Abstract
Δ9-Tetrahydrocannabinol, a major psychoactive constituent of marijuana, interacts with specific receptors, i.e. the cannabinoid receptors, thereby eliciting a variety of pharmacological responses. To date, two types of cannabinoid receptors have been identified: the CB1 receptor, which is abundantly expressed in the nervous system, and the CB2 receptor, which is predominantly expressed in the immune system. Previously, we investigated in detail the structure–activity relationship of various cannabinoid receptor ligands and found that 2-AG (2-arachidonoylglycerol) is the most efficacious agonist. We have proposed that 2-AG is the true natural ligand for both the CB1 and CB2 receptors. Despite the potential physiological importance of 2-AG, not much information is available concerning its biological activities towards mammalian tissues and cells. In the present study, we examined the effect of 2-AG on morphology as well as the actin filament system in differentiated HL-60 cells, which express the CB2 receptor. We found that 2-AG induces rapid morphological changes such as the extension of pseudopods. We also found that it provokes a rapid actin polymerization in these cells. Actin polymerization induced by 2-AG was abolished when cells were treated with SR144528, a CB2 receptor antagonist, and pertussis toxin, suggesting that the response was mediated by the CB2 receptor and Gi/o. A phosphoinositide 3-kinase, Rho family small G-proteins and a tyrosine kinase were also suggested to be involved. Reorganization of the actin filament system is known to be indispensable for a variety of cellular events; it is possible that 2-AG plays physiologically essential roles in various inflammatory cells and immune-competent cells by inducing a rapid actin rearrangement.
Keywords: actin polymerization, anandamide, 2-arachidonoylglycerol, cannabinoid, macrophage, morphological change
Abbreviations: 2-AG, 2-arachidonoylglycerol; Δ9-THC, Δ9-tetrahydrocannabinol; F-actin, filamentous actin; IL, interleukin; MAPK, mitogen-activated protein kinase; NBD-phallacidin, 7-nitrobenz-2-oxa-1,3-phallacidin; 1,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; PI3K, phosphoinositide 3-kinase; PTX, pertussis toxin
INTRODUCTION
A major psychoactive constituent of marijuana, Δ9-THC (Δ9-tetrahydrocannabinol), is known to elicit a variety of pharmacological responses in vitro and in vivo. The mechanism underlying these actions of Δ9-THC remained elusive until recently. In 1988, Devane et al. [1] demonstrated the occurrence of a specific binding site for cannabinoids in rat brain synaptosomes using [3H]CP55940, a radiolabelled synthetic cannabinoid. Soon after, Matsuda et al. [2] cloned a cDNA encoding a cannabinoid receptor (CB1), which is abundantly expressed in the brain. Later, Munro et al. [3] cloned a cDNA for another cannabinoid receptor (CB2), which is mainly expressed in the lymphoid tissues. The discovery of specific receptors for cannabinoids prompted the search for endogenous ligands. In 1992, Devane et al. [4] isolated N-arachidonoylethanolamine (anandamide) from pig brain as an endogenous cannabinoid receptor ligand. Anandamide has been shown to possess various cannabimimetic activities [5–8]. However, the levels of anandamide in various living tissues were usually very low [9]. Furthermore, anandamide was found to act as a partial agonist for the cannabinoid receptors [10–19]. These observations strongly suggested the existence of another endogenous ligand in mammalian tissues.
In 1995, we [20] and Mechoulam et al. [21] isolated 2-AG (2-arachidonoylglycerol) as the second endogenous ligand for the cannabinoid receptors. 2-AG exhibits a variety of cannabimimetic activities. Noticeably, the levels of 2-AG in various mammalian tissues are markedly higher than those of anandamide [9]. In fact, 2-AG can be rapidly formed from arachidonic acid-containing phospholipids in various stimulated tissues and cells [9,23]. Importantly, 2-AG was found to act as a full agonist at the cannabinoid receptors [10,11,14,17–19,22]. On the basis of these experimental results, we proposed that 2-AG, and not anandamide, is the intrinsic natural ligand for the cannabinoid receptors [9–11,22,23].
Despite the potential physiological significance of 2-AG, details of its exact physiological functions have not yet been fully elucidated. Several lines of evidence strongly suggested that 2-AG attenuates synaptic transmission by acting at the CB1 receptor expressed predominantly in the presynaptic terminals [24]; it is becoming evident that 2-AG is a novel type of neuromodulator. 2-AG has also been shown to possess cytoprotective properties in the brain [25]. On the other hand, the physiological function of the CB2 receptor still remains rather obscure. Recently, we found that 2-AG induces the activation of p42/44 MAPK (mitogen-activated protein kinase) [26], p38 MAPK and c-Jun N-terminal kinase [27]. We also found that 2-AG evoked the migration of HL-60 cells differentiated into macrophage-like cells [28]. Several investigators have also reported that 2-AG induces the migration of mouse splenocytes [29] and microglia cells [30]. Both stimulative and suppressive effects of 2-AG on lymphocyte proliferation have also been demonstrated [31,32]. Nevertheless, sufficient information has not yet been available concerning the biological activities of 2-AG towards inflammatory cells. In addition, it is not clear in some cases whether the effects are due to 2-AG itself or to arachidonic acid metabolites derived from 2-AG. It is essential to investigate the exact physiological and pathophysiological roles of 2-AG as well as the mechanism and mode of action of 2-AG in inflammatory reactions and immune responses, since the CB2 receptor is abundantly expressed in several types of inflammatory cells and immune-competent cells and is suggested to be involved in the regulation of these cellular functions [2,33–39].
