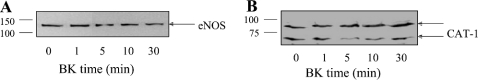Abstract
eNOS (endothelial nitric oxide synthase) catalyses the conversion of L-arginine into L-citrulline and NO. Evidence has been presented previously that eNOS is associated with the CAT (cationic amino acid transporter)-1 arginine transporter in endothelial caveolae, and it has been proposed that eNOS–CAT-1 association facilitates the delivery of extracellular L-arginine to eNOS. Definitive proof of a protein–protein interaction between eNOS and CAT-1 is lacking, however, and it is also unknown whether the two proteins interact directly or via an adaptor protein. In the present study, we raised a polyclonal antibody against CAT-1, and show using reciprocal co-immunoprecipitation protocols that eNOS and CAT-1 do indeed form a complex in BAECs (bovine aortic endothelial cells). In vitro binding assays with GST (glutathione S-transferase)–CAT-1 fusion proteins and eNOS show that the two proteins interact directly and that no single CAT-1 intracellular domain is sufficient to mediate the interaction. Overexpression of CAT-1 in BAECs by adenoviral-mediated gene transfer results in significant increases in both L-arginine uptake and NO production by the cells. However, whereas increased L-arginine transport is reversed completely by the CAT-1 inhibitor, L-lysine, increased NO release is unaltered, suggesting that NO production in this in vitro model is independent of CAT-1-mediated transport. Furthermore, eNOS enzymic activity is increased in lysates of CAT-1-overexpressing cells accompanied by increased phosphorylation of eNOS at Ser-1179 and Ser-635, and decreased association of eNOS with caveolin-1. Taken together, these data suggest that direct interaction of eNOS with CAT-1 enhances NO release by a mechanism not involving arginine transport.
Keywords: arginine paradox, arginine transport, CAT-1, endothelial nitric oxide synthase (eNOS), protein–protein interaction
Abbreviations: Ad-CAT-1, adenovirus carrying mouse full-length CAT-1; Ad-β-Gal, control adenovirus carrying β-galactosidase; BAEC, bovine aortic endothelial cell; CAT, cationic amino acid transporter; CMV, cytomegalovirus; eNOS, endothelial nitric oxide synthase; GST, glutathione S-transferase; Hsp90, heat-shock protein 90; ID, intracellular domain; MOI, multiplicity of infection; Ni-NTA, Ni2+-nitrilotriacetate; RASM, rat aortic smooth muscle cell; sGC, soluble guanylate cyclase
INTRODUCTION
The vascular homoeostatic enzyme, eNOS (endothelial nitric oxide synthase) catalyses the oxidation of L-arginine (hereafter referred to simply as arginine) to L-citrulline and nitric oxide (NO). NO, thus produced, plays a key role in regulation of vascular tone and in suppression of atherogenesis. eNOS is subject to tight regulation at the transcriptional, post-transcriptional (mRNA stability) and post-translational levels [1]. One important aspect of post-translational regulation involves covalent modification of eNOS by myristoylation, palmitoylation and phosphorylation. A second element of post-translational regulation occurs through protein–protein interactions. Among the proteins that have been suggested to interact directly or indirectly with eNOS in endothelial cells are Ca2+-calmodulin, the plasmalemmal caveolae structural protein, caveolin-1, certain G-protein-coupled receptors, such as the bradykinin B2 receptor, Hsp90 (heat-shock protein 90), Akt protein kinase, dynamin-2, the porin anion channel, sGC (soluble guanylate cyclase), two eNOS trafficking proteins known as NOSIP (nitric oxide synthase-interacting protein) and NOSTRIN (nitric oxide synthase trafficking inducer), and the CAT (cationic amino acid transporter) known as CAT-1 [2].
The transport of arginine and other cationic amino acids in different types of cells is mediated by the system y+ carrier [3]. In endothelial cells, system y+ functions through the CAT-1, which is responsible for 70–95% of arginine uptake [4]. CAT-1 is believed to exist in a complex with eNOS in endothelial cells based on a report by McDonald et al. [5] that showed that the two proteins are co-localized in endothelial plasmalemmal caveolae. These authors showed further that immunodepletion of endothelial cell lysates with an anti-eNOS antibody results in a reduction in the rate of arginine transport into proteoliposomes reconstituted from immunoprecipitation supernatants [5]. Alternative explanations for the results of McDonald et al. [5] are also possible. For example, CAT-1 activity could be regulated by S-nitrosylation, phosphorylation or other covalent modification of the CAT-1 protein, which could be reduced in endothelial lysates following eNOS immunodepletion. eNOS immunodepletion of lysates could also lead to an increased intraliposomal arginine concentration in reconstituted proteoliposomes, which could also decrease the rate of arginine uptake. We have therefore sought to obtain more definitive evidence for an eNOS–CAT-1 interaction in endothelial cells. We have raised and affinity-purified a polyclonal anti-CAT-1 antibody to test whether the antibody can co-immunoprecipitate the eNOS protein from endothelial cell lysates. Furthermore, it is known that the eNOS interaction with some proteins is indirect. For example, we have shown recently that the eNOS interaction with sGC is not direct, but rather occurs through Hsp90 acting as an adaptor protein for eNOS and sGC association [6]. We have therefore prepared GST (glutathione S-transferase) fusion proteins of full-length CAT-1 and each of the eight predicted intracellular domains of CAT-1 for use in in vitro binding assays with purified eNOS in order to determine whether the eNOS–CAT-1 interaction is direct or indirect and to determine whether or not a single CAT-1 intracellular domain is sufficient to mediate the interaction.
eNOS–CAT-1 association has been proposed as an explanation for the ‘arginine paradox’ [5]. The arginine paradox arises from the observation that, while the Km of eNOS for arginine is in the range 3–5 μM [7,8], the intracellular concentration of arginine in endothelial cells is in the range 100–800 μM [9–11]. The eNOS enzyme should thus be saturated with substrate at all times, and extracellular arginine availability should not affect endothelial cell NO release. However, a number of in vivo studies have shown that extracellular arginine administration, either via feeding or intravenous infusion, improves endothelium-dependent vascular relaxation and release of NO [12–15]. This circumstance, in which exogenous arginine drives endothelial NO production even though intracellular levels of arginine are in excess, has been termed the arginine paradox. McDonald et al. [5] have suggested that this paradox may be explained by the existence of an eNOS–CAT-1 complex that provides directed delivery of extracellular arginine to eNOS in endothelial caveolae.
