reliever inhalers (beta2‐agonists) used for exercise‐induced asthma and exercise‐induced bronchoconstriction
Review question
Physical exercise may trigger symptoms such as cough, chest tightness and shortness of breath in people with asthma that is not adequately treated (exercise‐induced asthma). Sometimes people who do not have asthma still experience asthma‐like symptoms during exercise; this is called exercise‐induced bronchoconstriction. We looked at both types of people in this review. The treatments we were interested in are called beta2‐agonists. These are drugs that are known to open up the airways (small tubes in the lungs), making it easier for people to breathe. Two kinds of beta2‐agonists are available: short‐acting (SABA, e.g. salbutamol and terbutaline) and long‐acting (LABA, e.g. formoterol and salmeterol).
What evidence did we find?
We found 53 trials consisting of 1139 participants. Forty‐eight studies used a cross‐over design, which meant that each person in the trial received two or more treatments-one or more active treatments, the beta‐agonist and a placebo in random order. The rest were parallel‐group trials, meaning that people received either the active treatment or a placebo. Most of the studies addressed the effect of a giving a single beta2‐agonist treatment before exercise and recorded the effect on lung function following exercise. Only eight focused on longer treatment-longer treatments would be needed to assess whether these treatments were harmful over the longer term.
Results
Studies in which people received a single administration of a beta‐agonist showed that FEV1 (a measure of lung function) fell significantly less for people taking SABA or LABA compared with placebo (mean difference (MD) ‐17.67%; 95% confidence interval (CI) ‐19.51% to ‐15.84%). Other lung function measures confirmed that beta2‐agonists were more beneficial compared with placebo. No significant difference in the number of side effects was noted in people taking SABA or LABA compared with people taking placebo. However, it is unlikely that people would be prescribed an inhaler for a single treatment, so we must consider longer‐term studies to get a true measure of the side effects that inhalers can cause.
We found that included longer‐term studies showed that beta2‐agonists were helpful in terms of lung function for the first dose of treatment. However, studies that provided longer‐term treatment with SABA or LABA showed that over time, people built up a tolerance to the effects of treatments, and the beneficial effects lasted for shorter periods of time.
Quality of the evidence
Overall, we believe that the evidence was of low to moderate quality.
Conclusions
This review shows that beta2‐agonists-both SABA and LABA-when administered in a single dose, are effective and safe in preventing the symptoms of EIA. Longer‐term administration of inhaled beta2‐agonists induces tolerance and lacks sufficient safety data. It is important to note that taking LABA without background inhaled steroids is considered unsafe and is not currently recommended in most of the clinical guidelines for asthma. We recommend that more studies are needed to determine whether it is safe to administer inhaled beta2‐agonists alone to people who experience asthma symptoms when exercising.
This review is current as of August 2013.
Summary of findings
Summary of findings for the main comparison. Beta2‐agonists compared with placebo (single administration) for exercise‐induced asthma.
| Beta2‐agonists compared with placebo (single administration) for exercise‐induced asthma | ||||||
| Patient or population: exercise‐induced asthma Intervention: beta2‐agonists Comparison: placebo (single administration) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo (single administration) | Beta2‐agonists | |||||
| Maximal percentage fall in FEV1 | The mean fall in FEV1 in the intervention group was MD 17.67 lower (19.51 lower to 15.84 lower)a | - | 799 (72 studies)a,b,c | ⊕⊕⊕⊝ moderated,e,f | The results in the subgroup of LABA and SABA were similar: MD 15.6 lower (18.29 lower to 12.92 lower) and MD 18.99 lower (21.38 lower to 16.6 lower) in 44 and 28 studies, respectively | |
| Number of participants with an FEV1 fall > 10% | 843 per 1000 (84.3)% | 300 per 1000 (243 to 410) | OR 0.08 (0.06 to 0.13) | 773 (19 studies) | ⊕⊕⊕⊝ moderated,e | |
| Maximal percentage fall in PEF | The mean maximal percentage fall in PEF in the intervention group was MD 24.61 lower (37.57 lower to 11.65 lower)1 | - | 92 (14 studies)b | ⊕⊕⊝⊝ lowd,e,g | ||
| Maximal percentage fall in FEF25‐75% | The mean maximal percentage fall in FEF25‐75% in the intervention group was MD 20.75 lower (27.17 lower to 14.32 lower)1 | - | 106 (8 studies)b | ⊕⊕⊝⊝ lowd,e,g | ||
| Side effects | 50 per 1000 (5.0)% | 42 per 1000 (22 to 77) | OR 0.83 (0.43 to 1.59) | 2165 (55 studies)h | ⊕⊕⊝⊝ lowe,g,I | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEF25‐75%: forced expiratory flow 25–75%; FEV1: forced expiratory volume in 1 minute; LABA: long‐acting beta2‐agonist; MD: mean difference; OR: odds ratio; peak expiratory flow (PEF); SABA: short‐acting beta2‐agonist. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aLower indicates that beta2‐agonists are better than placebo.
bIn 51 studies that provided data for subgroup analysis, no difference was observed in the maximal percentage fall in FEV1, but the heterogeneity of the effect was seen primarily in the paediatric population.
cThese represent 72 study arms from 53 studies.
dIt is unclear how directly pulmonary function measures relate to what participants feel.
eThere was concern about lack of concealment, loss to follow‐up and reporting bias.
fInconsistency was moderate to high and was explained in part by subgroup analyses of adults and children.
gSmall numbers of participants were included with resulting wide confidence intervals.
hThese represent 55 study arms rather than studies.
iThis represents a mix of outcomes, and not all of them are of equal importance to patients.
Background
Exercise‐induced asthma (EIA) is the term commonly used to describe the transient increase in airway resistance that follows vigorous exercise (Anderson 1997). However a recent position paper suggested a preferred definition of exercise‐induced bronchoconstriction (EIB) with or without asthma, depending on the presence of underlying clinical asthma (Weiler 2010). Despite this new nomenclature, most of the relevant studies were published before these terms were proposed and often do not provide sufficient information to distinguish between the two conditions. We therefore decided to adopt the term exercise‐induced asthma (EIA) throughout the review for the sake of better consistency and clarity.
The prevalence of EIA ranges from 5% to 20% in the general population, to even 100% in people with uncontrolled asthma. This huge variability depends not only on the criteria used for diagnosis, but also on the population samples studied. EIA is, in fact, reported to be particularly frequent in children (Randolph 2008), in people with rhinitis (Brozek 2010) and in athletes, with percentages varying according to different sport disciplines (Carlsen 2008).
The diagnosis of EIA is usually made by exercise testing, either in the field or in the laboratory; this second option allows more standardised procedures (Rundell 2000). An individual's response to exercise is generally expressed by the maximal percent fall in forced expiratory volume in one second (FEV1). The maximal percent fall is considered an expression of severity of EIA and is calculated by subtracting the lowest FEV1 value from the pre‐exercise value and expressing it as a percentage of the pre‐exercise value. Both European Respiratory Society (ERS) and American Respiratory Society (ATS) recommendations set a fall threshold of 10% as a diagnostic criterion for EIA and a value greater than 30% as a marker of severe bronchial hyperreactivity, particularly if the person is treated with inhaled steroids (Sterk 1993). Other indirect tests such as eucapnic voluntary hyperpnoea (EVH) and mannitol challenge are usually considered surrogate tests for the diagnosis of EIA because they induce similar pathophysiological changes in the airways (Anderson 2003).
The main principle of treating EIA involves reversing the bronchial obstruction induced by exercise with bronchodilators or preventing it with daily use of either controller drugs in people with asthma (Koh 2007; Bateman 2008) or drugs that inhibit symptoms and improve pulmonary function immediately before exercise. Pretreatment before exercise includes mast cell stabilisers (Kelly 2000; Spooner 2003), leukotriene antagonists (Peroni 2011), short‐acting beta2‐agonists (SABA) and, more recently, long‐acting beta2‐agonists (LABA), especially in endurance athletes (Shapiro 2002).
Both SABA and LABA, administered at standard doses immediately before exercise, have been shown to reduce the fall in FEV1 by 70% to 80% in most people with EIA (Anderson 2006). The mechanism of this protection is believed to be related to beta2‐receptor-induced relaxation of bronchial smooth muscle, which opposes the contractile effects of the various mediators of bronchoconstriction. Protection from EIA is also afforded by beta2‐receptor-induced inhibition of mediator release from mast cells.
At present, however, no consensus has been reached about the efficacy and safety of beta2‐agonists in the pretreatment of EIA. The role of these molecules in preventing EIA was questioned when patients taking beta2‐agonists daily reported breakthrough EIA within a dosing period. Several negative findings have been reported regarding the efficacy of daily treatment with beta2‐agonists in controlling the severity of bronchoconstriction and recovery from EIA. In fact, in a significant minority of people, EIA is not prevented by beta2‐agonists administered at the recommended dose (Anderson 1991; Weiler 2005). Furthermore, it has been reported how daily treatment with beta2‐agonists can enhance the severity of EIA (Hancox 2002) and decrease the duration of their protective effect, especially for LABA (Ramage 1994). In addition, recovery from EIA after a standard dose of beta2‐agonists is slower, and additional doses are often required when SABA or LABA are used daily (Hancox 2002).
On the other hand, the reported association between administration of LABA, not in combination with inhaled corticosteroids, and increased numbers of severe cardiovascular side effects and sudden deaths (Nelson 2006; Salpeter 2010) induced the U.S. Food and Drug Administration (FDA) to set a "black box" on these drugs, highlighting the urgent need to promote clear studies of pharmacovigilance (Martinez 2005).
Objectives
To assess the effects of inhaled short‐ and long‐acting beta2‐agonists, compared with placebo, in the pretreatment of children and adults with exercise‐induced asthma (or exercise‐induced bronchoconstriction).
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind, randomised controlled trials (RCTs) of any study design. Data published in abstract form only were excluded. At least one primary outcome of this systematic review had to be reported for a study to be considered eligible.
Types of participants
We included children and adults (aged 18 years or older) with a clear history of exercise‐induced asthma and/or a positive response to a standardised exercise challenge, defined according to ERS and ATS guidelines as a fall in FEV1 ≥ 10%. Studies that did not clearly state criteria for EIA diagnosis were excluded.
Types of interventions
Eligible interventions included inhaled beta2‐agonists administered, at any dose, as short‐term or long‐term prophylactic treatment before participants underwent a standardised exercise challenge. SABA and LABA had to be administered within a time period before exercise challenge that did not exceed their pharmacological half‐life (arbitrarily set at 1 hour for SABA, and at 12 hours for LABA). For studies with more than one drug arm, only the comparison with placebo was considered. Studies with more than one drug arm that evaluated different beta2‐agonist molecules were considered as separate trials.
Types of outcome measures
Primary outcomes
Mean max % fall in FEV1 (100 × (baseline pre‐exercise value - lowest postexercise value)/baseline pre‐exercise value) in people treated with a beta2‐agonist versus mean max % fall in FEV1 in people treated with placebo
Mean % protection afforded by beta2‐agonists (% protection = 100 × (max % fall FEV1 placebo - max % fall FEV1 beta2‐agonist)/max % fall FEV1 placebo)
Mean area under the curve (AUC) of time course changes in FEV1 after exercise in people treated with a beta2‐agonist versus mean AUC of time course changes in FEV1 after exercise in people treated with placebo
Secondary outcomes
Number of people with a max % fall in FEV1 < 10% (complete protection), < 15% and < 20%
Changes from baseline in symptom and sign scores
Mean max % fall in other pulmonary function parameters (peak expiratory flow (PEF), forced expiratory flow 25–75% (FEF), maximal expiratory flow at 50% (MEF) etc.)
Onset of tolerance (considered for long‐term administration studies and in relation to concomitant treatment with inhaled corticosteroids)
Outcomes of physical performance
Side/adverse effects
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of Trials, which is derived from systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, as well as handsearching of respiratory journals and meeting abstracts (see Appendix 1 for additional details). The Register was searched using the terms in Appendix 2 from the date of inception up to August 2013. No restriction was placed on language of publication.
Searching other resources
We screened reference lists of included studies, recent reviews and textbooks for relevant citations. We contacted authors of unpublished or 'in‐progress' studies and selected manufacturers of beta2‐agonists to identify additional studies. Furthermore, we searched national and international clinical trial websites (www.clinicalstudyresults.org; www.clinicaltrials.gov; www.fda.gov) for additional trials. Personal contacts with colleagues, collaborators and other trialists working in the field of asthma were at last made to identify further potentially relevant studies. No language or publication restrictions were applied to these searches.
Data collection and analysis
Selection of studies
Titles and abstracts of papers identified in the search were reviewed independently by two review authors (MB, CDM), and articles that appeared to fulfil the inclusion criteria were retrieved. From the full text of these papers, two review authors (MB, EC) independently established whether studies met the inclusion criteria. Studies that did not fulfil all of the inclusion criteria were excluded, and reasons for exclusion were reported. The percentage of agreement was recorded, and any disagreement was solved by consensus. If the two review authors did not reach an agreement, a third review author adjudication (MC) was used to resolve disagreements. In case of further uncertainty, study authors were contacted. Review authors were not blinded to authors, journals, results, etc.
Data extraction and management
Data extraction was performed independently by two review authors (MB, EC). Full texts were screened, and bibliographic details, as well as data regarding study design, participants, disease severity, intervention and outcomes, were recorded in predefined forms and entered into RevMan 5.2. All data, numerical calculations and graphic extrapolations were independently confirmed. We did not deal with missing data.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies as high, low or unclear using The Cochrane Collaboration’s 'Risk of bias' tool (Higgins 2011) and the following headings.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
Discussion or third party adjudication was used to resolve disagreements when necessary.
Measures of treatment effect
Treatment effects were measured as mean differences (MDs) for continuous outcomes and odds ratios (ORs) for dichotomous outcomes.
Unit of analysis issues
Many included studies were of cross‐over design, but many did not report the results of paired t‐tests for continuous outcomes in this review. Some studies provided raw data that allowed a calculation of the correlation between treatment periods on beta2‐agonists and placebo on maximum percentage fall in FEV1 (see Appendix 3 for the raw data). We used the average correlation from these studies (0.36) to impute an appropriate standard error for within‐‐participant differences (as described in Section 16.4.6.4 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). We carried out a meta‐analysis of the mean differences and their standard errors for this outcome using the Generic Inverse Variance method in RevMan 5.2.
Dealing with missing data
Missing data on the correlation between results from participants in the cross‐over studies were imputed using the average correlation from studies that reported appropriate raw data. Cross‐over studies provided information on the number of participants in each arm who experienced a given drop in FEV1, but not the number of participants whose FEV1 dropped on both interventions (active treatment and placebo). We were able to calculate marginal odds ratios for these outcomes but could not adjust the standard error to take advantage of the cross‐over design.
Assessment of heterogeneity
To assess the level of heterogeneity, the Chi2 test and the I2 statistic were used. In establishing the level of heterogeneity, we considered the following rules for interpretation of results (Higgins 2011).
0 to 30% as low heterogeneity.
30% to 60% as moderate heterogeneity worthy of investigation.
60% to 90% as severe heterogeneity worthy of understanding.
90% to 100% as allowing aggregation only with major caution.
Assessment of reporting biases
Funnel plots were used to investigate the possibility of publication bias.
Data synthesis
Data were entered into RevMan 5.2. For continuous measures, individual and pooled statistics were reported as mean difference (MD) of treatment effect with 95% confidence intervals (95% CIs) using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
Heterogeneity worthy of investigation was examined using the following predefined subgroup analyses.
Type of beta2‐agonist (SABA vs LABA).
Molecule of beta2‐agonist (formoterol vs salmeterol).
Age of participants (children vs adults).
Concomitant treatments (beta2‐agonist monotherapy vs concomitant inhaled corticosteroid treatment).
Results
Description of studies
See:Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search identified 2400 articles, and from these, 289 papers were independently selected by two review authors (k = 0.95) as being of potential interest on the basis of titles and abstracts. After non‐ interventional studies (n = 27) and articles published as abstract only (n = 52) were removed, we retrieved the remaining articles in full text and from these identified 51 studies for inclusion in the meta‐analysis (Figure 1). Two further articles were added through the cross‐checking reference process. We excluded 158 studies with reasons provided in Characteristics of excluded studies; one study is listed under Studies awaiting classification to be considered for inclusion when the review is next updated.
1.
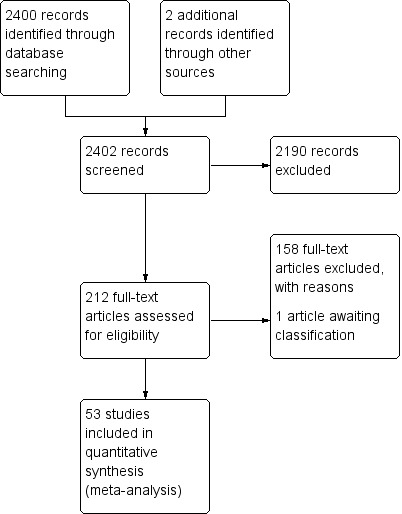
Study flow diagram.
Included studies
Full details can be found in the Characteristics of included studies tables.
Study features and design
All 53 included studies were double‐blind, placebo‐controlled, randomised trials. Forty‐eight studies used a cross‐over design, and five were performed in a parallel ‐group design. In the cross‐over studies, washout periods ranged between 1 and 21 days. Nine studies did not mention duration of washout, and in two papers, no washout was performed. The exercise challenges involved treadmill (n = 35), cycle ergometer (n = 13) and free running (n = 5). All challenges were standardised and met recommended testing criteria. Trials were conducted between 1976 and 2010 in 12 different countries: Europe (N = 27), United States/Canada (N = 22) and Australia (N = 4). All articles except one (in Spanish) were written in English.
Population
Collectively, included studies reported data on 1139 participants. Population sample size ranged from 10 to 161 participants (55 in a single drug arm of parallel‐group studies and 46 in cross‐over studies-the highest number of enrolled participants). Studies included children and adults (age range 4 to 64 years). A total of 20 studies were performed in children, 18 in adults and 12 in both children and adults. Three papers did not provide sufficient information to allocate people according to age. Nine studies provided only information about ethnicity, and Caucasian was the most represented race.
Interventions
Of 53 included studies, 45 addressed beta2‐agonist short‐term administration, and eight focused on long‐term treatment. Articles were grouped on the basis of the type of beta2‐agonist drugs evaluated: SABA (N = 42; short‐term administration n = 40 and long‐term administration n = 2) and LABA (N = 27, short‐term administration n = 21 and long‐term administration n = 6). Among different beta2‐agonists, salbutamol (n = 27), salmeterol (n = 14), formoterol (n = 13) and terbutaline (n = 6) represented the molecules most frequently investigated. Beta2‐agonists were delivered through different devices (nebulisers, metered‐dose inhalers (MDIs) and inhalers).
Details on dosage and types of beta2‐agonist administration are summarised in Table 2, together with timing in relation to exercise.