In the present study, we examined the effects of 2-AG on morphology as well as the organization of the actin filament system in HL-60 cells differentiated into macrophage-like cells, which are known to be a useful model of macrophages [40]. We found that 2-AG induces rapid morphological changes such as the extension of pseudopods. We also found that 2-AG induces rapid actin polymerization in differentiated HL-60 cells in a CB2 receptor-dependent manner. The physiological meaning of the 2-AG-induced actin rearrangement in macrophage-like cells is discussed.
MATERIALS AND METHODS
Chemicals
Arachidonic acid, essentially fatty acid-free BSA, 1,25(OH)2D3 (1α, 25-dihydroxyvitamin D3) and Clostridium difficile toxin B were purchased from Sigma (St. Louis, MO, U.S.A.). Formaldehyde, lysophosphatidylcholine, wortmannin and herbimycin A were obtained from Wako Pure Chemical Industries (Osaka, Japan). CP55940 and Y-27632 were purchased from Tocris (Bristol, U.K.). WIN55212-2 and WIN55212-3 were obtained from RBI (Natick, MA, U.S.A.). PD98059, SB203580 and Ro-31-8220 were acquired from Calbiochem–Novabiochem (San Diego, CA, U.S.A.). PTX (pertussis toxin) and C. botulinum C3 exoenzyme were obtained from List Biological Laboratories (Campbell, CA, U.S.A.). SR144528 was a gift from SANOFI (Montpellier, France). NBD-phallacidin (7-nitrobenz-2-oxa-1,3-phallacidin) was purchased from Molecular Probes (Junction City, OR, U.S.A.). 1,3-Benzylideneglycerol was prepared as described in [10]. 2-AG was prepared from 1,3-benzylideneglycerol and arachidonic acid as described earlier [10].
Cells
Human promyelocytic leukaemia HL-60 cells were grown at 37 °C in RPMI 1640 medium (Asahi Technoglass, Chiba, Japan) supplemented with 10% (v/v) fetal bovine serum in an atmosphere of 95% air and 5% CO2. Cells were differentiated into macrophage-like cells by treatment with 100 nM 1,25(OH)2D3 for 5 days.
Morphological changes
HL-60 cells differentiated into macrophage-like cells (1×106) were then stimulated with 1 μM 2-AG. Morphological changes were examined by phase-contrast microscopy (Olympus IX71).
Fluorescence microscopy
HL-60 cells differentiated into macrophage-like cells (1×106) were suspended in 0.2 ml of 25 mM Hepes-Tyrode solution (pH 7.4) containing 1 mM CaCl2 and 0.1% BSA. After incubation at 37 °C for 5 min, the cells were challenged with 1 μM 2-AG or the vehicle (DMSO) (final concentration of DMSO, 0.2%) at 37 °C for 10 s. Cells were then simultaneously fixed, permeabilized and labelled for F-actin (filamentous actin) by the addition of an equal volume of PBS (pH 7.4) containing 3.7% (w/v) formaldehyde, 0.1 mg/ml of lysophosphatidylcholine, 0.1% BSA and 0.16 μM NBD-phallacidin. After keeping at room temperature (25 °C) for 1 h in the dark, cells were washed twice with PBS (pH 7.4) containing 0.1% BSA and analysed using an Olympus CX41 fluorescence microscope. Emission from NBD-phallacidin (465 nm) was detected after excitation at 488 nm.
Flow cytometric analysis of actin polymerization
HL-60 cells differentiated into macrophage-like cells (1×106) were suspended in 0.2 ml of 25 mM Hepes-Tyrode solution (pH 7.4) containing 1 mM CaCl2 and 0.1% BSA. After incubation at 37 °C for 5 min, cells were stimulated with 2-AG (1 μM) or other cannabinoid receptor ligands (1 μM) at 37 °C for 10 s. Cells were then simultaneously fixed, permeabilized and labelled for F-actin by the addition of an equal volume of PBS (pH 7.4) containing 3.7% formaldehyde, 0.1 mg/ml of lysophosphatidylcholine, 0.1% BSA and 0.16 μM NBD-phallacidin. After keeping at 4 °C overnight in the dark, cell-associated fluorescence was measured with a flow cytometer (ELITE; Beckman Coulter, Fullerton, CA, U.S.A.), using a 15 mW argon-ion laser for excitation at 488 nm. Fluorescence was detected through a 530 nm band-pass filter. Data from 5000 events per sample were acquired in a list mode and analysed using ELITE software. In the experiments where the effects of various inhibitors were examined, cells were pretreated with inhibitors at 37 °C before the challenging with 2-AG (5 min for SR144528, 1 h for various enzyme inhibitors, 12 h for toxin B, 16 h for PTX and 24 h for C3 exoenzyme).
