Abstract
Mesial temporal lobe epilepsy (MTLE) is a common cause of seizures, and hippocampal sclerosis (HS) is the predominant subtype. BRAFV600E mutations in MTLE-HS have only been reported infrequently. Herein, we illustrate the neurologic, radiological, and histopathological details of a patient with MTLE-HS and BRAFV600E mutant neurons. A 31-year-old male with medically refractory epilepsy presented with magnetic resonance imaging (MRI) and electroencephalography (EEG) findings typical of mesial temporal sclerosis without a mass lesion. The surgical specimens showed ILAE Type 1 HS with neurons immunopositive for BRAFV600E mutant protein distributed along the Cornu Ammonis (CA) curvature. Instead of the normal mostly perpendicular orientation of pyramidal neurons relative to the hippocampal surface, the BRAF mutant neurons were often oriented in a parallel manner. On CD34 immunostaining, sparse clusters or nodules of CD34+ stellate cells and single immunopositive stellate cells were identified. BRAFV600E or CD34 immunopositive cells were less than 1 % of total cells. The patient responded well to surgery with no further seizures after 2 years and occasional auras. Hippocampal BRAF mutant non-expansive lesion (HBNL) has been used to describe such lesions with preserved cytoarchitecture and without overt tumor mass. Others may argue for the dual pathology of HS with early ganglioglioma. Whether pre-neoplastic lesions or early tumors, these cases are important for understanding early glioneuronal tumorigenesis and suggest that BRAFV600E studies should be routinely performed on MTLE-HS cases in the setting of clinical trials. With next-generation sequencing, a FANCL deletion was detected in almost half of the alleles in our case, suggesting that many of the histologically normal-appearing cells of the hippocampus contain this alteration. FANCL mutations can result in cytogenetic anomalies and defective DNA repair and therefore may underlie the development of a low frequency BRAF alteration.
Keywords: Hippocampal sclerosis, Epilepsy, BRAF, FANCL, CD34, MTLE, Brain tumor
Abbreviations
CA - Cornu Ammonis; EEG - Electroencephalography; FANCL - Fanconi Anemia Complementation Group L; GTC - Generalized Tonic-Clonic; HBNL - Hippocampal BRAF mutant non-expansive lesion; HS - Hippocampal Sclerosis; IHC - Immunohistochemistry; ILAE - International League Against Epilepsy; IPI - Inciting Precipitating Injury; LEATs - Low-grade Epilepsy-Associated Tumors; MRI - Magnetic Resonance Imaging; MTLE - Mesial Temporal Lobe Epilepsy; PCR - Polymerase Chain Reaction; PLNTY - polymorphous low-grade neuroepithelial tumor of the young; sEEG - stereoelectroencephalography; T1 MP-RAGE - T1-weighted Magnetization Prepared - RApid Gradient Echo; T2/FLAIR - T2-weighted-Fluid-Attenuated Inversion Recovery.
Introduction
Among the various causes of epilepsy, mesial temporal lobe epilepsy (MTLE) is noteworthy for its variability in presentation and resistance to anti-epileptic drug treatment.1-3 While MTLE has myriad etiologies, including focal cortical dysplasia, vascular lesions, tumors, and ischemia, hippocampal sclerosis (HS) remains the most common.4 The natural history of this entity classically involves an inciting precipitating injury (IPI) and subsequent development of epilepsy after a variable latency period.1 The disease is heterogeneous in clinical presentation, response to therapy, ictal semiology, and ictal electroencephalography (EEG).5 Similarly, the histopathological findings are diverse and include different patterns of granule cell layer alterations, neuronal loss, and gliosis in different regions of the hippocampus and amygdala.6 In 2013, the International League Against Epilepsy (ILAE) defined several patterns of HS: Type 1 involves severe neuronal loss and gliosis in the Cornu Ammonis (CA) 1 and 4 regions; Type 2 involves neuronal loss and gliosis predominantly in CA1; and Type 3 involves loss mainly in CA4.7,8 The fourth category, no-HS, shows reactive gliosis without neuron loss.8 Immunohistochemistry (IHC) for CD34 may highlight multipolar or stellate cells. In a very small fraction of HS cases, BRAFV600E immunostaining of neurons with cytoarchitectural preservation of the hippocampus has been reported and named BRAF+ HS or hippocampal BRAF mutant nonexpansive lesion (HBNL).6,9 However, this entity is controversial, as some consider such lesions to represent dual pathology (hippocampal sclerosis with ganglioglioma) and requires further study.