1. Summary of study interventions.
| Study | Intervention (single dose) | Study type (single‐dose test or chronic [duration]) | When administered before challenge/exercise (min, h) |
|
Anderson 2001 Salb Disk; Anderson 2001 Salb MDI |
Salbutamol diskus 200 mcg Salbutamol MDI |
Single dose | 30 min |
|
Blake 1999 Salb 180 Blake 1999 Salm 25 Blake 1999 Salm 50 |
Albuterol 180 mcg Salmeterol diskus 25 mcg Salmeterol diskus 50 mcg |
Single dose | 30 min, 5 h 30, 11 h 30 |
|
Boner 1994 Salb 200 Boner 1994 Form 12 |
Salbutamol 200 mcg Formoterol 12 mcg |
Single dose | 3 h, 12 h |
| Boulet 1989 Salb | Salbutamol 200 mcg | Single dose | 30 min |
|
Bronski 1995 Salb MDI Bronski 1995 Salb Pwd |
Albuterol MDI 180 mcg Albuterol rotacaps 200 mcg |
Single dose | 15 min |
|
Bronski 1999 Salm Disk Bronski 1999 Salm Diskhal |
Salmetrol discus 50 mcg Salmeterol diskhaler 50 mcg |
Single dose | 30 min, 5 h 30, 11 h 30 |
|
Bronski 2002 Salb Bronski 2002 Form 12 Bronski 2002 Form 24 |
Albuterol 180 mcg Formoterol 12 mcg Formoterol 24 mcg |
Single dose | 15 min, 4 h, 8 h, 12 h |
|
Carlsen 1995 Salm 25 Carlsen 1995 Salm 50 |
Salmeterol diskhaler 25 mcg Salmeterol diskhaler 50 mcg |
Single dose | 10‐12 h |
|
Cavagni 1993 Salb MDI Cavagni 1993 Salb Jet |
Salbutamol MDI 200 mcg Salbutamol jet disposable 200 mcg |
Single dose | 10 min |
| Clarke 1990 Fen | Fenoterol 100 mcg | Single dose | 10 min |
|
Daugbjerg 1996 Salb Daugbjerg 1996 Form 12 |
Salbutamol 400 mcg Formoterol 12 mcg |
Single dose | 3 h, 12 h |
| Debelic 1988 Reproterol | Reproterol 1 mg | Single dose | 15 min |
|
DeBenedictis 1996 Salm 25 DeBenedictis 1996 Salm 50 |
Salmeterol 25 mcg Salmeterol 50 mcg |
Single dose | 1 h, 2 h |
| DeBenedictis 1998 Salb | Salbutamol 200 mcg | Single dose | 20 min |
|
Del Col 1993 Salb MDI Del Col 1993 Salb Jet |
Salbutamol MDI 200 mcg Salbutamol jet device 200 mcg |
Single dose | 10 min |
| Dinh Xuan 1989 Terb | Terbutaline 500 mcg | Single dose | 15 min |
| Egglestone 1981 Terb 250 | Terbutaline 250 mcg | Single dose | 1 h |
| Ferrari 2000 Form 12 | Formoterol 12 mcg | Single dose | 15 min, 4 h |
| Garcia 2001 Form 12 | Formoterol 12 mcg twice daily | Long‐term (4 weeks) | 30 min, 12 h at days 1, 14 and 28 |
| Green 1992 Salm 50 | Salmeterol 50 mcg | Single dose | 1 h, 5 h, 9 h |
|
Gronnerod 2000 Terb 500 Gronnerod 2000 Form 9 Gronnerod 2000 Form 4.5 |
Terbutaline 500 mcg Formoterol 9 mcg Formoterol 4.5 mcg |
Single dose | 15 min, 4 h, 8 h |
| Hancox 2002 | Salbutamol 800 mcg daily | Long‐term (1 week) | 8 h |
|
Hawksworth 2002 Salb HFA Hawksworth 2002 Salb MDI |
Salbutamol 180 HFA Salbutamol 180 mcg MDI |
Single dose | 30 min |
| Henricksen 1983 Terb | Terbutaline 32.5 mcg | Single dose | 15 min |
|
Henricksen 1992 Salb Henriksen 1992 Form 12 |
Salbutamol 200 mcg Formoterol 12 mcg |
Single dose | 30 min, 3 h, 5 h 30, 8 h |
|
Hills 1976 Salb Hills 1976 Salmefamol |
Salbutamol 200 mcg Salmefamol 200 mcg |
Single dose | 20 min |
| Inman 1996 | Salbutamol 800 mcg daily | Long‐term (81 weeks) | 24 h |
|
Kemp 1994 Salb Kemp 1994 Salm 42 |
Salbutamol 180 mcg Salmeterol 42 mcg |
Single dose | 30 min, 5 h 30, 11 h 30 |
| Konig 1981 Metaprot | Metaproterenol 130 mcg | Single dose | 10 min, 1 h |
| Larsson 1982 Fen | Fenoterol 400 mcg | Single dose | 10 min |
|
McAlpine 1990 Salb McAlpine 1990 Form 12 |
Salbutamol 200 mcg Formoterol 12 mcg |
Single dose | 2 h, 4 h |
| McFadden 1986 Salb (I) | Salbutamol 200 mcg | Single dose | 15 min |
| McFadden 1986 Salb (II) | Salbutamol 180 mcg | Single dose | 15 min |
| Morton 1989 Rimet | Rimeterol 400 mcg | Single dose | 2 min |
| Nelson 1998 | Salmeterol 84 mcg daily | Long‐term (29 days) | 30 min, 9 h |
|
Newnham 1993 Salb 200 Newnham 1993 Salm 50 |
Salbutamol 200 mcg Salmeterol 50 mcg |
Single dose | 1 h, 6 h, 12 h |
|
Patel 1986 Salb 200 Patel 1986 Tulob 200 Patel 1986 Tulob 400 |
Salbutamol 200 mcg Tolobuterol 200 mcg Tolobuterol 400 mcg |
Single dose | 20 min |
|
Patessio 1991 Salb 200 Patessio 1991 Form 24 |
Salbutamol 200 mcg Formoterol 24 mcg |
Single dose | 2 h, 8 h |
|
Pearlman 2006 Salb 180 Pearlman 2006 Form 12 Pearlman 2006 Form 24 |
Salbutamol 180 mcg Formoterol 12 mcg Formoterol 24 mcg |
Single dose | 15 min, 4 h, 8 h, 12 h |
| Pearlman 2007 Salb 90 | Salbutamol 90 mcg | Single dose | 20 min |
| Philip 2007 Salm 50 | Salmeterol 50 mcg | Single dose | 2 h, 8 h 30, 24 h |
| Ramage 1994 | Salmeterol 100 mcg daily | Long‐term (28 days) | 6 h, 12 h |
|
Richter 2002 Terb 500 Richter 2002 Form 12 Richter 2002 Salm 50 |
Terbutaline 500 mcg Formoterol 12 mcg Salmeterol 50 mcg |
Single dose | 5 min, 30 min, 1 h |
| Shapiro 2002 | Salbutamol 180 mcg Formoterol 12 mcg Formoterol 24 mcg |
Single dose | 15 min, 4 h, 8 h, 12 h |
| Simons 1997 | Salmeterol 50 mcg | Long‐term (28 weeks) | 1 h, 9 h |
| Stelmach 2008 | Formoterol 9 mcg daily | Long‐term (28 weeks) | 1 h, 9 h |
| Storms 2004 | Salmeterol 100 mcg daily | Long‐term (28 weeks) | 1 h, 9 h |
|
Sturani 1983 Fen 400 Sturani 1983 Salb 200 |
Fenoterol 400 mcg Salbutamol 200 mcg |
Single dose | 30 min |
| VanHaitsma 2010 Salb | Salbutamol 180 mcg | Single dose | 15 min |
| Vasquez 1984 Salb 400 | Salbutamol 400 mcg | Single dose | 15 min |
| Walker 1986 Bitolterol | Bitolterol 1050 mcg | Single dose | 45 min |
| Wolley 1990 Terb 500 | Terbutaline 500 mcg | Single dose | 25 min, 2 h, 4 h, 6 h |
Outcomes
As per inclusion criteria, all 53 included studies reported data on at least one primary outcome of this review.
Excluded studies
Risk of bias in included studies
An assessment of the risk of bias is presented in the Characteristics of included studies tables and is summarised in a risk of bias figure (Figure 2).
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most of the included studies were judged at unclear risk for selection bias. Lack of information provided on random sequence generation and on allocation concealment may be explained by the high number of papers (40/53) conducted before 2000, when reporting of these risk of bias criteria was less common.
Blinding
Risks of performance and detection bias were minimised by the narrow inclusion criteria adopted, which allow inclusion in the systematic review of only randomised, at least double‐blind, placebo‐controlled trials.
Incomplete outcome data
Three drug arms ( Konig 1984 Fen 0.4; Konig 1984 Fen 0.8; Boulet 1989 Salb) reported incomplete data on outcomes as specified in this systematic review and were therefore assigned a high risk of bias.
Selective reporting
All studies except one (Ferrari 2000 Form 12) were rated as having unclear risk for reporting bias.
Other potential sources of bias
The possibility of publication bias was investigated in the funnel plot shown in Figure 3. The presence of other potential sources of bias was rated as unclear risk because of the scarce information provided.
3.
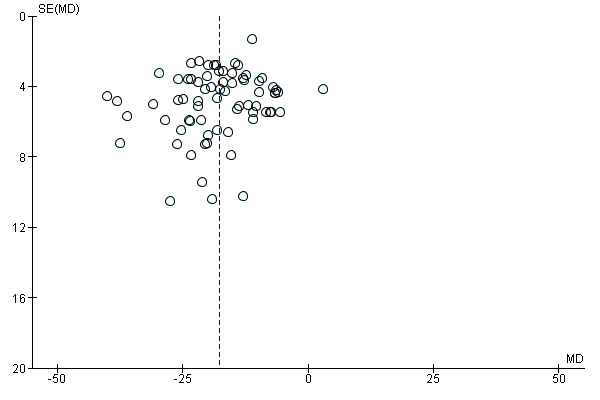
Funnel plot of comparison: 1 Beta2‐agonists versus placebo (single administration), outcome: 1.1 Maximal percentage fall in FEV1.
Several papers reported data derived from industry‐funded studies.
Effects of interventions
See: Table 1
Effects of intervention were separately assessed for short‐term (single administration) and long‐term beta2‐agonist administration.
Short‐term administration
The 45 studies evaluating short‐term beta2‐agonist administration included 77 arms of active treatment (49 SABA and 28 LABA) in comparison with placebo.
Primary outcomes
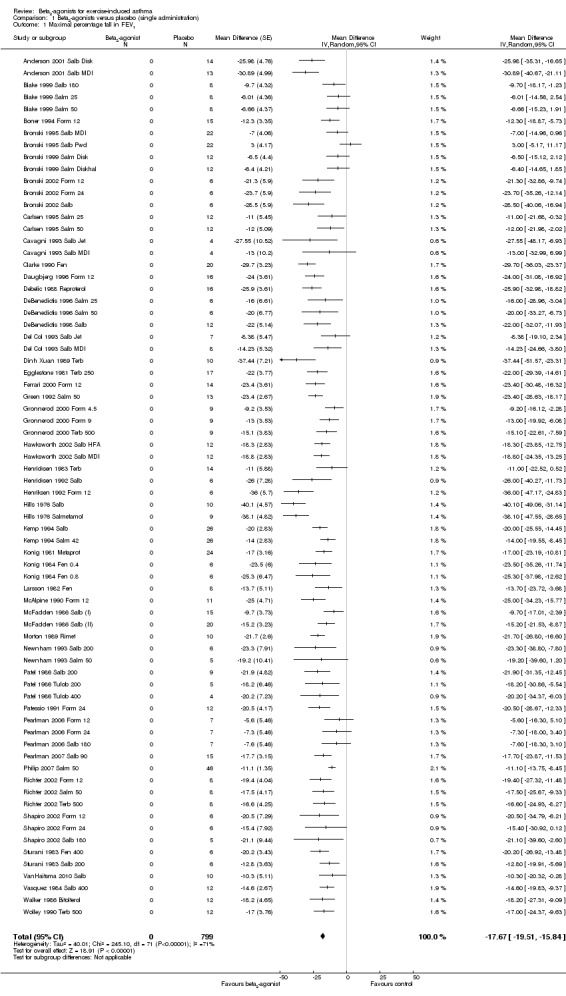
Data on max % fall in FEV1 were provided by 77 arms. Effects of SABA short‐term administration were evaluated beyond the pharmacological half‐life (1 hour) in five studies, which were therefore excluded. Analysis of the remaining 72 arms, including 799 participants (Figure 4), showed a significant protective effect of beta2‐agonists compared with placebo (MD ‐17.67%, 95% CI ‐19.51% to ‐15.84%; P = 0.00001). In particular, 58 study arms favoured the active treatment, and 14 trial arms reported no significant difference. Heterogeneity was, however, high (I2 = 71%).
4.
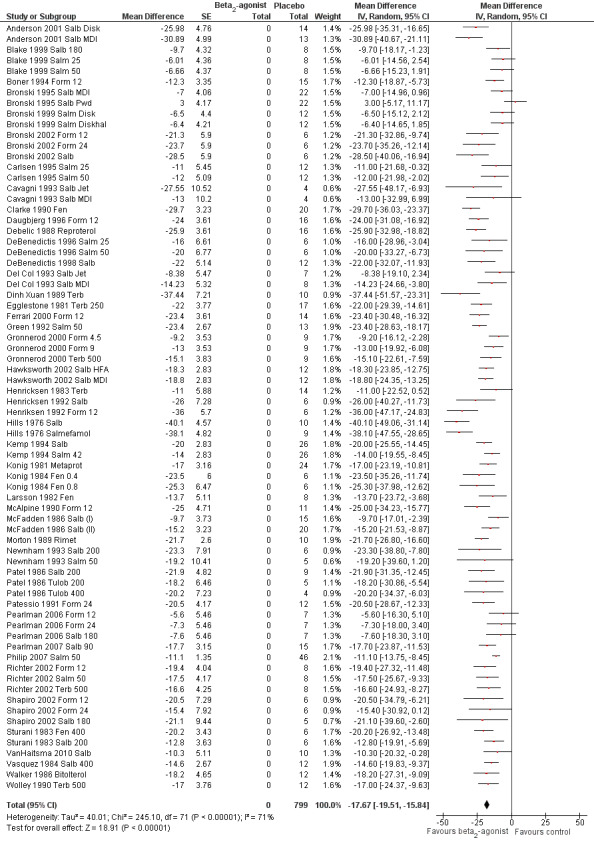
Forest plot of comparison: 1 beta2‐agonists versus placebo (single administration), outcome: 1.1 Maximal percentage fall in FEV1.
Mean % protection was 66% (range 29% to 91%). In seven studies, beta2‐agonist administration not only completely protected participants from EIA but also induced a bronchodilator effect compared with baseline values. In only one case (Bronski 1995 Salb Pwd), placebo offered greater protection compared with the active treatment.
Seventeen studies evaluating LABA short‐term administration at different time points showed an FEV1 % fall AUC that favoured the active treatment. Different study designs prevented merging of the data in a unique comprehensive analysis.
Secondary outcomes
Secondary outcomes on pulmonary function parameters confirmed a positive protective effect of beta2‐agonists on EIA compared with placebo. Complete protection from EIA as assessed by numbers of participants with an FEV1 % fall < 10% was evaluated in 19 studies (Analysis 1.2). A significant difference was noted in the number of participants completely protected (OR 0.08, 95% CI 0.06 to 0.13; P = 0.00001). Similar results were obtained for thresholds of FEV1 fall set at 15% (OR 0.06, 95% CI 0.03 to 0.15) and 20% (OR 0.09, 95% CI 0.06 to 0.14) (Analysis 1.3; Analysis 1.4). Max PEF and FEF25‐75 % fall were assessed, respectively, in 14 and in 8 studies (Analysis 1.5; Analysis 1.6). However, statistical analysis for these two outcomes was based on a limited number of trials because dispersion data were lacking in most of the studies considered. Only three arms (Vasquez 1984 Salb 400; Carlsen 1995 Salm 25; Carlsen 1995 Salm 50) reported data on max MEF25‐50 % fall. No study provided information on changes in symptoms and sign scores and effects on physical performance.
1.2. Analysis.
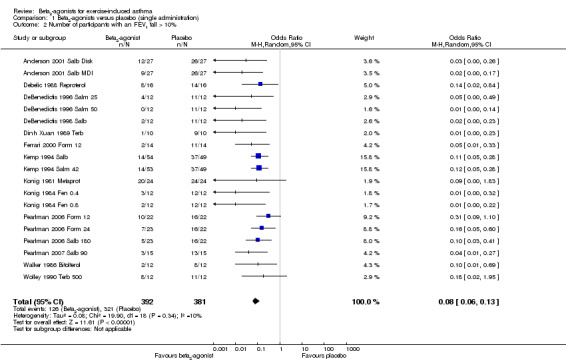
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 2 Number of participants with an FEV1 fall > 10%.
1.3. Analysis.
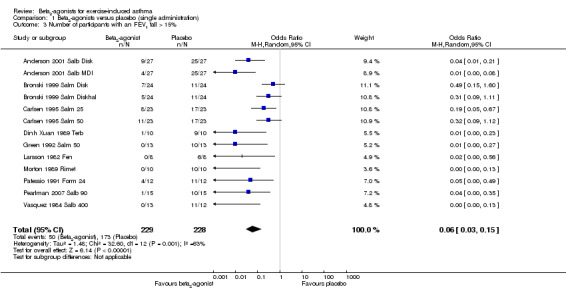
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 3 Number of participants with an FEV1 fall > 15%.
1.4. Analysis.
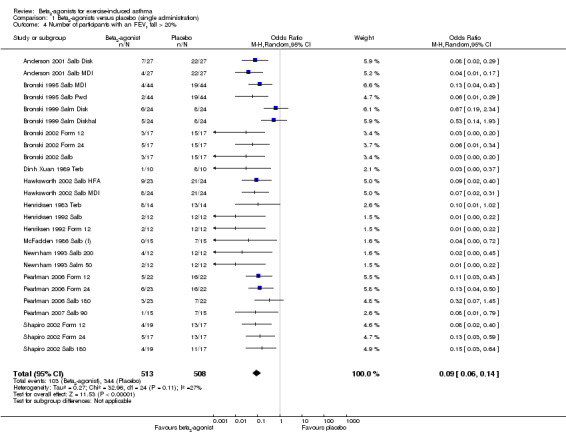
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 4 Number of participants with an FEV1 fall > 20%.
1.5. Analysis.
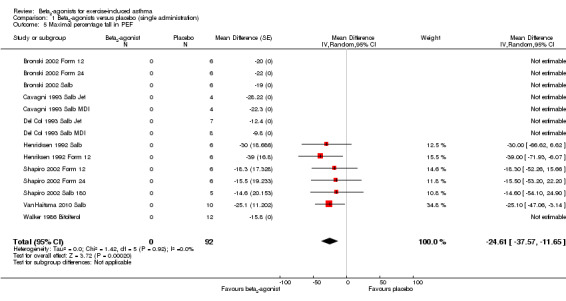
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 5 Maximal percentage fall in PEF.
1.6. Analysis.
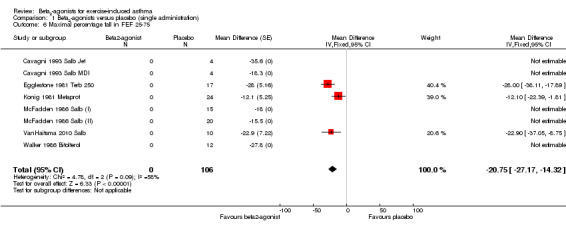
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 6 Maximal percentage fall in FEF 25‐75.
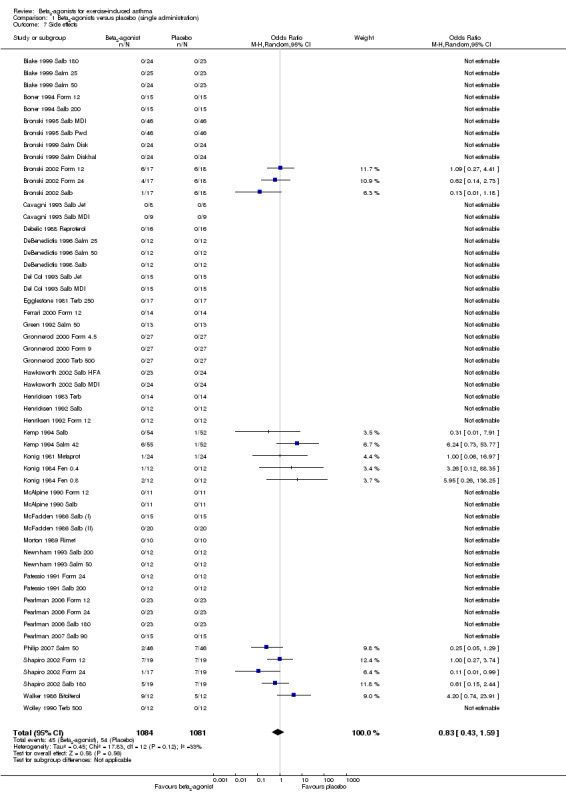
As far as they concern secondary outcomes related to safety, side effects were assessed in 55 trials (Figure 5). Among these, 42 arms reported no adverse event for either active or placebo treatment. Analysis of the remaining 13 trials showed no significant difference between beta2‐agonists and placebo.
5.
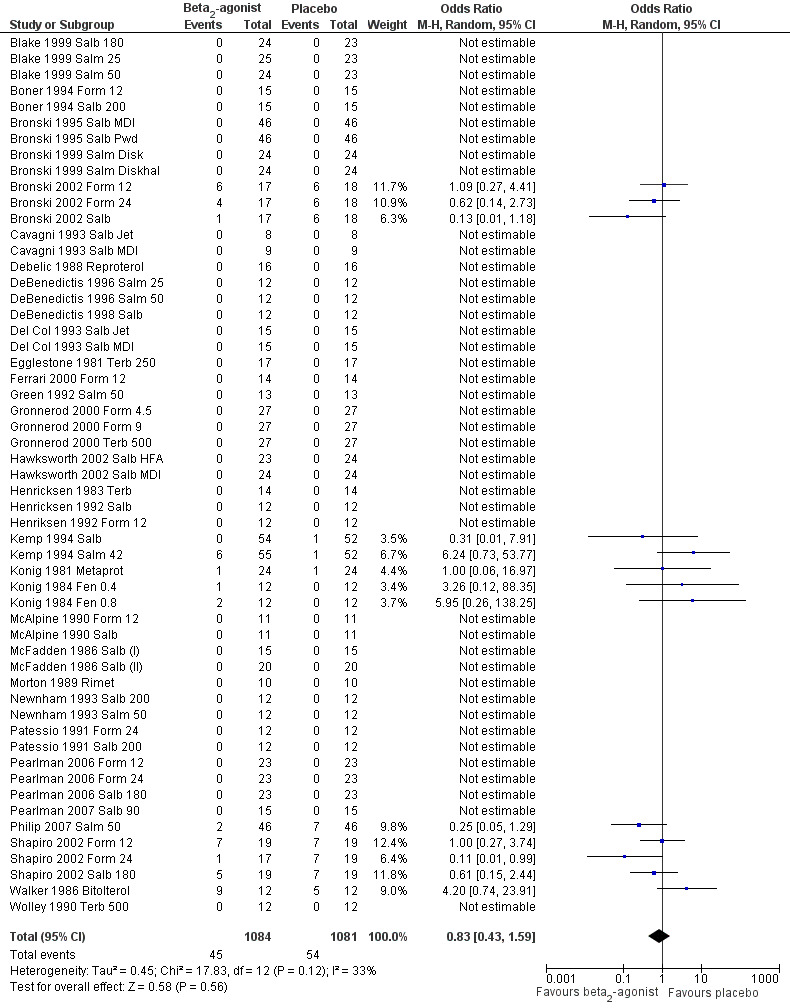
Forest plot of comparison: 1 Beta2‐agonists versus placebo (single administration), outcome: 1.1 Side effects.