Statistical analysis
The statistical analysis was performed using Student's t test. A P<0.05 was considered to be significant.
RESULTS
Effect of 2-AG on the morphology of HL-60 cells differentiated into macrophage-like cells
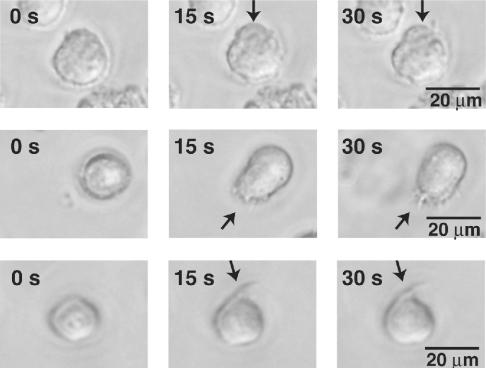
First, we examined the effect of 2-AG on the morphology of HL-60 cells differentiated into macrophage-like cells. Figure 1 shows representative photographs of differentiated HL-60 cells after stimulation with 2-AG. We found that the exposure of cells to 1 μM 2-AG provoked rapid morphological changes such as the extension of pseudopods. In contrast with 2-AG-stimulated cells, DMSO-treated control cells did not exhibit apparent morphological changes (results not shown).
Figure 1. Effects of 2-AG on the morphology of HL-60 cells differentiated into macrophage-like cells.
HL-60 cells were differentiated into macrophage-like cells by treatment with 1,25(OH)2D3 for 5 days. They were then challenged with 1 μM 2-AG, and examined for morphological changes by phase-contrast microscopy (400×). Arrows indicate morphological changes observed when the cells were challenged with 2-AG.
Effects of 2-AG on the organization of actin filaments
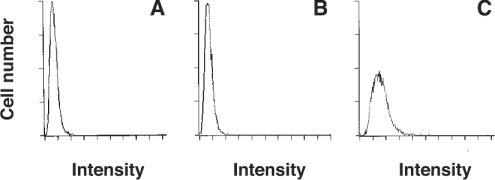
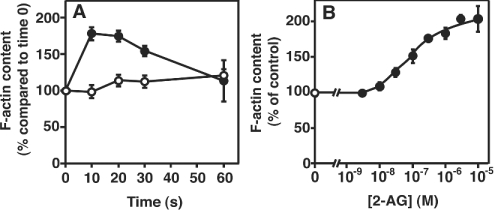
That 2-AG induces rapid morphological changes strongly suggests that a rapid actin rearrangement takes place in 2-AG-stimulated cells. We then examined the effects of 2-AG on the organization of actin filaments using NBD-phallacidin as a probe and a fluorescence microscope. We found that the fluorescence intensity was markedly increased in 2-AG-stimulated cells compared with the control (results not shown). The formation of F-actin was further investigated using a flow cytometer. Figure 2 shows a representative histogram. When cells were stimulated with 1 μM 2-AG, the flow cytometric profile was shifted to the right (Figure 2C). On the other hand, no change was observed in DMSO-treated control cells (Figure 2B), indicating that the stimulation of cells with 2-AG triggered the formation of F-actin. The increase in cellular F-actin in response to 2-AG reached a peak 10 s after the addition of 2-AG and decreased to the basal level after 1 min (Figure 3A). The formation of F-actin in 2-AG-stimulated cells increased dose-dependently (Figure 3B): the effect of 2-AG was observed from 10 nM and reached a peak at 10 μM.
Figure 2. Effect of 2-AG on the fluorescent distribution of HL-60 cells differentiated into macrophage-like cells stained with NBD-phallacidin.
Differentiated HL-60 cells were challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and then stained with an NBD-phallacidin mixture as described in the Materials and methods section. Results are representative histograms of flow-cytometric analysis. (A) Time 0; (B) vehicle (DMSO) alone; and (C) 2-AG (1 μM). The result is representative of four separate experiments.
Figure 3. Time- and dose-dependencies of 2-AG-induced actin polymerization in HL-60 cells differentiated into macrophage-like cells.
Differentiated HL-60 cells were challenged with 2-AG (1 μM) or the vehicle (DMSO) and then stained with the NBD-phallacidin mixture. The F-actin content was determined as described in the Materials and methods section. (A) The incubation was performed for the indicated periods of time. ●, 2-AG; and ○, vehicle (DMSO) alone. (B) Differentiated HL-60 cells were challenged with various concentrations of 2-AG or the vehicle (DMSO) for 10 s. ●, 2-AG; and ○, vehicle (DMSO) alone. Results are expressed as a percentage of the F-actin content in unstimulated cells (zero time or vehicle alone). Results are means±S.D. for four determinations.
