Abstract
GSTP1 (glutathione S-transferase pi) is involved in stress responses and in cellular proliferation pathways as an inhibitor of JNK (c-Jun N-terminal kinase). It has been proposed that monomeric GSTP1 functions as a JNK inhibitor. All of the studies to date have been performed using rodent cells, and it is unclear if monomeric GSTP1 exists in human cells. Monomeric GSTP1 was sought in human gastric cancer cells (Kato III) and in normal human erythrocytes using gel filtration, ELISA and Western blots. Monomeric GSTP1 was found in conditioned medium, in cytosol of Kato III cells and in cytosol of erythrocytes. GSTP1 subunits from Kato III cells and erythrocytes were heterogeneous when analysed by MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS, suggesting that there were post-translational modifications to GSTP1. One post-translational modification, phosphorylation of a serine residue in the C-terminal portion of GSTP1 where JNK binds, was identified in GSTP1 purified from Kato III cells, but not in GSTP1 purified from human erythrocytes. Therefore normal and malignant human cells contain GSTP1 monomers with post-translational modifications, and it is likely that GSTP1 monomers regulate JNK activity in human cells in the same manner as in rodent cells.
Keywords: glutathione S-transferase (GST), c-Jun N-terminal kinase (JNK), monomer, MS, post-translational modification
Abbreviations: amu, atomic mass unit(s); ASK, apoptosis signal-regulating kinase; CM, conditioned medium; DTT, dithiothreitol; GST, glutathione S-transferase; GSTA2, GST alpha; GSTM1, GST mu; GSTP1, GST pi; JNK, c-Jun N-terminal kinase; LC-MS/MS, liquid chromatography-tandem MS; MALDI–TOF MS, matrix-assisted laser-desorption ionization–time-of-flight MS
INTRODUCTION
The GSTs (glutathione S-transferases; EC 2.5.1.18) comprise a family of mostly cytosolic, dimeric enzymes that are widely distributed in all mammalian cell types. Based on their structures, the GSTs are placed into alpha, kappa, mu, omega, pi, sigma, theta and zeta classes [1–4]. The GSTs catalyse the nucleophilic addition of reduced glutathione (GSH) to electrophilic centres of heterogeneous compounds, both xenobiotic and metabolically generated, facilitating their elimination from the cell [5,6]. The GSTs are also thought to function non-enzymically by binding non-substrate ligands and reactive compounds, leading to enhanced elimination of these molecules [1,7].
More recently, a third role has been suggested for GSTs. Mouse fibroblasts were known to contain an inhibitor of JNK (c-Jun N-terminal kinase), and, when the inhibitor was purified, it was shown to be GSTP1 (GST pi). Despite the fact that GSTs have been characterized as dimeric proteins, it appeared that JNK was binding to a single subunit or monomeric form of GSTP1 [8]. Subsequent studies have shown that GSTP1 acts as a stress-response protein, which multimerizes through disulphide cross-links following oxidative stress (e.g. UV irradiation), and it was proposed that by multimerizing, it loses its ability to bind JNK, causing an increase in JNK activity [9]. Also, mouse hepatocytes and fibroblasts lacking GSTP1 have higher JNK activity and higher proliferative rates [10,11]. The increase in proliferation is due to activation of both JNK and STAT (signal transducer and activator of transcription) pathways [12]. Finally, the C-terminal region of JNK is known to bind to human GSTP1 in vitro [13]. All of these studies support a role for GSTP1 in the regulation of JNK activity. Another form of GST, mu, may also be involved in regulating signal transduction through direct protein–protein interaction. Mouse GSTM1 (GST mu) is an inhibitor of ASK (apoptosis signal-regulating kinase) 1 by binding to ASK1 within the C-terminal portion of GSTM1 [14]. Therefore the GSTs, through direct interactions with ASK and JNK, and other factors [15], may not only regulate cell proliferation but may also regulate apoptosis [16,17].
Although GSTP1 is expressed in many adult cell types, GSTP1 is a foetal enzyme in rat and human hepatocytes, because it is not expressed in adult, differentiated hepatocytes [3]. The expression levels of GSTP1 are increased in a variety of malignant tumours, including renal and bladder cancers, malignant tumours of the gastrointestinal tract and lung, squamous cell carcinoma and ovarian tumours, and cancers from other tissues, findings that are consistent with the suggestion that expression of GSTP1 is a marker of de-differentiation [18–22]. For these reasons, GSTP1 has been advocated as a tumour marker, and, because of its increased activity, it has been implicated in the development of drug-resistant tumours [21,23–25].
Although studies with mouse fibroblasts suggest that JNK binds to constitutively present monomeric GSTP1 [8], studies with human cells are not clear about the presence of monomers within the cytosol. A study of cultured human cells (gastric cancer cell line Kato III and human colon cancer cell line M7609) and human platelets failed to find monomeric GSTP1 in cytosol by gel filtration and ELISA. However, GSTP1 monomers were found in the medium from the cultured cells and in medium after platelets were activated [26]. The source of the monomer in the medium was unclear, given its absence in the cytosol. Therefore the data from mice suggest that the GSTP1 monomer is an important regulator of JNK activity, whereas proof is lacking that GSTP1 has the same function in human cells.
In the present study, we looked for monomeric GSTP1 in human gastric cancer cells (Kato III) and in human erythrocytes using gel filtration, ELISA and Western blotting. MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS was used to characterize GSTP1 monomers. Cytosol and medium from Kato III cells, and cytosol of erythrocytes, were found to contain both monomeric and dimeric GSTP1. The monomer in both Kato III cells and in erythrocytes was post-translationally modified, and one of the modifications present in Kato III, but not erythrocyte GSTP1, was phosphorylation of a serine residue at the site on the subunit where JNK binds.