Subgroup analysis
The high heterogeneity (I2 = 71%) found for the primary outcome max FEV1 % fall was investigated through the preplanned subgroup analysis.
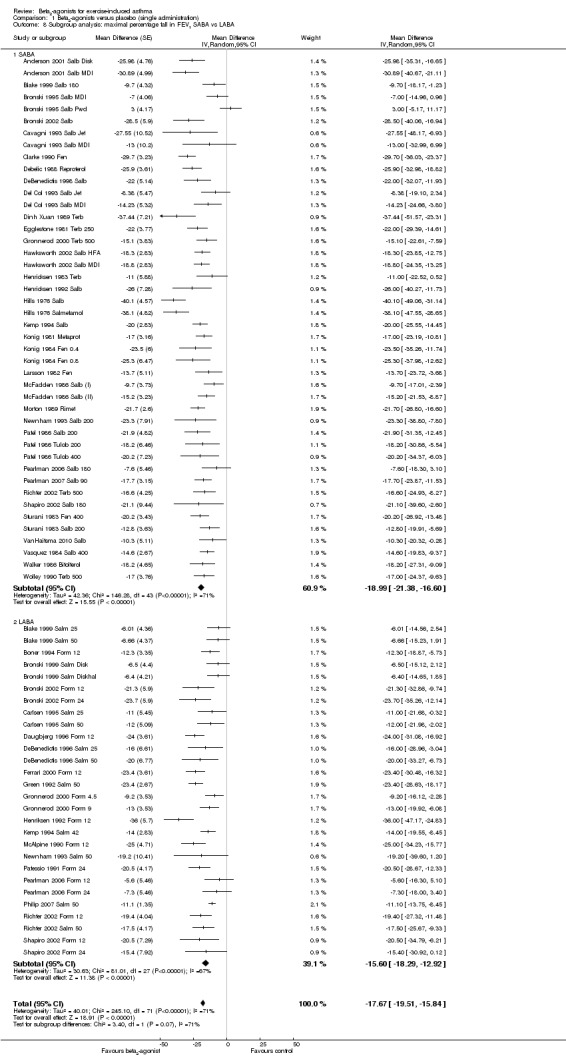
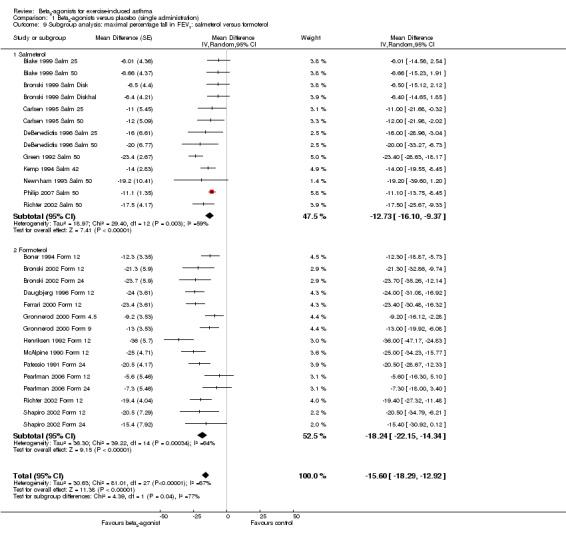
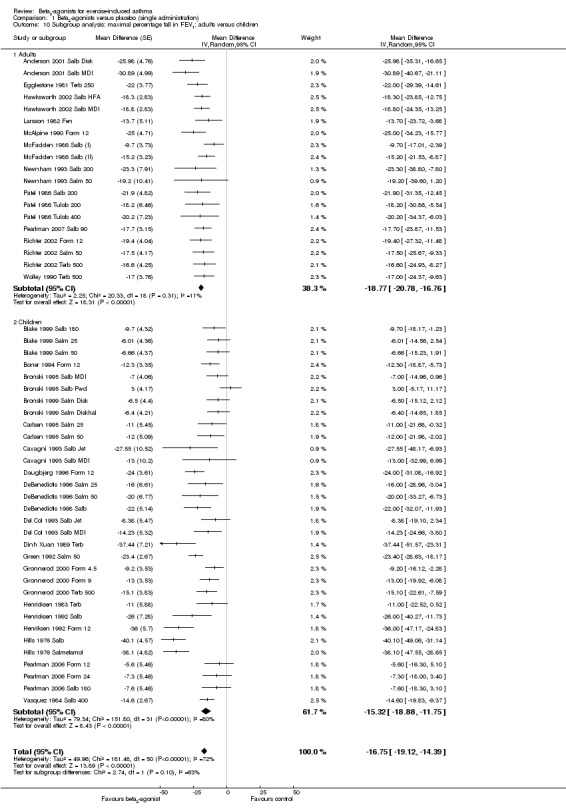
We performed a subgroup analysis according to types of beta2‐agonists (SABA vs LABA; Figure 6). Although subgroup analysis confirmed a significant bronchoprotective effect against EIA for both classes compared with placebo, it was not able to explain the marked heterogeneity observed in the entire population sample: SABA (I2 = 71%) and LABA (I2 = 67%). Accordingly, non‐significant differences emerged from the analysis of the different beta2‐agonist molecules administered (Figure 7). This evaluation was plotted only for the comparison between formoterol and salmeterol, because the number of studies evaluating different SABA molecules, apart from salbutamol, appeared to be too small for a reliable investigation. It is interesting to note that analysis of studies performed only in adults (n = 19) compared with those performed only in children (n = 32) showed that high heterogeneity was largely confined to studies in children (I2 = 11% and 80%, respectively), despite the comparable mean bronchoprotective effect (Figure 8). Furthermore all studies that failed to show a positive protective effect against EIA of beta2‐agonists compared with placebo dealt with the paediatric population.
6.
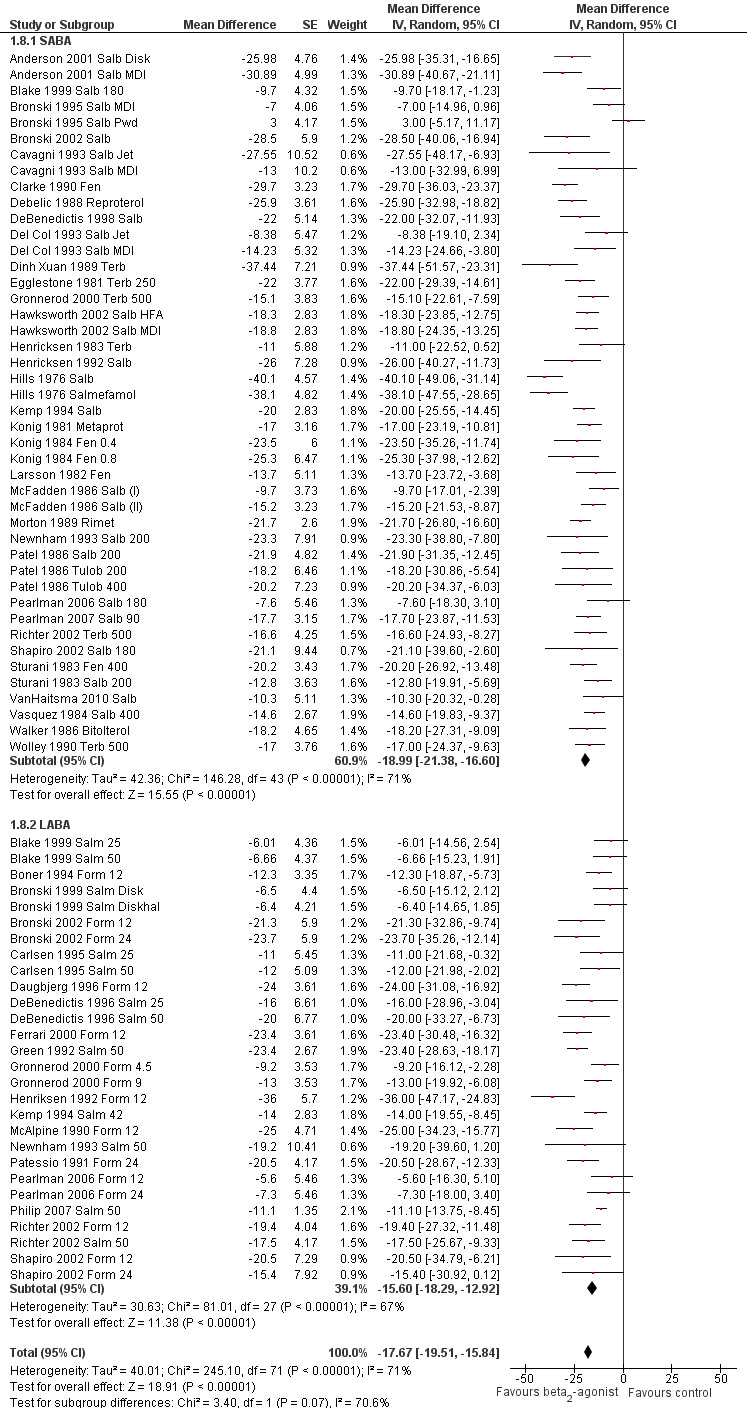
Forest plot of comparison: 1 Beta2‐agonists versus placebo (single administration), outcome: 1.8 Subgroup analysis: maximal percentage fall in FEV1 SABA versus LABA.
7.
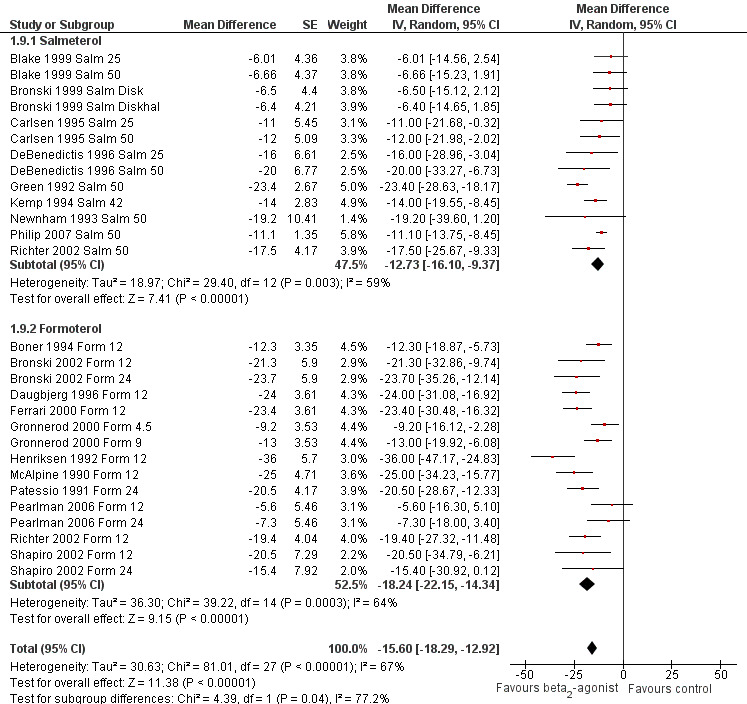
Forest plot of comparison: 1 Beta2‐agonists versus placebo (single administration), outcome: 1.9 Subgroup analysis: maximal percentage fall in FEV1: salmeterol versus formoterol.
8.
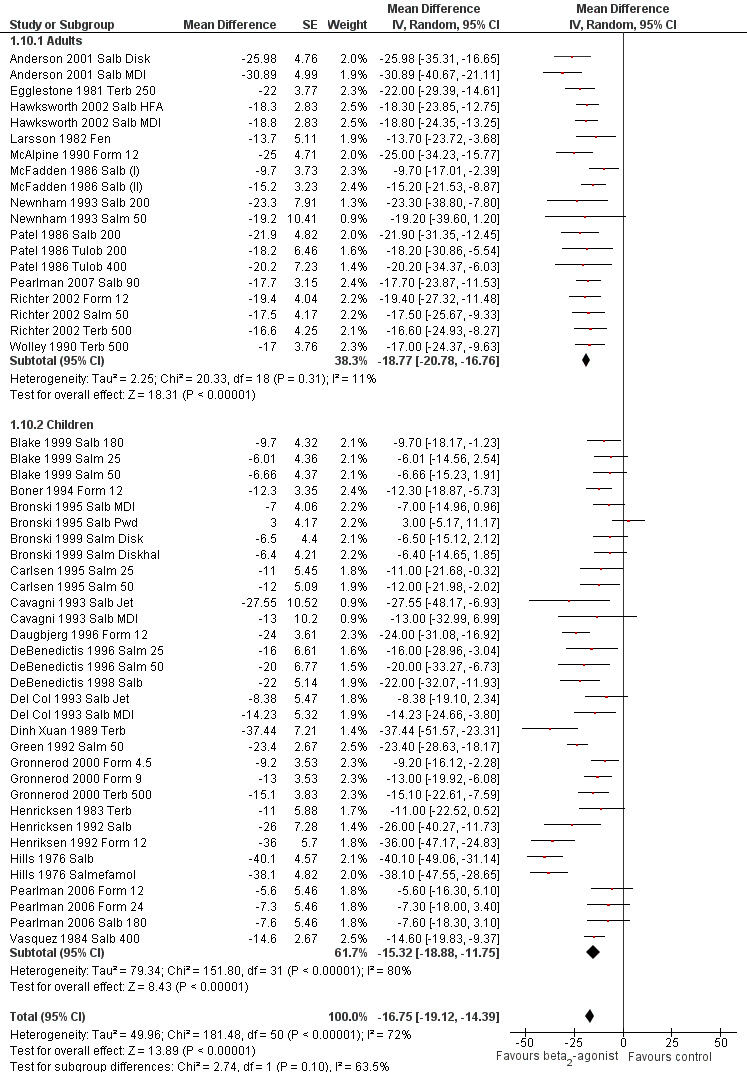
Forest plot of comparison: 1 Beta2‐agonists versus placebo (single administration), outcome: 1.10 Subgroup analysis: maximal percentage fall in FEV1: adults versus children.
The different study designs used and the limited information provided prevented assessment of the potential role of concomitant treatment with inhaled corticosteroids.
Long‐term administration
Long‐term beta2‐agonist administration was addressed in only eight papers. Five trials were performed with a cross‐over design in a total of 64 people (50 adults and 14 children). Three studies adopted a parallel‐group design and included 69 people (49 adults and 20 children) in the active arms and 73 (53 adults and 20 children) placebo controls. Treatment periods range from 7 to 29 days. Effects of SABA were evaluated in two protocols, and LABA administration was assessed in six studies. The limited number of trials, the small population samples and the different study designs and drugs tested allow only a descriptive approach and prevent plotting of data in a meta‐analysis.
Garcia and coauthors (Garcia 2001 Form 12) evaluated the effects of 28‐day formoterol administration in a parallel‐group design (10 people in the beta2‐agonist arm and 9 people in the placebo arm). The protective effect of salmeterol 12 mcg twice daily was assessed on the 1st, 14th and 28th study days, and results were not significantly greater than those provided by placebo at any time point. Furthermore, tachyphylaxis to the beta2‐agonist effect was developed already after two weeks of treatment, although it was not progressive.
Hancox (Hancox 2002) studied the effect of 200 mcg once daily of salbutamol in eight adults treated for seven days in a cross‐over study. Results showed not only an increased max FEV1 % fall after the exercise challenge, but also a sub‐optimal bronchodilator response to further beta2‐agonist administration at the end of the treatment period in the active group.
Ten adults who inhaled 200 mcg of salbutamol or placebo four times a day for seven days were studied by Inman and O'Byrne (Inman 1996) in a cross‐over study. One week of regular inhaled salbutamol resulted in worsening of EIA.
A cross‐over design was also adopted by Nelson (Nelson 1998) in 20 adults treated for a month with inhaled salmeterol 42 mcg twice a day. Significant beta2‐agonist protection against EIA was maintained for the entire study period. However, the length of time that the drug remained active after a single dose significantly decreased. Furthermore, the number of participants for whom salmeterol did not offer complete protection against EIA (FEV1 % fall < 10%) increased from two on study day 1 to 11 on day 29 (P = 0.02)
Ramage and coworkers (Ramage 1994) studied 12 adults treated with inhaled salmeterol 50 mcg twice daily for 4 weeks in a cross‐over manner. The significant protection provided by the first dose of salmeterol against EIA at 6 and 12 hours was no longer present at the end of the treatment period.
Fourteen children were studied by Simons (Simons 1997) in a four‐week cross‐over study. The first dose of salmeterol had an excellent bronchoprotective effect against EIA at 1 and 9 hours. At the end of the study, however, the bronchoprotective effect was significantly greater than that of placebo only at 1 hour.
A parallel‐group study design was adopted by Stelmach (Stelmach 2008) to compare the effects of formoterol 9 mcg daily against placebo in two arms, each consisting of 20 children. Both groups of people were receiving concomitant inhaled corticosteroid (ICS) treatment with budesonide 100 mcg daily. At the end of the four‐week treatment period, bronchoconstriction induced by a standardised treadmill exercise challenge was significantly diminished in the active group compared with the placebo group,
At last, Storms (Storms 2004) in a four‐week parallel‐group study compared the protective effect of salmeterol 50 mcg twice daily against placebo. All enrolled people were receiving concomitant 100 mcg twice‐daily fluticasone treatment. The protective effect against EIA was evaluated at weeks 1 and 4 and was not different between the two groups.
Only one study (Simons 1997) took into consideration safety aspects of long‐term beta2‐adrenergic administration. Reported side effects were minor, were poorly related to study drug and anyway were not different between active and placebo groups.
The overall evaluation of presented studies seems to confirm the beta2‐agonist bronchoprotective effect for the first dose of treatment. However, long‐term use of both SABA and LABA induced the development of tolerance and decreased the duration of drug effect, even after short‐term treatment. The few available data on concomitant therapy with inhaled corticosteroids did not allow a firm statement about their potential influence on the response to beta2‐agonists.
Discussion
Summary of main results
Evidence emerging from the meta‐analysis of 45 short‐term (single administration) studies shows that both short‐ and long‐acting beta2‐agonists administered as preventive treatment (within the time‐effect period set at one hour for SABA and at 12 hours for LABA) prevent exercise‐induced asthma, as shown by the primary outcomes related to the FEV1 fall. This pharmacological effect appears to be clinically relevant and independent of the exercise challenge adopted (treadmill, cycle ergometer, free run). The assessment of secondary outcomes considered shows that the beta2‐agonist preventive effect is also documented by the number of participants protected (complete protection detectable in 68% of participants) and by other pulmonary function variables (PEF, FEF 25%‐75%, MEF 50%) and beta2‐agonists did not cause side effects.
Overall completeness and applicability of evidence
The choice to include only double‐blind randomised trials may influence the completeness of the present review but was thought to reinforce the quality of evidence.
Quality of the evidence
The quality of the evidence gathered for the primary outcome of maximal percentage fall in FEV1 and the number of participants with a fall in FEV1 greater than 10% were moderate owing to some concerns about risk of bias (unclear allocation concealment) and detected and not completely explained inconsistency and indirectness (relation of FEV1 to patient‐important outcomes). The same concerns apply to all other outcomes reported in the 'Summary of findings' (SoF) Table. In addition, for the outcomes of maximal percentage fall in PEF, maximal percentage fall in FEF 25‐75 and side effects, the quality was lowered further by the small numbers of participants for these outcomes and the resulting wide confidence intervals. It can be argued that this review started with the premise that pulmonary function measures are patient‐important outcomes and that, therefore, the quality of evidence should not be lowered for indirectness because the primary outcome was measured in these studies. However, despite these considerations, the meaning of the degree of change in FEV1 for patients remains unclear (as well as how it relates to their well‐being). Furthermore, (small) concerns about inconsistency and risk of bias justify an overall rating of quality as presented in the SoF Table. Further research should focus on patient‐important outcomes and the imprecision that was encountered for many of the secondary outcomes.
Potential biases in the review process
The review process was protected from bias by adherence to a prepublished protocol. We tried to prevent bias in our search process by using comprehensive search terms and by asking study authors to identify other published and non‐published studies. We minimised bias by assessing studies independently and resolving differences of opinion by discussion. Extraction of data and assessment of risk of bias were performed in duplicate as well. We performed only subgroup analyses that were specified a priori in the protocol.
Agreements and disagreements with other studies or reviews
Our results appear to be in agreement with those obtained by Spooner and coworkers in a Cochrane review and meta‐analysis published in 2009 and focused on quantitative comparison of the effects of inhaling mast cell stabilisers (nedocromil sodium or sodium cromoglycate) versus beta2‐agonists (Spooner 2003). However, the review authors confined their review to single‐dose administration and to short‐acting beta2‐agonist molecules. As far as we know, no recent systematic reviews have specifically addressed the efficacy and safety of both SABA and LABA, in short‐term and long‐term administration, for prevention of exercise‐induced asthma.
Authors' conclusions
Implications for practice.
Beta2‐agonists, both SABA and LABA, when administered in a single dose before exercise is undertaken, are effective and safe in preventing exercise‐induced asthma. Long‐term regular administration of inhaled beta2‐agonists induces tolerance and lacks sufficient safety data. This finding appears to be of particular clinical relevance in view of the potential for regular prolonged use of beta2‐agonists as monotherapy in the pretreatment of EIA, despite the drug agencies' warning on LABA.
Implications for research.
Further research should focus on the following.
Distinguishing between EIA (exercise‐induced bronchoconstriction with asthma) from exercise‐induced bronchoconstriction without coexisting asthma as different phenotypes, in relation to clinical features, pathophysiological mechanisms, patterns of inflammation and response to treatments, including beta2‐agonists.