Comparison of the abilities of several cannabinoid receptor ligands and their analogues to induce actin polymerization
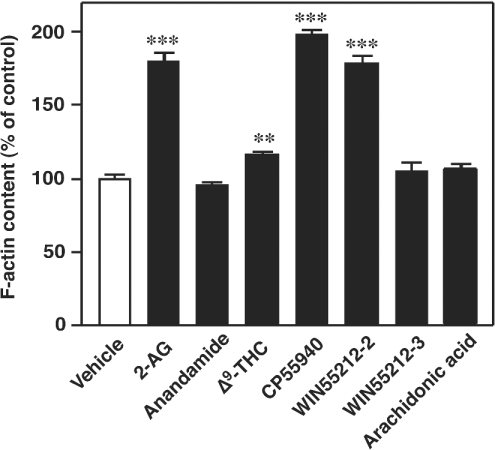
The abilities of several cannabinoid receptor ligands and their analogues to induce actin polymerization were examined next (Figure 4). As mentioned above, the addition of 1 μM 2-AG induced a rapid actin polymerization in cells. In contrast with 2-AG, the activity of 1 μM anandamide was found to be almost negligible. The activity of Δ9-THC was also very low. On the other hand, 1 μM CP55940, a synthetic cannabinoid, possessed activity comparable with that of 1 μM 2-AG. WIN55212-2 (1 μM), a cannabimimetic aminoalkylindole, also possessed appreciable activity similar to that of CP55940, whereas 1 μM WIN55212-3, an inactive isomer of WIN55212-2, failed to induce actin polymerization. Free arachidonic acid (1 μM) also did not exhibit any activity.
Figure 4. Effects of various cannabinoid receptor ligands on the actin filament system in differentiated HL-60 cells.
Cells were incubated with various cannabinoid receptor ligands (1 μM each) or the vehicle (DMSO) at 37 °C for 10 s. They were then stained with the NBD-phallacidin mixture as described in the Materials and methods section. Results are expressed as a percentage of the F-actin content in unstimulated cells (vehicle alone). Results are means±S.D. for four determinations; **P<0.01 and ***P<0.001.
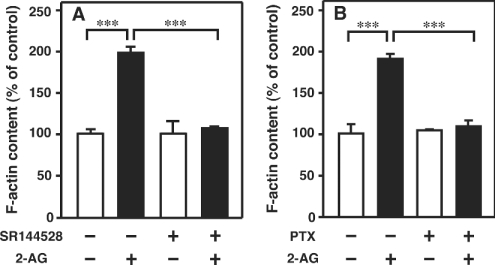
Effects of SR144528, a CB2 receptor-specific antagonist, and PTX-treatment on the actin polymerization induced by 2-AG
We next examined whether the CB2 receptor is involved in the 2-AG-induced actin polymerization. We found that the addition of SR144528, a CB2 receptor-specific antagonist, abrogated the actin polymerization induced by 2-AG (Figure 5A). It is apparent, therefore, that the 2-AG-induced actin polymerization is mediated through the CB2 receptor. The effect of pretreatment of the cells with PTX on 2-AG-induced actin polymerization was then examined. As shown in Figure 5(B), pretreatment of the cells with PTX abolished actin polymerization evoked by 2-AG, indicating that Gi/o is involved in the 2-AG-induced actin polymerization.
Figure 5. Effects of the CB2 receptor antagonist SR144528 and PTX-treatment on the actin polymerization in HL-60 cells differentiated into macrophage-like cells.
(A) Differentiated HL-60 cells were pretreated with SR144528 (1 μM) for 5 min. They were then challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and stained with the NBD-phallacidin mixture as described in the Materials and methods section. (B) Cells were pretreated with PTX (160 nM) for 16 h. They were then challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and stained with the NBD-phallacidin mixture as described in the Materials and methods section. Results are expressed as a percentage of the F-actin content in unstimulated cells (vehicle alone). Results are means±S.D. for four determinations; ***P<0.001.
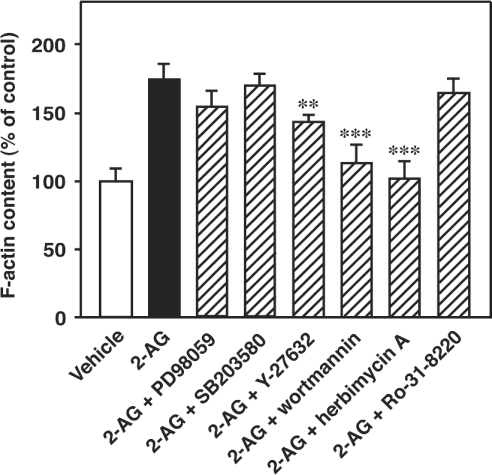
Effects of several inhibitors on 2-AG-induced actin polymerization
The effects of several inhibitors of intracellular signal transduction on 2-AG-induced actin polymerization were next investigated. As shown in Figure 6, the treatment of the cells with wortmannin [a PI3K (phosphoinositide 3-kinase) inhibitor, 200 nM] and herbimycin A (a tyrosine kinase inhibitor, 20 μM) markedly suppressed the actin polymerization induced by 2-AG, suggesting that a PI3K(s) and a tyrosine kinase(s) are closely involved in 2-AG-induced actin polymerization. On the other hand, the treatment of the cells with Y-27632 (a Rho kinase inhibitor, 20 μM) only slightly reduced the actin polymerization and the treatment of the cells with PD98059 (a p42/44 MAPK kinase inhibitor, 20 μM), SB203580 (a p38 MAPK inhibitor, 20 μM) and Ro-31-8220 (a protein kinase C inhibitor, 20 μM) did not remarkably affect the actin polymerization induced by 2-AG.