MATERIALS AND METHODS
Materials
Human gastric carcinoma cell line Kato III was obtained from the A.T.C.C. (Manassas, VA, U.S.A.). Reagents were from Sigma–Aldrich (St. Louis, MO, U.S.A.), Fisher Scientific (Houston, TX, U.S.A.) and VWR Scientific (Willard, OH, U.S.A.). Biogel P30 and Biospin 30 protein-desalting columns were from Bio-Rad (Richmond, CA, U.S.A.); Centricon and Microcon spin columns were from Amicon (Beverley, MA, U.S.A.). A Western blot antiserum to human GSTP1 and the ELISA for human GSTP1 were purchased from Biotrin (Dublin, Ireland), and a separate antiserum to human GSTP1 was purchased from Dako Cytomation (Carpinteria, CA, U.S.A.). Human GSTP1 was expressed in bacteria from cloned cDNA and purified as described previously [27].
Cell culture and erythrocytes
Kato III cells were maintained in Iscove's MDM (modified Dulbecco's medium) containing penicillin, streptomycin, glutamine and 10% (v/v) foetal bovine serum in air with 5% CO2 at 37 °C, with periodic scraping, pelleting (500 g for 5 min) and resuspension into fresh medium. Since the cells exhibit clumping and partial attachment to the substrate, they were trypsinized and replated 1 day before an experiment to achieve greater uniformity. Erythrocytes were obtained from two of the authors through a protocol that was approved by the Human Subjects Protection Program of the University of Arizona. Blood was collected by veni-puncture into tubes that contained 0.105 M buffered sodium citrate and centrifuged at 1200 g for 15 min to pellet the erythrocytes. Plasma and the buffy coat were discarded, and the erythrocytes were filtered through cotton wool to remove remaining white blood cells. Erythrocytes were washed four times with 6 vol. of ice-cold 10 mM potassium phosphate buffer with 140 mM NaCl (pH 7.0), and then lysed with 6 vol. of ice-cold 5 mM potassium phosphate buffer with 1.4 mM 2-mercaptoethanol (pH 7.0), and centrifuged for 1 h at 100000 g. The supernatant was dialysed against 22 mM potassium phosphate buffer with 1.4 mM 2-mercaptoethanol (pH 7.0) at 4 °C [28]. The dialysate was concentrated over a 5000 Da nominal molecular mass cut-off ultrafiltration membrane (PBCC, Amicon/Millipore Corporation) under nitrogen and diafiltered into 50 mM sodium phosphate buffer, 150 mM NaCl, 1 mM DTT (dithiothreitol) (pH 7.0) for gelfiltration chromatography.
Purification of GSTP1 by GSH-affinity chromatography
Kato III cells were grown in 7 ml of medium for either 6 or 16 h, and cells were collected by scraping, rinsed three times with PBS (10 mM phosphate buffer and 140 mM NaCl, pH 7.4), and homogenized in ice-cold PBS containing 2 mM EDTA and 5 mM DTT. All of the following steps were performed at 4 °C. The homogenate was centrifuged at 12000 g for 15 min, followed by centrifugation at 100000 g for 1 h. The Kato cell CM (conditioned medium) was centrifuged at 100 g for 6 min to remove cell debris, and the supernatant was centrifuged at 12000 g for 15 min. Cytosol, CM or erythrocyte cytosol was adjusted to 20 mM sodium phosphate buffer, 2 mM EDTA and 5 mM DTT (pH 7.4) and applied to a 1–2 ml GSH–Sepharose affinity column, washed with 5 column vol. of buffer (20 mM sodium phosphate buffer, 140 mM NaCl, 2 mM EDTA and 5 mM DTT, pH 7.4) and eluted with 2 column vol. of elution buffer (50 mM Tris/HCl and 10 mM GSH, pH 8.2). The eluates were concentrated 10–20-fold with Centricon microconcentrators. For MS, concentrated GST was exchanged into 20 mM sodium phosphate buffer, 50 mM NaCl, 2 mM EDTA and 5 mM DTT (pH 7.4).
Gel electrophoresis and Western Blots
Proteins were resolved on 12% polyacrylamide gels (with or without SDS) in 25 mM Tris/HCl buffer plus 250 mM glycine (pH 8.3). After electrophoresis, the gel was either stained with AgNO3 (SilverQuest Ag staining kit; Invitrogen, Carlsbad, CA, U.S.A.) or electroblotted on to Immunoblot PVDF membrane (Bio-Rad) using the above buffer plus 10% methanol. Western blots were performed with rabbit anti-GSTP1 primary antiserum (1:2000; Biotrin or Dako) and anti-rabbit donkey antibody conjugated to horseradish peroxidase (1:2000; Amersham Biosciences, Piscataway, NJ, U.S.A.) with ECL® (enhanced chemiluminescence) detection (Amersham Biosciences).
Gel-filtration chromatography
Gel filtration was performed with a Superdex 75 HR 10/30 column on an ÄktaPurifier chromatography system (Amersham Biosciences) at 0.5 ml/min in 50 mM sodium phosphate buffer, 150 mM NaCl and 1 mM DTT (pH 7.0) at 4 °C. A mixture of BSA, ovalbumin, chymotrypsinogen A and RNase A (Amersham Biosciences) in running buffer was used as molecular-mass standards. Fractions were assayed for GSTP1 by anti-(human GSTP1) ELISA and by Western blotting, and for GSTP1 enzymic activity (see below).
