Abstract
SV40 (simian virus 40)-infected CV1 cells were permeabilized with Staphylococcus aureus α-toxin for small molecules (<2 kDa) in a medium that supports DNA replication. Incorporation of [α-32P]dATP was shown to proceed at an essentially constant rate for at least 1 h. 32P-labelled DNA replication intermediates and products were analysed by alkaline sucrose density centrifugation. The results suggested that SV40 DNA replication in α-toxin-permeabilized CV1 cells occurred essentially as in vivo. After bromodeoxyuridine 5′-triphosphate-labelling and isopycnic banding, significant amounts of DNA density-labelled in both strands were detected from 110 min of permeabilization onwards, indicating repeated rounds of viral DNA replication in the permeabilized cells. Incubation of permeabilized SV40-infected cells under hypoxic culture conditions caused inhibition of SV40 DNA replication. As seen in unpermeabilized cells, SV40 DNA replication was inhibited at the stage of initiation. The inhibition of DNA replication induced by hypoxia was mimicked by AA (antimycin A), an inhibitor of mitochondrial respiration, and also by the replacement of glutamate, a substrate of mitochondrial respiration, by Hepes in the permeabilization medium. Inhibition of DNA replication was not mediated by intracellular ATP depletion. AA also inhibited SV40 DNA replication in unpermeabilized, normoxically incubated cells. Moreover, as in hypoxically incubated cells, the addition of glucose to SV40-infected cells incubated for several hours with AA induced a burst of new initiations followed by a nearly synchronous round of viral DNA replication. Taken together, these results indicate that mitochondria are involved in the oxygen-dependent regulation of SV40 DNA replication.
Keywords: DNA replication, hypoxia, mitochondria, oxygen, permeabilized cell, simian virus 40
Abbreviations: AA, antimycin A; BrdU, bromodeoxyuridine; BrdUTP, bromodeoxyuridine 5′-triphosphate; hU, haemolytic unit(s); LL-, HL-, HH-DNA, DNA substituted with BrdU in no, one or both strands respectively; PEP, phosphoenol pyruvate; p.i., post-infection; p.p.m., parts per million; ROS, reactive oxygen species; SV40, simian virus 40
INTRODUCTION
DNA replication in mammalian cells is inhibited when the O2 concentration in the cellular environment falls below 0.1%. Inhibition primarily concerns initiation, but, frequently, elongation of DNA replication is also affected. So far, this oxygen-dependent regulation of DNA replication was demonstrated to occur in cellular genome replication of several mammalian cell lines [1,2] and also in SV40 (simian virus 40) DNA replication in virus-infected CV1 cells [3,4]. Glucose has been shown to prevent the hypoxia-induced inhibition of initiation of DNA replication, at least in SV40-infected CV1 cells and HeLa cells, but probably also in other cell lines [5].
Examination of the molecular basis of this oxygen-dependent regulation of DNA replication in living cells is hampered by the fact that cellular ATP generation depends on either oxygen or glucose, both of which can potentially release the inhibition of DNA replication under hypoxia. To circumvent these difficulties, permeabilized cells and an ATP-regenerating system can be used. Although cells permeabilized by the usual membrane distorting methods were shown to replicate their DNA at the nuclear matrix [6] in a semi-conservative manner [7,8], initiation of cellular DNA replication was reported to be impaired in cells permeabilized thus [7] and, therefore, their DNA synthesis generally represents elongation of replicons initiated before permeabilization. On the other hand, initiation of SV40 DNA replication was shown to be possible in lysolecithin-permeabilized, SV40-infected CV1 cells [9].
In the present study, we used α-toxin, produced by Staphylococcus aureus (see [10–12] and references therein), for permeabilization. α-Toxin, a water-soluble protein of 34 kDa, hexamerizes in the cell membrane and creates stable transmembrane pores of 1–2 nm effective diameter [11], which are permeable to low-molecular-mass compounds. The toxin exclusively enters the plasma membrane and does not reach intracellular organelles or cytoplasmic components. The pores created by α-toxin are too small to allow proteins to escape from the cell. Complex cellular machinery depending on a high local concentration of respective proteins, like the DNA replication apparatus, is therefore expected to remain intact over extended periods. Molecules of up to 1000–2000 Da, on the other hand, are easily exchanged with the surrounding medium.
After adaptation of the permeabilization medium according to a cell-free system of SV40 DNA synthesis [13], SV40 DNA replication was found to proceed essentially as in vivo, at least for the first hour after permeabilization. Hypoxic incubation of permeabilized cells inhibited SV40 DNA replication at the stage of initiation. This effect of hypoxia could be mimicked by AA (antimycin A), an inhibitor of mitochondrial respiration, or by the replacement of glutamate, a substrate of the mitochondrial electron-transport chain, by Hepes in the permeabilization medium. AA also mimicked the effect of hypoxia in unpermeabilized, normoxically incubated cells. Taken together, these results suggest that mitochondria are involved in the fast O2-dependent regulation of SV40 DNA replication in virus-infected CV1 cells.
EXPERIMENTAL
Preparation of α-toxin and determination of haemolytic activity
α-Toxin was prepared from the culture fluid of Staph. aureus (strain Wood 46, ATCC 10832). Bacteria were grown in 1 litre of tryptic soy broth (30 g/l, pH 7.3; Difco Laboratories, Augsburg, Germany) to an absorbance of approx. 7.0 at 578 nm. Sodium azide (8 mM), EDTA (2 mM) and acetic acid (pH 5.0) were added. The suspension was diluted with 1.5 litres of 8 mM sodium azide and 10 mM sodium acetate (pH 5.0). After 3 h at 4 °C, bacteria and contaminating protein were removed by centrifugation (9000 g, 15 min, 4 °C). Then, 200 g of preswollen cationic exchanger CM52 (Whatman, Maidstone, U.K.), equilibrated in 10 mM sodium acetate (pH 5.0) were added to the clear supernatant. After 2 h, the column was filled with cationic exchanger and successively washed with 700 ml of sodium azide (8 mM)/sodium acetate (10 mM) solution (pH 5.0) and 600 ml of sodium azide (8 mM)/sodium phosphate (10 mM) solution (pH 6.5). Elution was performed with 200 ml of a solution containing 8 mM sodium azide, 500 mM NaCl, 1 mM imidazole and 50 mM sodium phosphate (pH 7.0). The eluate was collected in fractions of 10 ml. Haemolytic activity of fractions was determined semi-quantitatively as described below. Fractions exhibiting haemolytic activity were pooled and further purified on a chelating Sepharose fast-flow column (Amersham Biosciences, Freiburg, Germany), which was prepared as follows: 50 ml of chelating Sepharose in 8 mM sodium azide, 50 mM Tris (pH 8.0) and 20% (v/v) ethanol was packed into a column of 30 mm diameter and washed with 150 ml of double-distilled water. The column was washed successively with 54 ml of solution A (25 mM CuSO4, 1 M NaCl and acetic acid, pH 3.4); 50 ml of solution B (500 mM NaCl, 8 mM sodium azide, 1 mM imidazole and 50 mM sodium acetate, pH 4.5); 50 ml of solution C (500 mM NaCl, 8 mM sodium azide, 300 mM imidazole and 50 mM sodium acetate, pH 4.5); and again with 150 ml of solution B.
The collected eluate of the cationic exchange column was loaded on to the chelating Sepharose column and washed with 500 mM NaCl, 8 mM sodium azide, 11 mM imidazole and 50 mM sodium acetate (pH 4.5, 350 ml). Elution of α-toxin was performed by gradually increasing the imidazole concentration from 11 mM (150 ml) to 50.5 mM (300 ml) and 300 mM (200 ml). The eluate was collected in 10 ml fractions, which were analysed for haemolytic activity (see below).