Figure 6. Effects of various inhibitors on 2-AG-induced actin polymerization in HL-60 cells differentiated into macrophage-like cells.
Differentiated HL-60 cells were treated with various inhibitors (20 μM for PD98059, SB203580, Y-27632, herbimycin A and Ro-31-8220, and 200 nM for wortmannin) at 37 °C for 1 h. They were then challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and stained with the NBD-phallacidin mixture as described in the Materials and methods section. Results are expressed as a percentage of the F-actin content in unstimulated cells (vehicle alone). Results are means±S.D. for five determinations; **P<0.01 and ***P<0.001 (compared with 2-AG alone).
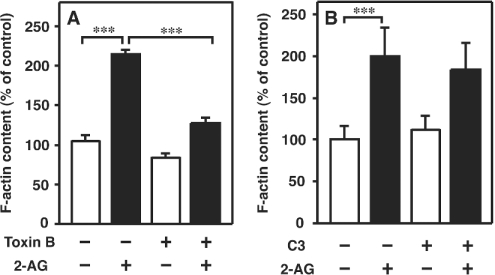
We also examined the effects of pretreatment of the cells with Rho family small G-protein inhibitors. First, we examined the effect of C. difficile toxin B [an inhibitor of Rho family small G-proteins (Rho, Rac and Cdc42)] on 2-AG-induced actin polymerization. As demonstrated in Figure 7(A), pretreatment of the cells with toxin B (100 ng/ml) markedly reduced the actin polymerization induced by 2-AG. It is apparent, therefore, that Rho family small G-proteins are involved in 2-AG-induced actin polymerization. We then examined the effect of pretreatment of the cells with C. botulinum C3 exoenzyme (a Rho inhibitor, 20 μg/ml). As depicted in Figure 7(B), the response induced by 2-AG was not markedly affected by treatment with C3 exoenzyme. These results suggest that rapid actin polymerization induced by 2-AG was mediated mainly by Rac and/or Cdc42.
Figure 7. Effects of toxin B and C3 exoenzyme on 2-AG-induced actin polymerization in HL-60 cells differentiated into macrophage-like cells.
(A) Differentiated HL-60 cells were treated with toxin B (100 ng/ml) at 37 °C for 12 h. They were then challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and stained with the NBD-phallacidin mixture as described in the Materials and methods section. Results are expressed as a percentage of the F-actin content in unstimulated cells (vehicle alone). Results are means±S.D. for four determinations. (B) Differentiated HL-60 cells were treated with C3 exoenzyme (20 μg/ml) at 37 °C for 24 h. They were then challenged with 2-AG (1 μM) or the vehicle (DMSO) for 10 s and stained with the NBD-phallacidin mixture as described in the Materials and methods section. Results are expressed as a percentage of the F-actin content in unstimulated cells (vehicle alone). Results are means±S.D. for five determinations; ***P<0.001.
DISCUSSION
In the present study, we investigated in detail the effect of 2-AG on the organization of the actin filament system in HL-60 cells that have been differentiated into macrophage-like cells. We found that 2-AG induced rapid actin polymerization in differentiated HL-60 cells. It is apparent that the 2-AG-induced actin polymerization is mediated through the CB2 receptor for the following reasons: (i) SR144528, a CB2 receptor-specific antagonist, blocked the response induced by 2-AG (Figure 5A); (ii) CP55940 and WIN55212-2 induced actin polymerization similar to 2-AG (Figure 4); (iii) in contrast with WIN55212-2, WIN55212-3, a stereoisomer of WIN55212-2 lacking cannabimimetic activities, did not exhibit appreciable activity (Figure 4); (iv) free arachidonic acid did not induce actin polymerization (Figure 4), providing evidence that arachidonic acid and its metabolites, which may be generated from 2-AG during incubation, are not involved in the response.
Several types of receptor ligands such as various chemoattractants are known to induce actin polymerization [41–46]. For example, fMLP (N-formylmethionyl-leucylphenylalanine) induces rapid actin polymerization in neutrophils [41,42] and HL-60 cells [43,44] and IL-8 (interleukin 8) induce actin polymerization in neutrophils [45,46]. However, it is not known whether cannabinoids as well as endogenous cannabinoid receptor ligands induce actin polymerization. Previously, Zimmerman and co-workers demonstrated that high concentrations of Δ9-THC (≥10 μM) disrupt the microfilament network in PtK2 cells [47], rabbit aortic endothelial cells [47] and Chinese-hamster ovary cells [48]. They also reported that high concentrations of Δ9-THC and HU210 disrupt microfilaments in PC12 cells [49,50]. However, they did not describe whether cannabinoids provoke actin polymerization. Derkinderen et al. [51] have reported that anandamide induces the phosphorylation of focal adhesion kinase, although they did not examine whether anandamide induces actin polymerization. 2-AG has been shown to rearrange the actin and vimentin in control and endothelin-1-stimulated human brain endothelial cells, yet polymerization was not studied [52]. To our knowledge, this is the first report showing that cannabinoid receptor ligands, including 2-AG, induce rapid actin polymerization.