MALDI–TOF MS
MALDI–TOF MS of GSH-affinity-purified GST was performed by the Proteomics Core Facility at the University of Arizona on a MALDI–TOF L/R Mass Spectrometer (Waters Corporation, Milford, MA, U.S.A.) in the positive-ion linear mode using 50–75% of laser intensity as required. The GSTP1 protein, dissolved in 0.1% trifluoroacetic acid to a final concentration of 10–20 μM, was mixed with matrix (sinapinic acid) at a molar ratio of either 1:1000 or 1:10000, and spotted on a sample plate. Spectra were acquired with a 337 nm N2 laser at a firing rate of 5 Hz with a 2 ns sampling period; ten shots per spectrum were accumulated. The data were analysed with MassLynx software (Micromass).
LC-MS/MS (liquid chromatography-tandem MS)
LC-MS/MS of peptides from trypsin or chymotrypsin digests [29] of human GSTP1 that was purified from Kato III cells or from erythrocytes by GSH-affinity chromatography or of digests of human GSTP1 in bands excised from SDS gels was performed by the Proteomics Core Facility at the University of Arizona. Extracted peptides were analysed in a ThermoFinnigan LCQ Classic quadrupole ion-trap mass spectrometer (San Jose, CA, U.S.A.) with a nanospray ion source (University of Washington, Seattle, WA, U.S.A.) and a Michrom MAGIC2002 HPLC (Auburn, CA, U.S.A.). LC-MS/MS spectra of peptides were analysed with TurboSequest (Thermo Electron Corporation) to assign peptide sequences. TurboSequest analyses were performed against the non-redundant and human GST protein databases for modifications such as O-phosphorylation of serine/threonine/tyrosine residues and for the loss of H3PO4, which is a signature when a parent ion containing phosphoserine or phosphothreonine undergoes fragmentation in the spectrometer. Sequences were verified by correlating the experimentally obtained masses with those predicted theoretically from the sequence.
GST enzyme assays
GST enzyme assays were performed in 100 mM potassium phosphate buffer (pH 6.5), 1 mM EDTA, 1 mM chlorodinitrobenzene and 1 mM GSH in a final volume of 1 ml at room temperature (24 °C), as described previously [30]. Reactions were initiated by addition of enzyme, and the initial velocity was measured by following the increase in A340 over time.
RESULTS
Purification and identification of intracellular GSTP1
To identify monomeric GSTP1, we first performed Western blotting of the cytosol from the Kato III cells after native gel electrophoresis. Although dimeric GSTP1 in the cytosol was readily identified, two Western blotting antisera to GSTP1 were unable to identify monomeric GSTP1 in the absence of a denaturing agent. After non-reducing SDS/PAGE, both monomeric and dimeric forms of GSTP1 were identified by Western blotting (Figure 1).
Figure 1. Western blot identification of GSTP1 in Kato III cell cytosol.
(a) Native 12% polyacrylamide gel. (b) SDS/12% polyacrylamide gel.
Gel filtration and ELISA of Kato III medium and cytosol
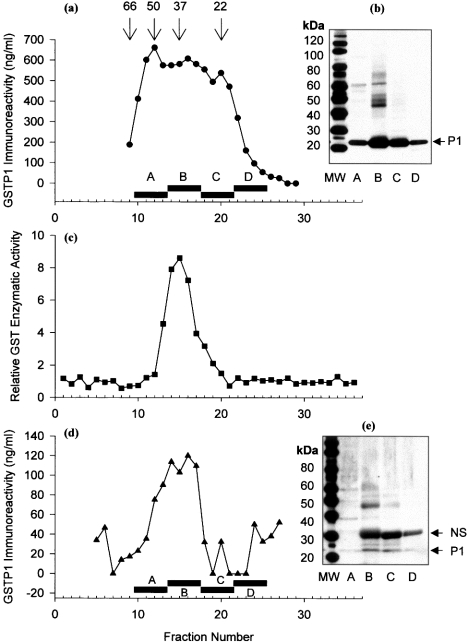
Proteins in cytosol from Kato III cells were fractionated by gel filtration, and the fractions were assayed for GST enzymic activity and for GSTP1 protein immunoreactivity by ELISA. Enzymic activity was present in fractions 13–17, with a peak at fraction 15 (retention time of 20 min), while immunoreactive protein was present in a wider range of fractions (9–23), with several apparent peaks (Figure 2a). The enzymic activity of GSTP1 from Kato III cells corresponded only to protein that was eluted with a molecular mass of that of the dimer (Figure 2c). Human GSTP1 expressed and GSH-affinity-purified from bacteria was also eluted with a single peak of enzymic activity in fraction 15 (results not shown). To prove that there was in fact GSTP1 in the additional fractions indicated by ELISA, we pooled fractions 9–12 (pool A), 13–16 (pool B), 17–20 (pool C) and 21–25 (pool D), and performed Western blotting on proteins in the pooled fractions after SDS/PAGE (Figure 2b). Immunoreactive GSTP1 was present in all of the pools, and corresponded with results from ELISA. Of note, pools A–C contained non-covalently associated GSTP1 subunits (23.5 kDa) and higher-molecular mass covalently cross-linked GSTP1 subunits, whereas pool D, which corresponded to the elution position of the monomer, contained only 23.5 kDa subunits with no evidence of covalent cross-linking.
Figure 2. Monomeric and dimeric GSTP1 in cytosol of Kato III cells and erythrocytes.
(a) GSTP1 in Kato III cell cytosol was fractionated by gel filtration and identified by ELISA. Numbers above arrows show retention times of molecular-mass markers (kDa) and bars show pooled fractions for Western blot. (b) Kato III proteins in gel filtration fractions were pooled and precipitated, run on a 4–12% gel by SDS/PAGE, transferred on to a PVDF membrane and identified with antiserum to GSTP1; the arrow indicates GSTP1 band. MW, molecular mass (in kDa) (c) Enzymic activity in cytosol of Kato III cell GST fractionated by gel filtration. (d) GSTP1 in erythrocyte cytosol was fractionated by gel filtration and identified by ELISA. (e) Erythrocyte proteins in gel-filtration fractions were pooled and precipitated, run on a 4–12% gel by SDS/PAGE, transferred on to a PVDF membrane, and identified with antiserum to GSTP1. The arrow shows monomer of GSTP1; NS, non-specific band. MW, molecular mass (in kDa). Higher-molecular-mass immunoreactive bands in fraction pool B in both (b) and (e) probably represent covalently cross-linked forms of GSTP1.
