Abstract
Background
Shared decision making (SDM) improves the likelihood that patients will receive care in a manner consistent with their priorities. To facilitate SDM, decision aids (DA) are commonly used, both to prepare a patient before their clinician visit, as well as to facilitate discussion during the visit. However, the relative efficacy of patient-focused or encounter-based DAs on SDM and patient outcomes remains largely unknown. We aim to directly estimate the comparative effectiveness of two DA’s on SDM observed in encounters to discuss stroke prevention strategies in patients with atrial fibrillation (AF).
Methods
The study aims to recruit 1200 adult patients with non-valvular AF who qualify for anticoagulation therapy, and their clinicians who manage stroke prevention strategies, in a 2×2 cluster randomized multi-center trial at six sites. Two DA’s were developed as interactive, online, non-linear tools: a patient decision aid (PDA) to be used by patients before the encounter, and an encounter decision aid (EDA) to be used by clinicians with their patients during the encounter. Patients will be randomized to PDA or usual care; clinicians will be randomized to EDA or usual care.
Results
Primary outcomes are quality of SDM, patient decision making, and patient knowledge. Secondary outcomes include anticoagulation choice, adherence, and clinical events.
Conclusion
This trial is the first randomized, head-to-head comparison of the effects of an EDA versus a PDA on SDM. Our results will help to inform future SDM interventions to improve patients’ AF outcomes and experiences with stroke prevention strategies.
Background
Atrial Fibrillation (AF) is the most common heart arrhythmia worldwide and puts patients at risk for having debilitating or fatal strokes.1–3 Oral anticoagulants are highly effective at reducing stroke and mortality in patients with non-valvular AF and additional stroke risk factors (assessed using the CHA2DS2-VASc risk stratification score).4–6 However, they remain highly under-utilized in practice.7–11 Up to 50% of patients prescribed an anticoagulant do not initiate therapy; an additional 30% to 50% discontinue usage within 1 year.10 The trade-off for taking anticoagulants is an increased risk of bleeding; with anticoagulants being the most common cause of adverse drug events resulting in emergency room visits or hospitalizations.12–14 Additionally, the choice of which anticoagulant to take is not straightforward. While warfarin is inexpensive and has been used since the 1950s, it has a narrow-therapeutic range. This range can be difficult to maintain and requires frequent monitoring, which can be burdensome to patients.15 However, direct oral anticoagulants (DOAC) have standardized dosing and no monitoring, but they can be unaffordable for many patients, particularly those without adequate drug coverage. Therefore, it is important for patients with AF to thoroughly consider the risks and benefits surrounding the initiation and choice of anticoagulant.
Shared decision making (SDM) is a purposeful collaborative conversation between patients and their clinicians regarding health care decisions, and is particularly useful when decisions are complex and are influenced by individual patient values.16, 17 Models of SDM stress clear communication to patients of the risks and benefits of all treatment options, including the option of no treatment, while allowing patients to share their treatment preferences, relevant values, and goals of care with clinicians. SDM yields both short-and long-term benefits; patients who actively participate in their medical decisions tend to be more satisfied with their care, potentially more adherent to initial treatment decisions, and report improved quality of life compared to those who do not.18–20 The latest American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) and European Society of Cardiology (ESC) guidelines for management of patients with AF recommend SDM for decisions surrounding anticoagulant use.11, 21 SDM is part of the “Atrial Fibrillation Better Care” pathway; a clinical management program recommended by the ESC for improving clinical outcomes in patients with AF.11, 22
Decision aids (DA) are tools intended to support patients and clinicians in SDM. They provide accurate balanced information in an organized easy to understand format, clarify patients’ values, and improve SDM skills.23 DAs have been shown to increase patient knowledge of diagnostic and treatment options, satisfaction with the decision-making process, involvement in SDM, concordance between patient preference and treatment received, and communication with clinicians.24 Additionally, DAs can improve patient decision making by reducing decisional conflict and uncertainty.24
There are currently two types of decision aids: (1) patient decision aids (PDA) that are used by the patient before meeting with their clinician to prepare them for their encounter, and (2) encounter decision aids (EDA) that are used during an encounter between the patient and their clinician. A large body of research supports the use of PDAs; furthermore, evidence showing the value of EDAs has increased in recent years.25–29 However, a recent Randomized Control Trial (RCT) evaluating the use of an EDA alone during patient visits to discuss anticoagulation for stroke prevention in AF failed to demonstrate a significant impact on patient decisions or knowledge transfer.30
To date there have been no studies exploring the effectiveness of combining a PDA and an EDA, nor any head-to-head studies of the effectiveness of a PDA vs an EDA. This comparison furthers the science of SDM, as it will be important for understanding if one method is better suited to support SDM. PDAs can help patients prepare for a visit by clarifying values and giving them more information, while allowing the patient to engage with the information how they would like. However, PDAs do not provide the clinician with any support during a visit. EDAs are used during the actual conversation between the patient and provider, but a lack of preparation may lead to less involvement from the patient. Additionally, different settings and workflows may affect the implementation of one DA or the other, depending on the situation, such as inability to give a PDA to a patient ahead of time, or clinicians not using an EDA due to concerns of adding time to a visit. By developing complementary DAs, we will be able to compare the two types of Das directly. The aim of the current study is to evaluate the effectiveness of two SDM tools, one PDA and one EDA, in combination and separately, in promoting high-quality SDM pertaining to OAC for at-risk patients with non-valvular AF.