Almost all of the evidence for the existence of an arginine paradox has come from in vivo studies. The dependence of eNOS on extracellular arginine has been much more difficult to demonstrate in vitro. For example, in the very first report that demonstrated that endothelial cell NO production was dependent on arginine, Palmer et al. [16] showed that arginine did not stimulate NO production from cultured cells unless the cells were deprived of the amino acid for 24 h. Similarly, Arnal et al. [8] showed that bradykinin-stimulated NO release from cultured endothelial cells was not significantly increased by inclusion of arginine in the cell culture medium compared with that from cells grown in the complete absence of arginine [8]. In addition, in vitro treatment of aortic rings with arginine does not appear to improve relaxations to acetylcholine [17,18]. Indeed, if endothelium-dependent relaxation of blood vessel rings was dependent on extracellular arginine, it would be necessary to include arginine in the bathing medium in such studies, a practice that is not commonly followed. Because many in vitro studies do not support the existence of an arginine paradox in endothelial cells, it has been suggested that arginine might stimulate vasodilation in vivo by a mechanism that has nothing to do with its serving as a substrate for eNOS [18]. One such mechanism has been demonstrated by Giugliano et al. [19] to involve the long-known effect of arginine to stimulate insulin release from pancreatic β-cells. Insulin is a well-known vasodilating hormone and stimulator of endothelial cell NO release; however, it remains possible that insulin release does not completely account for the arginine paradox. In order to address this issue further, in the present study, we have used adenovirus-mediated gene transfer of CAT-1 into endothelial cells in order to determine whether increased arginine transport resulting from CAT-1 overexpression results in increased NO release.
EXPERIMENTAL
Preparation and purification of an anti-CAT-1 polyclonal antibody
A GST fusion protein containing mouse CAT-1 intracellular domain (ID) 6 (GST–CAT-1-ID6) was expressed and purified as described below. Rabbits were injected initially with 500 μg of protein followed by four booster injections of 250 μg each at two week intervals (Covance Research Products, Richmond, CA, U.S.A.). Serum was obtained 2 weeks after the final injection, and anti-CAT-1 antibody was purified by a two-step process of depletion of anti-GST antibody and affinity purification of anti-CAT-1 antibody. Depletion matrix was prepared by binding 500 mg of total soluble proteins from Escherichia coli expressing GST to 12 g of CNBr-activated Sepharose 4B (Amersham Biosciences, Piscataway, NJ, U.S.A.). Affinity matrix was prepared by binding 40 mg of total soluble proteins from E. coli expressing GST–CAT-1-ID6 to 3 g of CNBr-activated Sepharose 4B. A 4 ml volume of crude serum was incubated with an 8 ml slurry of depletion matrix. After shaking at room temperature (25 °C) for 1.5 h, the mixture was centrifuged at 200 g for 2 min at 4 °C, and the supernatant was transferred to a 1 ml slurry of affinity matrix. Following incubation with rocking for 2 h at room temperature, the affinity matrix and depleted serum were passed through a 3 ml syringe with glass cotton at the bottom. After washing with 25 ml of TTBS [10 mM Tris/HCl, pH 8.0, 150 mM NaCl and 0.05% (v/v) Tween 20], the anti-CAT-1-ID6 polyclonal antibody was eluted from the matrix with 0.15 mM glycine, pH 2.5. Antibody solution was stored in aliquots in 10% (v/v) glycerol at −80 °C.
Cell culture
Primary cultures of BAECs (bovine aortic endothelial cells) were obtained from VEC Technologies (Rensselaer, NY, U.S.A.) and used in experiments during passages 2–5. Cultures were maintained in M199 medium supplemented with 10% (v/v) foetal bovine serum, 5% (v/v) iron-supplemented calf serum, 20 μg/ml L-glutamine, 0.6 μg/ml thymidine, 500 units/ml penicillin and 500 μg/ml streptomycin. Primary cultures of RASMs (rat aortic smooth muscle cells) were also obtained from VEC Technologies, and were used in experiments during passages 2–5. Cultures were maintained in DMEM (Dulbecco's modified Eagle's medium) that contained 10% (v/v) foetal bovine serum, 500 units/ml penicillin and 500 μg/ml streptomycin.
Immunoprecipitation and immunoblotting
Confluent BAEC cultures in 100 mm dishes were lysed in ice-cold lysis buffer containing 50 mM Tris/HCl, pH 7.4, 100 mM NaF, 15 mM Na4P2O7, 1 mM Na3VO4, 1% (v/v) Triton X-100, 1 mM PMSF, 10 μg/ml pepstatin A and 5 μg/ml aprotinin. Lysates were centrifuged at 10000 g to remove insoluble material and were then pre-cleared by adding 50 μl of Protein A/G–agarose (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), followed by incubation for 2 h at 4 °C with rocking. The agarose beads were then pelleted by centrifugation at 1000 g. Anti-CAT-1-ID6 antibody (30 μl) or anti-eNOS antibody (10 μl) (BD Biosciences, San Diego, CA, U.S.A.) was then added to the pre-cleared lysate, and samples were incubated overnight at 4 °C with rocking. Beads were subsequently washed twice with ice-cold lysis buffer. Immunoprecipitated proteins were eluted from the beads by boiling for 5 min in SDS sample buffer. Anti-CAT-1 immunoprecipitates were immunoblotted with anti-eNOS antibody (1:1000 dilution), and anti-eNOS immunoprecipitates were immunoblotted with anti-CAT-1-ID6 antibody (1:4000 dilution). In some experiments, lysates were subjected to immunoblotting without immunoprecipitation. Antibodies used in these experiments were anti-CAT-1 (1:4000 dilution), anti-phospho-Ser-1179 eNOS (1:1000 dilution) (Upstate Biotechnology, Waltham, MA, U.S.A.), anti-phospho-Ser-635 (1:1000 dilution) (Upstate Biotechnology) and anti-caveolin-1 (1:1000 dilution) (BD Biosciences).
Detection of glycosylation and deglycosylation
CAT-1 was immunoprecipitated from BAEC lysates, subjected to SDS/PAGE, and electroblotted on to nitrocellulose membranes. Glycosylation of proteins on the blots were detected by the periodic acid–Schiff method [20] using a commercially available Glycoprotein Detection Kit (Sigma–Aldrich, St. Louis, MO, U.S.A.), according to the manufacturer's instructions. Deglycosylation of CAT-1 proteins was detected using a commercially available Enzymatic Protein Deglycosylation Kit (Sigma–Aldrich), according to the manufacturer's instructions. Reaction buffer (5×) was added to BAEC lysates. PNGase F (peptide N-glycosidase F), O-glycosidase and α-2 (3,6,8,9) neuraminidase solution, or the same volume of sterile water (control), were added to the lysate, and the mixture was incubated for 3 days at 37 °C. An aliquot of each reaction mixture was immunoblotted with anti-CAT-1-ID6 antibody (1:4000 dilution).
Construction and purification of GST–CAT-1 fusion proteins
The cDNA for mouse CAT-1 was generously provided by Dr James M. Cunningham (Harvard University, Boston, MA, U.S.A.) and was used as the template for PCR amplification of full-length CAT-1 (residues 1–622) and CAT-1 ID1 (residues 1–36), ID2 (residues 83–102), ID3 (residues 184–191), ID4 (residues 261–280), ID5 (residues 352–377), ID6 (residues 423–487), ID7 (540–552) and ID8 (residues 603–622). Primers used for PCR contained 5′ EcoRI and SalI restriction enzyme sites to facilitate subcloning into the GST fusion protein cloning vector, pGEX-4T-1 (Amersham Biosciences). The DNA sequence encoding each of the fusion proteins was verified by sequencing in the Molecular Biology Core Facility of the Medical College of Georgia. GST–CAT-1 fusion proteins and a GST non-fusion protein were expressed in E. coli and were purified by affinity binding to glutathione–Sepharose 4B (Amersham Biosciences) as described previously [21,22].