Not much information is available regarding the biological activities of 2-AG as a CB2 receptor ligand. Several investigators have examined the effects of 2-AG on inflammatory cells and immune-competent cells. For example, Lee et al. [31] have reported that 2-AG affects lymphocyte proliferation. Ouyang et al. [32] also demonstrated that 2-AG suppresses IL-2 gene expression in murine T-lymphocytes through down-regulation of the nuclear factor. On the other hand, Gallily et al. [53] reported that 2-AG suppresses the production of tumour-necrosis factor α in lipopolysaccharide-stimulated mouse macrophages in vitro and in lipopolysaccharide-administered mice in vivo. Chang et al. [54] also demonstrated that 2-AG inhibited the production of IL-6 in J774 macrophage-like cells. However, it remains unclear in some cases whether these effects of 2-AG are mediated through the cannabinoid receptor.
On the other hand, we found that 2-AG induces a rapid transient increase in the intracellular free Ca2+ concentration ([Ca2+]i) in HL-60 cells [11]. The rapid increase in [Ca2+]i induced by 2-AG was blocked by pretreatment of the cells with SR144528. We also found that 2-AG induces a rapid phosphorylation of the p42/44 MAPK in HL-60 cells [24]. The 2-AG-induced phosphorylation of the p42/44 MAPK was abolished when the cells were pretreated with SR144528. These results clearly indicate that 2-AG induces the Ca2+ transient and the phosphorylation of the p42/44 MAPK by acting on the CB2 receptor. Importantly, 2-AG was found to act as a full agonist at the CB2 receptor, whereas anandamide acted as a weak partial agonist [11,26,28]. The fact that anandamide acts as a partial agonist at the CB2 receptor has been reported by several investigators as well [13,17]. In addition to anandamide, Δ9-THC is also known to act as a weak partial agonist at the CB2 receptor [11,55]. On the basis of the results of structure–activity relationship experiments, we concluded that 2-AG, and not anandamide, is the true natural ligand for the CB2 receptor [9,11,23]. The failure of anandamide as well as Δ9-THC to induce prominent actin polymerization (Figure 4) is thus consistent with the previous observations mentioned above.
Possible signalling mechanisms underlying 2-AG-induced actin polymerization were investigated using several inhibitors. We found that the treatment of the cell with PTX, wortmannin, herbimycin A and toxin B reduced the response induced by 2-AG (Figures 5B, 6 and 7A). These observations suggest that Gi/o, PI3K, Rho family small G-proteins such as Rac and Cdc42 and tyrosine kinase(s) are involved in the response. Several investigators have demonstrated that a pathway, involving Gi/o, PI3K, Akt/protein kinase B and Rho family small G-proteins and their downstreams such as WASP (Wiskott–Aldrich syndrome protein), N-WASP (neuronal WASP), WAVE (WASP family Verprolin-homologous protein) and phosphatidylinositol-4-phosphate 5-kinase, participate in actin polymerization [44,46,56,57]. It is plausible that similar mechanisms operate in 2-AG-stimulated cells, although other signal transduction pathways and molecules may also take part in the induction of the response. Further studies are required for a full understanding of the molecular mechanism responsible for 2-AG-induced actin polymerization.
What then is the physiological meaning of the 2-AG-induced actin polymerization? Recently, several investigators have demonstrated that 2-AG augments cell motility; Jorda et al. [29] have reported that 2-AG induced the migration of mouse splenocytes and 32D/G-CSF-R cells transfected with CB2 receptor mRNA, and Walter et al. [30] have demonstrated that 2-AG evoked the migration of mouse microglia cells. We also found that 2-AG induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes [28]. It seems quite probable that the 2-AG-induced actin polymerization shown in the present study is closely involved in the 2-AG-induced cell migration, since the reorganization of actin filaments is essential to induce cell migration.
As mentioned above, Rho family small G-proteins such as Rac and Cdc42 were suggested to be involved in 2-AG-induced actin polymerization in HL-60 cells differentiated into macrophage-like cells (Figure 7A). In addition to being involved in actin polymerization, Rac and Cdc42 are known to participate in diverse cellular processes such as neurite outgrowth, cell adhesion, cell polarity, cell division, endocytosis, regulation of gene expression and generation of reactive oxygen species [56,58]. Considering that the Rho family small G-proteins such as Rac and Cdc42 are some of the downstream molecules of signalling pathways triggered by 2-AG, it is conceivable that 2-AG elicits some of these cellular responses, in addition to actin polymerization, when added to cells. At present, however, not much information is available regarding the biological activities of 2-AG; this is probably due to the fact that much attention has been directed to anandamide rather than to 2-AG. Further detailed studies are necessary to unveil unknown biological activities of 2-AG in various mammalian tissues and cells.
The CB2 receptor is abundantly expressed in various types of inflammatory cells and immune-competent cells [3,33,34]. Evidence is gradually accumulating which shows that the CB2 receptor plays some essential role in inflammatory reactions and immune responses [35–39], yet the details of the physiological functions of the CB2 receptor still remain to be clarified. A thorough elucidation of the physiological and pathophysiological roles of 2-AG and the CB2 receptor would thus greatly contribute to a comprehensive understanding of the precise regulatory mechanisms of inflammatory reactions and immune responses.