Methods
Design
A cluster-randomized trial design with initial randomization of providers was selected to avoid possible contamination caused by providers switching between use and non-use of the EDA. Patients further will be independently randomized to use or non-use of the PDA. This randomization created 4 study arms: use of a PDA (Arm 1), use of an EDA (Arm 2), use of both DAs (Arm 3), and usual care using neither DA (control) (Arm 4). We will assess the comparative effectiveness of each arm in achieving SDM Figure. outlines the overall design and flow of the study. The study has been registered on clinicaltrials.gov (NCT04357288).
Figure 1.
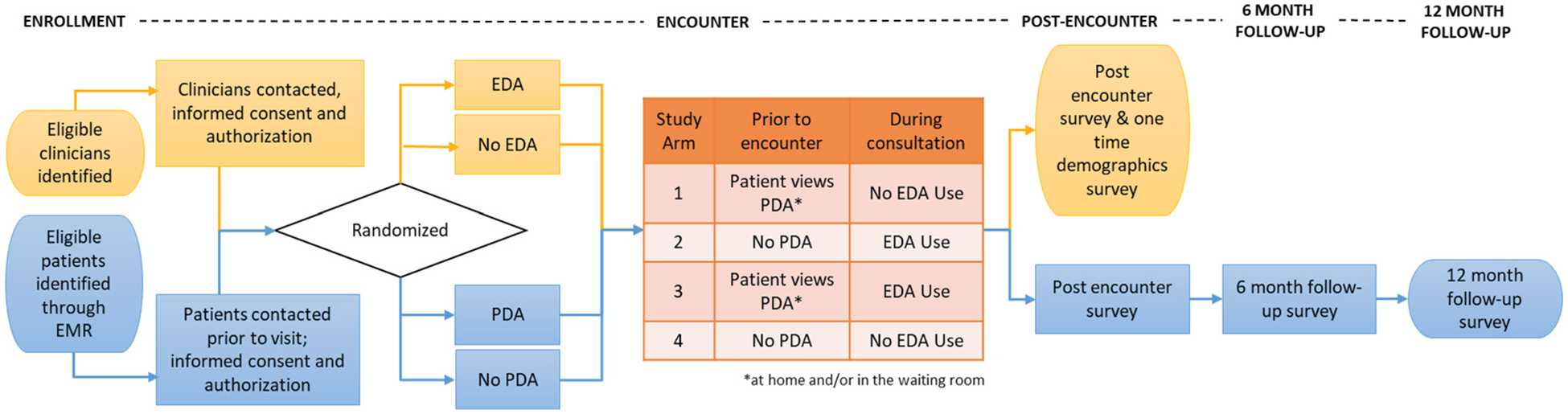
Outline of Randomized Evaluation of Decision Support Interventions for Atrial Fibrillation (RED-AF) methodology design. EDA, encounter decision aid; EMR, electronic medical records; PDA, patient decision aid.
Setting
This multi-center study will be conducted at six large academic centers in the United States: University of Utah, Mayo Clinic, Northwestern University, Vanderbilt University Medical Center, University of Alabama Birmingham, and University of Michigan. A variety of health care settings will be utilized, including but not limited to outpatient cardiology and electrophysiology clinics, emergency departments, inpatient services, and anticoagulation services.
Telehealth
Originally, study encounters were planned to be conducted entirely in-person. However, due to the COVID-19 pandemic, the protocol was updated to allow sites to use telehealth encounters as needed. Each site will utilize their health system’s telehealth capabilities and provide access to the EDA during a visit through screen sharing. Video/audio recording of telehealth encounters will be accomplished using HIPAA compliant video conferencing software (eg, Zoom, Microsoft Teams, Skype). The same pre-specified data elements and outcomes will be collected for both telehealth and in-person encounters.
Participants
Adult patients (18+) scheduled for a clinical appointment where stroke prevention strategies for AF are likely to be discussed will be invited to participate. In order to be eligible, patients need to have been diagnosed with AF, be aware of their diagnosis, and have at least one non-sex related additional risk factor for thromboembolic events (ie, CHA2DS2-VASc ≥1 for men, ≥2 for women). We will exclude those determined by their clinician to be ineligible for anticoagulation therapy. We will further exclude those unable to provide informed consent due to cognitive deficits, sensory input, or language that are significant enough to impede shared decision making and/or written informed consent. Any clinicians that manage or prescribe anticoagulation for these eligible patients with AF will be eligible for participation including post-graduate trainees, registered nurses, pharmacists, and advanced practice clinicians.
Cohorts
Two cohorts of patients will be recruited for study participation based on prior history of anticoagulation. The initiation cohort includes patients who have either: (a) no prior use of an anticoagulant; (b) are taking daily aspirin instead of an anticoagulant; or (c) have terminated anticoagulation usage (for any reason) more than 6 months prior to trial participation. Patients will be considered part of the initiation cohort within the first 60 days of starting an anticoagulant. The monitor cohort includes patients currently on anticoagulation, either warfarin or DOAC, for more than 60 days and at least one of the following: (a) experiencing issues with their current anticoagulation therapy (eg, adherence with international normalized ratio (INR) monitoring, perceived or actual side effects, ability to pay for medication, labile INR control); (b) emerging evidence supports reevaluation of prior relative contraindications to DOAC therapy (eg, apixaban use in renal dysfunction, obesity);31–34 or (c) changes in medical condition that may impact stroke prevention in AF (eg, declining renal function, new coronary stent). Anticoagulated patients who are newly establishing care at one of the sites may also qualify for the monitor group.