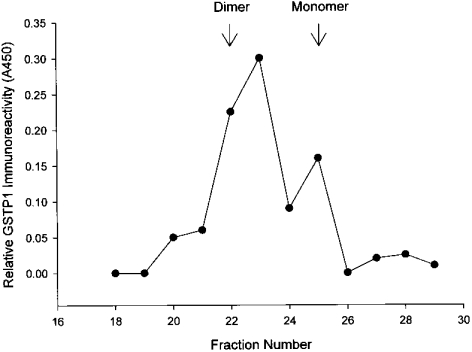
We examined CM from the Kato III cells because of a previous report that monomeric GSTP1 was secreted from Kato III cells [26]. Concentrated CM from Kato III cells was fractionated by gel filtration, and both dimeric and monomeric forms of human GSTP1 were identified in CM by ELISA (Figure 3). To characterize the molecular forms of GSTP1 in a normal human cell type, human erythrocyte cytosol was fractionated by gel filtration, and multimeric, dimeric and monomeric forms of human GSTP1 were identified (Figures 2d and 2e).
Figure 3. Monomeric and dimeric GSTP1 in CM from Kato III cells.
CM was concentrated and fractionated by gel filtration, and GSTP1 was identified in fractions by ELISA. Arrows show retention times of molecular-mass standards that correspond to monomeric and dimeric GSTP1.
Characterization of intracellular and extracellular GSTP1 from Kato III cells
GSTs in Kato III cell cytosol and CM were purified by GSH-affinity chromatography. The eluted proteins were resolved by non-reducing SDS/12% PAGE and stained with AgNO3. Bands corresponding to the monomeric GSTP1 subunit were excised from the gel and were subjected to in-gel trypsin and chymotrypsin digestion, followed by LC-MS/MS. A Sequest comparison was made between the human GSTP1 sequences from the human protein database with the sequences from the LC-MS/MS spectra. A protein sequence of mass 23356 amu (atomic mass units) (average) with a 94.5% match with GSTP1 confirmed the identity of GSTP1 in Kato III cytosol [31].
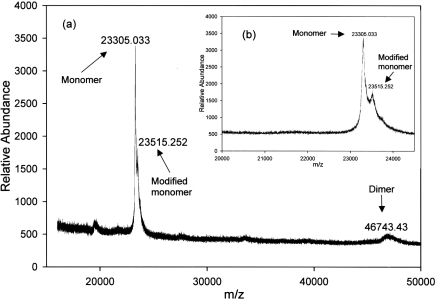
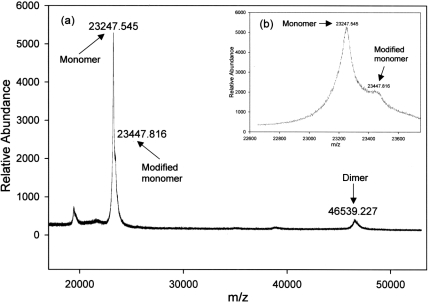
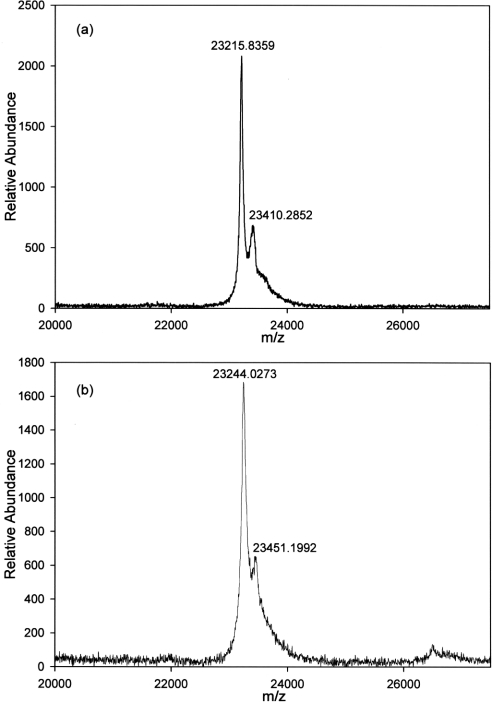
It is important to note that MALDI–TOF MS was performed under denaturing conditions, and GST dimers are dissociated into monomers to an unknown extent. Also, peak heights or areas do not correlate with the amount of a given species present. MALDI–TOF MS of GSTP1 that was GSH-affinity-purified from Kato III CM identified two molecular species, one with a mass of 23305 amu, and the other with a mass of 46743 amu (Figure 4). The peak at 46743 amu is consistent with the mass of dimeric GSTP1. There was no evidence using MALDI–TOF MS of other classes of GST, most of which have molecular masses greater than 25000 amu [32]. MALDI–TOF MS of GSH-affinity-purified GSTP1 from Kato III cytosol also identified two prominent species, one with a mass of 23248 amu and another with a mass of 46539 amu (Figure 5). The calculated mass of monomeric GSTP1 is 23224 amu [32]. Therefore the values of 23305 and 23248 amu measured using MALDI–TOF are very similar to the calculated value. The monomer peaks from both cytosol and CM were heterogeneous, with second peaks that differed from the primary peak by 200–210 amu (Figures 4 and 5).
Figure 4. MALDI–TOF MS spectrum of GSTP1 from Kato III cell CM.