Active fractions were pooled, and (NH4)2SO4 was added to 90% saturation (at 0 °C) and EDTA to 5 mM. The precipitate formed was collected by centrifugation, washed with a solution containing (NH4)2SO4 (90% saturated at 0 °C) and EDTA (2 mM) and frozen in a humid state at −80 °C. For the determination of haemolytic activity or cell permeabilization, aliquots were thawed, dissolved in 300 mM ammonium acetate (pH 7.0) and dialysed against 500 ml of 0.5 mM Tris and 0.05 mM EDTA (pH 8.0) for 2–3 h.
Activity of α-toxin was determined by haemolysis of rabbit erythrocytes, essentially as described in [10,12].
Semi-quantitative estimation of haemolytic activity was performed by applying aliquots of 10 μl to holes in sheep blood agar plates (Oxoid, Wesel, Germany) and evaluating the area of haemolysis surrounding the holes after incubation for 18 h at 37 °C.
Radioactive labelling of cells and viruses
African green monkey CV1 cells (ATCC CCL 70) were grown in plastic flasks in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) foetal calf serum, 100 units/ml penicillin G and 100 mg/ml streptomycin, under standard tissue culture conditions. Infection with SV40 was performed as described in [14]. SV40-infected cells were incubated normoxically (20% O2, 5% CO2, N2 to 100%) and labelled with [methyl-3H]-deoxythymidine as described previously [4].
Permeabilization of SV40-infected CV1 cells
For a typical permeabilization experiment, 300000 cells were seeded on to plastic Petri dishes (35 mm; Greiner, Frickenhausen, Germany) in 1.5 ml of Dulbecco's modified Eagle's medium and, 24 h later, infected with SV40. From 24 to 36 h p.i. (post-infection), the culture supernatant was removed by aspiration and replaced by 300 μl of prewarmed permeabilization solution, containing the following: potassium glutamate (120 mM), EGTA (0.4 mM), nitrilotriacetic acid (4 mM), CTP, GTP, UTP (100 μM), ATP (2.8 mM), dATP, dCTP, dGTP, dTTP (100 μM), MgCl2 (8.5 mM), CaCl2 (1 mM), Pipes (16 mM), α-toxin (3000 hU/ml), creatine kinase (0.1 mg/ml), phosphocreatine (50 mM) and dithiothreitol (0.5 mM), pH 7.8, where hU stands for haemolytic unit(s) (1 hU=3.6 ng of α-toxin=0.1 pmol). In some experiments, a permeabilization medium containing Hepes (120 mM) instead of glutamate was used. Permeabilization components were frozen in aliquots to avoid repeated freezing and thawing.
After permeabilization, cells were incubated at 37 °C and gassed with artificial air (20% O2, 80% N2, no CO2) or with 0.015% O2, Ar to 100% (hypoxic incubation). For radioactive labelling, [α-32P]dATP or [α-33P]dATP (specific radioactivity, 3 μCi/pmol) were either added directly to the permeabilized cells or, in the case of hypoxic incubation, by putting a spatula carrying the appropriate quantity in dried form into the culture medium. To stop incubations, the permeabilization solution was withdrawn by aspiration and the Petri dishes were washed once with ice-cold KG buffer, containing 150 mM potassium glutamate, 0.5 mM EGTA, 5 mM nitrilotriacetic acid and 10 mM Pipes (pH 7.8). The radioactivity incorporated was determined as described by Probst et al. [15].
Determination of ATP and glucose
Luminometric determination of cellular ATP was performed essentially as described in [5]. Permeabilization medium was removed by aspiration and cells were briefly washed once with ice-cold KG buffer. Immediately thereafter, 1 ml of hot (90 °C) buffer (50 mM Tris/HCl and 4 mM EDTA, pH 7.8) was added. The cells were subsequently boiled for 5 min and ATP was determined in the supernatant using the ATP Bioluminescence CLC assay (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Glucose concentration of the cell culture supernatant was determined using an enzymic glucose assay (Sigma, München, Germany).
Alkaline sedimentation analysis of labelled DNA
Alkaline sedimentation analysis was performed as described previously [4]. First, 12 ml of 15–30% or 5–40% (w/v) linear sucrose gradients in 0.25 M NaOH, 0.6 M NaCl, 1 mM EDTA, 0.1% sodium lauroyl sarcosinate (w/v) were built up over a 1.5 ml cushion of 30 or 40% (w/v) sucrose solution in centrifuge tubes for the Beckman SW 40 rotor. After radioactive labelling, permeabilized cells were lysed in 0.2 M NaOH (200 μl) and laid on the top of the gradients. After 1 h, gradients were centrifuged at 163000 g for 16 h at 23 °C. Fractions of 0.6 ml each were collected from the top and processed for determination of acid-insoluble radioactivity as described in [15].
BrdUTP (bromodeoxyuridine 5′-triphosphate) density labelling and isopycnic CsCl centrifugation
SV40-infected cell cultures were permeabilized, 24 h p.i. as described above. dTTP in the permeabilization medium was, however, replaced by BrdUTP (400 μM)/dTTP (100 μM). For radioactive labelling, [α-32P]dATP (30 μCi/ml; final specific activity, 0.3 μCi/nmol dATP) was added to the cells 20 min after permeabilization.
BrdUTP-labelled cells were processed essentially as described in [16]. Cells were washed with ice-cold KG buffer and lysed in 2 ml of lysis buffer [20 mM Tris/HCl, 10 mM EDTA, 0.5% sodium lauroyl sarcosinate, 100 μg/ml proteinase K (Merck, Darmstadt, Germany), pH 8.0] for 1 h at 37 °C. A lysate of reference cells, prepared in the same way from approx. 60000 SV40-infected CV1 cells labelled with [methyl-3H]deoxythymidine (10 μCi/ml; 90 μCi/nmol deoxythymidine) for 1 h, was added. The combined lysates were sheared by ten passages through a 25-gauge injection needle followed by five passages through a 27-gauge injection needle. The lysates were diluted with 10 mM Tris/HCl and 1 mM EDTA (pH 8.0) to 6 ml, mixed with 8.15 g of solid CsCl (resulting density, approx. 1.74 g/ml) and then, after being overlaid with paraffin oil, centrifuged in the Beckman 70 Ti rotor at 37000 rev./min (93500 g) for 60 h at 20 °C. The gradients fractions were collected from below into 40 fractions of 200 μl, which were analysed for acid-precipitable 32P- and 3H-radioactivity according to [15].
Detection of SV40 form U
SV40 form U was detected as described previously [4], and experiments were performed 24 h p.i. throughout. Briefly, washed cells were lysed and digested for 3 h at 55 °C in 0.25 M EDTA (pH 8.0), 1% sodium lauroyl sarcosinate and 100 μg/ml proteinase K. The lysate was extracted twice with phenol/chloroform and dialysed against 1 mM Tris/HCl and 0.1 mM EDTA (pH 8.0) at 4 °C overnight. After digestion with RNase A (100 μg/ml; 1 h at 37 °C), the concentration of isolated SV40 DNA was determined spectrometrically. Additionally, integrity and concentration were checked by electrophoretic separation of 50 ng of isolated DNA on an analytical agarose gel [17]. Thereafter, 100 ng of isolated DNA was loaded on to a 25 cm×20 cm agarose gel containing 20 μg/ml chloroquine (gel buffer: 30 mM NaH2PO4, 36 mM Tris and 1 mM EDTA). Electrophoresis was performed at 2 V/cm and 4 °C for 20 h. After Southern blotting under alkaline conditions [17], SV40 DNA was detected by hybridization with a 32P-labelled SV40 DNA [18].