Recruitment and consent
Eligible patients will be approached, recruited, and consented: (a) in person, (b) via telephone, (c) via email or US mail, or (d) via telehealth prior to the encounter with their clinician.
Interventions and comparators
Patient decision aid
Patients randomized to the PDA arms will be asked to review the PDA prior to their encounter with a clinician. Patients who do not have access to the internet or have low digital health literacy are provided options to review the tool in a clinical setting with a study coordinator before their encounter. The PDA walks the patient through what AF is, what it means in their life, includes various risk calculators (CHA2DS2-VASc and HAS-BLED) to show individualized risk for stroke and bleeding events, compares differences between warfarin and DOACs (bleeding, medication routine, cost, drug and dietary interactions), and helps the patient plan for their clinician encounter. It is an interactive, non-linear online tool that allows patients to explore topics that interest them in the order they would like. There are two different pathways to present similar information, one for the initiation cohort that takes an introductory approach and one for the monitor cohort that takes a review approach. However, patients have autonomy on which pathway they choose. A report summarizing patients’ medication selections and questions to discuss during their clinician encounter is generated upon completion of the tool.
Encounter decision aid
Clinicians randomized to the EDA arm will use the EDA to augment stroke prevention discussions during the patient encounter. The EDA is an interactive online tool intended for use within patient-clinician decision making conversations. It can be shared with patients both during in-person or telehealth encounters (via screen sharing). The content and visual presentation of the EDA is similar to that of the PDA, but it does not include the introductory review describing AF, nor does it include the section summarizing questions or issues to discuss with the clinician that are a part of the PDA.
Combination PDA and EDA
Arm 3 will include patients randomized to the PDA and clinicians randomized to the EDA. Patients and clinicians will interact with the PDA and the EDA as described previously.
Usual care
Patients in Arm 4 will not use either the PDA or EDA. As DAs are not routinely used in AF clinical practice, the clinician will conduct the encounter per their standard of care. The EDA will not be available to these clinicians to minimize potential for contamination.
Randomization
Clinicians will be randomized to EDA use or non-use, and patients will be randomized to PDA use or non-use with equal allocation to the four study arms.35 Both patient and clinician randomization will be stratified by study site, and patients will additionally be stratified by CHA2DS2-VASc score (≥2 or < 2 in males and ≥3 or < 3 in females), and cohort (initiation or monitor). The chosen cut-off for CHA2DS2-VASc score is due to variation in the cut-point for initiating anticoagulation therapy for stroke prevention between different guidelines. Complete blinding of participants is not possible due to the nature of the interventions. Randomization to the EDA at the clinician level minimizes the risk of bias due to cross-contamination, such that each participating clinician either uses the EDA or not. Clinicians randomized to the EDA arm will be trained on how to use it.
Data collection
Encounters
When possible, video and/or audio recordings will be made of the study encounters. Telehealth encounters will capture screen recordings of the EDA when applicable. Participants can opt out of any or all recording of the encounter. Although the entire encounter will be recorded when possible, only the portion where the EDA is used or anticoagulation is discussed will be analyzed.
Surveys
Patient self-reported outcomes will be collected via online survey at the end of the initial encounter, and at 6- and 12-months follow-up surveys (Figure). Patient surveys will include questions about sociodemographics, and patient characteristics (Table I), and primary and secondary outcome measures (Tables II and III). Brief clinician outcome surveys will be collected at the end of each clinical encounter which collects the decision made concerning anticoagulation, clinician satisfaction (Table III), and who they feel had the most say in the decision, the patient, the referring clinician, or themselves. Clinicians will complete an additional survey detailing sociodemographics and practice characteristics during the study period.
Table I.
Patient characteristics and descriptions
| Patient characteristics | |
|---|---|
| Measure | Description |
| Demographics | Consists of age, highest education level, ethnicity, and race. |
| Health services and insurance | Consists of insurance coverage, location of primary healthcare (at study site or not), and driving time to regular healthcare location. |
| Health literacy36, 37 | Measured by two single-item health literacy screeners asking about needing help reading material from doctors or the pharmacy and confidence in filling out medical forms by themselves. |
| Numeracy38, 39 | Measured by two scales, it includes 6 items combined from a 3-item subjective numeracy scale and a modified 8-question subjective numeracy scale measuring people’s beliefs about their skill in math and preferences for numeracy presentation. |
| Digital literacy | Measures confidence one has in using computers, smartphones, or other electronic devices to accomplish the things patients need to do online. |
Table II.