References
- 1.Devane W. A., Dysarz F. A., III, Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 2.Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 5.Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim. Biophys. Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 6.Piomelli D., Beltramo M., Giuffrida A., Stella N. Endogenous cannabinoid signaling. Neurobiol. Dis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R., Fride E., Di Marzo V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V., De Petrocellis L., Bisogno T., Berger A., Mechoulam R. Biology of endocannabinoids. In: Onaivi E. S., editor. Biology of Marijuana. London: Taylor & Francis; 2002. pp. 125–174. [Google Scholar]
- 9.Sugiura T., Kobayashi Y., Oka S., Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 10.Sugiura T., Kodaka T., Nakane S., Miyashita T., Kondo S., Suhara Y., Takayama H., Waku K., Seki C., Baba N., et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T., Kondo S., Kishimoto S., Miyashita T., Nakane S., Kodaka T., Suhara Y., Takayama H., Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor. J. Biol. Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 12.Mackie K., Devane W. A., Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- 13.Bayewitch M., Levy T. R., Barg J., Mechoulam R., Vogel Z. The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett. 1995;375:143–147. doi: 10.1016/0014-5793(95)01207-u. [DOI] [PubMed] [Google Scholar]
- 14.Sugiura T., Kodaka T., Kondo S., Tonegawa T., Nakane S., Kishimoto S., Yamashita A., Waku K. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochem. Biophys. Res. Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 15.Burkey T. H., Quock R. M., Consroe P., Ehlert F. J., Hosohata Y., Roeske W. R., Yamamura H. I. Relative efficacies of cannabinoid CB1 receptor agonists in the mouse brain. Eur. J. Pharmacol. 1997;336:295–298. doi: 10.1016/s0014-2999(97)01255-7. [DOI] [PubMed] [Google Scholar]
- 16.McAllister S. D., Griffin G., Satin L. S., Abood M. E. Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium channels in a xenopus oocyte expression system. J. Pharmacol. Exp. Ther. 1999;291:618–626. [PubMed] [Google Scholar]
- 17.Gonsiorek W., Lunn C., Fan X., Narula S., Lundell D., Hipkin R. W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol. Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- 18.Hillard C. J. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 19.Savinainen J. R., Jarvinen T., Laine K., Laitinen J. T. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 21.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R., et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura T., Kodaka T., Kondo S., Nakane S., Kondo H., Waku K., Ishima Y., Watanabe K., Yamamoto I. Is the cannabinoid CB1 receptor a 2-arachidonoylglycerol receptor? Structural requirements for triggering a Ca2+ transient in NG108-15 cells. J. Biochem. 1997;122:890–895. doi: 10.1093/oxfordjournals.jbchem.a021838. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T., Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem. Phys. Lipids. 2000;108:89–106. doi: 10.1016/s0009-3084(00)00189-4. [DOI] [PubMed] [Google Scholar]
- 24.Oka S., Ikeda S., Waku K., Ishima Y., Sugiura T. Endocannabinoids as retrograde messengers in synaptic transmission. In: Onaivi E. S., Sugiura T., Di Marzo V., editors. Endocannabinoids. Boca Raton: CRC Press; 2005. in the press. [Google Scholar]
- 25.Panikashvili D., Simeonidou C., Ben-Shabat S., Hanus L., Breuer A., Mechoulam R., Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature (London) 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi Y., Arai S., Waku K., Sugiura T. Activation by 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, of p42/44 mitogen-activated protein kinase in HL-60 cells. J. Biochem. 2001;129:665–669. doi: 10.1093/oxfordjournals.jbchem.a002904. [DOI] [PubMed] [Google Scholar]
- 27.Sugiura T., Kishimoto S., Oka S., Gokoh M., Waku K. Metabolism and physiological significance of anandamide and 2-arachidonoylglycerol, endogenous cannabinoid receptor ligands. In: Fonteh A. N., Wykle R. L., editors. Arachidonate Remodeling and Inflammation. Basel: Birkhauser Verlag; 2004. pp. 211–237. [Google Scholar]
- 28.Kishimoto S., Gokoh M., Oka S., Muramatsu M., Kajiwara T., Waku K., Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J. Biol. Chem. 2003;278:24469–24475. [Google Scholar]
- 29.Jorda M. A., Verbakel S. E., Valk P. J., Vankan-Berkhoudt V., Maccarrone M., Finazzi-Agro A., Lowenberg B., Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- 30.Walter L., Franklin A., Witting A., Wade C., Xie Y., Kunos G., Mackie K., Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M., Yang K. H., Kaminski N. E. Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J. Pharmacol. Exp. Ther. 1995;275:529–536. [PubMed] [Google Scholar]