(a) GSTP1 from cytosol of Kato III cells affinity-purified on GSH–agarose. (b) Enlarged spectrum showing two forms of monomer at 23305 and 23515 amu.
Figure 5. MALDI/TOF MS spectrum of GSTP1 from Kato III cell cytosol.
(a) GSTP1 from CM of Kato III cells affinity-purified on GSH–agarose. (b) Enlargement of spectrum shown in (a) showing two forms of monomer at 23248 and 23448 amu.
MALDI–TOF MS analysis of GSTP1 purified from erythrocytes and of cloned GSTP1
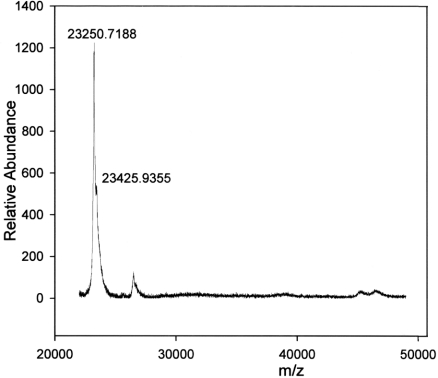
We were interested in whether the heterogeneity of GSTP1 subunits, seen with material purified from Kato III cells, was present in normal human cells and in cloned human GSTP1 expressed in bacteria. LC-MS/MS sequence analysis of GST purified from erythrocytes confirmed the identity of GSTP1 (results not shown). MALDI–TOF MS of GSTP1 purified from erythrocytes showed individual variation in GSTP1 subunits from different donor subjects, with a mass of 23216 amu in one and a mass of 23244 amu in the other. The variation is most likely due to a known amino acid polymorphism at A113V (Ala113→Val) that results in a mass difference of 28 amu [33]. Both samples also showed heterogeneity, with secondary peaks at 23410 amu (a difference of 194 amu) and at 23451 amu (a difference of 207 amu) respectively (Figure 6). Dimeric GSTP1 peaks were also different between subjects, with masses of 46413 and 46382 amu respectively (results not shown). Cloned GSTP1 subunits were heterogeneous, with peaks at 23251 amu and at 23426 amu (a difference of 175 amu) (Figure 7).
Figure 6. MALDI–TOF MS spectra of GSTP1 purified by GSH–agarose chromatography from erythrocytes of two different blood donors (a, b).
Figure 7. MALDI–TOF MS spectrum of human GSTP1 expressed in bacteria and affinity-purified on GSH–agarose.
LC-MS/MS analysis of GSTP1 purified from Kato III cells and erythrocytes
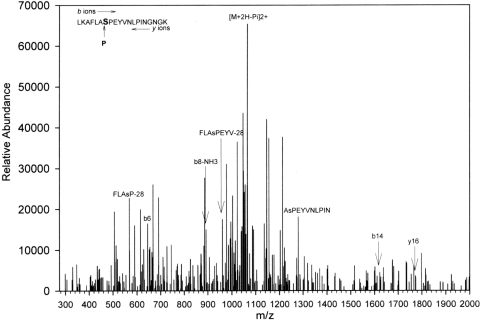
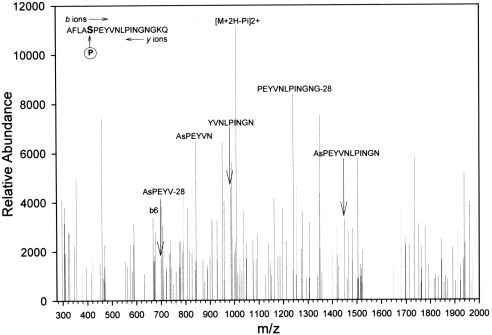
The findings from MALDI–TOF MS suggested that GSTP1 in Kato III cells was post-translationally modified. Fragments from the enzymic digests of the purified protein were subjected to LC-MS/MS to identify modifications. GSTP1 was purified from Kato III CM, and 133 amino acids matched those of human GSTP1 (64% sequence coverage). The C-terminal domain of GSTP1 has been implicated in JNK binding [13], and we therefore scanned this region for possible phosphorylation using Salsa [31] and identified a peptide, LKAFLASPEYVNLPINGNGKQ, that was phosphorylated. The spectrum, shown in Figure 8, revealed seven internal fragment ions in the 20-residue region that are specific for the phosphopeptide, and Ser196 was identified as the phosphate acceptor. This conclusion is supported further by the strong signal from the product ion [M+2H−H3PO4]2+ signal, which resulted from the loss of phosphoric acid as a neutral fragment from the doubly charged precursor. LC-MS/MS analysis of GSTP1 purified from the cytosol of the Kato III cells identified the same Ser196 phosphorylation modification (Figure 9). There also was a strong signal from the product ion [M+2H−H3PO4]2+, due to the loss of phosphoric acid. However, LC-MS/MS analysis of GSTP1 purified from erythrocytes identified no phosphorylation of Ser196 in samples from either donor subject (results not shown).
Figure 8. LC-MS/MS spectrum of the [M+H]+ and [M+2H]2+ ions of the phosphopeptide LKAFLASPEYVNLPINGNGK (mass=2226.49) of GSTP1 from Kato III cell CM.
Only the positively identified b and y ions that are also specific for the phosphoserine are labelled. Other ions, although positively identified, are omitted for clarity. Symbol NH3 indicates loss of NH3 moiety from the product ion. The complete sequence of the peptide was obtained. The first lysine residue persists due to missed cleavage, which occurs when two lysine residues are nearby. amu values are mass-averaged. Charge state is +1 for all ions, except for product ion, which is +2.
Figure 9. LC-MS/MS spectrum of the [M+H]+ and [M+2H]2+ ions of the phosphopeptide AFLAsPEYVNLPINGNGK (mass=2113.29) of GSTP1 from Kato III cell cytosol.