Evaluating the potential influence of different genotypes (i.e. beta2‐adrenergic receptor polymorphisms) and phenotypes on exercise‐induced bronchoconstriction severity and response to beta2‐adrenergic treatment.
Better defining response to therapy, not only according to functional parameters, but also on the basis of clinical endpoints (symptoms, disease control and patient‐related outcomes), markers of inflammation and omic approaches.
Defining standardised operational procedures (i.e. exercise challenges, diagnostic thresholds, outcome measures) for clinical trials in EIA.
Performing additional independent trials to address long‐term beta2‐agonist administration in EIA, with special reference to concomitant inhaled corticosteroid treatment, to better assess the risk/benefit ratio between drug efficacy and potential cardiovascular side effects and onset of tolerance.
Establishing whether pretreatment of EIA with beta2‐agonists may exert, beyond the bronchodilator effect, an anti‐inflammatory action, as suggested by recent findings on the role of mechanical factors in inflammation and airways remodelling.
Designing protocols specifically addressed to clarify the potential role of generics and different devices in influencing the efficacy and safety of beta2‐agonist prevention of EIA.
Developing systematic reviews and meta‐analyses to assess which is the most appropriate and effective treatment strategy, among those available, for prevention of exercise‐induced asthma.
Acknowledgements
We thank Brian Rowe for conceiving the original idea for this review and drafting the first version of the protocol.
The authors would like to thank the staff of the Cochrane Airways Group, especially Toby Lasserson and Emma Welsh, who provided invaluable assistance in developing and refining the systematic review. Special thanks to Chris Cates for statistical support, Elizabeth Stovold for her precious assistance in the electronic search and retrieval of papers and Barbara Prediger for her kind help with the SoF Tables.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma & Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Cochrane Airways Group Register search strategy
(physical* OR exercis* OR exert* or train* or bronchoconstrict* or bronchospasm* or EIB or EIA OR athlet*)
AND
(bronchodilat* or ((beta* or B2) and (agonist* or adrenergic*)) or salmeterol or formoterol or salbutamol or albuterol or terbutaline or clenbuterol)
[Limited to records coded as 'asthma']
Appendix 3. Raw data for the maximal percent fall in FEV1 calculations
| Study ID | Beta‐agonist arm | Placebo arm | Correlation | ||||
| Mean | SD | N | Mean | SD | N | ||
| Anderson 2001 Salb Disk | 13.42 | 13.23 | 27 | 39.4 | 17.58 | 27 | 0.46 |
| Anderson 2001 Salb MDI | 8.51 | 13.75 | 27 | 39.4 | 17.58 | 27 | 0.46 |
| Blake 1999 Salb 180 | 3.8 | 7.5 | 25 | 13.5 | 12.7 | 24 | |
| Blake 1999 Salm 25 | 7.99 | 10.2 | 26 | 14.0 | 11.5 | 23 | |
| Blake 1999 Salm 50 | 7.34 | 10.3 | 24 | 14.0 | 11.5 | 23 | |
| Boner 1994 Form 12 | 2.2 | 8.3 | 15 | 14.5 | 13.4 | 15 | |
| Bronski 1995 Salb MDI | 16.0 | 11.0 | 44 | 23.0 | 20.0 | 44 | |
| Bronski 1995 Salb Pwd | 26.0 | 13.0 | 44 | 23.0 | 20.0 | 44 | |
| Bronski 1999 Salm Disk | 5.6 | 10.2 | 24 | 12.1 | 15.6 | 24 | |
| Bronski 1999 Salm Diskhal | 5.7 | 6.36 | 24 | 12.1 | 15.6 | 24 | |
| Bronski 2002 Form 12 | 17.0 | 0.0 | 17 | 38.3 | 0.0 | 18 | |
| Bronski 2002 Form 24 | 14.6 | 0.0 | 17 | 38.3 | 0.0 | 18 | |
| Bronski 2002 Salb | 8.6 | 0.0 | 17 | 37.1 | 0.0 | 18 | |
| Carlsen 1995 Salm 25 | 19.0 | 16.7 | 23 | 30.0 | 16.7 | 23 | |
| Carlsen 1995 Salm 50 | 18.0 | 14.3 | 23 | 30.0 | 16.7 | 23 | |
| Cavagni 1993 Salb Jet | 7.15 | 4.9 | 8 | 34.7 | 22.3 | 8 | |
| Cavagni 1993 Salb MDI | 15.9 | 9.3 | 9 | 28.9 | 21.8 | 9 | |
| Clarke 1990 Fen | ‐19.9 | 0.0 | 20 | 9.8 | 0.0 | 20 | |
| Daugbjerg 1996 Form 12 | 11.0 | 0.0 | 16 | 35.0 | 0.0 | 16 | |
| Debelic 1988 Reproterol | 12.6 | 0.0 | 16 | 38.5 | 0.0 | 16 | |
| DeBenedictis 1996 Salm 25 | 19.0 | 12.0 | 12 | 35.0 | 16.0 | 12 | 0.28 |
| DeBenedictis 1996 Salm 50 | 15.0 | 13.0 | 12 | 35.0 | 16.0 | 12 | 0.33 |
| DeBenedictis 1998 Salb | 3.7 | 4.4 | 12 | 25.7 | 18.9 | 12 | |
| Del Col 1993 Salb Jet | 20.76 | 2.1 | 15 | 29.14 | 15.1 | 15 | |
| Del Col 1993 Salb MDI | 12.37 | 5.1 | 15 | 26.6 | 16.1 | 15 | |
| Dinh Xuan 1989 Terb | ‐2.24 | 17.7 | 10 | 35.2 | 22.1 | 10 | |
| Egglestone 1981 Terb 250 | 10.0 | 8.24 | 17 | 32.0 | 16.49 | 17 | |
| Ferrari 2000 Form 12 | 5.9 | 7.2 | 14 | 29.3 | 14.3 | 14 | |
| Green 1992 Salm 50 | 3.2 | 4.8 | 13 | 26.6 | 10.27 | 13 | 0.30 |
| Gronnerod 2000 Form 4.5 | 9.2 | 8.5 | 27 | 18.4 | 10.1 | 27 | |
| Gronnerod 2000 Form 9 | 5.4 | 8.5 | 27 | 18.4 | 10.1 | 27 | |
| Gronnerod 2000 Terb 500 | 3.3 | 10.2 | 27 | 18.4 | 10.1 | 27 | |
| Hawksworth 2002 Salb HFA | 15.4 | 9 | 23 | 33.7 | 8.3 | 24 | |
| Hawksworth 2002 Salb MDI | 14.9 | 9 | 24 | 33.7 | 8.3 | 24 | |
| Henricksen 1983 Terb | 26.0 | 22.4 | 14 | 37.0 | 14.9 | 14 | |
| Henricksen 1992 Salb | 18.0 | 17.3 | 12 | 44.0 | 13.8 | 12 | |
| Henriksen 1992 Form 12 | 8.0 | 10.4 | 12 | 44.0 | 13.8 | 12 | |
| Hills 1976 Salb | ‐4.6 | 0.0 | 19 | 35.5 | 0.0 | 19 | |
| Hills 1976 Salmefamol | ‐2.6 | 0.0 | 19 | 35.5 | 0.0 | 19 | |
| Kemp 1994 Salb | 7.0 | 0.0 | 54 | 27.0 | 0.0 | 52 | |
| Kemp 1994 Salm 42 | 13.0 | 0.0 | 53 | 27.0 | 0.0 | 52 | |
| Konig 1981 Metaprot | 19.0 | 12.0 | 24 | 36.0 | 15.0 | 24 | |
| Konig 1984 Fen 0.4 | 4.3 | 10.1 | 12 | 27.8 | 14.9 | 12 | |
| Konig 1984 Fen 0.8 | 2.5 | 13.0 | 12 | 27.8 | 14.9 | 12 | |
| Larsson 1982 Fen | ‐2.7 | 0.0 | 8 | 11.0 | 0.0 | 8 | |
| McAlpine 1990 Form 12 | 7.7 | 8.6 | 11 | 32.7 | 16.5 | 11 | |
| McFadden 1986 Salb (I) | 1.1 | 0.0 | 15 | 10.8 | 0.0 | 15 | |
| McFadden 1986 Salb (II) | ‐1.1 | 0.0 | 20 | 14.1 | 0.0 | 20 | |
| Morton 1989 Rimet | 2.8 | 5.5 | 10 | 24.5 | 8.4 | 10 | |
| Newnham 1993 Salb 200 | 3.8 | 18.2 | 11 | 27.1 | 15.9 | 11 | |
| Newnham 1993 Salm 50 | 12.8 | 16.9 | 12 | 32.0 | 23.2 | 11 | |
| Patel 1986 Salb 200 | 6.0 | 0.0 | 9 | 27.9 | 0.0 | 9 | |
| Patel 1986 Tulob 200 | 9.7 | 0.0 | 9 | 27.9 | 0.0 | 9 | |
| Patel 1986 Tulob 400 | 7.7 | 0.0 | 9 | 27.9 | 0.0 | 9 | |
| Patessio 1991 Form 24 | 10.0 | 0.0 | 12 | 30.5 | 0.0 | 12 | |
| Pearlman 2006 Form 12 | 7.6 | 0.0 | 22 | 13.2 | 0.0 | 20 | |
| Pearlman 2006 Form 24 | 5.9 | 0.0 | 23 | 13.2 | 0.0 | 20 | |
| Pearlman 2006 Salb 180 | 3.5 | 0.0 | 22 | 11.1 | 0.0 | 19 | |
| Pearlman 2007 Salb 90 | 4.8 | 10.8 | 15 | 22.5 | 10.8 | 15 | 0.39 |
| Philip 2007 Salm 50 | 10.7 | 8.1 | 46 | 21.8 | 8.1 | 46 | |
| Richter 2002 Form 12 | 5.7 | 5.3 | 24 | 25.1 | 12.2 | 24 | |
| Richter 2002 Salm 50 | 7.6 | 7.5 | 24 | 25.1 | 12.2 | 24 | |
| Richter 2002 Terb 500 | 8.5 | 8.3 | 24 | 25.1 | 12.2 | 24 | |
| Shapiro 2002 Form 12 | 12.4 | 14.6 | 19 | 32.9 | 16.8 | 17 | |
| Shapiro 2002 Form 24 | 17.5 | 17.5 | 17 | 32.9 | 16.8 | 17 | |
| Shapiro 2002 Salb 180 | 10.0 | 18.6 | 19 | 31.1 | 18.7 | 17 | |
| Sturani 1983 Fen 400 | 15.8 | 7.9 | 12 | 36.0 | 6.9 | 12 | |
| Sturani 1983 Salb 200 | 23.2 | 8.6 | 12 | 36.0 | 6.9 | 12 | |
| VanHaitsma 2010 Salb | 4.0 | 16.4 | 10 | 14.3 | 11.1 | 10 | |
| Vasquez 1984 Salb 400 | ‐0.3 | 4.9 | 13 | 14.3 | 9.8 | 12 | |
| Walker 1986 Bitolterol | 5.0 | 11.4 | 12 | 23.2 | 16.2 | 12 | |
| Wolley 1990 Terb 500 | 17.0 | 6.9 | 12 | 34.0 | 13.8 | 12 | |
Data and analyses
Comparison 1. Beta2‐agonists versus placebo (single administration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximal percentage fall in FEV1 | 72 | 799 | Mean Difference (Random, 95% CI) | ‐17.67 [‐19.51, ‐15.84] |
| 2 Number of participants with an FEV1 fall > 10% | 19 | 773 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.06, 0.13] |
| 3 Number of participants with an FEV1 fall > 15% | 13 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.03, 0.15] |
| 4 Number of participants with an FEV1 fall > 20% | 25 | 1021 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.06, 0.14] |
| 5 Maximal percentage fall in PEF | 14 | 92 | Mean Difference (Random, 95% CI) | ‐24.61 [‐37.57, ‐11.65] |
| 6 Maximal percentage fall in FEF 25‐75 | 8 | 106 | Mean Difference (Fixed, 95% CI) | ‐20.75 [‐27.17, ‐14.32] |
| 7 Side effects | 55 | 2165 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.59] |
| 8 Subgroup analysis: maximal percentage fall in FEV1 SABA vs LABA | 72 | Mean Difference (Random, 95% CI) | ‐17.67 [‐19.51, ‐15.84] | |
| 8.1 SABA | 44 | Mean Difference (Random, 95% CI) | ‐18.99 [‐21.38, ‐16.60] | |
| 8.2 LABA | 28 | Mean Difference (Random, 95% CI) | ‐15.60 [‐18.29, ‐12.92] | |
| 9 Subgroup analysis: maximal percentage fall in FEV1: salmeterol versus formoterol | 28 | Mean Difference (Random, 95% CI) | ‐15.60 [‐18.29, ‐12.92] | |
| 9.1 Salmeterol | 13 | Mean Difference (Random, 95% CI) | ‐12.73 [‐16.10, ‐9.37] | |
| 9.2 Formoterol | 15 | Mean Difference (Random, 95% CI) | ‐18.24 [‐22.15, ‐14.34] | |
| 10 Subgroup analysis: maximal percentage fall in FEV1: adults versus children | 51 | Mean Difference (Random, 95% CI) | ‐16.75 [‐19.12, ‐14.39] | |
| 10.1 Adults | 19 | Mean Difference (Random, 95% CI) | ‐18.77 [‐20.78, ‐16.76] | |
| 10.2 Children | 32 | Mean Difference (Random, 95% CI) | ‐15.32 [‐18.88, ‐11.75] |
1.1. Analysis.
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 1 Maximal percentage fall in FEV1.
1.7. Analysis.
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 7 Side effects.
1.8. Analysis.
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 8 Subgroup analysis: maximal percentage fall in FEV1 SABA vs LABA.
1.9. Analysis.
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 9 Subgroup analysis: maximal percentage fall in FEV1: salmeterol versus formoterol.
1.10. Analysis.
Comparison 1 Beta2‐agonists versus placebo (single administration), Outcome 10 Subgroup analysis: maximal percentage fall in FEV1: adults versus children.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 2001 Salb Disk.
| Methods | Study design: Randomized, double blind, cross over Study location: 2 centres, Australia Wash‐out: 1‐14 days Exercise challenge: Cycle‐ergometer for 8 min up to 50‐60% of MVV Criteria for EIB diagnosis: Positive history, FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 29 % of males: 40% Age range: 18‐40 years Ethnicity: Not reported Withdrawal or drop out: 2 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min. Intervention: Salbutamol MDI 200 mcg; Salbutamol diskus 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed on the study day |
|
| Outcomes | Primary available: max FEV1 % fall, % protection Secondary available: Number of patients with a max FEV1 % fall <10%, <15%, <20% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Anderson 2001 Salb MDI.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Anderson 2001 Salb Disk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Blake 1999 Salb 180.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 3‐14 days Exercise challenge: Treadmill for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 26 % of males: 65% Age range: 4‐11 years Ethnicity: 81% Caucasians, 15% Blacks, 4% Hispanic Withdrawal or drop out: 3 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min, 5:30 hours, 11:30 hours Intervention: Albuterol 180 mcg, Salmeterol 25 mcg Diskus, Salmeterol 50 mcg Diskus Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: max FEV1 % fall, % protection, FEV1 fall AUC Secondary available: Side effects |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Blake 1999 Salm 25.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Blake 1999 Salb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Blake 1999 Salm 50.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Blake 1999 Salb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Boner 1994 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: 2‐10 days Exercise challenge: Treadmill for 6 min at 90±4% of max HR Criteria for EIB diagnosis: Positive history, asthma according to ATS, FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 16 % of males: 68% Age range: 6‐12 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 3 hours, 12 hours Intervention: Salbutamol 200 mcg, Formoterol 12 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: max FEV1 % fall, % protection, FEV1 fall AUC Secondary available: Side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Boner 1994 Salb 200.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Boner 1994 Form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Boulet 1989 Salb.
| Methods | Study design: Randomized, double blind, cross over Study location: Canada Wash‐out: >2 days Exercise challenge: Ergometer for 6 min at 80% of VO2 max Criteria for EIB diagnosis: Positive history, asthma according to ATS, FEV1 fall >10% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 36% Age range: 19‐49 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min. Intervention: Salbutamol 200 mcg Control: Placebo Other drug arms: Ipratroprium bromide, Sodium cromoglycate Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: % protection Secondary available: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on primary and secondary outcomes are reported incompletely |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 1995 Salb MDI.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 2‐7 days Exercise challenge: Treadmill for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 46 % of males: 59% Age range: 4‐11 years Ethnicity: 87% Caucasians, 13% Others Withdrawal or drop out: 2 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min. Intervention: Albuterol MDI 180 mcg, Albuterol rotacaps 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: max FEV1 % fall, % protection Secondary available: Side effects, Number of patients with a max FEV1 % fall <20% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 1995 Salb Pwd.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Bronski 1995 Salb MDI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 1999 Salm Disk.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 2‐14 days Exercise challenge: Treadmill for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 24 % of males: 58% Age range: 4‐11 years Ethnicity: 91% Caucasians, 9% Blacks Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min, 5:30 hours, 11:30 hours Intervention: Salmeterol 50 mcg Diskus, Salmeterol 50 mcg Diskhaler Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: max FEV1 % fall, % protection, FEV1 fall AUC Secondary available: Side effects, Number of patients with a max FEV1 % fall <15%, <20% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 1999 Salm Diskhal.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Bronski 1999 Salm Disk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 2002 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 3‐7 days Exercise challenge: Treadmill for 6 min at 90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 18 % of males: 78% Age range: 13‐36 years Ethnicity: 88% Caucasians, 12% Others Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min, 4 hours, 8 hours, 12 hours Intervention: Albuterol 180 mcg, Formoterol 12 mcg, Formoterol 24 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: max FEV1 % fall; % protection; FEV1 fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <20%; Max PEF % fall |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 2002 Form 24.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Bronski 2002 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Bronski 2002 Salb.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Bronski 2002 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Carlsen 1995 Salm 25.
| Methods | Study design: Randomized, double blind, cross over Study location: Norway Wash‐out: 2‐14 days Exercise challenge: Treadmill for 6 min up to 170‐180 bpm Criteria for EIB diagnosis: Positive history, FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 23 % of males: 47% Age range: 8‐16 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: between 10‐12 hours Intervention: Salmeterol diskhaler 25 mcg, Salmeterol diskhaler 50 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Number of patients with a max FEV1 % fall <15%; Max MEF25‐50 % fall |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Carlsen 1995 Salm 50.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Carlsen Salm 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Cavagni 1993 Salb Jet.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: Not reported Exercise challenge: Treadmill for 6 min up to 170‐180 bpm Criteria for EIB diagnosis: Positive history, FEV1 fall >15% after exercise challenge, FEV1 >15% after bronchodilator |
|
| Participants | Number of subjects: 9 % of males: 66% Age range: 5‐9 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min. Intervention: Salbutamol MDI 200 mcg, Salbutamol jet disposable 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Side effects; Max PEF % fall; Max FEF25‐75 % fall |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Cavagni 1993 Salb MDI.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Cavagni 1993 Salb Jet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Clarke 1990 Fen.
| Methods | Study design: Randomized, double blind, cross over Study location: Australia Wash‐out: <14 days Exercise challenge: Treadmill at 15° inclination for 6 min. up to 150 bpm Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 20 % of males: 70% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min. Intervention: Fenoterol 100 mcg Control: Placebo Other drug arms: Sodim cromoglycate 20 mg; Sodium cromoglycate 20 mg + Fenoterol 100 mcg Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: None |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Daugbjerg 1996 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: Denmark Wash‐out: Not reported Exercise challenge: Treadmill for 6 min. up to 150 bpm Criteria for EIB diagnosis: FEV1 fall >22% after exercise challenge |
|
| Participants | Number of subjects: 16 % of males: 81% Age range: 10‐14 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 3 hours, 12 hours Intervention: Salbutamol 400 mcg, Formoterol 12 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Daugbjerg 1996 Salb.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Daugbjerg 1996 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Debelic 1988 Reproterol.
| Methods | Study design: Randomized, double blind, cross over Study location: Germany Wash‐out: Not reported Exercise challenge: Free running for 6 min up to 160‐180 bpm Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 16 % of males: Not reported Age range: 8‐20 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min. Intervention: Reproterol 1 mg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Any drug suspended 12 hours before exercise challenge |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
DeBenedictis 1996 Salm 25.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: 2‐10 days Exercise challenge: Treadmill for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 83% Age range: 7‐14 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 1 hour, 12 hours Intervention: Salmeterol 25 mcg, Salmeterol 50 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
DeBenedictis 1996 Salm 50.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: De Benedictis 1996 Salm 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
DeBenedictis 1998 Salb.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: <10 days Exercise challenge: Treadmill for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 66% Age range: 7‐13 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 20 min. Intervention: Salbutamol 200 mcg Control: Placebo Other drug arms: Salbutamol 200 mcg + Nedocromil 4 mg Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Del Col 1993 Salb Jet.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: 2‐6 days Exercise challenge: Treadmill for 6 min at 90±4% of max HR Criteria for EIB diagnosis: Positive history |
|
| Participants | Number of subjects: 15 % of males: 60% Age range: 9‐13 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min. Intervention: Salbutamol MDI 200 mcg, Salbutamol Jet device 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Max PEF % fall |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Del Col 1993 Salb MDI.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Del Col 1993 Salb Jet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Dinh Xuan 1989 Terb.