RESULTS
Incorporation of [α-32P]dATP into permeabilized SV40-infected CV1 cells
All experiments described below were confirmed by repetition. All repetitions strictly yielded the evidence presented in the text. Nevertheless, mean values and error bars are usually not shown since the results obtained from repetitions of one and the same experiment were not sufficiently identical, but differed quantitatively. In particular, SV40 DNA sedimentation profiles obtained after alkaline sucrose gradient centrifugation (see below) varied with respect to total [32P]dATP incorporated into DNA or the position of peak maxima. In other words, it was not possible to keep the experimental conditions as constant as necessary. Therefore we selected representative results of the respective experiments for presentation.
The medium used here to permeabilize SV40-infected CV1 cells was based on a medium described by Ahnert-Hilger et al. [12]. Complete permeabilization, evaluated by the determination of ATP released from the cells, occurred at α-toxin concentrations above 1000 hU/ml (results not shown). For standard permeabilization experiments, 3000 hU/ml were used. To sustain SV40 DNA replication, the permeabilization medium was supplemented in analogy to described cell-free systems of SV40 DNA synthesis [13].
Continuous labelling of permeabilized SV40-infected CV1 cells with [α-32P]dATP (5 μCi/ml; 0.05 μCi/nmol dATP) resulted in a linear increase of incorporation of 32P-radioactivity into DNA between 2 min and approx. 60 min after permeabilization (Figure 1A). Thereafter, the incorporation usually slowed down. Extension of incubation beyond 3 h revealed that SV40 DNA replication frequently resumed, for unknown reasons, between 3 and 5 h after permeabilization (results not shown). Since the reproducibility of the course of incorporation of [α-32P]dATP into DNA was greatest within the first 90 min after permeabilization, we restricted our experiments to this time span.
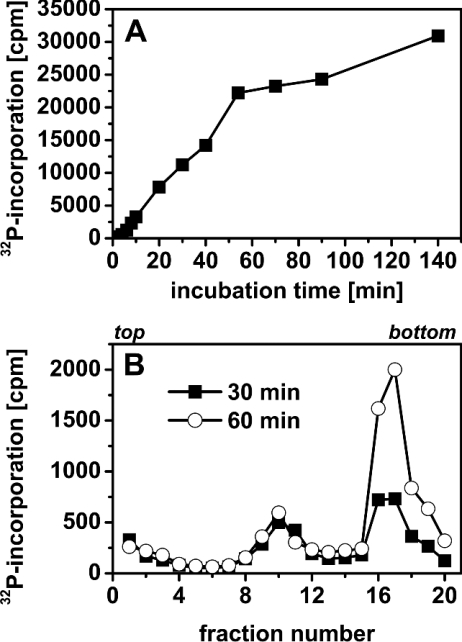
Figure 1. SV40 DNA synthesis in α-toxin-permeabilized CV1 cells.
(A) Temporal course of SV40 DNA replication. SV40-infected cells were incubated in permeabilization medium and labelled with [α-32P]dATP (5 μCi/ml, 0.05 μCi/nmol dATP). [α-32P]dATP incorporation into acid-insoluble material was determined at the indicated times. (B) Alkaline sedimentation profiles of permeabilized SV40-infected CV1 cells. Cells were permeabilized and labelled with [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP) for 30 or 60 min. Thereafter, DNA was separated according to size by sedimentation in a 5–40% alkaline sucrose gradient. Sedimentation direction was from left to right; the top and bottom of the gradients are indicated.
On the other hand, incorporation of [methyl-3H]deoxythymidine (10 μCi/ml, 2 μM or 4.7 μCi/nmol deoxythymidine), added to the cell cultures 10 min before permeabilization and thereafter present in the permeabilization medium, ceased approx. 10 min after permeabilization (results not shown). This indicated that the dTTP of the permeabilization medium was taken up into the cells and effectively competed with dTTP salvaged from deoxythymidine for incorporation into DNA.
Characterization of 32P-labelled SV40 DNA was performed by alkaline sedimentation analysis. The permeabilized SV40-infected cells were labelled for 30 or 60 min with [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP). Thereafter, they were processed for centrifugation in a 5–40% alkaline sucrose gradient.
Sedimentation profiles of viral DNA are shown in Figure 1(B). Under the sedimentation conditions used, growing daughter chains up to full-length (5.2 kb, 16 S) and relaxed circular DNA (18 S) appeared in gradient fractions 7–12, and closed circular, supercoiled duplexes (form I, approx. 50 S) sedimented to fractions 15–19.
As shown in Figure 1(B), the amount of mature supercoiled SV40 DNA significantly increased between 30 and 60 min after permeabilization, whereas the amount of growing daughter strands in fractions 7–12 remained unchanged. This indicates unrestricted viral DNA replication during the whole incubation time. Similar sedimentation profiles (results not shown) were obtained when unpermeabilized, SV40-infected cells were labelled with [methyl-3H]deoxythymidine [3,4], suggesting that SV40 DNA replication in permeabilized cells proceeds essentially as in vivo.
Comparison of replicated viral DNA of permeabilized and unpermeabilized SV40-infected cells by two-dimensional neutral/alkaline agarose gel electrophoresis as described in [19] also revealed no significant differences (results not shown).
BrdUTP density labelling of permeabilized cells
Generally, prolonged DNA replication and initiations of new replicons have not been observed in permeabilized cells so far [7]. To examine whether initiations occur in α-toxin-permeabilized SV40-infected cells, we used a BrdUTP-labelling schedule as given in [16].
Repeated rounds of viral DNA replication result in viral DNA substituted with bromodeoxyuridine in either one (HL-DNA) or both strands (HH-DNA). The differently density-labelled DNA can be detected by CsCl gradient centrifugation [16]. For the demonstration of repeated rounds of viral replication, incubation for up to several hours after permeabilization was necessary.
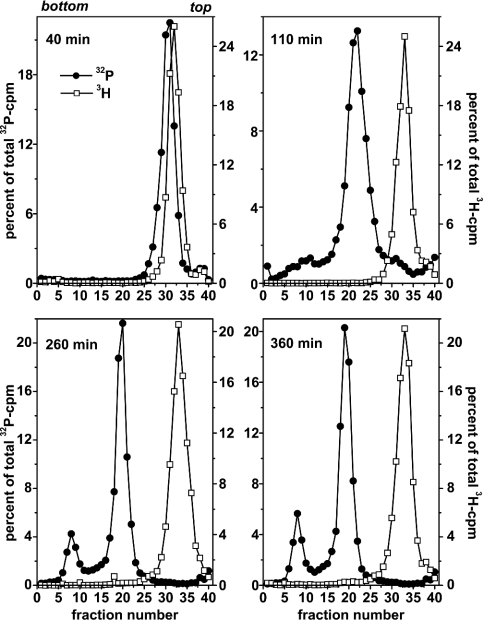
Figure 2 shows isopycnic profiles obtained from permeabilized cells that were labelled with BrdUTP for 40, 110, 260 or 360 min. Labelling with [α-32P]dATP was performed 20 min after permeabilization until the end of incubation. HH-DNA (fractions 6–10) could be detected after 110 min of BrdUTP labelling and it increased further thereafter. This demonstrates that initiations occurred in the permeabilized cells and the viral DNA produced was rereplicated at least once.
Figure 2. Occurrence of multiple replication rounds of viral DNA in permeabilized SV40-infected CV1 cells.
Cells were permeabilized with medium containing BrdUTP/dTTP (400 μM/100 μM). Labelling with [α-32P]dATP (30 μCi/ml, 0.3 μCi/nmol dATP) was started 20 min after permeabilization and continued until the end of incubation. Total time periods of incubation are indicated. After lysis, 3H-labelled control DNA (not density-labelled) was added and total DNA was separated according to density by isopycnic banding using CsCl gradient centrifugation. Density increases from right to left; the top and bottom of the gradients are indicated. Total 32P-incorporation recovered from the gradients was 10785 (40 min), 14427 (110 min), 95117 (260 min) and 172136 c.p.m. (360 min).