Only the positively identified b and y ions that are also specific for the phosphoserine are labelled. Other ions, although positively identified, are omitted for clarity. Symbol NH3 indicates loss of NH3 moiety from the product ion. The complete sequence of the peptide was obtained. amu are mass-averaged. Charge state is +1 for all ions, except for product ion, which is +2.
DISCUSSION
Results from a number of studies have suggested that GSTP1 is an important inhibitor of JNK activity and may function as a regulator of cell proliferation and apoptosis [11,16,17]. The ability of GSTP1 to function as an inhibitor of JNK appears to be dependent upon the presence of GSTP1 monomer in the cytosol of cells [8]. However, previous work had suggested that monomer, although present in rodent cells, was not present within human cells, raising the question of whether or not GSTP1 is a regulator of JNK activity in human cells [26]. Therefore we looked for GSTP1 monomer in a human cell line both intracellularly and extracellularly using gel filtration, ELISA and Western blotting. It was not possible to identify monomers in cell extracts using Western blotting and non-denaturing gels, because either several Western blot antisera to GSTP1 do not recognize the native monomer or the amount of monomer was below the limits of detection. We conclude that the antisera do not recognize native monomer because the ELISA assay, which uses a different antiserum from those used for Western blotting, demonstrates that monomeric GSTP1 is present in cytosolic extracts of Kato III cells and exists as a significant fraction of the total immunoreactivity (see Figure 2a). This finding is similar to a previous report in which dimers, but not monomers, of prostate-specific membrane antigen were recognized by a monoclonal antibody [34]. However, when GSTP1 was denatured, the antisera readily recognized monomers (Figure 1). The presence of monomers in the non-reduced SDS gels does not, however, prove that monomers are present in cells, because SDS dissociates dimeric GSTP1 into monomers. This appears to be the case, at least in part, because most of the dimers seen by native gel electrophoresis were lost in the presence of SDS alone (Figure 1). Using gel filtration, we identified native GSTP1 monomers, dimers and covalently, as well as non-covalently, associated dimers in the cytosol and medium of Kato III cells, and monomers and dimers in the cytosol of human erythrocytes. Therefore both normal and malignant human cells have GSTP1 monomers and dimers. It is unclear why previous investigators did not detect monomers within the cytosol of Kato III cells when they could detect it in the medium of these cells [26]. They used different antibodies for their ELISA from the ones we used in the present study, and it is possible that assay conditions were such that only monomers were seen in medium and only dimers seen in cytosol. As reported previously, the monomeric form is enzymically inactive, and only dimeric GSTP1 has enzymic activity [26].
In addition to identifying monomers of GSTP1 in Kato III cells and in erythrocytes, we also observed heterogeneity of monomers by MALDI–TOF MS. The principal peak obtained by MALDI–TOF MS gave a molecular mass for the monomer that was the same as predicted by sequence analysis. We therefore believe the heterogeneity represented post-translational modifications of the protein. To test this idea, we examined cloned human GSTP1 enzyme expressed in bacteria for heterogeneity using MALDI–TOF MS. We had sequenced the cloned protein previously, and a significant portion of the protein contained an N-terminal methionine (molecular mass of 131 amu). In addition, a portion was also N-formylated (29 amu). We therefore expected MALDI–TOF MS to show two peaks with a mass difference of 160 amu. The cloned protein was heterogeneous by MALDI–TOF MS, and the second peak was 175 amu greater than the first peak. Therefore the subunit heterogeneity in cloned GSTP1 corresponds with known modifications of the bacterially expressed protein.
MALDI–TOF MS identified unmodified GSTP1 subunits in Kato III cells and normal human erythrocytes, based on the similarity of the masses of the subunits to the calculated mass of GSTP1. Secondary peaks with masses that were 194–210 amu larger than that of the unmodified subunit were identified in GSTP1 subunits from both the CM and cytosol of Kato III cells and in human erythrocytes. To identify the post-translational modifications that account for the mass differences, we examined proteolytic fragments of GSTP1 from Kato III cells for modifications with LC-MS/MS. We first compared the amino acid sequences obtained with the known sequence of GSTP1, and confirmed that we were analysing only GSTP1. We then looked for phosphorylation of the protein near its C-terminus, because other studies suggest that JNK binds to the C-terminus of mouse GSTP1 [35]. We found that Ser196 was phosphorylated in GSTP1 purified from either Kato III cell cytosol or CM. A serine residue followed by a proline or threonine is the preferred phosphorylation motif for MAPKs (mitogen-activated protein kinases) such as JNK, and, in human GSTP1, Ser196 is followed by a proline residue. Therefore, since JNK binds to this region of GSTP1, it is possible that it phosphorylates Ser196. However, Ser196 in GSTP1 purified from erythrocytes was not phosphorylated. Whether phosphorylation of Ser196 is unique to malignant cells or occurs in other human cell types is unknown.
There are known genetic polymorphisms of GSTP1, including A113V, with a difference of 28 amu, and I104V (Ile104→Val), with a difference of 14 amu [33]. These differences alone cannot account for the observed 194–210 amu subunit mass differences observed between the modified and unmodified GSTP1 subunits, and therefore other unidentified post-translational modifications must be present. The addition of a single phosphorylated serine residue (the mass of PO3 is 80 amu) to GSTP1 also cannot account for the difference in mass between subunits. Even if a second amino acid is phosphorylated, the difference would be 160 amu, which is less that the observed difference. More importantly, the 194–210 amu differences occur in erythrocyte GSTP1 subunits that are not phosphorylated. Therefore there are additional post-translational modifications to GSTP1 that have not been identified. Previous reports have suggested that GSTP1 purified from human malignant cell lines is glycosylated [36], or that a fatty acid may be bound to the protein [37]. In the latter report, they also had evidence for the phosphorylation of both the serine and threonine residues of GSTP1. However, we found no evidence that GSTP1 either was glycosylated or had a fatty acid bound to it. The presence of a C18 fatty acid would increase the mass by 284 amu, greater than that observed by MALDI–TOF MS. In addition, the fatty acid was not covalently bound to the protein and therefore would not affect the mass as determined by MALDI–TOF MS. Previous reports found that the glycosylated form of GSTP1 had a pI of approx. 6.0 [36]. We performed isoelectric focusing/Western blotting and found the pI for GSTP1 from Kato III CM to be pH~5.0, and no other forms were seen with higher pI values (results not shown). In addition, the mass of each sugar residue is 180 amu, which cannot account for the mass difference observed by MALDI–TOF MS of 194–210 amu.