Expression and purification of eNOS
Bovine eNOS was expressed in a baculovirus/Sf9 insect cell system, and was purified to apparent homogeneity by affinity binding to 2′,5′-ADP–Sepharose as described previously [23].
In vitro binding of eNOS to GST–CAT-1 fusion proteins
GST, full-length GST–CAT-1 and GST fusion proteins containing ID1, ID2, ID3, ID4, ID5, ID6, ID7 or ID8 of CAT-1 (100 pmol of each), pre-bound to glutathione–agarose beads, were incubated overnight (at 4 °C with shaking) with 100 pmol of purified eNOS in 1 ml of buffer containing 50 mM Tris/HCl, pH 7.4, 20% (v/v) glycerol and the following protease inhibitors: 1% PMSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin A and 5 μg/ml aprotinin. After overnight incubation, beads were washed six times in 1 ml of buffer containing 50 mM Hepes, pH 7.5, 1 M NaCl, 1 mM EDTA, 0.5% (w/v) CHAPS and protease inhibitors. Proteins that remained bound to the beads after washing were eluted with 100 mM reduced glutathione, 50 mM Tris/HCl, pH 8.0, 1 mM EDTA and 1% (v/v) Triton X-100, plus protease inhibitors. Eluted proteins were immunoblotted with anti-eNOS antibody (1:1000 dilution).
Construction and purification of Ad-CAT-1 (adenovirus carrying mouse full-length CAT-1)
Ad-CAT-1 was generated by the procedure of He et al. [24]. Briefly, mouse CAT-1 cDNA was first amplified by PCR with primers containing 5′ EcoRI and SalI to facilitate subcloning into a pAd-Track-CMV (cytomegalovirus) shuttle vector. The correct DNA sequence was verified by sequencing in the Molecular Biology Core Facility of the Medical College of Georgia. The pAd-Track-CMV-CAT-1 construct was linearized with PmeI and subsequently co-transformed into E. coli BJ5183 cells with an adenoviral backbone plasmid, pAdEasy-1. Recombinants were selected by kanamycin resistance and verified by restriction enzyme digestion. The confirmed recombinant plasmid was then transfected into the adenoviral packaging HEK-293 (human embryonic kidney) cell line. Viral production was monitored over 7–10 days by visualization of green fluorescent protein expression. After 7–10 days, virus was harvested and purified by banding on a CsCl gradient. The purified virus was then dialysed and stored at −80 °C.
Expression of Ad-CAT-1 in endothelial cells
BAECs were infected with various MOIs (multiplicities of infection) of adenovirus for 24 h to determine the titre that gave maximal expression of CAT-1 without significant cell death. Cell viability was assessed by Trypan Blue exclusion. In subsequent experiments using gene transfer of CAT-1, confluent BAECs in 100-mm-diameter dishes were infected for 24 h at an MOI of 100.
Measurement of arginine transport
BAECs were grown to confluence in 12-well plates. Cells were serum-starved for 2 h and infected with either control Ad-β-Gal (adenovirus carrying β-galactosidase) or Ad-CAT-1 (MOI of 100) for 24 h at 37 °C. Cells were then washed at room temperature with 1 ml of buffer containing 25 mM Hepes, pH 7.5, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.8 mM MgSO4 and 5 mM D-glucose. The same buffer (0.25 ml/well) was then added containing 100 μM arginine and 0.25 μCi of [3H]arginine. After a 5 min incubation at room temperature, cells were washed twice with 1 ml of ice-cold buffer and solubilized in 0.25 ml of 0.4 M NaOH. Samples were then analysed for [3H]arginine by liquid-scintillation counting.
Measurement of NO release
NO release from BAECs was measured by the bioassay procedure first described by Ishii et al. [25], with the modifications described previously [26,27]. Confluent BAECs were treated as described above for the arginine transport experiments, except that radioactive arginine was omitted from the uptake buffer and 20 units/ml superoxide dismutase and 0.3 mM 3-isobutyl-1-methylxanthine was included. After 5 min, the BAEC bathing medium was transferred to confluent RASM reporter cells, which were incubated for 3 min and then lysed in ice-cold 20 mM sodium acetate, pH 4.0. cGMP concentrations in the lysates were quantified using an enzyme immunoassay kit (Cayman Chemical Co., Ann Arbor, MI, U.S.A.). cGMP production by the reporter cells was completely blocked by incubation of BAECs with 1 mM L-NAME (NG-nitro-L-arginine methyl ester), confirming that it was authentic NO that was being measured.
Measurement of eNOS enzymic activity
eNOS activity of BAEC lysates and purified eNOS was determined by the method of Bredt and Synder [28], which monitors the rate of formation of [14C]citrulline from [14C]arginine (100 μM) in the presence of excess cofactors including Ca2+ (2 mM), calmodulin (200 units), NADPH (1 mM), FAD (4 μM), FMN (4 μM) and tetrahydrobiopterin (40 μM). Product was separated from substrate on AG 50W-X8 cation-exchange columns (Bio-Rad Laboratories, Hercules, CA, U.S.A.).
Construction and purification of His6-tagged CAT-1
The cDNA for full-length mouse CAT-1 was PCR-amplified with primers containing 5′ EcoRI and SalI restriction enzyme sites to facilitate subcloning into the baculovirus transfer vector, pVL1393 (BD Biosciences). The upstream primer also contained a coding sequence for six consecutive histidine residues in-frame with the CAT-1 coding sequence. Recombinant transfer vector was then co-transfected with Baculogold viral DNA (BD Biosciences) into Sf9 insect cells, and a high-titre recombinant viral stock was obtained and used for subsequent infection of Sf9 cells. Histidine-tagged CAT-1 protein was purified from cell lysates by affinity binding to Ni-NTA (Ni2+-nitrilotriacetate)–agarose (Life Technologies, Rockville, MD, U.S.A.) and dialysed at 4 °C against buffer containing 20 mM Tris/HCl, pH 7.5, and 20% (v/v) glycerol.