Total 32P-incorporation recovered from the gradients showed an unexpectedly small increase (10785 versus 14427 c.p.m.) between 40 and 110 min of incubation (probably caused by the partial cell loss after the end of the 110 min incubation) and a subsequent marked increase with an approximate doubling between 260 and 360 min of incubation (95117 versus 172136 c.p.m.). This again indicates that SV40 DNA replication is possible even after several hours of permeabilization. As mentioned above, however, the best reproducibility was obtained when incubation times were limited to ≤90 min after permeabilization.
Hypoxic incubation of permeabilized SV40-infected cells
Figures 1 and 2 demonstrate that essentially unrestricted SV40 DNA replication is possible in permeabilized CV1 cells, at least within the first hour after permeabilization. We next examined whether the response of SV40 DNA replication to hypoxia, i.e. inhibition of viral replicon initiations, is also observed in permeabilized cells.
In a first experiment, we compared the incorporation of [α-32P]-dATP into viral DNA in hypoxically [150 p.p.m. (parts per million)] and normoxically treated, permeabilized cells. As hypoxically incubated cells tend to get lost during aspiration and washing after the end of incubation, cells were prelabelled with [methyl-3H]deoxythymidine for 2 h immediately before permeabilization. For normalization according to cell number, the recovered 32P-radioactivity was divided by 3H-radioactivity for each sample.
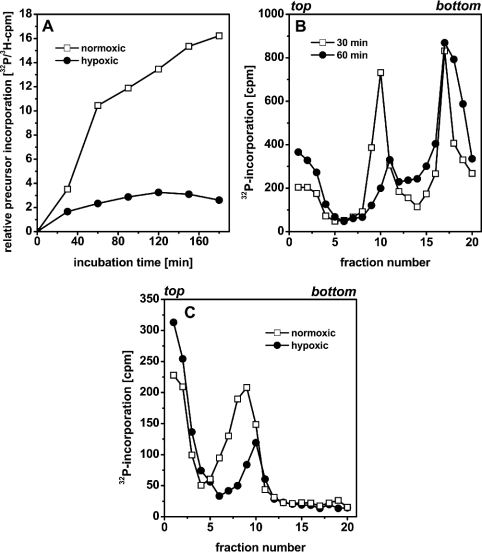
In normoxically incubated cells, incorporation showed a similar course as in Figure 1(A), i.e. a large increase until 60 min after permeabilization and a slowing down thereafter (Figure 3A). In hypoxic cells, on the other hand, incorporation of [α-32P]dATP ceased within the first 30 min of incubation.
Figure 3. Hypoxic incubation of permeabilized SV40-infected CV1 cells.
(A) Temporal course of [α-32P]dATP incorporation. Cells were prelabelled with [methyl-3H]-deoxythymidine (10 μCi/ml, 2 h, 90 μCi/nmol deoxythymidine) and permeabilized thereafter. [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP) was added to the permeabilization medium. (B) Alkaline sedimentation analysis of 32P-labelled SV40-DNA. Cells were permeabilized and labelled with [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP) for 30 or 60 min under hypoxic culture conditions. Thereafter, DNA was separated according to size by sedimentation in a 5–40% alkaline sucrose gradient. (C) Permeabilized SV40-infected cells were incubated normoxically or hypoxically for 30 min and then labelled with [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP) for 5 min. Thereafter, DNA was separated according to size by sedimentation in a 15–30% alkaline sucrose gradient. Sedimentation in (B, C) was from left to right; the top and bottom of the gradients are indicated.
We further analysed the growth of SV40 DNA daughter strands in hypoxically cultivated, permeabilized cells by means of alkaline sedimentation analysis. In analogy to the experiment shown in Figure 1(B), SV40-infected cells were permeabilized in the presence of [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP) and subsequently incubated hypoxically for 30 or 60 min. 32P-labelled DNA was analysed by centrifugation through a 5–40% sucrose gradient. Figure 3(B) shows that the amount of closed circular supercoiled SV40 DNA, represented by fractions 16–20, only slightly increased between 30 and 60 min of hypoxic incubation. The amount of growing daughter strands (fractions 8–12), on the other hand, decreased between 30 and 60 min. The shift of the peak from fraction 10 (30 min) to fraction 11 (60 min), i.e. the disappearance of low-molecular-mass SV40 DNA strands, moreover, suggests that initiations were inhibited under hypoxia.
Suppression of initiations was also confirmed by comparison of the SV40 DNA of permeabilized cells pulse-labelled for 5 min after 30 min incubation under hypoxic or normoxic culture conditions (Figure 3C). For the separation of labelled SV40 DNA, a 15–30% alkaline sucrose gradient, which better resolves low-molecular-mass DNA but also results in pelleting of mature supercoiled viral genomes, was used. Whereas DNA from hypoxic cells sedimented to fractions 8–12, DNA from normoxic cells spanned fractions 5–12. This demonstrates that labelled small SV40 DNA strands were underrepresented in the hypoxically incubated permeabilized cells, also indicating that new initiations of the viral replicons were missing. The high c.p.m. values in fractions 1–3 most probably represent unincorporated 32P-deoxynucleotides.
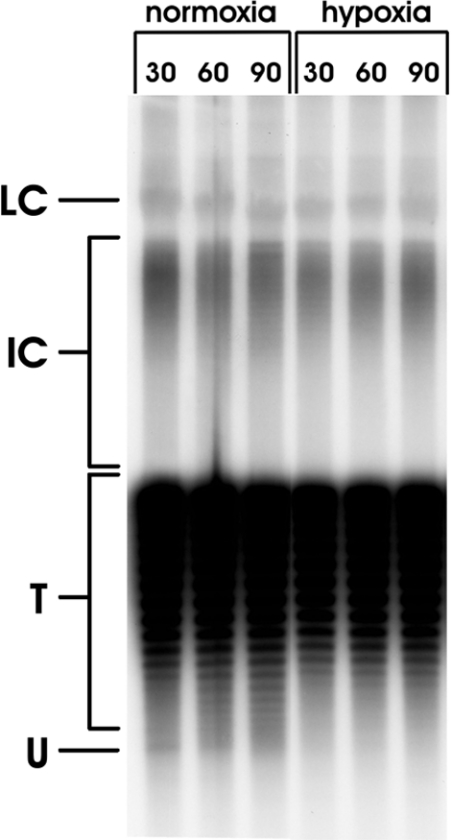
Finally, we also determined whether the highly underwound SV40 topoisomer, form U, was absent from SV40-infected permeabilized cells under hypoxic incubation conditions. SV40 form U represents the small fraction of SV40 genomes just engaged in initiation of DNA replication [4,20]. Since SV40 replication proceeds at an almost linear rate between 20 and 40 h p.i. [21], the amount of form U remains constant, whereas the amount of mature SV40 DNA increases. At 24 h p.i., form U only represents approx. 1% of the total SV40 DNA. The high background produced by the remaining SV40 genomes not engaged in DNA replication, indicated by ‘T’ in Figure 4, impede the demonstration of form U, especially in asynchronously growing SV40-infected cells [3,4]. To enhance the signal of form U, we treated the permeabilized cells with aphidicolin (2 μg/ml) for the last 15 min of hypoxic or normoxic incubation. This inhibits the elongation of newly initiated SV40 DNA molecules and thus leads to accumulation of form U [4].
Figure 4. Formation of SV40 form U in permeabilized SV40-infected cells.
Cells were permeabilized and incubated normoxically or hypoxically for the indicated times. For the last 15 min of each incubation, aphidicolin (2 μg/ml) was added. Thereafter, incubation was stopped and isolated viral DNA was separated on a chloroquine-containing agarose gel and detected after Southern blotting using 32P-labelled SV40-DNA as the probe. LC, late Cairns SV40 DNA; IC, intermediate Cairns SV40 DNA; T, topoisomers of mature SV40 DNA (form I); U, form U.