All of the post-translational modifications previously described for GSTP1 were observed in malignant cell lines. Work using GSTP1 purified from normal rat tissues has failed to show modification of the proteins [38]. Similarly, human GSTP1 purified from normal tissue does not appear to be modified [32]. However, it is unclear whether both monomer and dimer were obtained in the purification schemes used in the previous studies, and it is possible that, if only monomeric GSTP1 is modified, then it would not have been identified if it did not co-purify with dimeric GSTP1.
In addition to GSTP1, both mouse GSTM1 and human GSTA2 (GST alpha) also appear to regulate kinase activity [14,39]. Little is known about how GSTA2 inhibits kinase activity, but mouse GSTM1 dimer appears to be the inhibitor [14]. In addition, although it is the C-terminal portions of both GSTM1 and GSTP1 that interact with the kinases, there is little similarity between the amino acid sequences of the two enzymes, suggesting different mechanisms of interaction. Finally, GST enzymic activity appears to play no role in the interaction of GSTs with kinases, because a monomer is catalytically inactive, and only non-catalytic portions of either enzyme are required for inhibition of kinase activity [13,14]. Therefore both GSTM1 and GSTP1 influence the kinase cascade at different points, and may act in synergy during the stress response.
In conclusion, malignant and normal human cells contain GSTP1 monomers and dimers. Therefore GSTP1 may serve as a stress-response protein in humans in a manner similar to that described in rodents. In addition, GSTP1 is phosphorylated in the region of the protein where JNK binds, supporting the idea that the interaction between JNK and rodent and human forms of GSTP1 occurs in the C-terminal portion of the transferase [38]. Finally, human GSTP1 is post-translationally modified in ways that remain to be determined, but which may serve important regulatory functions in protein–protein interactions.
Acknowledgments
We are grateful to Mr L. T. Tucker for secretarial assistance and to Donna B. Webb for painless phlebotomy. This work was supported in part by grant numbers GM31555 (to T. D. B.) and ES06694 (to Dr G. Tsaprailis) from the National Institutes of Health (NIH), Bethesda, MD, U.S.A. We thank Dr G. Tsaprailis, Director, University of Arizona Proteomics Core Facility, and Andrea Hunt, for generating MS data, and for their advice.
References
- 1.Hayes J. D., Pulford D. J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. CRC Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 2.Mannervik B., Danielson U. H. Glutathione transferases – structure and catalytic activity. CRC Crit. Rev. Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- 3.Whalen R., Boyer T. D. Human glutathione S-transferases. Semin. Liver Dis. 1998;18:345–358. doi: 10.1055/s-2007-1007169. [DOI] [PubMed] [Google Scholar]
- 4.Board P. G., Coggan M., Chelvanayagam G., Easteal S., Jermiin L. S., Schulte G. K., Danley D. E., Hoth L. R., Griffor M. C., Kamath A. V., et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 5.Rushmore T. H., Pickett C. B. Glutathione S-transferases, structure, regulation, and therapeutic implications. J. Biol. Chem. 1993;268:11475–11478. [PubMed] [Google Scholar]
- 6.Hayes J. D., Strange R. C. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radical Res. 1995;22:193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 7.Boyer T. D., Vessey D. A., Holcomb C., Saley N. Studies of the relationship between the catalytic activity and binding of non-substrate ligands by the glutathione S-transferases. Biochem. J. 1984;217:179–185. doi: 10.1042/bj2170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler V., Yin Z., Fuchs S. Y., Benezra M., Rosario L., Tew K. D., Pincus M. R., Sardana M., Henderson C. J., Wolf C. R., et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tew K. D., Ronai Z. GST function in drug and stress response. Drug Resist. Updates. 1999;2:143–147. doi: 10.1054/drup.1999.0086. [DOI] [PubMed] [Google Scholar]
- 10.Ruscoe J. E., Rosario L. A., Wang T., Gate L., Arifoglu P., Wolf C. R., Henderson C. J., Ronai Z., Tew K. D. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J. Pharmacol. Exp. Ther. 2001;298:339–345. [PubMed] [Google Scholar]
- 11.Elsby R., Kitteringham N. R., Goldring C. E., Lovatt C. A., Chamberlain M., Henderson C. J., Wolf C. R., Park B. K. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J. Biol. Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 12.Gate L., Majumdar R. S., Lunk A., Tew K. D. Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J. Biol. Chem. 2004;279:8608–8616. doi: 10.1074/jbc.M308613200. [DOI] [PubMed] [Google Scholar]
- 13.Wang T., Arifoglu P., Ronai Z., Tew K. D. Glutathione S-transferase P1–1 (GSTP1–1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001;276:20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 14.Cho S. G., Lee Y. H., Park H. S., Ryoo K., Kang K. W., Park J., Eom S. J., Kim M. J., Chang T. S., Choi S. Y., et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 15.Cumming R. C., Lightfoot J., Beard K., Youssoufian H., O'Brien P. J., Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat. Med. 2001;7:814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 16.Gilot D., Loyer P., Corlu A., Glaise D., Lagadic-Gossmann D., Atfi A., Morel F., Ichijo H., Guguen-Guillouzo C. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J. Biol. Chem. 2002;277:49220–49229. doi: 10.1074/jbc.M207325200. [DOI] [PubMed] [Google Scholar]