RESULTS AND DISCUSSION
Preparation and characterization of an anti-CAT-1 polyclonal antibody
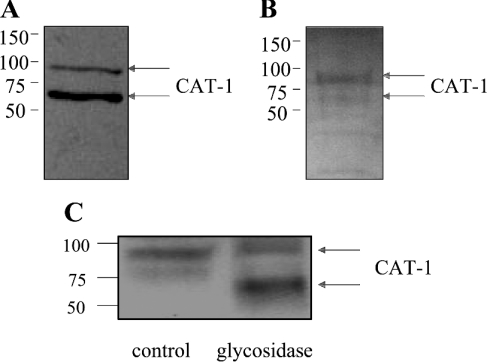
For use as a tool to investigate eNOS–CAT-1 interactions, we raised and purified a polyclonal antibody directed against CAT-1. The CAT-1 protein is predicted to contain 14 transmembrane-spanning domains, seven extracellular domains, and eight intracellular domains [29]. Among the CAT family of transporters (which also includes CAT-2A, CAT-2B and CAT-3), ID6 is the CAT domain that shares the least amino acid similarity [3]. We therefore prepared a GST fusion protein containing mouse CAT-1 ID6 (GST–CAT-1-ID6) for injection into rabbits and production of a polyclonal antibody that is not likely to cross-react with other CAT proteins. Anti-CAT-1 antibody was then purified from crude serum by a two-step process of depletion of anti-GST antibody and affinity purification of anti-CAT-1 antibody. Purified antibody was found to recognize two different forms of CAT-1 on immunoblots of BAEC lysates that migrate at apparent molecular masses of 70 kDa and 90 kDa respectively (Figure 1A). These two forms probably correspond to two differentially glycosylated forms of the CAT-1 protein based on the following observations. First, when CAT-1 was immunoprecipitated from BAEC lysates and probed for glycosylation by the periodic acid–Schiff method, two glycosylated proteins of approx. 70 kDa and 90 kDa were detected, with the upper band giving a stronger signal for carbohydrate content (Figure 1B). Secondly, when BAEC lysates were treated for 3 days with glycosidase, much of the 90 kDa band was converted into a lower-molecular-mass form of approx. 65 kDa (Figure 1C). The 70 kDa band appears to be susceptible to proteolytic degradation, because it was lost during the time of the incubation in both the treated and untreated samples (Figure 1C).
Figure 1. Immunoblotting of BAEC lysates with anti-CAT-1-ID6 antibody, and glycoprotein detection and deglycosylation of CAT-1 proteins.
Cultured BAECs were lysed, and lysates were immunoblotted with anti-CAT-1-ID6 antibody (A). Lysates were also subjected to immunoprecipitation with anti-CAT-1-ID6 antibody, and glycosylation of immunoprecipitated proteins was detected by the periodic acid–Schiff method (B). To investigate deglycosylation, lysates were also incubated without or with glycosidase and then immunoblotted with anti-CAT-1-ID6 (C). Similar results were obtained in three different experiments. Molecular masses are indicated in kDa.
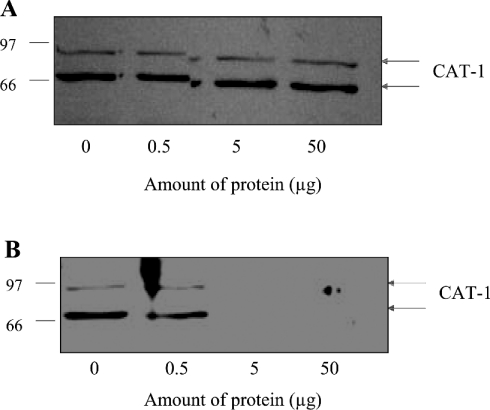
Whether or not CAT-1 occurs in differentially glycosylated forms appears to depend on the animal species and the vascular bed from which the endothelial cells are derived. For example, Dye et al. [30] reported recently the detection of two glycosylated forms of CAT-1 in human placental microvascular endothelial cells of apparent molecular masses of 79 kDa and 90 kDa. In contrast, the same authors detected a single protein species of 67 kDa in human umbilical vein endothelial cells that agrees with the predicted size of the non-glycosylated CAT-1 protein [30]. Likewise, Zharikov and Block [31], detected a single, sharp protein band smaller than 80 kDa in lysates of porcine aortic endothelial cells. Closs [32], on the other hand, reported that mouse and human CAT-1 proteins run as a smear between 80 and 100 kDa. In order to confirm the specificity of the antibody used in the present study, the antibody was pre-incubated with various concentrations of GST–CAT-1-ID6 or GST protein before carrying out immunoblots of BAEC lysates. The reactivity of the antibody with both forms of CAT-1 was blocked completely by pre-adsorption to GST–CAT-1-ID6 (Figure 2B), but not by pre-adsorption to GST only (Figure 2A), demonstrating that the antibody is specific for CAT-1 and that both bands on the blot represent authentic CAT-1 protein.
Figure 2. Effects of pre-adsorption of anti-CAT-1-ID6 antibody with either GST only or GST-CAT-1-ID6 on reactivity of the antibody in immunoblots of BAEC lysates.
Anti-CAT-1-ID6 antibody was pre-incubated with 0, 0.5, 5 and 50 μg of purified GST only (A) or GST-CAT-1-ID6 (B), and then used for immunoblotting of BAEC lysates. The experiment was carried out three times with similar results. Molecular masses are indicated in kDa.
CAT-1 interactions with eNOS in endothelial cells
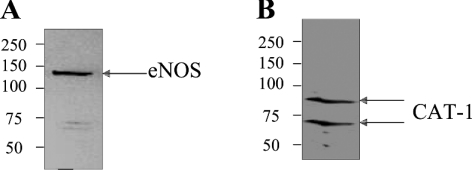
A previous study by McDonald et al. [5] used an immunodepletion approach to show that CAT-1 and eNOS may be physically associated in endothelial cells. This conclusion, however, had not been confirmed previously by co-immunoprecipitation. In order to determine whether CAT-1 interacts with eNOS either directly or indirectly in endothelial cells, we lysed BAECs in membrane-solubilizing buffer containing 1% (v/v) Triton X-100, and subjected the solubilized proteins in the lysates to immunoprecipitation with anti-CAT-1-ID6 and anti-eNOS antibodies. Anti-CAT-1-ID6 immunoprecipitates were then immunoblotted with anti-eNOS antibody, and anti-eNOS immunoprecipitates were immunoblotted with anti-CAT-1-ID6 antibody. As shown in Figure 3(A), eNOS (130 kDa band) was specifically co-immunoprecipitated by the anti-CAT-1-ID6 antibody. In addition, both forms of CAT-1 were specifically co-immunoprecipitated by the anti-eNOS antibody (Figure 3B). These results confirm that CAT-1 and eNOS do indeed exist in a protein–protein complex in endothelial cells. Because eNOS is almost exclusively membrane-bound, both glycosylated forms of CAT-1 appear to be inserted into the membrane in BAECs, thus allowing their interaction with eNOS.
Figure 3. Co-immunoprecipitation of CAT-1 and eNOS from endothelial cells.
BAEC lysates were immunoprecipitated with either anti-CAT-1-ID6 or anti-eNOS antibody. Anti-CAT-1-ID6 immunoprecipitates were immunoblotted with anti-eNOS antibody (A), and anti-eNOS immunoprecipitates were immunoblotted with anti-CAT-1-ID6 antibody (B). Results shown are representative of six different experiments. Molecular masses are indicated in kDa.