As shown in Figure 4, form U was detectable in normoxic cells but hardly at all in cells incubated hypoxically after permeabilization.
Mitochondria are involved in the regulation of SV40 DNA replication
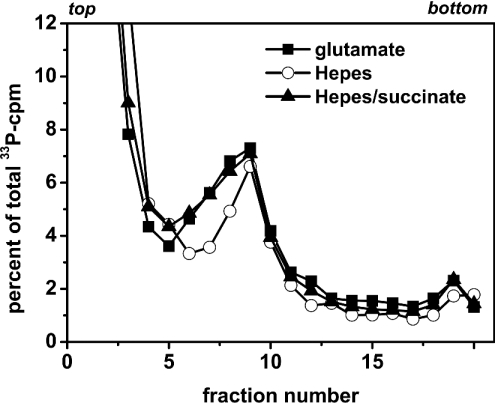
Adaptation of the permeabilization medium with respect to optimal SV40 DNA replication revealed that a medium containing potassium glutamate (120 mM) sustained SV40 DNA replication under normoxic culture conditions, whereas a medium containing Hepes instead did not. Since glutamate is a substrate delivering electrons to site I of mitochondrial respiration, we tested whether succinate, also a substrate of mitochondrial respiration, was able to rescue SV40 DNA replication in Hepes-containing permeabilization medium.
Figure 5 shows the DNA sedimentation profiles of cells labelled with Hepes buffer in the presence or absence of succinate (20 mM). For comparison, the profile of cells cultivated in glutamate buffer is also shown. Addition of succinate (20 mM) to the Hepes-containing medium significantly ameliorated SV40 DNA replication. Succinate not only increased the overall incorporation of [α-33P]dATP into growing SV40 DNA (2870 versus 1863 c.p.m. total 33P-incorporation, shown as a percentage of total 33P-incorporation in Figure 5), but especially augmented the incorporation into small-sized DNA, represented by fractions 6–8. This indicates that the addition of succinate stimulated initiation of SV40 DNA replication. Indeed, the DNA sedimentation profiles of cells cultivated either in Hepes buffer in the presence of succinate or in glutamate buffer are almost identical, indicating that DNA replication proceeds similarly in both buffers. As mentioned above, the high c.p.m. values in fractions 1–3 are probably due to unincorporated 33P-labelled deoxynucleotides.
Figure 5. SV40 DNA replication in Hepes-containing permeabilization medium.
SV40-infected CV1 cells were permeabilized either in standard permeabilization medium containing glutamate or in a medium containing Hepes instead of glutamate; the cells were incubated normoxically thereafter. Succinate (20 mM) was added to one (Hepes) culture, 15 min after permeabilization. All cultures were labelled for 5 min with [α-33P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP), 30 min after permeabilization, and processed for alkaline sedimentation on a 15–30% sucrose gradient. Sedimentation was from left to right; the top and bottom of the gradients are indicated. Total incorporation (fractions 5–20) was 2137 (glutamate buffer), 1863 (Hepes buffer) and 2870 c.p.m. (Hepes buffer+succinate).
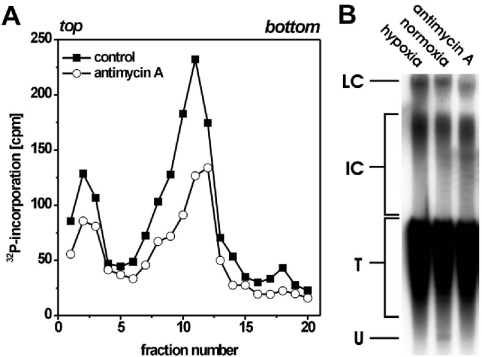
We next examined whether inhibition of mitochondrial respiration by AA in glutamate-containing permeabilization medium resulted in a similar inhibition of SV40 DNA replication as replacement of glutamate by Hepes.
As shown in Figure 6(A), the inhibitor strongly decreased the overall incorporation of [α-32P]dATP into viral DNA. Especially, diminished incorporation into small-sized DNA was seen in AA-treated permeabilized cells. Moreover, analysis of the formation of SV40 form U indicated that, as in hypoxic cells, replicon initiations did not occur in AA-inhibited permeabilized cells (Figure 6B). As mentioned above, form U represents the very small amount of total SV40 DNA that is actually initiated. Most of the SV40 genomes, represented by SV40 topoisomers (T) in Figure 6(B), are not engaged in DNA replication.
Figure 6. AA inhibits SV40 DNA replication in normoxically cultivated permeabilized cells.
(A) SV40-infected cells were permeabilized and incubated normoxically in the presence or absence of AA (2 μM). Cultures were labelled for 5 min with [α-32P]dATP (10 μCi/ml, 0.1 μCi/nmol dATP), 30 min after permeabilization, and processed for alkaline sedimentation on a 15–30% sucrose gradient. Sedimentation was from left to right; the top and bottom of the gradients are indicated. (B) Formation of SV40 form U. SV40-infected cells were permeabilized and incubated normoxically in the presence or absence of AA (2 μM) or hypoxically for 30 min. For the last 15 min, aphidicolin (2 μg/ml) was added. Thereafter, incubation was stopped and isolated viral DNA was separated on a chloroquine-containing agarose gel, blotted and detected using 32P-labelled SV40-DNA as probe. LC, late Cairns SV40 DNA; IC, intermediate Cairns SV40 DNA; T, topoisomers of mature SV40 DNA (form I); U, form U.
A similar restriction of SV40 DNA replication was obtained when rotenone, an inhibitor of site I mitochondrial respiration, was used instead of AA (results not shown).
Intracellular ATP concentration is not altered in permeabilized cells after inhibition of mitochondrial respiration
In a previous report, we showed that inhibition of SV40 DNA replication under hypoxia is not correlated with a diminished intracellular ATP concentration in unpermeabilized cells [5]. In the permeabilization system used here, ATP is regenerated extracellularly by creatine kinase and phosphocreatine. ATP depletion is therefore only possible if extracellular regeneration cannot keep track of the intracellular demand. Supposing this is the case, inhibition of mitochondrial ATP generation would probably intensify intracellular ATP shortage, possibly leading to inhibition of DNA replication.
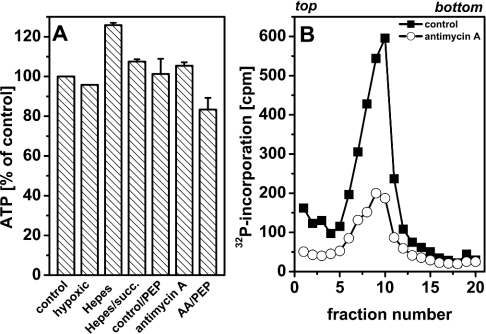
To determine whether inhibition of SV40 DNA replication in permeabilized cells is correlated with a possible intracellular ATP depletion as a result of impaired mitochondrial respiration, we measured the ATP remaining in the cells after a quick washing. Untreated permeabilized cells and permeabilized cells incubated with PEP (phosphoenol pyruvate) to allow intracellular ATP regeneration (see below) were taken as controls. After incubation, cells were briefly rinsed to keep diffusion of ATP to a minimum and quickly lysed thereafter. Figure 7(A) shows that the overall ATP recovered was not significantly changed when mitochondrial respiration was inhibited by different means.
Figure 7. ATP concentrations in SV40-infected permeabilized cells.