Primary outcome domains and descriptions of outcome measures
| Primary outcomes | |
|---|---|
| Measure | Description |
| Encounter outcome | |
| Shared decision making | Degree of patient involvement in decision making measured using audiovisual recordings of the visits. The involvement of patients by the clinician will be assessed using the clinician version of the OPTION12 scale41, 42 The clinician version consists of 12 items scored from 0, “the behavior is not observed,” to 4, “the behavior is observed and executed to a high standard.” The 12 items are summed and converted to a 100-point scale. The scoring is done by trained observers watching the audio and/or video encounters at a later date. |
| Decision making outcomes | |
| Decisional conflict scale (DCS)43, 44 | Uncertainty in choosing an option as measured by 16 items scored on a 0–4 scale divided by 16 and multiplied by 25. Scale is from 0 to 100 where higher scores reflect more uncertainty. Scores lower than 25 are associated with carrying out a decision, and scores above 37.5 are associated with feeling unsure about making a decision or decision delay. |
| Knowledge outcome | |
| Patient knowledge | Consists of 7 questions about AF and anticoagulation designed by the study team. Each question uses a response format of “true/false/do not know.” All questions are answered with full access to applicable decision aids. If a patient answers at least 1 question, they will be assessed for this outcome with missing responses coded as incorrect. Questions are listed in the Appendix. |
Abbreviation: AF, atrial fibrillation.
Table III.
Secondary outcomes measures and their descriptions
| Secondary outcomes | |
|---|---|
| Measure | Description |
| Decision making outcomes | |
| Decision regret49 | Measured by asking patients to reflect on the decision they made about initiating anticoagulation and/or anticoagulant choice after their encounter with their clinician. The measure consists of 5 items assessed on a 5-point Likert scale from “Strongly agree” to “Strongly disagree.” Higher scores are indicative of higher regret. |
| Preparation for decision making scale50 | Assesses patients’ perspectives of decision aid usefulness in preparing them to communicate with their clinicians and for SDM with 10 questions measured on a 5-point Likert scale from “Not at all” to “A great deal.” Higher scores indicate feeling better prepared for decision making. |
| Quality of communication51 | Measured using the 3-item modified version of the CAHPS Clinician and Group survey.28 Determines the extent to which communication is patient-centered, contains technical information that is easily understood, and is respectful. Each individually reported item is assessed on a 3-point scale (Yes, definitely; Yes, somewhat; and No). The higher the score, the better quality of communication. |
| 9-Item Shared Decision-Making Questionnaire (SDMQ9)52 | Assesses quality of patient involvement in the process of decision making with their clinician from the perspective of the patient. Comprised of 9 statements measured on a 6-point scale from “Completely disagree” to “Completely agree.” Higher scores are associated with a greater extent of SDM. |
| Control preference scale53 | Assesses patients’ desire to participate in SDM with one question. Asks if the patient prefers to make the decision alone, with their clinician, or have their clinician decide using a 5-point scale. Adapted from the Degnar & Sloan’s Control Preference Scale.54 |
| Patient satisfaction with decision aid55, 56 | Consists of 3 questions that ask about patients’ likelihood to recommend the PDA (1) “Not at all” [likely] to (5) “Extremely” [likely], the amount of information in the PDA (1) “Too little” to (5) “Too much”), and if the PDA was biased toward a certain treatment outcome or medication (Warfarin, DOAC, No treatment, Balanced). Higher scores indicated a higher level of satisfaction. |
| Adapted illness intrusiveness ratings57, 58 | Assesses the extent to which disease (AF) and treatment interfere with meaningful daily living among those affected by chronic disease using a modified version of the Illness Intrusiveness Ratings scale. It consists of 13 questions asking how much AF or stroke prevent treatments affects the patient’s life using a scale from (1) “Not very much” to (7) “Very much,” with an option for “Not applicable.” Higher scores are associated with a higher impact of AF on daily living. |
| Min-Max scale59 | Determines if patients have medical minimizer or maximizer tendencies. Medical minimizers prefer to do as little as possible when it comes to medical treatments and their health, whereas medical maximizers prefer active and aggressive medical treatments both of which can lead to appropriate or inappropriate use of health care (ie, Minimizers avoiding low-benefit health care procedures, but also high-benefit procedures, and maximizers participating in both kinds).60 It consists of one question on a 6-point Likert scale from (1) “I strongly lean toward waiting and seeing” to (6) “I strongly lean toward taking action.” |
| Values trade-off scale41 | Assesses patients’ perceptions of the trade-offs between increased bleeding or stroke risk by asking patients to choose between taking an anticoagulant every day, which has a risk of causing serious bleeding (Scenario A) or not taking an anticoagulant every day, even though stroke risk is higher (Scenario B). This will be assessed on a 5-point Likert scale from “Strongly prefer Scenario A” to “Strongly prefer Scenario B” measuring which scenario is preferred. |
| Collaborative agreement | Assesses decision concordance between the patient and the clinician. Both the patient and clinician will be asked to report about decisions made during the index encounter; take a stroke prevention medication (yes/no), and if yes, which stroke prevention medication was chosen (warfarin, DOAC, antiplatelet). Decisions will also be abstracted from electronic medical records (EMR) and through assessment of audiovisual recordings by research staff. Agreement will be calculated between all four sources and reported. |
| Clinician satisfaction | Assessed using: (1) a 5-point Likert scale (Very satisfied to Very unsatisfied) pertaining to clinician satisfaction with anticoagulant discussion; and (2) a question pertaining to whether clinician would recommend the approach used to other clinicians (yes/no/not sure). Higher scores are indicative of higher satisfaction, |