CAT-1 interactions with eNOS in in vitro binding assays of the purified proteins
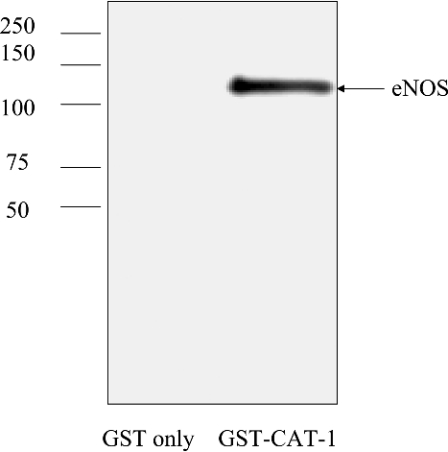
eNOS interactions with certain proteins are indirect. For example, eNOS interaction with sGC is indirect with the Hsp90 protein acting as a ‘bridge’ or adaptor protein that mediates eNOS–sGC association [6]. We have also shown previously the eNOS interactions with the seven-transmembrane-spanning bradykinin B2 receptor is mediated by only one of four different intracellular domains of the B2 receptor [33]. In contrast, eNOS interactions with caveolin-1 occur through two different caveolin-1 intracellular domains [22,34]. Therefore, in order to determine whether the eNOS–CAT-1 interaction is direct or indirect and to determine further whether a single CAT-1 intracellular domain is sufficient to mediate the interaction, we prepared GST fusion proteins of full-length CAT-1 and each of the eight individual intracellular domains of CAT-1 for use in in vitro binding assays with purified eNOS. GST fusion proteins containing full-length mouse CAT-1 (residues 1–622), and CAT-1 ID1 (residues 1–36), ID2 (residues 83–102), ID3 (residues 184–191), ID4 (residues 261–280), ID5 (residues 352–377), ID6 (residues 423–487), ID7 (residues 540–552) and ID8 (residues 603–622) were each expressed in E. coli. In addition a GST non-fusion protein was expressed as a control. The GST fusion proteins and GST alone were purified by affinity binding to glutathione–agarose. GST fusion proteins and GST alone, pre-bound to agarose beads, were then used in in vitro binding assays with recombinant bovine eNOS, expressed and purified from a baculovirus system [23]. Beads were incubated with eNOS at 4 °C overnight and then washed six times in buffer containing 1 M NaCl. Proteins remaining bound to the beads after washing were eluted with reduced glutathione, and were immunoblotted with anti-eNOS antibody. As shown in Figure 4, eNOS bound specifically to the full-length CAT-1 GST fusion protein, but not to GST alone, demonstrating that CAT-1 and eNOS bind directly without the need for an adaptor protein such as Hsp90. Furthermore, under the stringent, high-ionic-strength washing conditions of the binding assay, eNOS did not bind to any single CAT-1 intracellular domain by itself, indicating that high-affinity binding of eNOS to CAT-1 requires the interaction of eNOS with two or more CAT-1 intracellular domains.
Figure 4. In vitro binding of eNOS to a full-length-CAT-1 GST fusion protein.
GST or a GST-CAT-1 fusion protein was expressed in E. coli and purified by affinity binding to glutathione–agarose. Proteins, pre-bound to beads, were incubated overnight at 4 °C with recombinant bovine eNOS, expressed and purified from a baculovirus system. Beads were washed six times in buffer containing 1 M NaCl. Bound proteins were eluted with reduced glutathione and immunoblotted with anti-eNOS antibody. Results shown are representative of six separate experiments. Molecular masses are indicated in kDa.
Effects of agonist stimulation on eNOS–CAT-1 interactions in endothelial cells
eNOS interactions with certain other eNOS-interacting proteins are regulated in an agonist-dependent manner. For example, bradykinin stimulation of endothelial cells results in decreased association of eNOS with the B2 receptor [33] and increased association with Hsp90 [35] and sGC [6]. In order to determine whether bradykinin stimulation of endothelial cells affects eNOS interactions with CAT-1, BAECs were treated with bradykinin for 0, 1, 5, 10 and 30 min. Cells were lysed, and lysates were subjected to immunoprecipitation with anti-CAT-1-ID6 and anti-eNOS antibodies. Anti-CAT-1-ID6 immunoprecipitates were then immunoblotted with anti-eNOS antibody, and anti-eNOS immunoprecipitates were immunoblotted with anti-CAT-1-ID6 antibody. As shown in Figure 5, and in contrast with what has been shown concerning eNOS interactions with certain other proteins, such as the B2 receptor, Hsp90 and sGC, eNOS interactions with CAT-1 are not altered in response to bradykinin stimulation.
Figure 5. Effects of bradykinin (BK) on eNOS–CAT-1 complex formation in endothelial cells.
BAECs were treated with bradykinin (1 μM) for 0, 1, 5, 10 and 30 min. BAEC lysates were prepared and were immunoprecipitated with either anti-CAT-1-ID6 or anti-eNOS antibody. Anti-CAT-1-ID6 immunoprecipitates were immunoblotted with anti-eNOS antibody (A), and anti-eNOS immunoprecipitates were immunoblotted with anti-CAT-1-ID6 antibody (B). Similar results were obtained in three separate experiments. Molecular masses are indicated in kDa.
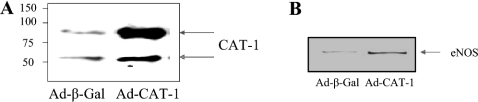
Overexpression of CAT-1 in endothelial cells by adenoviral-mediated gene transfer
To investigate further the role of the eNOS–CAT-1 interaction in endothelial NO release, we prepared an adenovirus that induces overexpression of the CAT-1 protein upon virus infection of BAECs. Cells were infected with either a negative control β-galactosidase adenovirus (Ad-β-Gal) or a mouse full-length CAT-1 adenovirus (Ad-CAT-1). Lysates were prepared from each infection, and equal quantities of lysate protein were immunoblotted with the anti-CAT-1-ID6 antibody. Two bands of approx. 70 kDa and 90 kDa were detected for both Ad-β-Gal and Ad-CAT-1 samples, with a clear increase (approx. 10-fold) in both bands in the Ad-CAT-1 sample (Figure 6A). These data indicate that Ad-CAT-1 can be used to significantly increase the level of CAT-1 protein expression in BAECs, and demonstrate further that both of the bands detected in lysates from uninfected cells (Figure 1) do indeed represent authentic CAT-1. In order to determine whether increased expression of CAT-1 produced by adenoviral-mediated gene transfer results in increased association of CAT-1 with eNOS, cells were infected with either Ad-β-Gal or Ad-CAT-1, and solubilized proteins in cell lysates were subjected to immunoprecipitation with anti-CAT-1-ID6 antibody. Under conditions in which solubilized CAT-1 was quantitatively immunoprecipitated from both types of cell lysates and immunoprecipitates were immunoblotted with anti-eNOS antibody, a significant increase in the amount of eNOS associated with CAT-1 was observed (Figure 6B). Densitometric analysis of immunoblots showed that the percentage of eNOS associated with CAT-1 increased from 8.3±0.8% in cells infected with Ad-β-Gal to 15.7±1.8% in cells infected with Ad-CAT-1. These values, however, may be underestimates due to incomplete solubilization of CAT-1 from the membrane by lysis in the lysis buffer containing 1% (v/v) Triton X-100. Furthermore, the detergent in the lysis buffer may also function to at least partially disrupt the complex.