- 32.Ouyang Y., Hwang S. G., Han S. H., Kaminski N. E. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol. Pharmacol. 1998;53:676–683. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- 33.Herkenham M. Localization of cannabinoid receptors in brain and periphery. In: Pertwee R. G., editor. Cannabinoid Receptors. London: Academic Press; 1995. pp. 145–166. [Google Scholar]
- 34.Galiegue S., Mary S., Marchand J., Dussossoy D., Carriere D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 35.Klein T. W., Newton C., Friedman H. Cannabinoid receptors and immunity. Immunol. Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- 36.Berdyshev E. V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 37.Parolaro D., Massi P., Rubino T., Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:319–332. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- 38.Cabral G. A. Marijuana and cannabinoid effects on immunity and AIDS. In: Onaivi E. S., editor. Biology of Marijuana. London: Taylor & Francis; 2002. pp. 282–307. [Google Scholar]
- 39.Klein T. W., Larsen C. K., Lu L., Perkins I., Nong L., Friedman H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy D. M., San Miguel J. F., Freake H. C., Green P. M., Zola H., Catovsky D., Goldman J. M. 1,25-Dihydroxyvitamin D3 inhibits proliferation of human promyelocytic leukaemia (HL60) cells and induces monocyte-macrophage differentiation in HL60 and normal human bone marrow cells. Leuk. Res. 1983;7:51–55. doi: 10.1016/0145-2126(83)90057-7. [DOI] [PubMed] [Google Scholar]
- 41.Howard T. H., Meyer W. H. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J. Cell Biol. 1984;98:1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts R. G., Howard T. H. Mechanisms for actin reorganization in chemotactic factor-activated polymorphonuclear leukocytes. Blood. 1993;81:2750–2757. [PubMed] [Google Scholar]
- 43.Rao K. M., Misukonis M. A., Cohen H. J., Weinberg J. B. Cooperative effect of tumor necrosis factor and gamma-interferon on chemotactic peptide receptor expression and stimulus-induced actin polymerization in HL-60 cells. Blood. 1988;71:1062–1067. [PubMed] [Google Scholar]
- 44.Cui Y. D., Inanami O., Yamamori T., Niwa K., Nagahata H., Kuwabara M. FMLP-induced formation of F-actin in HL60 cells is dependent on PI3-K but not on intracellular Ca2+, PKC, ERK or p38 MAPK. Inflamm. Res. 2000;49:684–691. doi: 10.1007/s000110050647. [DOI] [PubMed] [Google Scholar]
- 45.Sham R. L., Phatak P. D., Ihne T. P., Abboud C. N., Packman C. H. Signal pathway regulation of interleukin-8-induced actin polymerization in neutrophils. Blood. 1993;82:2546–2551. [PubMed] [Google Scholar]
- 46.Chodniewicz D., Zhelev D. V. Novel pathways of F-actin polymerization in the human neutrophil. Blood. 2003;102:2251–2258. doi: 10.1182/blood-2002-09-2936. [DOI] [PubMed] [Google Scholar]
- 47.Kiosses B. W., Tahir S. K., Kalnins V. I., Zimmerman A. M. The effects of delta-9-tetrahydrocannabinol on actin microfilaments. Cytobios. 1990;63:23–29. [PubMed] [Google Scholar]
- 48.Tahir S. K., Zimmerman A. M. Influence of marihuana on cellular structures and biochemical activities. Pharmacol. Biochem. Behav. 1991;40:617–623. doi: 10.1016/0091-3057(91)90372-9. [DOI] [PubMed] [Google Scholar]
- 49.Tahir S. K., Trogadis J. E., Stevens J. K., Zimmerman A. M. Cytoskeletal organization following cannabinoid treatment in undifferentiated and differentiated PC12 cells. Biochem. Cell Biol. 1992;70:1159–1173. doi: 10.1139/o92-162. [DOI] [PubMed] [Google Scholar]
- 50.Wilson R. G., Jr, Tahir S. K., Mechoulam R., Zimmerman S., Zimmerman A. M. Cannabinoid enantiomer action on the cytoarchitecture. Cell Biol. Int. 1996;20:147–157. doi: 10.1006/cbir.1996.0019. [DOI] [PubMed] [Google Scholar]
- 51.Derkinderen P., Toutant M., Burgaya F., Le Bert M., Siciliano J. C., de Franciscis V., Gelman M., Girault J. A. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science. 1996;273:1719–1722. doi: 10.1126/science.273.5282.1719. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y., McCarron R. M., Ohara Y., Bembry J., Azzam N., Lenz F. A., Shohami E., Mechoulam R., Spatz M. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ. Res. 2000;87:323–327. doi: 10.1161/01.res.87.4.323. [DOI] [PubMed] [Google Scholar]
- 53.Gallily R., Breuer A., Mechoulam R. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice. Eur. J. Pharmacol. 2000;406:R5–R7. doi: 10.1016/s0014-2999(00)00653-1. [DOI] [PubMed] [Google Scholar]
- 54.Chang Y. H., Lee S. T., Lin W. W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. Biochem. 2001;81:715–723. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- 55.Bayewitch M., Rhee M. H., Avidor-Reiss T., Breuer A., Mechoulam R. (–)-Delta9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J. Biol. Chem. 1996;271:9902–9905. doi: 10.1074/jbc.271.17.9902. [DOI] [PubMed] [Google Scholar]
- 56.Takai Y., Sasaki T., Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 57.Miki H., Takenawa T. Regulation of actin dynamics by WASP family proteins. J. Biochem. 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 58.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature (London) 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]