| Treatment choice | Assesses which treatment patient chose and whether their decision would be different if there were no out-of-pocket costs. |
| Encounter outcomes | |
| Encounter length | Length in minutes of the discussion about anticoagulation and of the total office encounter when available. |
| Fidelity of PDA and EDA tools | Assessed using checklist of key elements (ie, mentioning the tool, discussion of risk). Potential contamination will be assessed by reviewing video recordings. A sum of key elements will be calculated for each recording and across study arms 1–3. For encounters where audio/video recording was declined, a study coordinator may be present taking notes with the consent of the clinician and patient. |
| Anticoagulation outcomes | |
| Anticoagulation choice | Assessed by reviewing EMR, patient- and clinician-reported choice, and encounter recordings. Switches to another anticoagulant will be determined by comparing initial choice and patient self-reported medication use in the 12 months post intervention to pharmacy records. When available, documented reasons for initial choice and switching will be recorded. The individual who made the decision to switch medication will be noted when available (eg, patient, clinician) |
| Anticoagulation adherence61 | Assessed using: (1) a 100-point visual analogue scale where participants indicate the percentage of medication taken since the prior encounter; and (2) a self-reported 7-day recall of pill-taking behavior including how many days they took it and evaluation of any potential side effects. |
| Anticoagulation persistence | Patients will authorize access to pharmacy dispensing records from all pharmacies used to fill prescriptions. Persistence will be assessed using the percent days covered (PDC) (total days’ supply of anticoagulant filled / total days of observation from the first prescription fill date; range 0%-100%). For patients with prior history of using anticoagulation, pharmacy refill records for the 12 months prior to enrollment will also be accessed to allow calculation of persistence before and after study participation. |
| Warfarin use | Assessed using secondary measures of adherence: (1) the proportion of INR tests obtained/scheduled; and (2) percentage of time in the therapeutic international normalized ratio range (typically INR 2–3) using linear interpolation.45 |
| Health outcomes | |
| Clinical events | Assessed using manual review of the EMR to identify clinical outcome events including death from any cause, stroke, systemic embolism, transient ischemic attack, clinically-relevant non-major bleeding,47 and major bleeding.48 |
Abbreviations: AF, atrial fibrillation; DOAC, direct oral anticoagulant; EDA, encounter decision aid; EMR, electronic medical record; INR, international normalized ratio; PDA, patient decision aid; SDM, shared decision making.
Data abstraction
Sociodemographic, clinical, and prescription medication data will be abstracted from medical records for enrolled patients. Collected data will include adverse events, variables to estimate stroke and bleeding risks (CHA2DS2-VASc and HAS-BLED scores), location of primary healthcare delivery (whether different from the study site), and total number of current medications including use of anticoagulant and antiplatelet agents. For patients on warfarin at time of enrollment, INR data will be collected from 12 months prior to 12 months post enrollment. For a subset of patients, we plan on obtaining pharmacy records for 12 months prior to and 12 months after study enrollment. Data will be collected using an electronic case report form and randomization module hosted in Research Electronic Data Capture Redcap at the University of Utah.
Outcomes
Primary outcomes
The three co-primary outcome domains are: (1) quality of SDM, (2) patient decision making, and (3) knowledge. These outcomes will be measured by the OPTION scale, Decisional Conflict Scale, and a knowledge survey, respectively. Further description of these primary outcomes can be found in Table II. As these domains are conceptually different, separate comparisons will be made between the different arms and no multiple comparison adjustments will be applied across the three co-primary domains. The evaluation of all other outcome domains will be interpreted as exploratory. While clinical outcomes such as stroke are not primary outcomes, achieving high quality SDM is expected to improve adherence to the clinical decision. Therefore, we also expect SDM outcomes to be associated with more clinically important outcomes in the long term.
Analysis of primary outcomes
To control for Type 1 error due to multiple treatment groups, we designed a hierarchy of treatment comparisons as follows: (1) single primary treatment group of combined PDA and EDA vs usual care; (2) two main secondary comparisons of PDA vs usual care and EDA vs usual care; and (3) secondary comparisons of PDA vs EDA, combined PDA/EDA vs PDA alone, and combined PDA/EDA vs EDA alone.
The primary comparison will measure the combination of both DAs together vs usual care for each co-primary outcome. This comparison will test the hypothesis that maximal implementation of DAs at both the patient and encounter level will improve SDM outcomes the most. Using a serial gatekeeping strategy of sequential unadjusted tests, we will analyze the combination of EDA and PDA vs usual care. If this comparison is significant (at α = 0.05), then we will proceed to the comparison of EDA and PDA separately vs usual care. All other comparisons between EDA, PDA and usual care will be considered secondary comparisons. The two main secondary comparisons of each decision aid tool vs usual care, will use α = 0.01 for the PDA and α = 0.04 for evaluating the EDA to indicate significance.40 Any further comparisons between treatment arms will be considered exploratory.
This design enables us to control the study-wide risk of Type-1 error at 5% while maintaining high statistical power to infer benefits of the two DAs when applied jointly or individually (see Appendix). We chose a higher α for the EDA as clustering randomization by clinician is expected to reduce power for the EDA comparison much more than for the PDA comparison. Positive results for the two main secondary comparisons will be definitive only if the primary comparison achieves statistical significance.