Figure 6. Increased protein expression of CAT-1 and eNOS–CAT-1 association in endothelial cells by adenoviral-mediated gene transfer.
(A) BAECs were infected for 24 h with Ad-β-Gal (negative control) or Ad-CAT-1. Lysates were prepared, and equal quantities of lysate proteins from each condition were immunoblotted with anti-CAT-1-ID6 antibody. Three separate experiments gave similar results. Molecular masses are indicated in kDa. (B) Lysates were prepared, and equal quantities of lysate protein from each condition were immunoprecipitated with anti-CAT-1-ID6 antibody. Immunoprecipitates were then immunoblotted with anti-eNOS antibody. Results shown are representative of three experiments.
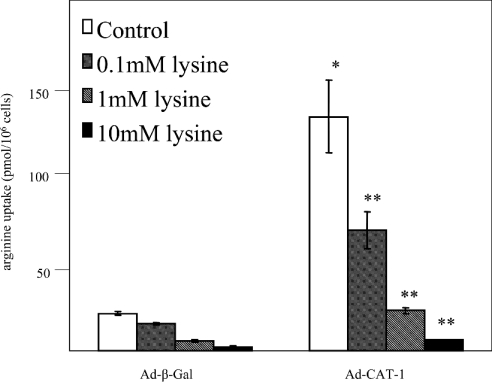
Effects of CAT-1 overexpression and L-lysine on arginine transport in endothelial cells
Uptake of cationic amino acids by endothelial cells is mediated by both high-affinity and low-affinity non-saturable systems [36–39]. At high concentrations of arginine (>1 mM), the low-affinity system (Km values near 20 mM) may participate. However, because plasma arginine levels are only approx. 50 μM, we carried out the arginine-uptake experiments with 100 μM arginine. Under these conditions, CAT-1 is responsible for up to 95% of arginine uptake [4]. BAECs were infected with either Ad-β-Gal or Ad-CAT-1, and the rate of transport of extracellular arginine (100 μM) into infected cells at pH 7.5 was determined by measuring [3H]arginine uptake. Arginine uptake under the conditions of the assay was first confirmed to be linear over at least a 30 min time period, and was comparable in magnitude with that reported previously by Preik-Steinhoff et al. [38] and Durante et al. [39] in other studies of arginine transport in BAECs. Cells were then incubated with [3H]arginine for 5 min in the absence or presence of increasing concentrations of the competitive inhibitor of CAT-1-mediated transport, L-lysine (hereafter referred to simply as lysine). Overexpression of CAT-1 by infection with Ad-CAT-1 increased arginine uptake 6.0±0.9-fold (mean±S.E.M., n=4) (Figure 7). Uptake was decreased by co-incubation with lysine in a dose-dependent manner in both control (Ad-β-Gal) and CAT-1-overexpressing (Ad-CAT-1) cells. The 6-fold increase in arginine uptake in CAT-1-overexpressing cells was completely reversed by 1 mM lysine, and was reduced to below the level of the control by 10 mM lysine.
Figure 7. Effects of CAT-1 overexpression and lysine on arginine uptake in endothelial cells.
BAECs were infected for 24 h with Ad-β-Gal (negative control) or Ad-CAT-1. Cells from both conditions were incubated in the absence or presence of various concentrations of lysine, and the amount of [3H]arginine taken up by the cells in a 5 min incubation was quantified by liquid-scintillation counting (means±S.E.M., n=4; *P<0.05 compared with Ad-β-Gal control, **P<0.05 compared with Ad-CAT-1 control, one-way ANOVA).
Effects of CAT-1 overexpression and lysine on NO release by endothelial cells
In order to determine whether CAT-1 overexpression increases basal NO production in endothelial cells, BAECs were infected with either Ad-β-Gal or Ad-CAT-1, and were then treated in an identical fashion as in the arginine-uptake experiments. NO release during a 5 min period in the absence or presence of 10 mM lysine was determined for control and CAT-1-overexpressing cells using the sensitive bioassay method of Ishii et al. [25] that we and others have used previously to quantify NO release from endothelial cells by measuring cGMP production in reporter cells [26,27,40,41]. In agreement with the results and conclusions reported by Closs et al. [42] for the human endothelial cell line, EA.hy926, lysine had no effect on basal (Ad-β-Gal) endothelial NO release, indicating that in the BAEC and EA.hy926 in vitro models, NO release is independent of CAT-1-mediated transport. In these two models, it appears that there exists an intracellular pool of arginine that is sufficient to drive eNOS. Furthermore, whereas CAT-1 overexpression (Ad-CAT-1) resulted in a 2.3±0.3-fold increase (mean±S.E.M., n=4) in NO release, this increase, unlike the increase in arginine transport, was not reversed by co-incubation with 10 mM lysine. Increased CAT-1 association with eNOS therefore appears to increase NO production by a mechanism not involving increased arginine transport.