| Methods | Study design: Randomized, double blind, cross over Study location: France Wash‐out: Not reported Exercise challenge: Cycle‐ergometer for 5 min at 90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 10 % of males: 70% Age range: 6‐16 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min. Intervention: Terbutaline 500 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Number of patients with a max FEV1 % fall <10%, <15% and <20% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Egglestone 1981 Terb 250.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: ≧2 days Exercise challenge: Treadmill for 5 min at 90% of max HR Criteria for EIB diagnosis: Positive history; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 17 % of males: Not reported Age range: 18‐32 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 1 hour Intervention: Terbutaline 250 mcg Control: Placebo Other drug arms: Isoproterenol 100 mcg Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Max FEF25‐75 % fall; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Ferrari 2000 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: ≧2 days Exercise challenge: Cycle‐ergometer for 7 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 14 % of males: 92% Age range: 12‐28 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min, 4 hours Intervention: Formoterol 12 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Statistical analysis and manuscript writing made by drug industry staff |
| Other bias | Unclear risk | Insufficient information |
Garcia 2001 Form 12.
| Methods | Study design: Randomized, double blind, parallel groups Study location: Spain Wash‐out: Not applicable Exercise challenge: Cycle‐ergometer for 6 min at 85% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 19 % of males: 42% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Chronic administration (4 weeks) Time of exercise challenge after drug administration: 30 min, 12 hours at day 1, 14 and 28 Intervention: Formoterol 12 mcg twice daily Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Onset of tolerance; Number of patients with a max FEV1 % fall <10% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Green 1992 Salm 50.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: 4‐10 days Exercise challenge: Treadmill for 8 min up to 170 bpm Criteria for EIB diagnosis: Positive history, FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 13 % of males: 61% Age range: 8‐15 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 1 hour, 5 hours, 9 hours Intervention: Salmeterol 50 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <15% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Gronnerod 2000 Form 4.5.
| Methods | Study design: Randomized, double blind, cross over Study location: Germany and Norway Wash‐out: ≧3 days Exercise challenge: Treadmill for 4‐8 min up to 180 bpm Criteria for EIB diagnosis: Asthma according ATS; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 27 % of males: 55% Age range: 8‐17 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min, 4 hours, 8 hours, 12 hours Intervention: Terbutaline 500 mcg; Formoterol 9 mcg; Formoterol 4.5 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Gronnerod 2000 Form 9.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Gronnerod 2000 Form 4.5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Gronnerod 2000 Terb 500.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Gronnerod 2000 Form 4.5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hancox 2002.
| Methods | Study design: Randomized, double blind, cross over Study location: Canada Wash‐out: No Exercise challenge: Cycle ergometer for 7 min at 80% of max work rate Criteria for EIB diagnosis: Positive history; FEV1 fall >15% after exercise challenge which was sustained >10% for at least 5 minutes |
|
| Participants | Number of subjects: 9 % of males: 11% Age range: 18‐44 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Chronic administration (1 week) Time of exercise challenge after drug administration: 8 hours Intervention: Salbutamol 800 mcg daily Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Tolerance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hawksworth 2002 Salb HFA.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: 1‐14 days Exercise challenge: Treadmill for 6 min at >80% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 24 % of males: 75% Age range: 18‐45 years Ethnicity: Caucasian 83%; Asian 17% Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min Intervention: Salbutamol 180 HFA; Salbutamol 180 mcg MDI Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <20% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hawksworth 2002 Salb MDI.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Hawksworth 2002 Salb HFA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Henricksen 1983 Terb.
| Methods | Study design: Randomized, double blind, cross over Study location: Denmark Wash‐out: No Exercise challenge: Free running for 6 min at 80‐85% of max work capacity Criteria for EIB diagnosis: PEF fall >20% after exercise challenge |
|
| Participants | Number of subjects: 14 % of males: Not reported Age range: 8‐15 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min Intervention: Terbutaline 32.5 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Max PEF % fall; Side effects; Number of patients with a max FEV1 % fall <20% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Henricksen 1992 Salb.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Henricksen 1992 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Henriksen 1992 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: Denmark Wash‐out: Not reported Exercise challenge: Treadmill for 6 min up to 180 bpm Criteria for EIB diagnosis: PEF or FEV1 fall >25% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: Not reported Age range: 8‐15 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min, 3 hours, 5.30 hours, 8 hours Intervention: Salbutamol 200 mcg; Formoterol 12 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Max PEF % fall; Side effects; Number of patients with a max FEV1 % fall <20% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hills 1976 Salb.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: < 7 days Exercise challenge: Free running for 8 min at max speed Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 19 % of males: 42% Age range: 5‐15 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 20 min Intervention: Salbutamol 200 mcg; Salmefamol 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Hills 1976 Salmefamol.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Hills 1976 Salb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Inman 1996.
| Methods | Study design: Randomized, double blind, cross over Study location: Canada Wash‐out: 7‐21 days Exercise challenge: Cycle ergometer for 5 min at 80% of max work rate Criteria for EIB diagnosis: Positive history |
|
| Participants | Number of subjects: 10 % of males: 70% Age range: 19‐37 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Chronic administration 81 week) Time of exercise challenge after drug administration: 24 hours Intervention: Salbutamol 800 mcg daily Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Onset of tolerance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Kemp 1994 Salb.
| Methods | Study design: Randomized, double blind, parallel groups Study location: United States Wash‐out: Not applicable Exercise challenge: Treadmill for 6 min at 80% of max HR Criteria for EIB diagnosis: Asthma according to ATS; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 161 % of males: 42% Age range: 12‐35 years Ethnicity: Not reported Withdrawal or drop out: 8 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min, 5.30 hours, 11.30 hours Intervention: Salbutamol 180 mcg; Salmeterol 42 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% and 20% |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Kemp 1994 Salm 42.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Kemp 1994 Salb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Konig 1981 Metaprot.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: Not reported Exercise challenge: Treadmill for 6 min at 90% of max HR Criteria for EIB diagnosis: Asthma according to ATS; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 24 % of males: 67% Age range: 17‐34 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min, 1 hour Intervention: Metaproterenol 130 mcg Control: Placebo Other drug arms: Oral metaproterenol Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Max FEF 25‐‐75 % fall; Side effects; Number of patients with a max FEV1 % fall <10%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Konig 1984 Fen 0.4.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: Not reported Exercise challenge: Treadmill up to 90% of max HR Criteria for EIB diagnosis: Asthma according to ATS; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 100% Age range: 17‐29 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min, 2 hours, 4 hours Intervention: Fenoterol 40 mcg; Fenoterol 80 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Max FEF 25‐‐75 % fall; Side effects; Number of patients with a max FEV1 % fall <10%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for max FEF 25‐‐75 % fall not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Konig 1984 Fen 0.8.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Konig 1984 Fen 0.4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for max FEF 25‐‐75 % fall not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Larsson 1982 Fen.
| Methods | Study design: Randomized, double blind, cross over Study location: Sweden Wash‐out: Not reported Exercise challenge: Cycle ergometer for 6‐9 min till exhaustion Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 8 % of males: 69% Age range: 29‐64 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 10 min Intervention: Fenoterol 400 mcg Control: Placebo Other drug arms: Oxitropium bromide; Ipratropium bromide Concomitant inhaled corticosteroid (ICS) treatment: Any anti‐asthmatic drug suspended 12 hours before the test |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Number of patients with a max FEV1 % fall <15%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
McAlpine 1990 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: 1‐7 days Exercise challenge: Treadmill for 5‐8 min up to 90% of max HR Criteria for EIB diagnosis: Documented exercise‐induced bronchoconstriction |
|
| Participants | Number of subjects: 12 % of males: 41% Age range: 19‐41 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 2 hours, 4 hours Intervention: Salbutamol 200 mcg; Formoterol 12 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
McAlpine 1990 Salb.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: McAlpine 1990 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
McFadden 1986 Salb (I).
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 1‐15 days Exercise challenge: Cycle ergometer for 4‐5 min till exhaustion Criteria for EIB diagnosis: Positive screening exercise challenge |
|
| Participants | Number of subjects: 20 % of males: 60% Age range: 21‐42 years Ethnicity: Not reported Withdrawal or drop out: 5 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min Intervention: Salbutamol 200 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Max FEF 25‐‐75 % fall; Side effects; Number of patients with a max FEV1 % fall <20%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
McFadden 1986 Salb (II).
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 1‐10 days Exercise challenge: Cycle ergometer for 4‐5 min till exhaustion Criteria for EIB diagnosis: Positive screening exercise challenge |
|
| Participants | Number of subjects: 20 % of males: 60% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min Intervention: Salbutamol 180 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Max FEF 25‐‐75 % fall; Side effects; Number of patients with a max FEV1 % fall <20%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Morton 1989 Rimet.
| Methods | Study design: Randomized, double blind, cross over Study location: Australia Wash‐out: 2‐7 days Exercise challenge: Treadmill for 8 min at 80% of anaerobic threshold Criteria for EIB diagnosis: Asthma according to ATS; FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 10 % of males: 70% Age range: 15‐30 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 2 min Intervention: Rimeterol 400 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Side effects; Number of patients with a max FEV1 % fall <15%; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Nelson 1998.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 7 days Exercise challenge: Cycle ergometer for 4 min at exhausting work Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 20 % of males: 45% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Chronic administration (29 days) Time of exercise challenge after drug administration: 30 min, 9 hours Intervention: Salmeterol 84 mcg daily Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Onset of tolerance; Number of patients with a max FEV1 % fall <10%; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Newnham 1993 Salb 200.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: ≥2 days Exercise challenge: Treadmill for 6 min up to 90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 50% Age range: 21‐33 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 1 hour, 6 hours, 12 hours Intervention: Salbutamol 200 mcg; Salmeterol 50 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <20%; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Newnham 1993 Salm 50.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Newnham 1993 Salb 200 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patel 1986 Salb 200.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: Not reported Exercise challenge: Treadmill for 6‐8 min Criteria for EIB diagnosis: Diagnosis of exercise‐induced bronchoconstriction |
|
| Participants | Number of subjects: 9 % of males: Not reported Age range: 19‐46 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 20 min Intervention: Salbutamol 200 mcg; Tolobuterol 200 mcg; Tolobuterol 400 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patel 1986 Tulob 200.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Patel 1986 Salb 200 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patel 1986 Tulob 400.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Patel 1986 Salb 200 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patessio 1991 Form 24.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: 1 day Exercise challenge: Treadmill for 7 min up to 90% of max HR Criteria for EIB diagnosis: Asthma according to ATS; FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects:12 % of males: 16% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 2 hours, 8 hours Intervention: Salbutamol 200 mcg; Formoterol 24 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <15%; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Patessio 1991 Salb 200.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Patessio 1991 Form 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Pearlman 2006 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: ≥3 days Exercise challenge: Treadmill for 6 min up to 80‐90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects:23 % of males: 30% Age range: 4‐11 years Ethnicity: Not reported Withdrawal or drop out: 2 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min, 4 hours, 8 hours, 12 hours Intervention: Salbutamol 180 mcg; Formoterol 12 mcg; Formoterol 24 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% and <20% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Pearlman 2006 Form 24.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Pearlman 2006 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Pearlman 2006 Salb 180.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Pearlman 2006 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Pearlman 2007 Salb 90.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 3‐7 days Exercise challenge: Treadmill for at least 4 min at 85% of max HR Criteria for EIB diagnosis: Exercise‐induced bronchoconstriction for at least 6 months; FEV1 fall >20% and <50% after exercise challenge |
|
| Participants | Number of subjects:15 % of males: 86% Age range: Not reported Ethnicity: 100% Caucasian Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 20 min Intervention: Salbutamol 90 mcg Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <10%, <15% and <20% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Philip 2007 Salm 50.
| Methods | Study design: Randomized, double blind, cross over Study location: South America Wash‐out: 3‐7 days Exercise challenge: Treadmill for 6 min up to 80‐90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% and <40% after exercise challenge |
|
| Participants | Number of subjects:47 % of males: 49% Age range: 15‐45 years Ethnicity: 55% Caucasian; 45% Others Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 2 hours, 8.30 hours, 24 hours Intervention: Salmeterol 50 mcg Control: Placebo Other drug arms: Oral Montelukast 10 mg Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: Side effects |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Ramage 1994.
| Methods | Study design: Randomized, double blind, cross over Study location: United Kingdom Wash‐out: 7 days Exercise challenge: Treadmill for 6 min up to 80‐90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% and <40% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 66% Age range: 19‐36 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Chronic administration (28 days) Time of exercise challenge after drug administration: 6 hours, 12 hours Intervention: Salmeterol 100 mcg daily Control: Placebo Other drug arms: No Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Onset of tolerance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Richter 2002 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: Germany Wash‐out: ≥2 days Exercise challenge: Cycle‐ergometer for 6 min up to 85% of max HR Criteria for EIB diagnosis: Positive history; Positive methacholine test (PC20 <8mg/ml); Positive exercise challenge |
|
| Participants | Number of subjects: 25 % of males: 66% Age range: 19‐36 years Ethnicity: Not reported Withdrawal or drop out: 1 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 5 min, 30 min, 1 hour Intervention: Terbutaline 500 mcg; Formoterol 12 mcg; Salmeterol 50 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: None |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Richter 2002 Salm 50.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Richter Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Richter 2002 Terb 500.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Richter Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Shapiro 2002 Form 12.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: 3‐7 days Exercise challenge: Treadmill for 6 min at 90% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 20 % of males: 45% Age range: 13‐41 years Ethnicity: Caucasians 90%; Others 10% Withdrawal or drop out: 3 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min, 4 hours, 8 hours, 12 hours Intervention: Salbutamol 180 mcg; Formoterol 12 mcg; Formoterol 24 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Side effects; Number of patients with a max FEV1 % fall <20%; Max PEF % fall; |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Shapiro 2002 Form 24.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Shapiro 2002 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Shapiro 2002 Salb 180.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Shapiro 2002 Form 12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Simons 1997.
| Methods | Study design: Randomized, double blind, cross over Study location: Canada Wash‐out: 14 days Exercise challenge: Treadmill for 8 min up to 90% of max HR or 180 bpm Criteria for EIB diagnosis: Asthma according to ATS; Positive exercise challenge |
|
| Participants | Number of subjects: 16 % of males: 41% Age range: 12‐16 years Ethnicity: Not reported Withdrawal or drop out: 2 |
|
| Interventions | Drug administration: Chronic administration (28 weeks) Time of exercise challenge after drug administration: 1 hour, 9 hours Intervention: Salmeterol 50 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: None |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Stelmach 2008.
| Methods | Study design: Randomized, double blind, parallel groups Study location: Poland Wash‐out: Not applicable Exercise challenge: Treadmill for six min at 95% of max HR Criteria for EIB diagnosis: FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 100 % of males: Not reported Age range: 6‐18 years Ethnicity: Not reported Withdrawal or drop out: 9 |
|
| Interventions | Drug administration: Chronic administration (28 weeks) Time of exercise challenge after drug administration: 1 hour, 9 hours Intervention: Formoterol 9 mcg daily Control: Placebo Other drug arms: Oral Montelukast Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Onset of tolerance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization described explicitly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Storms 2004.
| Methods | Study design: Randomized, double blind, parallel groups Study location: United States Wash‐out: Not applicable Exercise challenge: Treadmill for six min at 95% of max HR Criteria for EIB diagnosis: FEV1 fall >20% (or >15% if on ICS) after an exercise challenge in the last year |
|
| Participants | Number of subjects: 122 % of males: Not reported Age range: 15‐58 years Ethnicity: Not reported Withdrawal or drop out: 13 |
|
| Interventions | Drug administration: Chronic administration (28 weeks) Time of exercise challenge after drug administration: 1 hour, 9 hours Intervention: Salmeterol 100 mcg daily Control: Placebo Other drug arms: Oral Montelukast 10 mg Concomitant inhaled corticosteroid (ICS) treatment: Allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; FEV1 % fall AUC Secondary available: Onset of tolerance |
|
| Notes | Industry funded study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Sturani 1983 Fen 400.
| Methods | Study design: Randomized, double blind, cross over Study location: Italy Wash‐out: Not reported Exercise challenge: Free running for 6 min up to 85% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 58% Age range: 16‐42 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 30 min. Intervention: Fenoterol 400 mcg; Salbutamol 200 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection Secondary available: None |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Sturani 1983 Salb 200.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | See: Sturani 1983 Fen 400 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
VanHaitsma 2010 Salb.
| Methods | Study design: Randomized, double blind, cross‐over Study location: United States Wash‐out: ≥2 days Exercise challenge: Treadmill for at least 3 min at 85% of max HR Criteria for EIB diagnosis: Physician diagnosed asthma; FEV1 fall >10% after exercise challenge |
|
| Participants | Number of subjects: 10 % of males: 70% Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min. Intervention: Salbutamol 180 mcg Control: Placebo Other drug arms: None Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Max Pef % fall; Max FEF 25‐‐75 % fall |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Vasquez 1984 Salb 400.
| Methods | Study design: Randomized, double blind, parallel groups Study location: Spain Wash‐out: Not applicable Exercise challenge: Free running 5‐8 min at max speed (around 170 bpm) Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 25 % of males: Not reported Age range: Not reported Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 15 min. Intervention: Salbutamol 400 mcg Control: Placebo Other drug arms: Disodium cromoglycate 200 mcg; Ipratropium bromide 40 mcg; Concomitant inhaled corticosteroid (ICS) treatment: Not reported |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Number of patients with a max FEV1 % fall <15%; Max MEF50 % fall; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Walker 1986 Bitolterol.
| Methods | Study design: Randomized, double blind, cross over Study location: United States Wash‐out: Not reported Exercise challenge: Cycle‐ergometer for 6 min at 80% of max HR Criteria for EIB diagnosis: FEV1 fall >15% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 58% Age range: 14‐25 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 45 min. Intervention: Bitolterol 1050 mcg Control: Placebo Other drug arms: Isoproterenol 255 mcg Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <10%; Max PEF % fall; Max FEF25‐75 % fall; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Wolley 1990 Terb 500.
| Methods | Study design: Randomized, double blind, cross over Study location: Australia Wash‐out: Not reported Exercise challenge: Treadmill for 8 min at 60% of MVV Criteria for EIB diagnosis: Positive history, FEV1 fall >20% after exercise challenge |
|
| Participants | Number of subjects: 12 % of males: 58% Age range: 18‐28 years Ethnicity: Not reported Withdrawal or drop out: 0 |
|
| Interventions | Drug administration: Single dose Time of exercise challenge after drug administration: 25 min, 2 hours, 4 hours, 6 hours Intervention: Terbutaline 500 mcg Control: Placebo Other drug arms: Cromolyn sodium 2 mg; Cromolyn sodium 2 mg + Terbutaline 500 mcg Concomitant inhaled corticosteroid (ICS) treatment: Not allowed |
|
| Outcomes | Primary available: Max FEV1 % fall; % protection; Secondary available: Side effects; Number of patients with a max FEV1 % fall <10% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aebischer 1984 | No beta‐2 agonist pretreatment |
| Agostini 1983 I | Duplicate |
| Agostini 1983 II | No systematic review primary outcomes |
| Allegra 1976 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Anderson 1975 | No double blind |
| Anderson 1976 | No randomization |
| Anderson 1991 | No randomization |
| Aranda 1992 | No double blind |
| Bakran 1980 | No placebo control |
| Battistini 1980 | No inhaled beta‐2 agonist administration |
| Baur 1979 | No systematic review primary outcomes |
| Berkowitz 1986 | No double blind |
| Boner 1984 | No double blind |
| Boner 1987 | No placebo control |
| Boner 1988 | No inhaled beta‐2 agonist administration |
| Bratteby 1986 | No placebo control |
| Bundgaard 1980 | No systematic review primary outcomes |
| Bundgaard 1983 I | No randomization |
| Bundgaard 1983 II | No systematic review primary outcomes |
| Bundgaard 1983 III | No systematic review primary outcomes |
| Bye 1980 | No randomization |
| Ceugniet 1997 | No placebo control |
| Colice 1999 | No double blind |
| Coreno 2000 | No double blind |
| Corrias 1989 | No placebo control |
| Dal Col 1995 | No double blind |
| Del Bono 1979 | No double blind |
| Di Gioacchino 1987 | No inhaled beta‐2 agonist administration |
| Dockhorn 1997 | No double blind |
| Edelman 2000 | No placebo control |
| Eggleston 1981 | No inhaled beta‐2 agonist administration |
| Ferrari 2002 | No placebo control |
| Fogel 2010 | No placebo control |
| Francis 1980 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Freeman 1989 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Gibson 1978 | No randomization |
| Gimeno 1985 | No clear diagnosi of exercise‐induced bronchoconstriction |
| GlaxoSmithKline 2006 I | No placebo control |
| GlaxoSmithKline 2006 II | Duplicate |
| Godfrey 1975 | Duplicate |
| Godfrey 1976 | No inhaled beta‐2 agonist administration |
| Guerin 1992 | No placebo control |
| Gunawardena 2005 | No placebo control |
| Hermansen 2006 | No beta‐2 agonist pretreatment |
| Higgs 1983 | No placebo control |
| Ienna 1997 | No systematic review primary outcomes |
| Iikura 1988 | No randomization |
| Ioli 1986 | No double blind |
| Johnson 1986 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Koch 1972 | No randomization |
| Kumar 1988 | No randomization |
| Lopes Dos Santos 1991 | No beta‐2 agonist pretreatment |
| Machado 2012 | No placebo control |
| Macucci 2004 | No randomization |
| Magnussen 1984 | No double blind |
| Makela 2012 | No placebo control |
| Martinsson 1985 | No inhaled beta‐2 agonist administration |
| Merck 2005 I | Duplicate |
| Merck 2005 II | No placebo control |
| Mickleborough 2007 | No placebo control |
| Millqvist 2000 | No randomization |
| Morandini 1982 | No randomization |
| Morooka 1987 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Morse 1976 | No inhaled beta‐2 agonist administration |
| Morton 1992 | No double blind |
| Murray 2011 | No placebo control |
| Pearlman 2009 | No placebo control |
| Pfleger 2002 | No exercise challenge |
| Pichaipat 1995 | No randomization |
| Pichon 2005 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Poppius 1973 | No placebo control |
| Rabe 1993 | No systematic review primary outcomes |
| Raissy 2006 | No placebo control |
| Raissy 2008 | No placebo control |
| Revill 1998 | No placebo control |
| Robertson 1994 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Rohr 1987 | No placebo control |
| Sanguinetti 1986 | Exercise challenge beyond beta‐2 agonist pharmacological half‐life |
| Schaanning 1996 | No systematic review primary outcomes |
| Shah 1983 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Shapiro 1981 | No inhaled beta‐2 agonist administration |
| Shapiro 1990 | No double blind |
| Sichletidis 1993 | No placebo control |
| Silverman 1973 | No randomization |
| Singh 1992 | No randomization |
| Sly 1968 | No double blind |
| Sly 1975 | No systematic review primary outcomes |
| Sly 1982 | No inhaled beta‐2 agonist administration |
| Spada 1985 | No placebo control |
| Stark 1981 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Steinshamn 2004 | No placebo control |
| Svenonius 1983 | No randomization |
| Svenonius 1988 | No double blind |
| Svenonius 1994 | No beta‐2 agonist pretreatment |
| Tabas 1985 | No inhaled beta‐2 agonist administration |
| Tammivaara 1979 | No randomization |
| Unnithan 1994 | No clear diagnosi of exercise‐induced bronchoconstriction |
| Verini 1983 | No placebo control |
| Verini 1985 | No inhaled beta‐2 agonist administration |
| Verini 1999 | No placebo control |
| Villaran 1999 | No placebo control |
| Vilsvik 1991 | No beta‐2 agonist pretreatment |
| Vilsvik 2001 | No placebo control |
| Von Berg 2002 | No placebo control |
| Weiler 2005 | No placebo control |
| Weinberg 1982 | No double blind |
| Yeung 1980 | No placebo control |
| Zanconato 1990 | No placebo control |
| Zimmermann 2003 | No placebo control |
Differences between protocol and review
Compared with what was originally planned in the protocol, given the high number of papers retrieved in full and reviewed (N = 211), the review authors agreed to narrow the inclusion criteria, considering eligible only double‐blind trials that assessed at least one primary outcome of the review. Lack of data reported selected outcomes and the low rate of heterogeneity prevented or made unnecessary some of the subgroup analyses defined a priori. We prepared a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
MB updated the protocol and was responsible for drafting the full text of the review. MB, CDM and EC selected the studies to be included in the review and extracted and collected data. EC and MB entered data into the Review Manager software for statistical analysis. HS created the 'Summary of findings' table, acting as expert contact for the assessment of risk of bias and for evaluation of the quality of evidence. GWC, MAC and SD acted as independent review authors for solving disagreements among rating authors. All authors critically reviewed the protocol and were involved in revising the full text of the systematic review.