- 17.Bernardini S., Bernassola F., Cortese C., Ballerini S., Melino G., Motti C., Bellincampi L., Iori R., Federici G. Modulation of GST P1-1 activity by polymerization during apoptosis. J. Cell. Biochem. 2000;77:645–653. [PubMed] [Google Scholar]
- 18.Inoue T., Ishida T., Sugio K., Maehara Y., Sugimachi K. Glutathione S transferase Pi is a powerful indicator in chemotherapy of human lung squamous-cell carcinoma. Respiration. 1995;62:223–227. doi: 10.1159/000196451. [DOI] [PubMed] [Google Scholar]
- 19.Green J. A., Robertson L. J., Clark A. H. Glutathione S-transferase expression in benign and malignant ovarian tumours. Br. J. Cancer. 1993;68:235–239. doi: 10.1038/bjc.1993.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv. Cancer Res. 1989;52:205–255. doi: 10.1016/s0065-230x(08)60214-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Pavelic Z. P., Li Y., Gleich L., Gartside P. S., Pavelic L., Gluckman J. L., Stambrook P. J. Overexpression and amplification of glutathione S-transferase pi gene in head and neck squamous cell carcinomas. Clin. Cancer Res. 1997;3:111–114. [PubMed] [Google Scholar]
- 22.Satta T., Isobe K., Yamauchi M., Nakashima I., Takagi H. Expression of MDR1 and glutathione S transferase-pi genes and chemosensitivities in human gastrointestinal cancer. Cancer. 1992;69:941–946. doi: 10.1002/1097-0142(19920215)69:4<941::aid-cncr2820690418>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat. Rep. 1985;69:677–682. [PubMed] [Google Scholar]
- 24.Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 25.Ricci G., Caccuri A. M., Lo Bello M., Parker M. W., Nuccetelli M., Turella P., Stella L., Di Iorio E. E., Federici G. Glutathione transferase P1-1: self-preservation of an anti-cancer enzyme. Biochem. J. 2003;376:71–76. doi: 10.1042/BJ20030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kura T., Takahashi Y., Takayama T., Ban N., Saito T., Kuga T., Niitsu Y. Glutathione S-transferase-pi is secreted as a monomer into human plasma by platelets and tumor cells. Biochim. Biophys. Acta. 1996;1292:317–323. doi: 10.1016/0167-4838(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 27.Whalen R., Kempner E. S., Boyer T. D. Structural studies of a human pi class glutathione S-transferase: photoaffinity labeling of the active site and target size analysis. Biochem. Pharmacol. 1996;52:281–288. doi: 10.1016/0006-2952(96)00205-5. [DOI] [PubMed] [Google Scholar]
- 28.Awasthi Y. C., Singh S. V. Purification and characterization of a new form of glutathione S-transferase from human erythrocytes. Biochem. Biophys. Res. Commun. 1984;125:1053–1060. doi: 10.1016/0006-291x(84)91390-1. [DOI] [PubMed] [Google Scholar]
- 29.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 30.Hoesch R. M., Boyer T. D. Purification and characterization of hepatic glutathione S-transferases of rhesus monkeys: a family of enzymes similar to the human hepatic glutathione S-transferases. Biochem. J. 1988;251:81–88. doi: 10.1042/bj2510081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yates J. R., III, Eng J. K., McCormack A. L., Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal. Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 32.Rowe J. D., Nieves E., Listowsky I. Subunit diversity and tissue distribution of human glutathione S-transferases: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem. J. 1997;325:481–486. doi: 10.1042/bj3250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X., Herzog C., Zimniak P., Singh S. V. Differential protection against benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced DNA damage in HepG2 cells stably transfected with allelic variants of pi class human glutathione S-transferase. Cancer Res. 1999;59:2358–2362. [PubMed] [Google Scholar]
- 34.Schulke N., Varlamova O. A., Donovan G. P., Ma D., Gardner J. P., Morrissey D. M., Arrigale R. R., Zhan C., Chodera A. J., Surowitz K. G., et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12590–12595. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaco R., Friedman F. K., Hyde M. J., Chen J. M., Manolatus S., Adler V., Ronai Z., Koslosky W., Pincus M. R. Identification of a glutathione-S-transferase effector domain for inhibition of jun kinase, by molecular dynamics. J. Protein Chem. 1999;18:859–866. doi: 10.1023/a:1020679229110. [DOI] [PubMed] [Google Scholar]
- 36.Kuzmich S., Vanderveer L. A., Tew K. D. Evidence for a glycoconjugate form of glutathione S-transferase pi. Int. J. Peptide Protein Res. 1991;37:565–571. doi: 10.1111/j.1399-3011.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 37.Bulavin D. V., Karpishchenko A. I., Gubanov A. I., Reshetov A. V. Glutathione S-transferase P1-1 in normal and cancerous lung tissue: properties, function, and possible mechanisms for regulating activity. Biochemistry (Moscow) 1996;61:1015–1027. [PubMed] [Google Scholar]
- 38.Hsieh C. H., Tsai S. P., Yeh H. I., Sheu T. C., Tam M. F. Mass spectrometric analysis of rat ovary and testis cytosolic glutathione S-transferases (GSTs): identification of a novel class-Alpha GST, rGSTA6*, in rat testis. Biochem. J. 1997;323:503–510. doi: 10.1042/bj3230503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y., Cheng J. Z., Singhal S. S., Saini M., Pandya U., Awasthi S., Awasthi Y. C. Role of glutathione S-transferases in protection against lipid peroxidation: overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J. Biol. Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]