(A) Luminometric determination of ATP concentration. SV40-infected cells were permeabilized using glutamate- or Hepes-containing (Hepes or Hepes+succinate) permeabilization medium and incubated normoxically or hypoxically (hypoxic) in the presence or absence of the indicated effectors for 30 min. Concentrations were 20 mM for succinate (succ.), 5 mM for PEP and 2 μM for AA. Incubation was stopped by quickly rinsing the cells with ice-cold KG buffer and immediate lysis thereafter. Intracellular ATP concentration was determined luminometrically. Results are expressed as the means for two independent experiments and vertical bars indicate S.D. values. (B) SV40 DNA replication in the presence of PEP. SV40-infected cells were permeabilized in the presence or absence of AA (2 μM). PEP (5 mM) was added 10 min after permeabilization and, 20 min later, cultures were labelled with [α-32P]dATP (10 μCi/ml, 0.1 mCi/nmol dATP) for 5 min and processed for alkaline sedimentation on a 15–30% sucrose gradient. Sedimentation was from left to right; the top and bottom of the gradients are indicated.
In a further experiment, we tested whether addition of the glycolysis intermediate PEP (5 mM) can reverse the inhibitory effect of AA on SV40 DNA replication. PEP is capable of regenerating ATP from ADP within permeabilized cells, since pyruvate kinase is strongly expressed in SV40-infected CV1 cells (results not shown). Reversal of the effect of AA in this experiment may be a hint of ATP deficiency in the permeabilized cells.
Figure 7(B) shows, however, that inhibition of SV40 DNA replication by AA persisted in the presence of PEP. Taken together, these results indicate that alterations of intracellular ATP concentration are not responsible for the inhibition of SV40 DNA replication in AA-treated permeabilized cells. Instead, a mitochondrial function in the regulation of SV40 DNA replication, which is independent of ATP production, is probable.
Hypoxia-induced inhibition of SV40 DNA replication is mimicked by AA in unpermeabilized cells
The results presented above indicate that mitochondria are involved in the regulation of SV40 DNA replication. To demonstrate that the effects are not restricted to permeabilized cells, we examined whether AA is capable of mimicking the hypoxia-induced inhibition of SV40 DNA replication in normoxic unpermeabilized cells. As reported previously, inhibition of SV40 DNA replication under hypoxia only takes place under ‘low-glucose’ conditions [5].
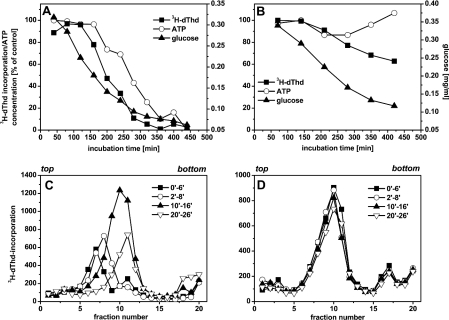
In a first experiment, SV40-infected cells were cultivated in the presence or absence of AA, pulse-labelled with [methyl-3H]-deoxythymidine at different times after the start of incubation and subsequently processed for the determination of acid-precipitable radioactivity. Concomitantly, intracellular ATP concentration and glucose concentration in the cell culture supernatant were determined.
Figure 8(A) shows that the incorporation of [methyl-3H]deoxythymidine decreased to almost zero after 7 h of incubation in the presence of AA. Intracellular ATP concentration also decreased. However, this decrease was distinctly delayed compared with the decrease of [methyl-3H]deoxythymidine incorporation. It is therefore unlikely that ATP depletion causes inhibition of DNA replication. On the other hand, the decrease of [methyl-3H]deoxythymidine incorporated into DNA began when the glucose concentration in the culture fluid decreased below approx. 0.2 mg/ml. Thus inhibition of SV40 DNA replication emerged at the same glucose concentration as that found earlier in hypoxically incubated CV1 cells [5].
Figure 8. SV40 DNA replication in unpermeabilized AA-treated cells.
(A, B) Temporal course of [methyl-3H]deoxythymidine (dThd) incorporation and of glucose and ATP concentrations. SV40-infected cells were incubated normoxically in the presence (A) or absence (B) of AA (2 μM), and the ATP and glucose concentrations as well as incorporation of [methyl-3H]deoxythymidine (10 μCi/ml, 2 μM≡4.7 μCi/nmol deoxythymidine) into SV40 DNA were determined at the indicated times. Glucose concentration at the start of incubation was 0.4 mg/ml. (C, D) Induction of a synchronous viral DNA replication round after the addition of glucose to AA-treated SV40-infected cells. Cell cultures containing 0.4 mg of glucose/ml were incubated in the presence (C) or absence (D) of AA (2 μM) for 7 h. Subsequently, glucose (1 mg/ml) was added, and cells were labelled with [methyl-3H]deoxythymidine (10 μCi/ml, 90 μCi/nmol deoxythymidine) at different times thereafter. Labelled DNA was separated by alkaline centrifugation using a 5–40% sucrose gradient. Sedimentation was from left to right; the top and bottom of the gradients are indicated.
In the absence of AA, incorporation of [methyl-3H]deoxythymidine only slightly decreased and ATP concentration remained constant for the first 5 h and then increased (Figure 8B).
Addition of glucose to 1 mg/ml after 7 h of incubation induced new initiations and a synchronous round of SV40 DNA replication in AA-treated cells (Figure 8C), but not in untreated cells (Figure 8D). This was indicated by an increased shifting of labelled DNA molecules to higher sedimentation fractions with increase in incubation times in the AA-treated cells. Essentially the same results had been obtained earlier when glucose was added to SV40-infected cells which were incubated hypoxically, and under glucose-limiting conditions for several hours [5]. Thus glucose exerts the same effect on SV40 DNA replication in AA-treated and hypoxic cells.
DISCUSSION
SV40-infected CV1 cells as well as other eukaryotic cells are subject to O2-dependent regulation of DNA replication [1–5]. To study further this phenomenon, we established an α-toxin-based permeabilization system capable of sustaining SV40 DNA replication in virus-infected CV1 cells. As the permeabilization medium contained creatine kinase and phosphocreatine for regenerating ATP, SV40 DNA replication is supposed to be independent of intracellular ATP generation, which may be impaired under hypoxic/hypoglycaemic incubation conditions. In the first part of the present study, we have provided evidence that SV40 DNA replication in α-toxin-permeabilized CV1 cells proceeds essentially as in vivo. Viral DNA synthesis, measured as [α-32P]dATP incorporation into acid-precipitable material, occurred for at least 60 min at a nearly constant rate in the permeabilized cells and slowed down during prolonged incubation (Figure 1).
Alkaline sedimentation analysis of 32P-labelled viral DNA demonstrated that incorporation of [α-32P]dATP into permeabilized cells represented replication of SV40 DNA.
The occurrence of complete repeated rounds of SV40 DNA replication in permeabilized cells was confirmed by means of isopycnic banding of SV40 DNA density-labelled with BrdUTP. A small peak of DNA density-labelled in both strands (HH-DNA), i.e. DNA that had been replicated at least twice during the permeabilization time, emerged after 110 min and further increased up to 360 min after the start of incubation (Figure 2).
Permeabilized SV40-infected cells incubated under hypoxic conditions showed a significant decrease in SV40 DNA replication. Analysis of growing SV40 DNA daughter strands by alkaline sucrose gradient centrifugation revealed that low-molecular-mass DNA strands were significantly underrepresented (Figure 3). Furthermore, generation of SV40 form U, indicative of initiation of viral DNA replication was distinctly decreased under hypoxia (Figure 4). Taken together, these results demonstrate that hypoxia exerts a similar inhibitory effect on SV40 DNA replication in permeabilized and in unpermeabilized cells.
The hypoxia-dependent decrease of SV40 DNA replication is most probably not caused by a depletion of intracellular ATP, because ATP is delivered by the ATP-regenerating system in the permeabilization medium. Determination of intracellular ATP concentration gave no hint that regeneration did not keep step with the intracellular ATP demand even when mitochondrial respiration was compromised (Figure 7). We demonstrated earlier that inhibition of SV40 DNA replication under hypoxia is not accompanied by intracellular ATP depletion in unpermeabilized cells [5].
A regulatory role of the concentration of dCTP or other dNTPs, as we have proposed for other cell lines in previously published studies [22,23], also appears unlikely, as these compounds are present in the permeabilization medium.