Secondary outcomes
Secondary outcomes fall under the domains of SDM (patient reported), patient decision making, anticoagulation use, and health outcomes (Table III). Health outcomes will be collected at 12-months post-enrollment. We will assess use of alternative stroke-prevention strategies (eg, left atrial appendage closure procedure), anticoagulation initiation and drug type (by prescription), adherence and persistence to anticoagulation therapy, and prescription refills. For patients taking warfarin, proportion of scheduled INR tests obtained, and time in therapeutic INR range (using linear interpolation) will be measured.45, 46 We will also review prescription refills 12 months prior to and/or 12 months after enrollment to compare adherence pre-and post-encounter for patients in the monitor cohort.46
We will monitor for major clinical outcomes such as stroke or bleeding using manual medical record review and patient-self reports. However, we predict a low incidence of these outcomes. Clinical outcomes will include transient ischemic attack, stroke, systemic embolism, clinically relevant non-major bleeding, major bleeding, and death.47, 48
Sub-group analysis
Outcomes will be compared in relevant sub-groups including but not limited to encounter type (telehealth vs other encounter types), patient literacy and numeracy (low vs high), age group, sex category, race category, comorbidities, stroke risk category, bleeding risk category, patient cohort (initiation vs monitor), and prior history of anticoagulation use. These sub-group analysis will utilize linear and logistic regression models as appropriate for the dependent outcome variable.
Sample size and power calculation
We plan to recruit 1200 patients across 6 sites, (N = 285 with non-missing outcomes per treatment arm). We assume that >95% of patients will have non-missing values for each of the primary and secondary outcomes which are collected immediately after the encounter or through observation of the encounter. The power and sample size calculations for the primary comparison are summarized in the supplemental appendix. Estimates shown are based on a max number of 30 patients per clinician and are based on intermediate inter-class correlation estimates.30, 62–65 A maximum number of 30 patients per clinician will limit loss of power that may occur due to correlated outcomes for patients seen by the same clinician. As of January 27, 2022, 475 patients have been enrolled.
Discussion
This study is poised to answer a significant question regarding the effectiveness of decision aids to facilitate SDM. While the latest update to the ACC/AHA guidelines in 2019 recommended that “anticoagulant therapy should be individualized on the basis of shared decision-making,” it did not report what SDM should look like, nor did it mention methods to increase the uptake of SDM.21 The ACC/AHA recommendation is also based on a low certainty evidence. No studies have shown an improvement in clinical outcomes using shared decision making in AF.66 While not primary outcomes, adherence and clinical outcomes will also be assessed to further evaluate the impact of SDM. Adoption of and adherence to anticoagulation therapy are known challenges in this population, if SDM and DAs can improve these measures, this could lead to improved clinical outcomes over time.9, 67
This study is among the first to undertake a head-to-head PDA vs EDA comparison. The proposed design will provide crucial feedback on what types of DAs to focus on in the future and how to best support SDM using DAs. Findings from a recent Randomized Control Trial (RCT) measuring the impact of a similar EDA on SDM in AF and anticoagulation choice have been incorporated into our study design.30 That study demonstrated that using an EDA assisted clinicians with better SDM engagement, and led to increased clinician satisfaction with encounters without lengthening the encounter. However, no differences were found in patient decisional conflict or knowledge. This outcome may be because patients were generally satisfied with their current anticoagulation therapy or had no difficulty making a decision (ie, high knowledge and low decisional conflict at baseline).30 Thus, in the proposed study we will only enroll patients who are new to anticoagulation or have experience with AF and are currently experiencing issues that warrant reevaluation of anticoagulation choice with their clinician. Clinicians will be randomized to either the EDA or control, which decreases potential contamination associated with clinicians having familiarity with the EDA.
We also have been able to adapt to using telehealth platforms for a larger proportion of patients due to COVID-19 restrictions and thus will be able to explore differences between in-person and telehealth SDM. Our results will help to inform and improve SDM interventions in the future as we seek to improve AF patients’ outcomes and experiences with their health care. If these interventions can be used in a telehealth setting as well as in-person, this will improve the flexibility and ability of health systems and clinicians to implement them into their general practice.
Supplementary Material
Funding
Research reported in this article, was funded by the American Heart Association (AHA) through a funding collaboration between AHA and the Patient-Centered Outcomes Research Institute (PCORI) Grant # 18SFRN34110489 to Dr Fagerlin (Center, STEP-UP AF), and Grant # 18SFRN34230142 to Dr Ozanne/(Project, RED-AF).
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Vanderbilt University Medical Center study staff is supported by an AHA SFRN Grant Number: 18SFRN34110369/201 to Dr Dan Rodin.
Northwestern University Feinberg School of Medicine study staff is supported by an AHA SFRN Grant Number: 18SFRN34250013 to Dr Rodney Passman.
Dr Steinberg is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156. Dr Witt is supported by the Agency for Health Research and Quality under Award Number R18HS027960-01. Dr Barnes is supported by the National Heart, Lung, and Blood Institute under Award Number K01HL135392. Dr Noseworthy receives research funding from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI] and the National Institute on Aging [NIA]), Agency for Healthcare Research and Quality (AHRQ), Food and Drug Administration (FDA), and the American Heart Association (AHA).