Sources of support
Internal sources
National Research Council, Institute of Translational Pharmacology (IFT), Italy.
External sources
Italian National Drug Agency (AIFA), Italy.
21st Century Canada Research Chairs Programme; Government of Canada (Ottawa, Ontario), Canada.
Declarations of interest
Disclosures of interest provided by the review authors did not imply any potential conflict of interest with reference to this review.
New
References
References to studies included in this review
Anderson 2001 Salb Disk {published data only}
- Anderson SD, Lambert S, Brannan JD, Wood RJ, Koskela H, Morton AR, et al. Laboratory protocol for exercise asthma to evaluate salbutamol given by two devices. Medicine & Science in Sports & Exercise 2001;33(6):893‐900. [DOI] [PubMed] [Google Scholar]
Anderson 2001 Salb MDI {published data only}
- Anderson SD, Lambert S, Brannan JD, Wood RJ, Koskela H, Morton AR, et al. Laboratory protocol for exercise asthma to evaluate salbutamol given by two devices. Medicine & Science in Sports & Exercise 2001;33(6):893‐900. [DOI] [PubMed] [Google Scholar]
Blake 1999 Salb 180 {published data only}
- Blake K, Pearlman DS, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma & Immunology 1999;82(2):205‐11. [DOI] [PubMed] [Google Scholar]
Blake 1999 Salm 25 {published data only}
- Blake K, Pearlman DS, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma & Immunology 1999;82(2):205‐11. [DOI] [PubMed] [Google Scholar]
Blake 1999 Salm 50 {published data only}
- Blake K, Pearlman DS, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma & Immunology 1999;82(2):205‐11. [DOI] [PubMed] [Google Scholar]
Boner 1994 Form 12 {published data only}
- Boner AL, Spezia E, Piovesan P, Chiocca E, Maiocchi G. Inhaled formoterol in the prevention of exercise‐induced bronchoconstriction in asthmatic children. American Journal of Respiratory and Critical Care Medicine 1994;149(4):935‐9. [DOI] [PubMed] [Google Scholar]
Boner 1994 Salb 200 {published data only}
- Boner AL, Spezia E, Piovesan P, Chiocca E, Maiocchi G. Inhaled formoterol in the prevention of exercise‐induced bronchoconstriction in asthmatic children. American Journal of Respiratory and Critical Care Medicine 1994;149(4):935‐9. [DOI] [PubMed] [Google Scholar]
Boulet 1989 Salb {published data only}
- Boulet LP, Turcotte H, Tennina S. Comparative efficacy of salbutamol, ipratropium, and cromoglycate in the prevention of bronchospasm induced by exercise and hyperosmolar challenges. Journal of Allergy and Clinical Immunology 1989;83(5):882‐7. [DOI] [PubMed] [Google Scholar]
Bronski 1995 Salb MDI {published data only}
- Bronsky EA, Spector SL, Pearlman DS, Justus SE, Bishop AL. Albuterol aerosol versus albuterol Rotacaps in exercise‐induced bronchospasm in children. Journal of Asthma 1995;32(3):207‐14. [DOI] [PubMed] [Google Scholar]
Bronski 1995 Salb Pwd {published data only}
- Bronsky EA, Spector SL, Pearlman DS, Justus SE, Bishop AL. Albuterol aerosol versus albuterol Rotacaps in exercise‐induced bronchospasm in children. Journal of Asthma 1995;32(3):207‐14. [DOI] [PubMed] [Google Scholar]
Bronski 1999 Salm Disk {published data only}
- Bronsky EA, Pearlman DS, Pobiner BF, Scott C, Wang Y, Stahl E. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of two salmeterol powder delivery devices. Pediatrics 1999;104:501‐6. [DOI] [PubMed] [Google Scholar]
Bronski 1999 Salm Diskhal {published data only}
- Bronsky EA, Pearlman DS, Pobiner BF, Scott C, Wang Y, Stahl E. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of two salmeterol powder delivery devices. Pediatrics 1999;104:501‐6. [DOI] [PubMed] [Google Scholar]
Bronski 2002 Form 12 {published data only}
- Bronsky EA, Yegen U, Yeh CM, Larsen LV, Della Cioppa G. Formoterol provides long‐lasting protection against exercise‐induced bronchospasm. Annals of Allergy, Asthma & Immunology 2002;89(4):407‐12. [DOI] [PubMed] [Google Scholar]
Bronski 2002 Form 24 {published data only}
- Bronsky EA, Yegen U, Yeh CM, Larsen LV, Della Cioppa G. Formoterol provides long‐lasting protection against exercise‐induced bronchospasm. Annals of Allergy, Asthma & Immunology 2002;89(4):407‐12. [DOI] [PubMed] [Google Scholar]
Bronski 2002 Salb {published data only}
- Bronsky EA, Yegen U, Yeh CM, Larsen LV, Della Cioppa G. Formoterol provides long‐lasting protection against exercise‐induced bronchospasm. Annals of Allergy, Asthma & Immunology 2002;89(4):407‐12. [DOI] [PubMed] [Google Scholar]
Carlsen 1995 Salm 25 {published data only}
- Carlsen KH, Roksund O, Olsholt K, Njå F, Leegaard J, Bratten G. Overnight protection by inhaled salmeterol on exercise‐induced asthma in children. European Respiratory Journal 1995;8(11):1852‐5. [DOI] [PubMed] [Google Scholar]
Carlsen 1995 Salm 50 {published data only}
- Carlsen KH, Roksund O, Olsholt K, Njå F, Leegaard J, Bratten G. Overnight protection by inhaled salmeterol on exercise‐induced asthma in children. European Respiratory Journal 1995;8(11):1852‐5. [DOI] [PubMed] [Google Scholar]
Cavagni 1993 Salb Jet {published data only}
- Cavagni G, Caffarelli C, Manni PL, Stapane I, Preti PAM, Cantini L. Salbutamol administered through a new spacer device to prevent exercise‐induced asthma. Advanced Therapy 1993;10(5):207‐16. [Google Scholar]
Cavagni 1993 Salb MDI {published data only}
- Cavagni G, Caffarelli C, Manni PL, Stapane I, Preti PAM, Cantini L. Salbutamol administered through a new spacer device to prevent exercise‐induced asthma. Advanced Therapy 1993;10(5):207‐16. [Google Scholar]
Clarke 1990 Fen {published data only}
- Clarke PS, Ratowsky DA. Effect of fenoterol hydrobromide and sodium cromoglycate individually and in combination on postexercise asthma. Annals of Allergy 1990;64:187‐90. [PubMed] [Google Scholar]
Daugbjerg 1996 Form 12 {published data only}
- Daugbjerg P, Nielsen KG, Skov M, Bisgaard H. Duration of action of formoterol and salbutamol dry‐powder inhalation in prevention of exercise‐induced asthma in children. Acta Paediatrica 1996;85(6):684‐7. [DOI] [PubMed] [Google Scholar]
Daugbjerg 1996 Salb {published data only}
- Daugbjerg P, Nielsen KG, Skov M, Bisgaard H. Duration of action of formoterol and salbutamol dry‐powder inhalation in prevention of exercise‐induced asthma in children. Acta Paediatrica 1996;85(6):684‐7. [DOI] [PubMed] [Google Scholar]
Debelic 1988 Reproterol {published data only}
- Debelic M, Hertel G, Konig J. Double‐blind crossover study comparing sodium cromoglycate, reproterol, reproterol plus sodium cromoglycate, and placebo in exercise‐induced asthma. Annals of Allergy 1988;61(1):25‐9. [PubMed] [Google Scholar]
DeBenedictis 1996 Salm 25 {published data only}
- Benedictis FM, Tuteri G, Pazzelli P, Niccoli A, Mezzetti D, Vaccaro R. Salmeterol in exercise‐induced bronchoconstriction in asthmatic children: comparison of two doses. European Respiratory Journal 1996;9(10):2099‐103. [DOI] [PubMed] [Google Scholar]
DeBenedictis 1996 Salm 50 {published data only}
- Benedictis FM, Tuteri G, Pazzelli P, Niccoli A, Mezzetti D, Vaccaro R. Salmeterol in exercise‐induced bronchoconstriction in asthmatic children: comparison of two doses. European Respiratory Journal 1996;9(10):2099‐103. [DOI] [PubMed] [Google Scholar]
DeBenedictis 1998 Salb {published data only}
- Benedictis FM, Tuteri G, Pazzelli P, Solinas LF, Niccoli A, Parente C. Combination drug therapy for the prevention of exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma & Immunology 1998;80(4):352‐6. [DOI] [PubMed] [Google Scholar]
Del Col 1993 Salb Jet {published data only}
- Col G, Spezia E, Richelli C, Piovesan P, Cantini L, Boner AL. Assessment of a new space device (Jet) for use with inhaled beta2‐agonists in children with exercise‐induced asthma. Pediatric Allergy and Immunology 1993;7(2):119‐26. [Google Scholar]
Del Col 1993 Salb MDI {published data only}
- Col G, Spezia E, Richelli C, Piovesan P, Cantini L, Boner AL. Assessment of a new space device (Jet) for use with inhaled beta2‐agonists in children with exercise‐induced asthma. Pediatric Allergy and Immunology 1993;7(2):119‐26. [Google Scholar]
Dinh Xuan 1989 Terb {published data only}
- Dinh Xuan AT, Lebeau C, Roche R, Ferriere A, Chaussain M. Inhaled terbutaline administered via a spacer fully prevents exercise‐induced asthma in young asthmatic subjects: a double‐blind, randomized, placebo‐controlled study. Journal of International Medical Research 1989;17(6):506‐13. [DOI] [PubMed] [Google Scholar]
Egglestone 1981 Terb 250 {published data only}
- Eggleston PA, Beasley PP. Bronchodilation and inhibition of induced asthma by adrenergic agonists. Clinical Pharmacology & Therapeutics 1981;29(4):505‐510. [DOI] [PubMed] [Google Scholar]
Ferrari 2000 Form 12 {published data only}
- Ferrari M, Balestreri F, Baratieri S, Biasin C, Oldani V, Lo Cascio V. Evidence of the rapid protective effect of formoterol dry‐powder inhalation against exercise‐induced bronchospasm in athletes with asthma. Respiration 2000;67(5):510‐3. [DOI] [PubMed] [Google Scholar]
Garcia 2001 Form 12 {published data only}
- Garcia R, Guerra P, Feo F, Galindo PA, Gómez E, Borja J, et al. Tachyphylaxis following regular use of formoterol in exercise‐induced bronchospasm. Journal of Investigational Allergology and Clinical Immunology 2001;11(3):176‐82. [PubMed] [Google Scholar]
Green 1992 Salm 50 {published data only}
- Green CP, Price JF. Prevention of exercise induced asthma by inhaled salmeterol xinafoate. Archives of Disease in Childhood 1992;67(8):1014‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gronnerod 2000 Form 4.5 {published data only}
- Gronnerod TA, Berg A, Schwabe G, Soliman S. Formoterol via Turbuhaler gave better protection than terbutaline against repeated exercise challenge for up to 12 hours in children and adolescents. Respiratory Medicine 2000;94(7):661‐7. [DOI] [PubMed] [Google Scholar]
Gronnerod 2000 Form 9 {published data only}
- Gronnerod TA, Berg A, Schwabe G, Soliman S. Formoterol via Turbuhaler gave better protection than terbutaline against repeated exercise challenge for up to 12 hours in children and adolescents. Respiratory Medicine 2000;94(7):661‐7. [DOI] [PubMed] [Google Scholar]
Gronnerod 2000 Terb 500 {published data only}
- Gronnerod TA, Berg A, Schwabe G, Soliman S. Formoterol via Turbuhaler gave better protection than terbutaline against repeated exercise challenge for up to 12 hours in children and adolescents. Respiratory Medicine 2000;94(7):661‐7. [DOI] [PubMed] [Google Scholar]
Hancox 2002 {published data only}
- Hancox RJ, Subbarao P, Kamada D, Watson RM, Hargreave FE, Inman MD. Beta2‐agonist tolerance and exercise‐induced bronchospasm. American Journal of Respiratory and Critical Care Medicine 2002;165(8):1068‐70. [DOI] [PubMed] [Google Scholar]
Hawksworth 2002 Salb HFA {published data only}
- Hawksworth RJ, Sykes AP, Faris M, Mant T, Lee TH. Albuterol HFA is as effective as albuterol CFC in preventing exercise‐induced bronchoconstriction. Annals of Allergy, Asthma & Immunology 2002;88(5):473‐7. [DOI] [PubMed] [Google Scholar]
Hawksworth 2002 Salb MDI {published data only}
- Hawksworth RJ, Sykes AP, Faris M, Mant T, Lee TH. Albuterol HFA is as effective as albuterol CFC in preventing exercise‐induced bronchoconstriction. Annals of Allergy, Asthma & Immunology 2002;88(5):473‐7. [DOI] [PubMed] [Google Scholar]
Henricksen 1983 Terb {published data only}
- Henriksen JM, Dahl R. Effects of inhaled budesonide alone and in combination with low‐dose terbutaline in children with exercise‐induced asthma. American Review of Respiratory Disease 1983;128(6):993‐7. [DOI] [PubMed] [Google Scholar]
Henricksen 1992 Salb {published data only}
- Henriksen JM, Agertoft L, Pedersen S. Protective effect and duration of action of inhaled formoterol and salbutamol on exercise‐induced asthma in children. Journal of Allergy and Clinical Immunology 1992;89(6):1176‐82. [DOI] [PubMed] [Google Scholar]
Henriksen 1992 Form 12 {published data only}
- Henriksen JM, Agertoft L, Pedersen S. Protective effect and duration of action of inhaled formoterol and salbutamol on exercise‐induced asthma in children. Journal of Allergy and Clinical Immunology 1992;89(6):1176‐82. [DOI] [PubMed] [Google Scholar]
Hills 1976 Salb {published data only}
- Hills EA, Davies S, Geary M. Salmefamol and salbutamol in exercise‐induced asthma in children. British Journal of Diseases of the Chest 1976;70(2):78‐82. [DOI] [PubMed] [Google Scholar]
Hills 1976 Salmefamol {published data only}
- Hills EA, Davies S, Geary M. Salmefamol and salbutamol in exercise‐induced asthma in children. British Journal of Diseases of the Chest 1976;70(2):78‐82. [DOI] [PubMed] [Google Scholar]
Inman 1996 {published data only}
- Inman MD, O'Byrne PM. The effect of regular inhaled albuterol on exercise‐induced bronchoconstriction. American Journal of Respiratory and Critical Care Medicine 1996;153(1):65‐9. [DOI] [PubMed] [Google Scholar]
Kemp 1994 Salb {published data only}
- Kemp JP, Dockhorn RJ, Busse WW, Bleecker ER, As A. Prolonged effect of inhaled salmeterol against exercise‐induced bronchospasm. American Journal of Respiratory and Critical Care Medicine 94;150:1612‐5. [DOI] [PubMed] [Google Scholar]
Kemp 1994 Salm 42 {published data only}
- Kemp JP, Dockhorn RJ, Busse WW, Bleecker ER, As A. Prolonged effect of inhaled salmeterol against exercise‐induced bronchospasm. American Journal of Respiratory and Critical Care Medicine 94;150:1612‐5. [DOI] [PubMed] [Google Scholar]
Konig 1981 Metaprot {published data only}
- Konig P, Eggleston PA, Serby CW. Comparison of oral and inhaled metaproterenol for prevention of exercise‐induced asthma. Clinical Allergy 1981;11(6):597‐604. [DOI] [PubMed] [Google Scholar]
Konig 1984 Fen 0.4 {published data only}
- Konig P, Hordvik NL, Serby CW. Fenoterol in exercise‐induced asthma: effect of dose on efficacy and duration of action. Chest 1984;85:462‐4. [DOI] [PubMed] [Google Scholar]
Konig 1984 Fen 0.8 {published data only}
- Konig P, Hordvik NL, Serby CW. Fenoterol in exercise‐induced asthma: effect of dose on efficacy and duration of action. Chest 1984;85:462‐4. [DOI] [PubMed] [Google Scholar]
Larsson 1982 Fen {published data only}
- Larsson K. Oxitropium bromide, ipratropium bromide and fenoterol in exercise‐induced asthma. Respiration 1982;43(1):57‐63. [DOI] [PubMed] [Google Scholar]
McAlpine 1990 Form 12 {published data only}
- McAlpine LG, Thomson NC. Prophylaxis of exercise‐induced asthma with inhaled formoterol, a long‐acting beta 2‐adrenergic agonist. Respiratory Medicine 1990;84(4):293‐5. [DOI] [PubMed] [Google Scholar]
McAlpine 1990 Salb {published data only}
- McAlpine LG, Thomson NC. Prophylaxis of exercise‐induced asthma with inhaled formoterol, a long‐acting beta 2‐adrenergic agonist. Respiratory Medicine 1990;84(4):293‐5. [DOI] [PubMed] [Google Scholar]
McFadden 1986 Salb (I) {published data only}
- McFadden ER Jr, Mills R. Prevention of exercise‐induced bronchospasm with aerosolized albuterol. Current Therapeutic Research, Clinical and Experimental 1986;39(1):112‐8. [Google Scholar]
McFadden 1986 Salb (II) {published data only}
- McFadden ER, Mills R. Inhaled albuterol powder for the prevention of exercise‐induced bronchospasm. Immunology and Allergy Practice 1986;8(6):199‐203. [Google Scholar]
Morton 1989 Rimet {published data only}
- Morton AR, Scott CA, Fitch KD. Rimiterol and the prevention of exercise‐induced asthma. Journal of Allergy and Clinical Immunology 1989;83(1):61‐5. [DOI] [PubMed] [Google Scholar]
Nelson 1998 {published data only}
- Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER Jr. Effect of long‐term salmeterol treatment on exercise‐induced asthma. New England Journal of Medicine 1998;339(3):141‐6. [DOI] [PubMed] [Google Scholar]
Newnham 1993 Salb 200 {published data only}
- Newnham DM, Ingram CG, Earnshaw J, Palmer JB, Dhillon DP. Salmeterol provides prolonged protection against exercise‐induced bronchoconstriction in a majority of subjects with mild, stable asthma. Respiratory Medicine 1993;87(6):439‐4. [DOI] [PubMed] [Google Scholar]
Newnham 1993 Salm 50 {published data only}
- Newnham DM, Ingram CG, Earnshaw J, Palmer JB, Dhillon DP. Salmeterol provides prolonged protection against exercise‐induced bronchoconstriction in a majority of subjects with mild, stable asthma. Respiratory Medicine 1993;87(6):439‐4. [DOI] [PubMed] [Google Scholar]
Patel 1986 Salb 200 {published data only}
- Patel KR. Bronchodilator activity of a new inhaled beta2‐adrenoceptor agonist, tulobuterol, and its protective effect in exercise‐induced asthma. British Journal of Clinical Pharmacology 1986;21(2):234‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 1986 Tulob 200 {published data only}