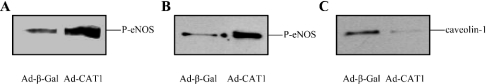
Effects of CAT-1 overexpression on eNOS activity of endothelial cell lysates
Increased NO production due to CAT-1 overexpression in endothelial cells that is not inhibited by lysine suggests that mechanisms other than arginine transport could account for the effect. For example, CAT-1 overexpression could induce increased eNOS gene expression. We therefore quantified the levels of eNOS protein expression by immunoblotting of BAEC lysates following infection with either Ad-β-Gal or Ad-CAT-1. These blots showed that eNOS protein expression was unaffected by CAT-1 overexpression. CAT-1 binding to eNOS could also conceivably increase eNOS enzymic activity due to a positive allosteric effect of CAT-1 on eNOS. Additionally, CAT-1 binding to eNOS could decrease eNOS interactions with inhibitory proteins, such as caveolin-1 or the B2 receptor, or increase eNOS interactions with activating proteins, such as Hsp90. To test the plausibility of the hypothesis that CAT-1 overexpression might increase eNOS activity through alterations in eNOS protein–protein interactions with either CAT-1 itself or with any of a number of other eNOS-interacting proteins, we prepared lysates from BAECs infected with either Ad-β-Gal or Ad-CAT-1. eNOS activities of lysates were measured by arginine-into-citrulline conversion assay in the presence of excess arginine, Ca2+ and enzyme cofactors. eNOS activity was increased 1.8±0.2-fold (mean±S.E.M., n=6) in lysates from CAT-1-overexpressing cells via a mechanism that apparently does not involve arginine transport. To determine whether increased eNOS enzymic activity could be due to a positive allosteric effect of CAT-1 on eNOS, we expressed and purified a His6-tagged CAT-1 protein from a baculovirus/Sf9 insect cell system by affinity chromatography on Ni-NTA–agarose. Incubation of purified eNOS with increasing amounts of purified CAT-1 had no effect on eNOS activity. In addition, incubation of purified eNOS with BAEC lysates did not increase activity of the purified enzyme. Combining the two proteins in a test tube thus does not recapitulate the effect of CAT-1 overexpression in increasing eNOS activity in intact cells or cell lysates. The mechanism whereby CAT-1 overexpression increases eNOS activity may involve alterations in the state of eNOS phosphorylation or changes in eNOS interactions with other proteins. To test this hypothesis, BAECs were infected with either Ad-β-Gal or Ad-CAT-1. Lysates were prepared and were immunoblotted with phospho-specific antibodies that recognize eNOS only when it is phosphorylated at either Ser-1179 or Ser-635. As shown in Figures 8(A) and 8(B), CAT-1 overexpression significantly increased phosphorylation of eNOS at Ser-1179 and Ser-635. When blots were stripped and reprobed with anti-eNOS antibody, no differences were again seen in levels of eNOS protein expression under the two conditions. Densitometric analysis revealed increases of 1.75±0.13-fold and 2.90±0.49-fold respectively (means±S.E.M., n=3). Lysates were also immunoprecipitated with anti-eNOS antibody and immunoblotted with anti-caveolin-1 antibody. This analysis showed that CAT-1 overexpression significantly decreased eNOS association with the eNOS-inhibitory protein, caveolin-1 (Figure 8C) (53±15%, mean±S.E.M., n=3). Because phosphorylation of eNOS at Ser-1179 and Ser-635, and dissociation of eNOS from caveolin-1 are known to increase eNOS activity [22,43], these three mechanisms may all contribute to the increases in NO release observed in intact cells and to the increases in eNOS enzymic activity observed in cell lysates. We cannot, however, rule out the possibility that CAT-1 overexpression could also induce increased expression of other high-affinity arginine transporters, such as y+LAT1/y+LAT2 and CAT-2. An additional potential mechanism of CAT-1 enhancement of eNOS activity that warrants further study is the possibility that increased CAT-1 insertion into the plasma membrane serves to recruit eNOS from the cytoplasmic face of the Golgi to plasmalemmal caveolae. Plasma membrane targeting of eNOS is known to result in increased eNOS activity due to increased constitutive phosphorylation of the enzyme at Ser-1179 [44].
Figure 8. Effects of CAT-1 overexpression on phosphorylation of eNOS at Ser-1179 and Ser-635, and eNOS association with caveolin-1.
BAECs were infected for 24 h with Ad-β-Gal (negative control) or Ad-CAT-1. Lysates were prepared, and equal quantities of lysate proteins were immunoblotted with anti-phospho-Ser-1179 eNOS (A) or anti-phospho-Ser-635 eNOS (B). Lysates were also immunoprecipitated with anti-eNOS antibody and immunoblotted with anti-caveolin-1 antibody (C). Similar results were obtained in at least three experiments.
Summary
The results of the present study provide several new insights into CAT-1 and the eNOS–CAT-1 interaction. CAT-1 appears to be expressed as two differentially glycosylated forms in BAEC cultures. Co-immunoprecipitation experiments demonstrate that both forms of CAT-1 exist in protein–protein complexes with eNOS in endothelial cells, and that agonist stimulation does not alter the degree of association of eNOS with CAT-1. In vitro binding assays with GST fusion proteins show that CAT-1 and eNOS interact directly, rather than indirectly, via an adaptor protein. Binding assays also demonstrate that at least two or more of the eight CAT-1 intracellular domains are required for high-affinity binding of CAT-1 to eNOS. Lack of inhibition by lysine of NO release from BAECs even in CAT-1-overexpressing cells supports the conclusion that NO release, at least in this in vitro model system, is independent of CAT-1-mediated transport. CAT-1 overexpression in BAECs and subsequent increased binding of CAT-1 to eNOS does, however, increase eNOS enzymic activity in BAEC lysates, suggesting that interaction of CAT-1 with eNOS enhances eNOS activity by a mechanism not involving arginine transport. CAT-1 overexpression also results in increased phosphorylation of eNOS at Ser-1179 and Ser-635, and decreased association of eNOS with the eNOS-inhibitory protein, caveolin-1: events known to be involved in the eNOS activation process. Because CAT-1 protein expression levels are known to be modulated over a very wide range, depending on amino acid and glucose availability [45,46], these mechanisms may make important contributions to eNOS regulation in endothelial cells.
Acknowledgments
This work was supported by National Institutes of Health Grants HL-57201 and HL-62152 (to R. C. V.). R. C. V. is an Established Investigator of the American Heart Association. M. B. H. is supported by a Scientist Development Grant from the American Heart Association.