In analogy to hypoxia, incubation of normoxically cultivated permeabilized cells with AA, a site III inhibitor of mitochondrial respiration, or replacement of glutamate by Hepes in the permeabilization medium also led to a significant inhibition of (initiation of) SV40 DNA replication (Figures 5 and 6). In unpermeabilized SV40-infected cells, AA inhibited the incorporation of [methyl-3H]deoxythymidine into viral DNA when the glucose concentration in the cell culture supernatant decreased below ≈0.2 mg/ml (Figure 8). Re-addition of glucose to AA-treated cells after several hours of (normoxic) incubation led to new SV40 replicon initiations, followed by a synchronous round of viral DNA replication. Essentially the same response was obtained when SV40-infected cells were incubated hypoxically for several hours and then treated with glucose under hypoxia [5].
These results strongly suggest that mitochondria are involved in the fast O2-dependent regulation of SV40 DNA replication in virus-infected CV1 cells. DNA replication under glucose-limiting conditions probably depends on respiring or at least energized mitochondria since inhibition of respiration by hypoxia or AA or by withdrawal of substrates of mitochondrial respiration apparently leads to inhibition of DNA replication.
Are mitochondria the cellular O2 sensors for O2-dependent regulation of (SV40) DNA replication? To be considered for such a function, the O2 range where mitochondrial respiration becomes impeded should be the same as that where DNA replication is decreased by the fast O2-dependent regulation, i.e. 1000–100 p.p.m. (0.1–0.01%) O2 [1]. This seems to be the case: Gnaiger et al. [24] showed that the oxygen concentration necessary to support half-maximal mitochondrial respiration is over the range of 250 p.p.m. in resting liver mitochondria and increases 2–3-fold on stimulation of respiration by ADP [25]. In Ehrlich ascites cells, half-maximal respiration was found at 250 p.p.m. oxygen [26]. Oxygen pressures of 0.3–0.4% are observed in the microenvironment of mitochondria in tissues under normoxia [25,27–29], indicating that these O2 concentrations are not yet completely unphysiological.
Another important question is how transduction of the hypothetical signal from mitochondria to the cell nucleus can be realized. One possibility is that ROS (reactive oxygen species) are involved in signal transduction. Besides their cell-damaging effects, ROS have been shown to act as signal transducers, which evoke several intracellular events, such as gene activation, induction of protein tyrosine phosphorylation and proliferation [30–32].
It is known that mitochondria are the main source of ROS in mammalian cells [33] and that mitochondrial ROS generation depends on substrates of the electron-transport chain and a high mitochondrial membrane potential [34,35]. Actually, mitochondria-generated ROS have been shown to act as second messengers in several hypoxia-induced processes [36–38].
Thus the following signal transduction for oxygen-dependent regulation of DNA replication seems possible: under normoxic conditions, mitochondrial respiration induces ROS generation, which in turn activates (by as yet unknown mechanisms) replicon initiation. Hypoxia (or inhibition of respiration by other means) causes decreased production of ROS and thereby inhibition of DNA replication. In support of this, ROS formation was reported to decrease under hypoxia compared with normoxia [39–41]. Our own experiments, although still preliminary, also indicated that ROS concentration decreases in hypoxically incubated SV40-infected cells (results not shown).
Paradoxically, however, the opposite, i.e. increased ROS formation, was also observed under hypoxia, and many effects are reported to depend on this increase of ROS concentration in the cell [37,38,42,43]. These discrepancies may be explained by different hypoxic incubation conditions. At moderate hypoxic O2 concentrations (5–0.2%) or for a short hypoxic incubation, ROS formation may be enhanced. On the other hand, prolonged incubation under severe hypoxia or anoxia, however, probably diminishes ROS formation because of substrate deficiency. Both an increase as well as a decrease of ROS after hypoxia may be used as a part of signal-transduction pathways for decreased intracellular O2 concentration [44], at least in distinct cell types. Interestingly, opposite to the effect we found for DNA replication under hypoxia, Schafer et al. [42] observed a stimulation in the proliferation of hypoxically incubated endothelial cells, and this stimulation was caused by an increased ROS formation compared with normoxia.
By analogy to hypoxia, inhibition of the electron-transport chain with AA produced different effects concerning generation of ROS. On one hand, AA and another inhibitor of site III respiration, myxothiazol, were reported to stimulate generation of ROS [45,46]. On the other, AA also showed the opposite effect, i.e. a suppression of ROS formation compared with untreated control cells [47].
At the present time, we cannot yet decide whether mitochondria-generated ROS are involved in the regulation of SV40 DNA replication in CV1 cells. The involvement of mitochondria themselves, however, is strongly supported by our results.
Acknowledgments
We thank G. Fuchs [Institut für Biologie II (Mikrobiologie), Universität Freiburg, Freiburg, Germany] and M. Gratzl (Anatomisches Institut, Ludwig-Maximilian Universität München, München, Germany) for providing Staph. aureus and H. J. Zoller for advice on the preparation of α-toxin. G. Probst (Physiologisch-chemisches Institut, Universität Tübingen, Tübingen, Germany) is acknowledged for a critical reading of this paper. This work was supported by the Deutsche Forschungsgemeinschaft (Pr 95/11-1).
References
- 1.Probst H., Schiffer H., Gekeler V., Kienzle-Pfeilsticker H., Stropp U., Stötzer K.-E., Frenzel-Stötzer I. Oxygen dependent regulation of DNA synthesis and growth of Ehrlich ascites cells in vitro and in vivo. Cancer Res. 1988;48:2053–2060. [PubMed] [Google Scholar]
- 2.Probst G., Riedinger H.-J., Martin P., Engelcke M., Probst H. Fast control of DNA replication in response to hypoxia and to inhibited protein synthesis in CCRF-CEM and HeLa cells. Biol. Chem. 1999;380:1371–1382. doi: 10.1515/BC.1999.177. [DOI] [PubMed] [Google Scholar]
- 3.Dreier T., Scheidtmann K. H., Probst H. Synchronous replication of SV40 DNA in virus infected TC 7 cells induced by transient hypoxia. FEBS Lett. 1993;336:445–451. doi: 10.1016/0014-5793(93)80853-m. [DOI] [PubMed] [Google Scholar]
- 4.Riedinger H.-J., van Betteraey M., Probst H. Hypoxia blocks in vivo initiation of simian virus 40 replication at a stage preceding origin unwinding. J. Virol. 1999;73:2243–2252. doi: 10.1128/jvi.73.3.2243-2252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedinger H.-J., van Betteraey-Nikoleit M., Hilfrich U., Eisele K.-H., Probst H. Oxygen-dependent regulation of in vivo replication of simian virus 40 DNA is modulated by glucose. J. Biol. Chem. 2001;276:47122–47130. doi: 10.1074/jbc.M106938200. [DOI] [PubMed] [Google Scholar]
- 6.Van der Velden H. M. V., Poot M., Wanka F. In vitro DNA replication in association with the nuclear matrix of permeable cells. Biochim. Biophys. Acta. 1984;782:429–436. doi: 10.1016/0167-4781(84)90050-2. [DOI] [PubMed] [Google Scholar]
- 7.Berger N., Petzold S. J., Johnson E. S. High molecular weight DNA intermediates synthesized by permeabilized cells. Biochim. Biophys. Acta. 1977;478:44–58. doi: 10.1016/0005-2787(77)90242-8. [DOI] [PubMed] [Google Scholar]