Conflicts of interest
Dr Steinberg reports research support from Abbott, Cardiva, AltaThera, Boston Scientific, and Janssen; and consulting to Janssen, AltaThera, Merit Medical, and Crowley Fleck, LLP; and speaking for NACCME (funded by Sanofi). Dr Witt reports research support from and consulting to Roche Diagnostics. Dr Barnes reports consulting for Pfizer/Bristol-Myers Squibb, Janssen, and Acelis Connected Health. Dr Noseworthy is a study investigator in an ablation trial sponsored by Medtronic, is involved in potential equity/royalty relationship with AliveCor, has served on an expert advisory panel for Optum, and has filed patents related to the application of AI to the ECG for diagnosis and risk stratification.
Appendix
Patient Knowledge – All statements presented in a matrix
Instructions: The following statements are about stroke prevention drug(s), which may be true or false. For each statement, please select whether you think it is true or false. Try not to guess if you are unsure; instead, select “I don’t know.”
Stroke prevention drugs are also known as anticoagulants, anti-clot medications, and blood thinners. These medications include: warfarin and DOACs (Direct Oral Anticoagulants, eg, apixaban (Eliquis), dabigatran (Pradaxa), edoxaban (Savaysa), rivaroxaban (Xarelto).
Afib is caused by abnormal electrical activity in the heart. True/False/I don’t know
AFib allows clots to form in the heart and these clots can cause a stroke. True/False/I don’t know
Taking a stroke prevention drug can lower my risk of stroke. True/False/I don’t know
In the future, I can change to a different stroke prevention drug or decide to stop taking a stroke prevention drug. True/False/I don’t know
All stroke prevention drugs require me to get regular blood tests. True/False/I don’t know
Taking a stroke prevention drug can increase my risk of bleeding. True/False/I don’t know
When using warfarin, I can change my diet without any concerns. True/False/I don’t know
Footnotes
Ethics and dissemination
The study was approved by the institutional review boards (IRBs) at all sites and recruitment is ongoing. This study is registered on clinicaltrials.gov and will be updated with any modifications and results. Any further updates to the protocol will be submitted to respective site IRBs and the sponsors. Sponsors are receiving regular updates on study progress. The outcomes of the study will be presented at scientific meetings and reported in peer-reviewed journals.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.ahj.2022.02.010.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009;104:1534–9. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290:2685–92. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492–501. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 7.Xian Y, O’Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA 2017;317:1057–67. doi: 10.1001/jama.2017.1371. [DOI] [PubMed] [Google Scholar]
- 8.Johansson C, Hagg L, Johansson L, Jansson JH. Characterization of patients with atrial fibrillation not treated with oral anticoagulants. Scand J Prim Health Care 2014;32:226–31. doi: 10.3109/02813432.2014.984952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friberg L, Hammar N, Ringh M, et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study). Eur Heart J. 2006;27:1954–64. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–645 e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 12.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007;147:755–65. [DOI] [PubMed] [Google Scholar]
- 13.Geller AI, Shehab N, Lovegrove MC, et al. Emergency visits for oral anticoagulant bleeding. J Gen Intern Med 2020;35:371–3. doi: 10.1007/s11606-019-05391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shehab N, Lovegrove MC, Geller AI, et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016;316:2115–25. doi: 10.1001/jama.2016.16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation Guide to Warfarin Therapy. Circulation 2003;107:1692–711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 16.Kon AA, Ackerson L, Lo B. How pediatricians counsel parents when no “best-choice” management exists: lessons to be learned from hypoplastic left heart syndrome. Arch Pediatr Adolesc Med 2004;158:436–41. doi: 10.1001/archpedi.158.5.436. [DOI] [PubMed] [Google Scholar]
- 17.Byrne PJ, Murphy A. Informed consent and hypoplastic left heart syndrome. Acta Paediatr 2005;94:1171–5. doi: 10.1111/j.1651-2227.2005.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 18.Moyer A, Salovey P. Patient participation in treatment decision making and the psychological consequences of breast cancer surgery. Womens Health 1998;4:103–16. [PubMed] [Google Scholar]
- 19.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet 1996;348:383–6. doi: 10.1016/s0140-6736(96)01073-2. [DOI] [PubMed] [Google Scholar]
- 20.Street RL Jr, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making 1997;17:298–306. doi: 10.1177/0272989X9701700306. [DOI] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the ‘Atrial Fibrillation Better Care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemostasis 2021. doi: 10.1055/a-1515-9630. [DOI] [PubMed] [Google Scholar]
- 23.Krug S. Rocket surgery made easy: the do-it-yourself guide to finding and fixing usability problems. Berkeley, California: New Riders; 2009. [Google Scholar]
- 24.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016;9:767–76. doi: 10.1161/CIRCOUTCOMES.116.002641. [DOI] [PubMed] [Google Scholar]