References
- 1.Fleming I., Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 2.Nedvetsky P. I., Sessa W. C., Schmidt H. H. H. W. There's NO binding like NOS binding: protein–protein interactions in NO/cGMP signaling. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16510–16512. doi: 10.1073/pnas.262701999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devés R., Boyd C. A. R. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol. Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 4.Zharikov S. I., Block E. R. Characterization of L-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim. Biophys. Acta. 1998;1369:173–183. doi: 10.1016/s0005-2736(97)00191-0. [DOI] [PubMed] [Google Scholar]
- 5.McDonald K. K., Zharikov S., Block E. R., Kilberg M. S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J. Biol. Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 6.Venema R. C., Venema V. J., Ju H., Harris M. B., Snead C., Jilling T., Dimitropoulou C., Maragoudakis M. E., Catravas J. D. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- 7.Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H. H. W., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnal J.-F., Münzel T., Venema R. C., James N. L., Bai C., Mitch W. E., Harrison D. G. Interactions between L-arginine and L-glutamine change endothelial NO production: an effect independent of NO synthase substrate availability. J. Clin. Invest. 1995;95:2565–2572. doi: 10.1172/JCI117957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baydoun A. R., Emery P. W., Pearson J. D., Mann G. E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 10.Hecker M., Sessa W. C., Harris H. J., Änggard E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. U.S.A. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block E. R., Herrera H., Couch M. Hypoxia inhibits L-arginine uptake by pulmonary artery endothelial cells. Am. J. Physiol. 1995;269:L574–L580. doi: 10.1152/ajplung.1995.269.5.L574. [DOI] [PubMed] [Google Scholar]
- 12.Aisaka K., Gross S. S., Griffith O. W., Levi R. L-Arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem. Biophys. Res. Commun. 1989;163:710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- 13.Cooke J. P., Andon N. A., Girerd X. J., Hirsch A. T., Creager M. A. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation. 1991;83:1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- 14.Creager M. A., Gallagher S. J., Girerd X. J., Coleman S. M., Dzau V. J., Cooke J. P. L-Arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J. Clin. Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fike C. D., Kaplowitz M. R., Rehorst-Paea L. A., Nelin L. D. L-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J. Appl. Physiol. 2000;88:1797–1803. doi: 10.1152/jappl.2000.88.5.1797. [DOI] [PubMed] [Google Scholar]
- 16.Palmer R. M. J., Ashton C. S., Moncada S. Vascular endothelial cells synthesize nitric oxide form L-arginine. Nature (London) 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 17.Mugge A., Harrison D. G. L-Arginine does not restore endothelial dysfunction in atherosclerotic rabbit aorta in vitro. Blood Vessels. 1991;28:354–357. doi: 10.1159/000158881. [DOI] [PubMed] [Google Scholar]
- 18.Kurz S., Harrison D. G. Insulin and the arginine paradox. J. Clin. Invest. 1997;99:369–370. doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giugliano D., Marfella R., Verrazzo G., Acampora R., Coppola L., Cozzolino D., D'Onofrio F. The vascular effects of L-arginine in humans: the role of endogenous insulin. J. Clin. Invest. 1997;99:433–438. doi: 10.1172/JCI119177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jay G. D., Culp D. J., Jahuke M. R. Silver staining of extensively glycosylated proteins on sodium dodecyl sulfate-polyacrylamide gels: enhancement by carbohydrate-binding dyes. Anal. Biochem. 1990;185:324–330. doi: 10.1016/0003-2697(90)90302-p. [DOI] [PubMed] [Google Scholar]
- 21.Frangioni J. V., Neel B. G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 22.Ju H., Zou R., Venema V. J., Venema R. C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 23.Venema R. C., Sayegh H. S., Arnal J.-F., Harrison D. G. Role of the enzyme calmodulin-binding domain in membrane association and phospholipid inhibition of endothelial nitric oxide synthase. J. Biol. Chem. 1995;270:14705–14711. doi: 10.1074/jbc.270.24.14705. [DOI] [PubMed] [Google Scholar]
- 24.He T.-C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii K., Sheng H., Warner T. D., Förstermann U., Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 1991;261:H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- 26.Marrero M. B., Venema V. J., Ju H., He H., Liang H., Caldwell R. B., Venema R. C. Endothelial nitric oxide synthase interactions with G-protein-coupled receptors. Biochem. J. 1999;343:335–340. [PMC free article] [PubMed] [Google Scholar]
- 27.Harris M. B., Ju H., Venema V. J., Liang H., Zou R., Michell B. J., Chen Z.-P., Kemp B. E., Venema R. C. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J. Biol. Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 28.Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 30.Dye J. F., Vause S., Johnston T., Clark P., Firth J. A., D'Souza S. W., Sibley C. P., Glazier J. D. Characterization of cationic amino acid transporters and expression of endothelial nitric oxide synthase in human placental microvascular endothelial cells. FASEB J. 2004;18:125–127. doi: 10.1096/fj.02-0916fje. [DOI] [PubMed] [Google Scholar]
- 31.Zharikov S., Block E. R. Association of L-arginine transporters with fodrin: implications for hypoxic inhibition of arginine uptake. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L111–L117. doi: 10.1152/ajplung.2000.278.1.L111. [DOI] [PubMed] [Google Scholar]
- 32.Closs E. I. Expression, regulation and function of carrier proteins for cationic amino acids. Curr. Opin. Nephrol. Hypertens. 2002;11:99–107. doi: 10.1097/00041552-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Ju H., Venema V. J., Marrero M. B., Venema R. C. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J. Biol. Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- 34.Venema V. J., Ju H., Zou R., Venema R. C. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle: identification of a novel caveolin scaffolding/inhibitory domain. J. Biol. Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 35.Harris M. B., Ju H., Venema V. J., Blackstone M., Venema R. C. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen. Pharmacol. 2001;35:165–170. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 36.Gazzola G. C., Franchi R., Saibene V., Ronchi P., Guidotti G. G. Regulation of amino acid transport in chick embryo heart cells. Biochim. Biophys. Acta. 1972;266:407–421. doi: 10.1016/0005-2736(72)90097-1. [DOI] [PubMed] [Google Scholar]
- 37.Christensen H. N., Handlogten M. E. Does the non-saturable cell entry apply to the charge-free form of amino acids? Biochim. Biophys. Acta. 1977;469:216–220. doi: 10.1016/0005-2736(77)90184-5. [DOI] [PubMed] [Google Scholar]
- 38.Preik-Steinhoff H., Zink S., Rösen P., Kelm M. Transport of L-arginine in arginine-deprived endothelial cells. Biochem. Biophys. Res. Commun. 1995;213:447–453. doi: 10.1006/bbrc.1995.2152. [DOI] [PubMed] [Google Scholar]
- 39.Durante W., Liao L., Schafer A. I. Differential regulation of L-arginine transport and inducible NOS in cultured vascular smooth muscle cells. Am. J. Physiol. 1995;268:H1158–H1164. doi: 10.1152/ajpheart.1995.268.3.H1158. [DOI] [PubMed] [Google Scholar]
- 40.García-Cardeña G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature (London) 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 41.Papapetropoulos A., Fulton D., Lin M. I., Fontana J., McCabe T. J., Zoellner S., García-Cardeña G., Zhou Z., Gratton J.-P., Sessa W. C. Vanadate is a potent activator of endothelial nitric-oxide synthase: evidence for the role of the serine/threonine kinase Akt and the 90-kDa heat shock protein. Mol. Pharmacol. 2004;65:407–415. doi: 10.1124/mol.65.2.407. [DOI] [PubMed] [Google Scholar]
- 42.Closs E. I., Scheld J.-S., Sharafi M., Förstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol. Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- 43.Michell B. J., Harris M. B., Chen Z., Ju H., Venema V. J., Blackstone M. A., Venema R. C., Kemp B. E. Identification of novel regulatory sites of phosphorylation of bovine endothelial nitric-oxide synthase at serines 617 and 635. J. Biol. Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 44.Fulton D., Babbitt R., Zoellner S., Fontana J., Acevedo L., McCabe T. J., Iwikiri Y., Sessa W. C. Targeting of endothelial nitric oxide synthase to the cytoplasmic face of the Golgi or plasma membrane regulates Akt- vs. calcium-dependent mechanisms for nitric oxide release. J. Biol. Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez J., Bode B., Koromilas A., Diehl J. A., Krukovets I., Snider M. D., Hatzoglou M. Translation mediated by the internal ribosome entry site of the cat-1 mRNA is regulated by glucose availability in the PERK kinase-dependent manner. J. Biol. Chem. 2002;277:11780–11787. doi: 10.1074/jbc.M110778200. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez J., Lopez A. B., Wang C., Mishra R., Zhou L., Yaman I., Snider M. D., Hatzolgou M. Transcriptional control of the arginine/lysine transporter, CAT-1, by physiological stress. J. Biol. Chem. 2003;278:50000–50009. doi: 10.1074/jbc.M305903200. [DOI] [PubMed] [Google Scholar]