- Patel KR. Bronchodilator activity of a new inhaled beta2‐adrenoceptor agonist, tulobuterol, and its protective effect in exercise‐induced asthma. British Journal of Clinical Pharmacology 1986;21(2):234‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 1986 Tulob 400 {published data only}
- Patel KR. Bronchodilator activity of a new inhaled beta2‐adrenoceptor agonist, tulobuterol, and its protective effect in exercise‐induced asthma. British Journal of Clinical Pharmacology 1986;21(2):234‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patessio 1991 Form 24 {published data only}
- Patessio A, Podda A, Carone M, Trombetta N, Donner CF. Protective effect and duration of action of formoterol aerosol on exercise‐induced asthma. European Respiratory Journal 1991;4(3):296‐300. [PubMed] [Google Scholar]
Patessio 1991 Salb 200 {published data only}
- Patessio A, Podda A, Carone M, Trombetta N, Donner CF. Protective effect and duration of action of formoterol aerosol on exercise‐induced asthma. European Respiratory Journal 1991;4(3):296‐300. [PubMed] [Google Scholar]
Pearlman 2006 Form 12 {published data only}
- Pearlman D, Milgrom H, Till D, Ziehmer B. Effect of formoterol fumarate treatment on exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma & Immunology 2006;97(3):382‐8. [DOI] [PubMed] [Google Scholar]
Pearlman 2006 Form 24 {published data only}
- Pearlman D, Milgrom H, Till D, Ziehmer B. Effect of formoterol fumarate treatment on exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma & Immunology 2006;97(3):382‐8. [DOI] [PubMed] [Google Scholar]
Pearlman 2006 Salb 180 {published data only}
- Pearlman D, Milgrom H, Till D, Ziehmer B. Effect of formoterol fumarate treatment on exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma & Immunology 2006;97(3):382‐8. [DOI] [PubMed] [Google Scholar]
Pearlman 2007 Salb 90 {published data only}
- Pearlman DS, Rees W, Schaefer K, Huang H, Andrews WT. An evaluation of levalbuterol HFA in the prevention of exercise‐induced bronchospasm. Journal of Asthma 2007;44(9):729‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philip 2007 Salm 50 {published data only}
- Philip G, Pearlman DS, Villarán C, Legrand C, Loeys T, Langdon RB, et al. Single‐dose montelukast or salmeterol as protection against exercise‐induced bronchoconstriction. Chest 2007;132(3):875‐83. [DOI] [PubMed] [Google Scholar]
Ramage 1994 {published data only}
- Ramage L, Lipworth BJ, Ingram CG, Cree IA, Dhillon DP. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respiratory Medicine 1994;88(5):363‐8. [DOI] [PubMed] [Google Scholar]
Richter 2002 Form 12 {published data only}
- Richter K, Janicki S, Jorres RA, Magnussen H. Acute protection against exercise‐induced bronchoconstriction by formoterol, salmeterol and terbutaline. European Respiratory Journal 2002;19(5):865‐71. [DOI] [PubMed] [Google Scholar]
Richter 2002 Salm 50 {published data only}
- Richter K, Janicki S, Jorres RA, Magnussen H. Acute protection against exercise‐induced bronchoconstriction by formoterol, salmeterol and terbutaline. European Respiratory Journal 2002;19(5):865‐71. [DOI] [PubMed] [Google Scholar]
Richter 2002 Terb 500 {published data only}
- Richter K, Janicki S, Jorres RA, Magnussen H. Acute protection against exercise‐induced bronchoconstriction by formoterol, salmeterol and terbutaline. European Respiratory Journal 2002;19(5):865‐71. [DOI] [PubMed] [Google Scholar]
Shapiro 2002 Form 12 {published data only}
- Shapiro GG, Kemp JP, DeJong R, Chapko M, Bierman CW, Altman LC, et al. Effects of albuterol and procaterol on exercise‐induced asthma. Annals of Allergy 1990;65(4):273‐6. [PubMed] [Google Scholar]
Shapiro 2002 Form 24 {published data only}
- Shapiro GG, Kemp JP, DeJong R, Chapko M, Bierman CW, Altman LC, et al. Effects of albuterol and procaterol on exercise‐induced asthma. Annals of Allergy 1990;65(4):273‐6. [PubMed] [Google Scholar]
Shapiro 2002 Salb 180 {published data only}
- Shapiro GG, Kemp JP, DeJong R, Chapko M, Bierman CW, Altman LC, et al. Effects of albuterol and procaterol on exercise‐induced asthma. Annals of Allergy 1990;65(4):273‐6. [PubMed] [Google Scholar]
Simons 1997 {published data only}
- Simons FE, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics 1997;99(5):655‐9. [DOI] [PubMed] [Google Scholar]
Stelmach 2008 {published data only}
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology 2008;121(2):383‐9. [DOI] [PubMed] [Google Scholar]
Storms 2004 {published data only}
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM, et al. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respiratory Medicine 2004;98(11):1051‐62. [DOI] [PubMed] [Google Scholar]
Sturani 1983 Fen 400 {published data only}
- Sturani C, Schiavina M, Tosi I, Gunella G. Comparison of inhaled fenoterol and salbutamol in the prevention of exercise‐induced asthma. European Journal of Respiratory Diseases 1983;128(Suppl):526‐8. [PubMed] [Google Scholar]
Sturani 1983 Salb 200 {published data only}
- Sturani C, Schiavina M, Tosi I, Gunella G. Comparison of inhaled fenoterol and salbutamol in the prevention of exercise‐induced asthma. European Journal of Respiratory Diseases 1983;128:526‐8. [PubMed] [Google Scholar]
VanHaitsma 2010 Salb {published data only}
- VanHaitsma TA, Mickleborough T, Stager JM, Koceja DM, Lindley MR, Chapman R. Comparative effects of caffeine and albuterol on the bronchoconstrictor response to exercise in asthmatic athletes. International Journal of Sports Medicine 2010;31(4):231‐6. [DOI] [PubMed] [Google Scholar]
Vasquez 1984 Salb 400 {published data only}
- Vazquez C, Fidalgo I, Virto MC, Labayru MT, Casas C. Efficacy of disodium chromoglycate, salbutamol and ipratroprium bromide on inhibition of exercise induced bronchospasm. Anales Espanoles de Pediatria 1984;20(8):756‐62. [PubMed] [Google Scholar]
Walker 1986 Bitolterol {published data only}
- Walker SB, Bierman CW, Pierson WE, Shapiro GG, Furukawa CT, Mingo TS. Bitolterol mesylate in exercise‐induced asthma. Journal of Allergy and Clinical Immunology 1986;77:32‐6. [DOI] [PubMed] [Google Scholar]
Wolley 1990 Terb 500 {published data only}
- Woolley M, Anderson SD, Quigley BM. Duration of protective effect of terbutaline sulfate and cromolyn sodium alone and in combination on exercise‐induced asthma. Chest 1990;97(1):39‐45. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aebischer 1984 {published data only}
- Aebischer JC, Benoit RC, Scherrer M. Pirbuterol and salbutamol aerosol for exercise‐induced bronchoconstriction. Journal Suisse de Medecine 1984;114(46):1660‐4. [PubMed] [Google Scholar]
Agostini 1983 I {published data only}
- Agostini M, Barlocco G, Mastella G. Protective effect of fenoterol spray, ipratropium bromide plus fenoterol spray, and oral clenbuterol, on exercise‐induced asthma in children. Double blind controlled and randomized clinical trial. European Journal of Respiratory Diseases 1983;128(2):529‐32. [PubMed] [Google Scholar]
Agostini 1983 II {published data only}
- Agostini M, Barlocco G, Mastella G. Protection against exercise induced asthma in children. Double blind controlled clinical trial with fenoterol spray, fenoterol plus ipratropium and oral clenbuterol. Rivista Italiana di Pediatria 1983;9(4):347‐52. [PubMed] [Google Scholar]
Allegra 1976 {published data only}
- Allegra J, Field J, Trautlein J, Gillin M, Zelis R. The pharmacologic effect of aerosolized terbutaline sulfate in exercise‐induced bronchospasm. Journal of Clinical Pharmacology 1976;16(8‐9):444‐7. [DOI] [PubMed] [Google Scholar]
Anderson 1975 {published data only}
- Anderson SD, Rozea PJ, Dolton R, Lindsay DA. Inhaled and oral bronchodilator therapy in exercise induced asthma. Australian and New Zealand Journal of Medicine 1975;5(6):544‐50. [DOI] [PubMed] [Google Scholar]
Anderson 1976 {published data only}
- Anderson SD, Seale JP, Rozea P, Bandler L, Theobald G, Lindsay D. Inhaled and oral salbutamol in exercise‐induced asthma. American Review of Respiratory Disease 1976;114(3):493‐500. [DOI] [PubMed] [Google Scholar]
Anderson 1991 {published data only}
- Anderson SD, Rodwell LT, Du Toit J, Young IH. Duration of protection by inhaled salmeterol in exercise‐induced asthma. Chest 1991;100(5):1254‐60. [DOI] [PubMed] [Google Scholar]
Aranda 1992 {published data only}
- Aranda PC, Merello AM, Power A, Reus M, Astudillo P. Prevention of exercise induced asthma by bronchodilator drug aerosol. Revista Chilena de Pediatria 1992;63(4):202‐5. [Google Scholar]
Bakran 1980 {published data only}
- Bakran I, Vrhovac B, Plavsić F. Aminophylline vs. salbutamol in exercise‐induced asthma. International Journal of Clinical Pharmacology, Therapy and Toxicology 1980;18(10):442‐6. [PubMed] [Google Scholar]
Battistini 1980 {published data only}
- Battistini A, Grzincich GL, Gorni A, Baroni AL. Asthma and bronchodilator drugs. Evaluation of a newly developed beta2‐agonist: clenbuterol. Rivista Italiana di Pediatria 1980;6(3):313‐9. [Google Scholar]
Baur 1979 {published data only}
- Baur X. Concerning the therapy of exercise‐induced asthma. Praxis und Klinik der Pneumologie 1979;33(6):791‐5. [PubMed] [Google Scholar]
Berkowitz 1986 {published data only}
- Berkowitz R, Schwartz E, Bukstein D, Grunstein M, Chai H. Albuterol protects against exercise‐induced asthma longer than metaproterenol sulfate. Pediatrics 1986;77(2):173‐8. [PubMed] [Google Scholar]
Boner 1984 {published data only}
- Boner AL, Zamo CR, Marchiori MM, Biancotto R, Antolini I, Vallone G. Comparison of nebulized ipratropium, salbutamol and cromoglicate solutions, cromoglicate inhaled powder, theophylline elixir and placebo in exercise induced asthma of the children. Giornale Italiano delle Malattie del Torace 1984;38(6):395‐9. [Google Scholar]
Boner 1987 {published data only}
- Boner AL, Stefano G, Niero E, Vallone G, Gaburro D. Salbutamol and ipratropium bromide solution in the treatment of bronchospasm in asthmatic children. Annals of Allergy 1987;58(1):54‐8. [PubMed] [Google Scholar]
Boner 1988 {published data only}
- Boner AL, Vallone G, Brighenti C, Schiassi M, Miglioranzi P, Richelli C. Comparison of the protective effect and duration of action of orally administered clenbuterol and salbutamol on exercise‐induced asthma in children. Pediatric Pulmonology 1988;4(4):197‐200. [DOI] [PubMed] [Google Scholar]
Bratteby 1986 {published data only}
- Bratteby LE, Foucard T, Lönnerholm G. Combined treatment with ipratropium bromide and beta‐2‐adrenoceptor agonists in childhood asthma. European Journal of Respiratory Diseases 1986;68(4):239‐47. [PubMed] [Google Scholar]
Bundgaard 1980 {published data only}
- Bundgaard AF, Rasmussen FV, Madsen L. Pretreatment of exercise‐induced asthma in adults with aerosols and pulverized tablets. Allergy 1980;35(8):639‐45. [DOI] [PubMed] [Google Scholar]
Bundgaard 1983 I {published data only}
- Bundgaard AF. Pretreatment of exercise‐induced asthma with beta‐2 agonists inhaled from RV to TLC or at TLC. A preliminary report. European Journal of Respiratory Diseases 1983;128(2):518‐20. [PubMed] [Google Scholar]
Bundgaard 1983 II {published data only}
- Bundgaard AF, Buch D, Schmidt A, Bach‐Mortensen N. Pretreatment of exercise‐induced asthma in children using disodium cromoglycate and fenoterol inhalation powder. European Journal of Respiratory Diseases 1983;130:36‐41. [PubMed] [Google Scholar]
Bundgaard 1983 III {published data only}
- Bundgaard A, Schmidt A. Double‐blind pretreatment of exercise‐induced asthma with sequential inhalations of fenoterol from an aerosol and as a powder; second of two parts. European Journal of Respiratory Diseases 1983;130:67‐72. [PubMed] [Google Scholar]
Bye 1980 {published data only}
- Bye PT, Anderson SD, Daviskas E, Marty JJ, Sampson D. Plasma cyclic AMP levels in response to exercise and terbutaline sulphate aerosol in normal and asthmatic subjects. European Journal of Respiratory Diseases 1980;61(5):287‐97. [PubMed] [Google Scholar]
Ceugniet 1997 {published data only}
- Ceugniet F, Cauchefer F, Fragneaud C, Evano‐Celli I. Prophylactic treatment of exercise‐induced asthma in children: salmeterol or sodium cromoglycate single dose before exercise?. Annales De Pediatrie 1997;44(9):625‐34. [Google Scholar]
Colice 1999 {published data only}
- Colice GL, Klinger NM, Ekholm BP, Dockhorn RJ. Proventil HFA prevents exercise‐induced bronchoconstriction in children. Journal of Asthma 1999;36(8):671‐6. [DOI] [PubMed] [Google Scholar]
Coreno 2000 {published data only}
- Coreno A, Skowronski M, Kotaru C, McFadden ER Jr. Comparative effects of long‐acting beta2‐agonists, leukotriene receptor antagonists, and a 5‐lipoxygenase inhibitor on exercise‐induced asthma. Journal of Allergy and Clinical Immunology 2000;106(3):500‐6. [DOI] [PubMed] [Google Scholar]
Corrias 1989 {published data only}
- Corrias A, Pelosi U, Corona GB, Minelli R, Peri M, Corda R. Efficacy of broxaterol vs salbutamol in asthma induced by physical exercise in children. Pediatria Medica e Chirurgica 1989;11:161‐3. [PubMed] [Google Scholar]
Dal Col 1995 {published data only}
- Dal Col G, Martinati L, Mingoni S, Boner A, Cantini L. Salbutamol powder, administered via a multidose and a single‐dose powder inhaler, in the prevention of exercise‐induced asthma in children. Pediatric Asthma, Allergy and Immunology 1995;9(3):165‐71. [Google Scholar]
Del Bono 1979 {published data only}
- Bono N, Quartieri F, Vibelli C. Aerosolized clenbuterol NAB 365 and salbutamol in exercise‐induced asthma. Current Medical Research & Opinion 1979;6(4):237‐43. [DOI] [PubMed] [Google Scholar]
Di Gioacchino 1987 {published data only}
- Gioacchino M, Mezzetti A, Mancini M, Guglielmi MD, Lo Medico E, Proietti Franceschilli G, et al. Study of the cardiovascular effects of clenbuterol in exercise‐induced asthma. Respiration 1987;51(3):205‐13. [DOI] [PubMed] [Google Scholar]
Dockhorn 1997 {published data only}
- Dockhorn RJ, Wagner DE, Burgess GL, Hafner KB, Letourneau K, Colice GL, et al. Proventil HFA provides protection from exercise‐induced bronchoconstriction comparable to proventil and ventolin. Annals of Allergy 1997;79(1):85‐8. [DOI] [PubMed] [Google Scholar]
Edelman 2000 {published data only}
- Edelman JL, Turpin JA, Bronsky EA, Grossman J, Kemp JP, Ghannam AF, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise‐induced bronchoconstriction. A randomized, double‐blind trial. Exercise Study Group. Annals of Internal Medicine 2000;132(2):97‐104. [DOI] [PubMed] [Google Scholar]
Eggleston 1981 {published data only}
- Eggleston PA, Beasley PP, Kindley RT. The effects of oral doses of theophylline and fenoterol on exercise‐induced asthma. Chest 1981;79(4):399‐405. [DOI] [PubMed] [Google Scholar]
Ferrari 2002 {published data only}
- Ferrari M, Segattini C, Zanon R, Bertaiola M, Balestreri F, Brotto E, et al. Comparison of the protective effect of formoterol and of salmeterol against exercise‐induced bronchospasm when given immediately before a cycloergometric test. Respiration 2002;69(6):509‐12. [DOI] [PubMed] [Google Scholar]
Fogel 2010 {published data only}
- Fogel BR, Rosario N, Aristizabal G, Loeys T, Noonan G, Gaile S, et al. Effect of montelukast or salmeterol added to inhaled fluticasone on exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma & Immunology 2010;104:511‐7. [DOI] [PubMed] [Google Scholar]
Francis 1980 {published data only}
- Francis PW, Krastins IR, Levison H. Oral and inhaled salbutamol in the prevention of exercise‐induced bronchospasm. Pediatrics 1980;66(1):103‐8. [PubMed] [Google Scholar]
Freeman 1989 {published data only}
- Freeman W, Packe GE, Cayton RM. Effect of nebulised salbutamol on maximal exercise performance in men with mild asthma. Thorax 1989;44(11):942‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 1978 {published data only}
- Gibson GJ, Greenacre JK, König P, Conolly ME, Pride NB. Use of exercise challenge to investigate possible tolerance to beta‐adrenoceptor stimulation in asthma. British Journal of Diseases of the Chest 1978;72(3):199‐206. [DOI] [PubMed] [Google Scholar]
Gimeno 1985 {published data only}
- Gimeno F, Veenen R, Steenhuis EJ, Berg WC. Comparison of disodium cromoglycate, terbutaline and thiazinamium in the prevention of exercise‐induced asthma and its relation to non‐specific bronchial responsiveness. Respiration 1985;48(2):108‐15. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2006 I {unpublished data only}
- GlaxoSmithKline. A study measuring asthma control in pediatric and adolescent subjects whose asthma is worsened by activity or exercise. clinicaltrials.gov.
GlaxoSmithKline 2006 II {unpublished data only}
- GlaxoSmithKline. A Stratified, Multicenter, Randomized, Double‐Blind, Parallel Group, 4‐Week Comparison of Fluticasone Propionate/Salmeterol DISKUS Combination Product 100/50 mcg BID versus Fluticasone Propionate DISKUS 100 mcg BID in Pediatric and in Adolescent Subjects With Activity‐Induced Bronchospasm. GlaxoSmithKline Clinical Trial Register.