- 26.Mann DM, Ponieman D, Montori VM, et al. The statin choice decision aid in primary care: a randomized trial. Patient Educ Couns 2010;80:138–40. doi: 10.1016/j.pec.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Montori VM, Shah ND, Pencille LJ, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med 2011;124:549–56. doi: 10.1016/j.amjmed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med 2009;169:1560–8. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 29.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med 2007;167:1076–82. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 30.Kunneman M, Branda ME, Hargraves IG, et al. Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern. Med 2020. doi: 10.1001/jamainternmed.2020.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanni C, Petrovitch E, Ali M, et al. Outcomes associated with apixaban vs warfarin in patients with renal dysfunction. Blood Advances 2020;4:2366–71. doi: 10.1182/bloodadvances.2019000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 2018;138:1519–29. doi: 10.1161/CIRCULATIONAHA.118.035418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanifer JW, Pokorney SD, Chertow GM, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation 2020;141:1384–92. doi: 10.1161/CIRCULATIONAHA.119.044059. [DOI] [PubMed] [Google Scholar]
- 34.Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother 2019;53:165–70. doi: 10.1177/1060028018796604. [DOI] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 37.Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, et al. Measuring numeracy without a math test: development of the subjective numeracy scale. Med Decis Making 2007;27:672–80. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 39.McNaughton CD, Cavanaugh KL, Kripalani S, et al. Validation of a short, 3-item version of the subjective numeracy scale. Med Decis Making 2015;35:932–6. doi: 10.1177/0272989X15581800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dmitrienko A, Tamhane AC, Wang X, Chen X. Stepwise gatekeeping procedures in clinical trial applications. Biom J 2006;48:984–91. doi: 10.1002/bimj.200610274. [DOI] [PubMed] [Google Scholar]
- 41.Allen LA, McIlvennan CK, Thompson JS, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: The DECIDE-LVAD randomized clinical trial. JAMA Intern. Med 2018;178:520–9. doi: 10.1001/jamainternmed.2017.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr PJ, O’Malley AJ, Tsulukidze M, et al. The psychometric properties of Observer OPTION(5), an observer measure of shared decision making. Patient Educ Couns 2015;98:970–6. doi: 10.1016/j.pec.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor AM. User Manual Decisional Conflict Scale (16 item statement format) [document on the Internet]. Ottawa: Ottawa Hospital Research Institute; ©1993. [updated 2010; cited 2019 May 23]. 16 p. Available from http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- 45.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemostasis 1993;69:236–9. [PubMed] [Google Scholar]
- 46.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119–26. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 48.Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 49.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 50.Bennett C, Graham ID, Kristjansson E, et al. Validation of a preparation for decision making scale. Patient Educ Couns 2010;78:130–3. doi: 10.1016/j.pec.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Patient Experience Measures from the CAHPS Clinician & Group Survey. Agency for Healthcare Research and Quality. Updated 6/1/2017. Accessed May 23, 2019, https://www.ahrq.gov/sites/default/files/wysiwyg/cahps/surveysguidance/cg/about/measures-cg30-2309.pdf.
- 52.Kriston L, Scholl I, Holzel L, et al. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns 2010;80:94–9. doi: 10.1016/j.pec.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 53.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res 1997;29:21–43. [PubMed] [Google Scholar]
- 54.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol 1992;45:941–50. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor AM, A. C. User Manual - Acceptability [document on internet]. 1996.
- 56.O’Connor AM, A. C. Sample Tool: Acceptability (Osteoporosis Therapy). https://decisionaid.ohri.ca/docs/develop/Tools/Acceptability_osteoporosis.pdf
- 57.Lorig KR, Sobel DS, Ritter PL, et al. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–62. [PubMed] [Google Scholar]
- 58.Adapted Illness Intrusiveness Ratings. Self-Management Resource Center. https://www.selfmanagementresource.com/docs/pdfs/English_-_illnessintrusiveness.pdf
- 59.Scherer LD, Zikmund-Fisher BJ. Eliciting medical maximizing-minimizing preferences with a single question: development and validation of the MM1. Med Decis Making 2020;40:545–50. doi: 10.1177/0272989X20927700. [DOI] [PubMed] [Google Scholar]
- 60.Scherer LD, Shaffer VA, Caverly T, et al. Medical maximizing-minimizing predicts patient preferences for high- and low-benefit care. Med Decis Making 2020;40:72–80. doi: 10.1177/0272989x19891181. [DOI] [PubMed] [Google Scholar]
- 61.Sevilla-Cazes J, Finkleman BS, Chen J, et al. Association between patient-reported medication adherence and anticoagulation control. Am J Med 2017;130:1092–8 e2. doi: 10.1016/jamjmed201703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metz M, Elfeddali I, Veerbeek M, et al. Effectiveness of a multi-facetted blended eHealth intervention during intake supporting patients and clinicians in Shared Decision Making: a cluster randomised controlled trial in a specialist mental health outpatient setting. PLoS One 2018;13. doi: 10.1371/journal.pone.0199795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res 2013;13:301. doi: 10.1186/1472-6963-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elwyn G, Edwards A, Hood K, et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract 2004;21:337–46. doi: 10.1093/fampra/cmh401. [DOI] [PubMed] [Google Scholar]
- 65.Krones T, Keller H, Sonnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med 2008;6:218–27. doi: 10.1370/afm.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung MK, Fagerlin A, Wang PJ, et al. Shared decision-making in cardiac electrophysiology procedures and arrhythmia management. Circ Arrhythm Electrophysiol. doi: 10.1161/CIRCEP.121.007958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost 2008;6:1500–6. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