- 8.Miller M. R., Castellot J. J., Jr, Pardee A. B. A permeable animal cell preparation for studying macromolecular synthesis. DNA synthesis and role of deoxynucleotides in S phase initiation. Biochemistry. 1978;17:1073–1080. doi: 10.1021/bi00599a021. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar N., List J. F., Banfalvi G. Replication of the origin region of simian virus 40 DNA in permeabilized monkey cells. Eur. J. Biochem. 1987;168:263–268. doi: 10.1111/j.1432-1033.1987.tb13415.x. [DOI] [PubMed] [Google Scholar]
- 10.Lind I., Ahnert-Hilger G., Fuchs G., Gratzl M. Purification of alpha-toxin from Staphylococcus aureus and application to cell permeabilization. Anal. Biochem. 1987;164:84–89. doi: 10.1016/0003-2697(87)90371-x. [DOI] [PubMed] [Google Scholar]
- 11.Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahnert-Hilger G., Stecher B., Beyer C., Gratzl M. Exocytotic membrane fusion as studied in toxin-permeabilized cells. Methods Enzymol. 1993;221:139–149. doi: 10.1016/0076-6879(93)21013-x. [DOI] [PubMed] [Google Scholar]
- 13.Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl H., Knippers R. Simian virus 40 large tumor antigen on replicating viral chromatin. J. Virol. 1983;47:65–76. doi: 10.1128/jvi.47.1.65-76.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Probst H., Hofstaetter T., Jenke H.-S., Gentner P. R., Müller-Scholz D. Metabolism and non-random occurrence of nonnascent short chains in the DNA of Ehrlich ascites cells. Biochim. Biophys. Acta. 1983;740:200–211. doi: 10.1016/0167-4781(83)90078-7. [DOI] [PubMed] [Google Scholar]
- 16.Probst H., Riedinger H.-J., Gekeler V. No significant overreplication occurs in Ehrlich ascites cells during and after reversal of hypoxia. Exp. Cell Res. 1989;180:563–568. doi: 10.1016/0014-4827(89)90084-0. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. Molecular Cloning: A Laboratory Manual; pp. 45–46. [Google Scholar]
- 18.Diddens H., Gekeler V., Neumann M., Niethammer D. Characterization of actinomycin-D-resistant CHO cell lines exhibiting a multidrug-resistance phenotype and amplified DNA sequences. Int. J. Cancer. 1987;40:635–642. doi: 10.1002/ijc.2910400511. [DOI] [PubMed] [Google Scholar]
- 19.Snapka R. M., Permana P. A., Marquit G., Shin C.-G. Two-dimensional agarose gel analysis of simian virus 40 DNA replication intermediates. Methods. 1991;3:73–82. [Google Scholar]
- 20.Dean F. B., Bullock P., Murakami Y., Wobbe C. R., Weissbach L., Hurwitz J. Simian virus 40 (SV40) DNA replication: SV40 large T antigen unwinds DNA containing the SV40 origin of replication. Proc. Natl. Acad. Sci. U.S.A. 1987;84:16–20. doi: 10.1073/pnas.84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirmbeck R., Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J. Virol. 1987;61:3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brischwein K., Engelcke M., Riedinger H.-J., Probst H. Role of ribonucleotide reductase and deoxynucleotide pools in the oxygen-dependent control of DNA replication in Ehrlich ascites cells. Eur. J. Biochem. 1997;244:286–293. doi: 10.1111/j.1432-1033.1997.00286.x. [DOI] [PubMed] [Google Scholar]
- 23.Probst H., Schiffer H., Gekeler V., Scheffler K. Oxygen dependent regulation of mammalian ribonucleotide reductase in vivo and possible significance for replicon initiation. Biochem. Biophys. Res. Commun. 1989;163:334–340. doi: 10.1016/0006-291x(89)92140-2. [DOI] [PubMed] [Google Scholar]
- 24.Gnaiger E., Mendez G., Hand S. C. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnaiger E., Lassnig B., Kuznetsov A. V., Margreiter R. Mitochondrial respiration in the low oxygen environment of the cell. Effect of ADP on oxygen kinetics. Biochim. Biophys. Acta. 1998;1365:249–254. doi: 10.1016/s0005-2728(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 26.Froese G. The respiration of ascites tumor cells at low oxygen concentrations. Biochim. Biophys. Acta. 1962;57:509–519. doi: 10.1016/0006-3002(62)91158-7. [DOI] [PubMed] [Google Scholar]
- 27.Jones D. P. Intracellular diffusion gradients of O2 and ATP. Am. J. Physiol. 1986;250:C663–C675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 28.Wittenberg B. A., Wittenberg J. B. Transport of oxygen in muscle. Annu. Rev. Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 29.Connett R. J., Honig C. R., Gayeski T. E. J., Brooks G. A. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J. Appl. Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar]
- 30.Masutani H., Ueno M., Ueda S., Yodoi J. Role of thioredoxin and redox regulation in oxidative stress response and signaling. In: Sen C. K., Sies H., Baeuerle P. A., editors. Antioxidant and Redox Regulation of Genes. San Diego, CA: Academic Press; 2000. pp. 297–310. [Google Scholar]
- 31.Nakamura H., Nakamura K., Yodoi J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y. S., Jin D. Q., Park S. H., Han S. Y., Kim H. S., Jeong T. C., Huh K., Kim J. A. 2,3,7,8-Tetrachlorobenzo-p-dioxin inhibits proliferation of SK-N-SH human neuronal cells through decreased production of reactive oxygen species. Free Radical Res. 2002;36:1283–1289. doi: 10.1080/1071576021000016517. [DOI] [PubMed] [Google Scholar]
- 33.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 34.Liu S. S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 35.Korshunov S. S., Skulachev V. P., Starkov A. A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 36.Waypa G. B., Chandel N. S., Schumaker P. T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ. Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 37.Chandel N. S., Schumaker P. T. Cellular oxygen sensing by mitochondria: old questions, new insight. J. Appl. Physiol. 2000;88:1880–1889. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 38.Chandel N. S., Maltepe E., Goldwasser E., Mathieu C. E., Simon M. C., Schumaker P. T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heberlein W., Wodopia R., Bartsch P., Mairbaurl H. Possible role of ROS as mediators of hypoxia-induced ion transport inhibition of alveolar epithelial cells. Am. J. Physiol. 2000;278:L640–L648. doi: 10.1152/ajplung.2000.278.4.L640. [DOI] [PubMed] [Google Scholar]
- 40.Lelkes P. I., Hahn K. L., Sukovich D. A., Karmiol S., Schmidt D. H. On the possible role of reactive oxygen species in angiogenesis. Adv. Exp. Med. Biol. 1998;454:295–310. doi: 10.1007/978-1-4615-4863-8_35. [DOI] [PubMed] [Google Scholar]
- 41.Yang W., Block E. R. Effect of hypoxia and reoxygenation on the formation and release of reactive oxygen species by porcine pulmonary artery endothelial cells. J. Cell. Physiol. 1995;164:414–423. doi: 10.1002/jcp.1041640222. [DOI] [PubMed] [Google Scholar]
- 42.Schafer M., Schafer C., Ewald N., Piper H. M., Noll T. Role of redox signaling in the autonomous proliferative response of endothelial cells to hypoxia. Circ. Res. 2003;92:1010–1015. doi: 10.1161/01.RES.0000070882.81508.FC. [DOI] [PubMed] [Google Scholar]
- 43.Duranteau J., Chandel N. S., Kulisz A., Shao Z., Schumaker P. T. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 44.Waypa G. B., Schumaker P. T. O2 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door re-opens. Respir. Physiol. Neurobiol. 2002;132:81–91. doi: 10.1016/s1569-9048(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 45.Jeong J. I., Lee Y. W., Kim Y. K. Chemical hypoxia-induced cell death in human glioma cells: role of reactive oxygen species, ATP depletion, mitochondrial damage and Ca2+ Neurochem. Res. 2003;28:1201–1211. doi: 10.1023/a:1024280429036. [DOI] [PubMed] [Google Scholar]
- 46.Young T. A., Cunningham C. C., Bailey S. M. Reactive oxygen species production by mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch. Biochem. Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong J. S., Jones D. P. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]