Godfrey 1975 {published data only}
- Godfrey S, König P. Suppression of exercise‐induced asthma by salbutamol, theophylline, atropine, cromolyn, and placebo in a group of asthmatic children. Pediatrics 1975;56(5 pt 2 suppl):930‐4. [PubMed] [Google Scholar]
Godfrey 1976 {published data only}
- Godfrey S, König P. Inhibition of exercise‐induced asthma by different pharmacological pathways. Thorax 1976;31(2):137‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerin 1992 {published data only}
- Guerin JC, Brambilla C, Godard P, Muizon H, Aubert B, Bons J. Prolonged effect against exercise‐induced bronchospasm: salmeterol versus sodium cromoglycate. Revue des Maladies Respiratoires 1992;9(1):27‐30. [PubMed] [Google Scholar]
Gunawardena 2005 {published data only}
- Gunawardena K, Palmer P, Das S, Hewitt A. Using exercise‐induced bronchoconstriction as a method to compare formoterol inhalers in children. Journal of Applied Therapeutic Research 2005;5(3):16‐23. [Google Scholar]
Hermansen 2006 {published data only}
- Hermansen MN, Nielsen KG, Buchvald F, Jespersen JJ, Bengtsson T, Bisgaard H. Acute relief of exercise‐induced bronchoconstriction by inhaled formoterol in children with persistent asthma. Chest 2006;129(5):1203‐9. [DOI] [PubMed] [Google Scholar]
Higgs 1983 {published data only}
- Higgs CM, Laszlo G. The duration of protection from exercise‐induced asthma by inhaled salbutamol, and a comparison with inhaled reproterol. British Journal of Diseases of the Chest 1983;77(3):262‐9. [PubMed] [Google Scholar]
Ienna 1997 {published data only}
- Ienna TM, McKenzie DC. The asthmatic athlete: metabolic and ventilatory responses to exercise with and without pre‐exercise medication. International Journal of Sports Medicine 1997;18(2):142‐8. [DOI] [PubMed] [Google Scholar]
Iikura 1988 {published data only}
- Iikura Y, Inui H, Obata T, Nagakura T, Sugimoto H, Lee TH, et al. Drug effects on exercise‐induced late asthmatic responses. New England and Regional Allergy Proceedings 1988;9(3):203‐7. [DOI] [PubMed] [Google Scholar]
Ioli 1986 {published data only}
- Ioli F, Donner CF, Fracchia C, Manini G, Patessio A, Spada EL, et al. A new bronchodilating agent, procaterol, in preventing exercise‐induced asthma. International Journal of Clinical Pharmacology Research 1986;6(5):389‐96. [PubMed] [Google Scholar]
Johnson 1986 {published data only}
- Johnson CE, Belfield PW, Davis S, Cooke NJ, Spencer A, Davies JA. Platelet activation during exercise induced asthma: effect of prophylaxis with cromoglycate and salbutamol. Thorax 1986;41(4):290‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koch 1972 {published data only}
- Koch G. Terbutaline in bronchial asthma. Effect on lung volumes, ventilatory performance, pulmonary gas exchange and circulation at rest and during exercise. Scandinavian Journal of Respiratory Diseases 1972;53(4):187‐95. [PubMed] [Google Scholar]
Kumar 1988 {published data only}
- Kumar AS. Salmeterol in exercise induced asthma. Indian Pediatrics 1988;35(7):681‐2. [PubMed] [Google Scholar]
Lopes Dos Santos 1991 {published data only}
- dos Santos JM, Costa H, Ståhl E, Wirén JE. Bricanyl Turbuhaler and Ventolinrho Rotahaler in exercise‐induced asthma in children. Allergy 1991;46(3):203‐5. [DOI] [PubMed] [Google Scholar]
Machado 2012 {published data only}
- Machado D, Pereira G, Tavares B, Loureiro G, Segorbe‐Luis A. Airways hyperresponsiveness to different inhaled combination therapies in adolescent asthmatics. European Annals of Allergy and Clinical Immunology 2012;44(1):12‐7. [PubMed] [Google Scholar]
Macucci 2004 {published data only}
- Macucci F, Guerrini L, Strambi M. Oral montelukast compared with inhaled salbutamol to prevent exercise‐induced asthma in children. Minerva Pneumologica 2004;43(1):41‐50. [Google Scholar]
Magnussen 1984 {published data only}
- Magnussen H, Reuss G. Blockade of exercise‐induced asthma by fenoterol. Klinische Wochenschrift 1984;62(4):168‐74. [DOI] [PubMed] [Google Scholar]
Makela 2012 {published data only}
- Makela MJ, Malmberg LP, Csonka P, Klemola T, Kajosaari M, Pelkonen AS. Salmeterol and fluticasone in young children with multiple‐trigger wheeze. Annals of Allergy, Asthma & Immunology 2012;109:65‐70. [DOI] [PubMed] [Google Scholar]
Martinsson 1985 {published data only}
- Martinsson A, Larsson K, Hjemdahl P. Reduced beta 2‐adrenoceptor responsiveness in exercise‐induced asthma. Chest 1985;88(4):594‐600. [DOI] [PubMed] [Google Scholar]
Merck 2005 I {unpublished data only}
- Merck. An investigational drug to prevent exercise‐induced bronchospasm. clinicaltrials.gov.
Merck 2005 II {unpublished data only}
- Merck. Two investigational drugs in the prevention of airway constriction brought on by exercise in asthmatic patients. clinicaltrials.gov.
Mickleborough 2007 {published data only}
- Mickleborough TD, Lindley MR, Turner LA. Comparative effects of a high‐intensity interval warm‐up and salbutamol on the bronchoconstrictor response to exercise in asthmatic athletes. International Journal of Sports Medicine 2007;28(6):456‐62. [DOI] [PubMed] [Google Scholar]
Millqvist 2000 {published data only}
- Millqvist EB, Bengtsson U, Löwhagen O. Combining a beta2‐agonist with a face mask to prevent exercise‐induced bronchoconstriction. Allergy 2000;55(7):672‐5. [DOI] [PubMed] [Google Scholar]
Morandini 1982 {published data only}
- Morandini GC. Protection of exercise‐induced asthma: comparative study of drugs with a different mechanism of action. Minerva Pneumologica 1982;21(2):75‐85. [Google Scholar]
Morooka 1987 {published data only}
- Morooka T, Nishima S, Ota S. Prevention of exercise‐induced bronchospasm in asthmatic children. Effect of aerosol and oral procaterol hydrochloride. Journal of Asthma 1987;24(6):335‐46. [DOI] [PubMed] [Google Scholar]
Morse 1976 {published data only}
- Morse J, Jones NL, Anderson GD. The effect of terbutaline in exercise‐induced asthma. American Review of Respiratory Disease 1976;113(1):89‐92. [DOI] [PubMed] [Google Scholar]
Morton 1992 {published data only}
- Morton AR, Papalia SM, Morton PS, Fitch KD. The efficacy of the nebuhaler method of administration of terbutaline sulphate in the prevention and amelioration of exercise induced asthma. Australian Journal of Science and Medicine in Sport 1992;24(4):103‐6. [Google Scholar]
Murray 2011 {published data only}
- Murray JJ, Waitkus‐Edwards KR, Yancey SW. Evaluation of fluticasone propionate and fluticasone propionate/salmeterol combination on exercise in pediatric and adolescent patients with asthma. The Open Respiratory Medicine Journal 2011;5:11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearlman 2009 {published data only}
- Pearlman D, Qaqundah P, Matz J, Yancey SW, Stempel DA, Ortega HG. Fluticasone propionate/salmeterol and exercise‐induced asthma in children with persistent asthma. Pediatric Pulmonology 2009;44(5):429‐35. [DOI] [PubMed] [Google Scholar]
Pfleger 2002 {published data only}
- Pfleger A, Eber E, Weinhandl E, Zach MS. Effects of nedocromil and salbutamol on airway reactivity in children with asthma. European Respiratory Journal 2002;20(3):624‐9. [DOI] [PubMed] [Google Scholar]
Pichaipat 1995 {published data only}
- Pichaipat V, Tongpenyai Y, Nerntong T, Sriprapachiranont C. The protective effect of inhaled terbutaline, sodium cromoglycate and budesonide on exercise‐induced asthma in children. Journal of the Medical Association of Thailand 1995;78(10):505‐8. [PubMed] [Google Scholar]
Pichon 2005 {published data only}
- Pichon A, Roulaud M, Denjean A, Bisschop C. Airway tone during exercise in healthy subjects: effects of salbutamol and ipratropium bromide. International Journal of Sports Medicine 2005;26(5):321‐6. [DOI] [PubMed] [Google Scholar]
Poppius 1973 {published data only}
- Poppius H, Salorinne Y. Comparative trial of salbutamol and an anticholinergic drug, SCH 1000, in prevention of exercise‐induced asthma. Scandinavian Journal of Respiratory Diseases 1973;54(3):142‐7. [PubMed] [Google Scholar]
Rabe 1993 {published data only}
- Rabe KF, Jörres R, Magnussen H. The effect of 10, 50 and 200 micrograms inhaled fenoterol on exercise induced asthma. Clinical and Experimental Allergy 1993;23(5):440‐5. [DOI] [PubMed] [Google Scholar]
Raissy 2006 {unpublished data only}
- Raissy HH. Exercise induced bronchospasm in children. clinicaltrials.gov.
Raissy 2008 {published data only}
- Raissy HH, Harkins M, Kelly F, Kelly HW. Pretreatment with albuterol versus montelukast for exercise‐induced bronchospasm in children. Pharmacotherapy 2008;28(3):287‐94. [DOI] [PubMed] [Google Scholar]
Revill 1998 {published data only}
- Revill SM, Morgan MD. The cardiorespiratory response to submaximal exercise in subjects with asthma following pretreatment with controlled release oral salbutamol and high‐dose inhaled salmeterol. Respiratory Medicine 1998;92(8):1053‐8. [DOI] [PubMed] [Google Scholar]
Robertson 1994 {published data only}
- Robertson W, Simkins J, O'Hickey SP, Freeman S, Cayton RM. Does single dose salmeterol affect exercise capacity in asthmatic men?. European Respiratory Journal 1994;7(11):1978‐84. [PubMed] [Google Scholar]
Rohr 1987 {published data only}
- Rohr AS, Siegel SC, Katz RM, Rachelefsky GS, Spector SL, Lanier R. A comparison of inhaled albuterol and cromolyn in the prophylaxis of exercise‐induced bronchospasm. Annals of Allergy 1987;59(2):107‐9. [PubMed] [Google Scholar]
Sanguinetti 1986 {published data only}
- Sanguinetti CM, Luca S, Gasparini S, Massei V. Evaluation of Duovent in the prevention of exercise‐induced asthma. Respiration 1986;50(2):181‐5. [DOI] [PubMed] [Google Scholar]
Schaanning 1996 {published data only}
- Schaanning J, Vilsvik J, Henriksen AH, Bratten G. Efficacy and duration of salmeterol powder inhalation in protecting against exercise‐induced bronchoconstriction. Annals of Allergy, Asthma & Immunology 1996;76(1):57‐60. [DOI] [PubMed] [Google Scholar]
Shah 1983 {published data only}
- Shah S, Johnston D, Woodcock AA, Johnson M, Geddes DM. Breathlessness and exercise tolerance in chronic airflow obstruction: 2‐hourly versus 4‐hourly salbutamol by inhalation. Current Medical Research & Opinion 1983;8(5):343‐9. [DOI] [PubMed] [Google Scholar]
Shapiro 1981 {published data only}
- Shapiro GG, McPhillips JJ, Smith K, Furukawa CT, Pierson WE, Bierman CW. Effectiveness of terbutaline and theophylline alone and in combination in exercise‐induced bronchospasm. Pediatrics 1981;67(4):508‐13. [PubMed] [Google Scholar]
Shapiro 1990 {published data only}
- Shapiro GG, Kemp JP, DeJong R, Chapko M, Bierman CW, Altman LC, et al. Effects of albuterol and procaterol on exercise‐induced asthma. Annals of Allergy 1990;65(4):273‐6. [PubMed] [Google Scholar]
Sichletidis 1993 {published data only}
- Sichletidis L, Daskalopoulou E, Kyriazis G, Kosmidou I, Koupidou S, Pechlivanidis T, et al. Comparative efficacy of salbutamol and salmeterol in exercise‐induced asthma. Journal of International Medical Research 1993;21(2):81‐8. [DOI] [PubMed] [Google Scholar]
Silverman 1973 {published data only}
- Silverman M, Konig P, Godfrey S. Use of serial exercise tests to assess the efficacy and duration of action of drugs for asthma. Thorax 1973;28(5):574‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1992 {published data only}
- Singh JP, Singh R, Gupta RC, Bharadwaja B. A comparative study of bronchodilator actions of ipratropium bromide (Atrovent) and salbutamol (Ventolin) on exercise induced bronchial asthma. Journal of the Association of Physicians of India 1992;40(8):545‐7. [PubMed] [Google Scholar]
Sly 1968 {published data only}
- Sly RM, Heimlich EM, Ginsburg J, Busser RJ, Strick L. Exercise‐induced bronchospasm: evaluation of metaproterenol. Annals of Allergy 1968;26(5):253‐8. [PubMed] [Google Scholar]
Sly 1975 {published data only}
- Sly RM, Puapan P, Ghazanshahi S, Midha R. Exercise‐induced bronchospasm: evaluation of albuterol aerosol. Annals of Allergy 1975;34(1):7‐14. [PubMed] [Google Scholar]
Sly 1982 {published data only}
- Sly RM, O'Brien SR. Effect of oral terbutaline on exercise‐induced asthma. Annals of Allergy 1982;48(3):151‐5. [PubMed] [Google Scholar]
Spada 1985 {published data only}
- Spada EL, Donner CF, Meriggi A, Vecchio C. Pharmacologic prevention of exercise‐induced asthma. Minerva Pneumologica 1985;24(2):159‐64. [Google Scholar]
Stark 1981 {published data only}
- Stark RD, Gambles SA. Effects of salbutamol, ipratropium bromide and disodium cromoglycate on breathlessness induced by exercise in normal subjects. British Journal of Clinical Pharmacology 1981;12(4):497‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinshamn 2004 {published data only}
- Steinshamn S, Sandsund M, Sue‐Chu M, Bjermer L. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise‐induced bronchoconstriction. Chest 2004;126(4):1154‐60. [DOI] [PubMed] [Google Scholar]
Svenonius 1983 {published data only}
- Svenonius E, Kautto R, Arborelius M Jr. Improvement after training of children with exercise‐induced asthma. Acta Paediatrica Scandinavica 1983;72(1):23‐30. [DOI] [PubMed] [Google Scholar]
Svenonius 1988 {published data only}
- Svenonius E, Arborelius M Jr, Wiberg R, Ekberg P. Prevention of exercise‐induced asthma by drugs inhaled from metered aerosols. Allergy 1988;43(4):252‐7. [DOI] [PubMed] [Google Scholar]
Svenonius 1994 {published data only}
- Svenonius E, Arborelius M, Wiberg R, Ståhl E, Svensson M. A comparison of terbutaline inhaled by Turbuhaler and by a chlorofluorocarbon CFC inhaler in children with exercise‐induced asthma. Allergy 1994;49(6):408‐12. [DOI] [PubMed] [Google Scholar]
Tabas 1985 {published data only}
- Tabas A, Rodríguez A, Lobera T, Diéguez I, Oehling A. Carbuterol, salbutamol and DSCG in exercise‐induced asthma. Allergologia et Immunopathologia 1985;13(6):493‐500. [PubMed] [Google Scholar]
Tammivaara 1979 {published data only}
- Tammivaara R. The efficacy of terbutaline and fenoterol aerosols on adult exercise‐induced asthma. Scandinavian Journal of Respiratory Diseases 1979;103:212‐3. [PubMed] [Google Scholar]
Unnithan 1994 {published data only}
- Unnithan VB, Thomson KJ, Aitchison TC, Paton JY. Beta 2‐agonists and running economy in prepubertal boys. Pediatric Pulmonology 1994;17(6):378‐82. [DOI] [PubMed] [Google Scholar]
Verini 1983 {published data only}
- Verini M, Chiarelli F, Tullio A, Morgese G, Pallotta R. Pharmacological prevention of exercise‐induced bronchospasm: review of the literature and trial of disodium cromoglycate, fenoterol and ipratropium bromide in a pediatric population. Pediatria Medica e Chirurgica 1983;5(6):501‐9. [PubMed] [Google Scholar]
Verini 1985 {published data only}
- Verini M, Ansaloni A, Vincenzo MG, Napoleone M, Morgese G. Evaluation of reproterol's effectiveness in preventing exercise‐induced bronchospasm in children. Journal of International Medical Research 1985;13(1):19‐23. [DOI] [PubMed] [Google Scholar]
Verini 1999 {published data only}
- Verini M, Verrotti A, Greco R, Chiarelli F. Functional effects of controlled physical activity in children and young adults affected by exercise‐induced asthma treated with corticosteroids and beta‐2 agonists. Clinical Drug Investigation 1999;17(6):467‐73. [Google Scholar]
Villaran 1999 {published data only}
- Villaran C, O'Neill SJ, Helbling A, Noord JA, Lee TH, Chuchalin AG, et al. Montelukast versus salmeterol in patients with asthma and exercise‐induced bronchoconstriction. Journal of Allergy and Clinical Immunology 1999;104(3):547‐53. [DOI] [PubMed] [Google Scholar]
Vilsvik 1991 {published data only}
- Vilsvik J, Schaanning J, Ståhl E, Holthe S. Comparison between Bricanyl Turbuhaler and Ventolin metered dose inhaler in the treatment of exercise‐induced asthma in adults. Annals of Allergy 1991;67(3):315‐8. [PubMed] [Google Scholar]
Vilsvik 2001 {published data only}
- Vilsvik J, Ankerst J, Palmqvist M, Persson G, Schaanning J, Schwabe G, et al. Protection against cold air and exercise‐induced bronchoconstriction while on regular treatment with Oxis. Respiratory Medicine 2001;95(6):484‐90. [DOI] [PubMed] [Google Scholar]
Von Berg 2002 {published data only}
- Berg A, Albrecht B, Darlath W, Vo HW, Berdel D. Intraindividual, randomised, double‐blind comparison between sodium cromoglycate and reproterol to assess the protective effect of the single drugs and their combination in children with exercise‐induced asthma. Allergologie 2002;25(11):557‐64. [Google Scholar]
Weiler 2005 {published data only}
- Weiler JM, Nathan RA, Rupp NT, Kalberg CJ, Emmett A, Dorinsky PM. Effect of fluticasone/salmeterol administered via a single device on exercise‐induced bronchospasm in patients with persistent asthma. Annals of Allergy, Asthma & Immunology 2005;94(1):65‐72. [DOI] [PubMed] [Google Scholar]
Weinberg 1982 {published data only}
- Weinberg EG. The effect of terbutaline sulphate on exercise‐induced asthma in children. South African Medical Journal 1982;61(16):587‐9. [PubMed] [Google Scholar]
Yeung 1980 {published data only}
- Yeung R, Nolan GM, Levison H. Comparison of the effects of inhaled SCH 1000 and fenoterol on exercise‐induced bronchospasm in children. Pediatrics 1980;66(1):109‐14. [PubMed] [Google Scholar]
Zanconato 1990 {published data only}
- Zanconato S, Baraldi E, Santuz P, Magagnin G, Zacchello F. Effect of inhaled disodium cromoglycate and albuterol on energy: cost of running in asthmatic children. Pediatric Pulmonology 1990;8(4):240‐4. [DOI] [PubMed] [Google Scholar]
Zimmermann 2003 {published data only}
- Zimmermann T, Gulyas A, Bauer CP, Steinkamp G, Trautmann M. Salmeterol versus sodium cromoglycate for the protection of exercise induced asthma in children-a randomised cross‐over study. European Journal of Medical Research 2003;8(9):428‐34. [PubMed] [Google Scholar]
References to studies awaiting assessment
Kupper 2012 {published data only}
- Küpper T, Goebbels K, Kennes LN, Netzer NC. Cromoglycate, reproterol, or both-what's best for exercise‐induced asthma?. Sleep and Breathing 2012;16(4):1229‐35. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1997
- Anderson SD. Exercise‐induced asthma. In: Kay AB editor(s). Allergy and Allergic Disease. Oxford: Blackwell Scientific Publications, 1997:692‐711. [Google Scholar]
Anderson 2003
- Anderson SD, Brannan JD. Methods for "indirect" challenge tests including exercise, eucapnic voluntary hyperpnea, and hypertonic aerosols. Clinical Reviews in Allergy & Immunology 2003;24(1):27‐54. [DOI] [PubMed] [Google Scholar]
Anderson 2006
- Anderson SD, Caillaud C, Brannan JD. Beta2‐agonists and exercise‐induced asthma. Clinical Reviews in Allergy & Immunology 2006;31(2‐3):163‐80. [DOI] [PubMed] [Google Scholar]
Bateman 2008
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal 2008;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Brozek 2010
- Brozek JL, Bousquet J, Baena‐Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. Journal of Allergy and Clinical Immunology 2010;126(3):466‐76. [DOI] [PubMed] [Google Scholar]
Carlsen 2008
- Carlsen KH, Anderson SD, Bjermer L, Bonini S, Brusasco V, Canonica W, et al. Exercise‐induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008;63(4):387‐403. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. www.cochranehandbook.org, 2011. [Google Scholar]
Kelly 2000
- Kelly KD, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise‐induced bronchoconstriction. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002731] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2007
- Koh MS, Tee A, Lasserson TJ, Irving LB. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002739.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2005
- Martinez FD. Safety of long‐acting beta‐agonists-an urgent need to clear the air. New England Journal of Medicine 2005;353:2637‐9. [DOI] [PubMed] [Google Scholar]
Nelson 2006
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, for the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15‐26. [DOI] [PubMed] [Google Scholar]
Peroni 2011
- Peroni DG, Pescollderungg L, Sandri M, Chinellato I, Boner AL, Piacentini GL. Time‐effect of montelukast on protection against exercise‐induced bronchoconstriction. Respiratory Medicine 2011;105(12):1790‐7. [DOI] [PubMed] [Google Scholar]
Randolph 2008
- Randolph C. Exercise‐induced bronchospasm in children. Clinical Reviews in Allergy & Immunology 2008;34(2):205‐16. [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan) Version 5.2. The Cochrane Collaboration, 2012.
Rundell 2000
- Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J. Exercise‐induced asthma screening of elite athletes: field versus laboratory exercise challenge. Medicine & Science in Sports & Exercise 2000;32(2):309‐16. [DOI] [PubMed] [Google Scholar]
Salpeter 2010
- Salpeter SR, Wall AJ, Buckley NS. Long‐acting beta‐agonists with and without inhaled corticosteroids and catastrophic asthma events. American Journal of Medicine 2010;123(4):322‐8. [DOI] [PubMed] [Google Scholar]
Shapiro 2002
- Shapiro GS, Yegen U, Xiang J, Kottakis J, Della Cioppa G. A randomized, double‐blind, single‐dose, crossover clinical trial of the onset and duration of protection from exercise‐induced bronchoconstriction by formoterol and albuterol. Clinical Therapeutics 2002;24(12):2077‐87. [DOI] [PubMed] [Google Scholar]
Spooner 2003
- Spooner CH, Spooner GR, Rowe BH. Mast‐cell stabilising agents to prevent exercise‐induced bronchoconstriction. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterk 1993
- Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, et al. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. European Respiratory Journal 1993;16(Suppl):53‐83. [PubMed] [Google Scholar]
Weiler 2010
- Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, et al. Pathogenesis, prevalence, diagnosis, and management of exercise‐induced bronchoconstriction: a practice parameter. Annals of Allergy, Asthma & Immunology 2010;105(6 suppl):1‐47. [DOI] [PubMed] [Google Scholar]