Prior studies have searched for associations between histopathology, IHC, clinical features, and outcomes in patients with MTLE-HS; these suggest that different histopathological patterns may be linked to outcomes after surgery.7,10 Based on limited early data, patients with BRAF mutant HS may have good seizure control with surgery, and diagnostic criteria to distinguish HBNL from existing BRAF mutant brain tumors have been proposed.9 In this report, we discuss the clinical and histopathologic findings in the case of a young male patient with MTLE-HS, whose resected tissue demonstrates ILAE Type 1 HS with neurons that are immunopositive for BRAFV600E (BRAFV600E+). We also argue that there is value in routine BRAFV600E testing of tissues in MTLE-HS clinical trials.
Case Presentation
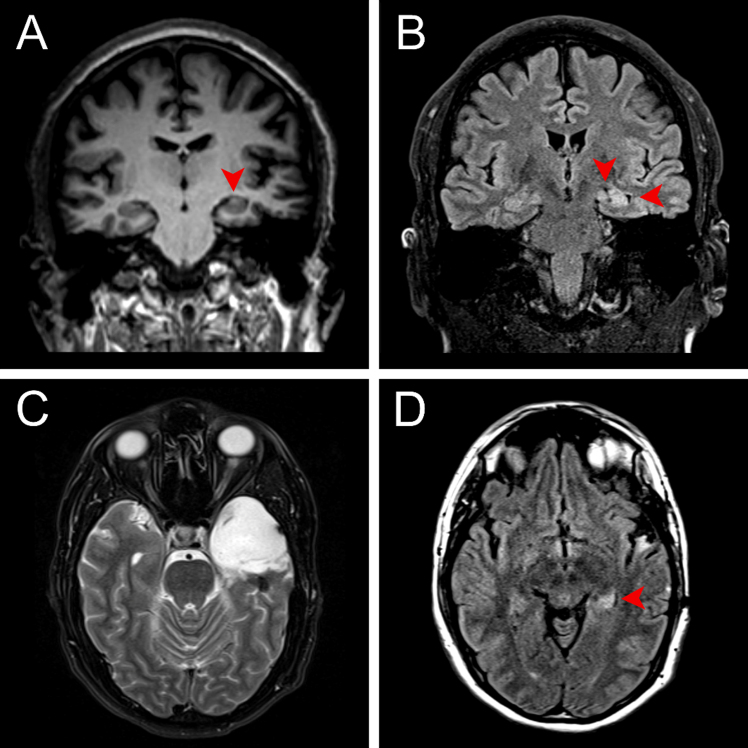
A 31-year-old right-handed male with a five-year history of focal epilepsy refractory to medical management presented to the Comprehensive Epilepsy Center for advanced monitoring and surgical planning. The patient’s seizures developed shortly after undergoing surgery for the removal of a bone spur that was complicated by a deep vein thrombosis requiring anticoagulation. The initial semiology included focal seizures with impaired awareness and generalized tonic-clonic (GTC) seizures with rightward head turn. Although the GTC seizures were controlled with valproic acid and lacosamide, he continued to have focal seizures without impaired awareness and failed a trial of levetiracetam. These seizures began with a sensation of déjà vu, followed by hyperventilation and oral automatisms. Magnetic resonance imaging (MRI) showed left hippocampal hyperintensity and mild volume loss, consistent with left mesial temporal lobe sclerosis, without evidence of mass lesion (Figure 1). After two unsuccessful attempts at electrographically characterizing seizures with noninvasive EEG, the patient underwent stereoelectroencephalography (sEEG) implantation, which localized the seizures to the left temporal lobe. A Wada test demonstrated the dominance of the left hemisphere for language and the right for memory. The case was discussed at the multidisciplinary epilepsy clinic, and after patient consent was obtained for surgery and involvement in research, he underwent left craniotomy for temporal lobectomy and amygdalohippocampectomy. The procedure was uncomplicated, and the patient went home on postoperative day 1. A neuropsychological evaluation performed 11 months after surgery showed improvement in measures of verbal memory and a cognitive profile essentially within normal limits. Two years after surgery, the patient has had occasional episodes of dysmnesic phenomena (déjà vu auras) but has not had any seizures, corresponding to Engel class IB (Supplementary Figure 1).
Figure 1.
Preoperative coronal MRI at the level of the hippocampal body (A) T1 MP-RAGE demonstrating left hippocampal atrophy and architectural distortion, and (B) T2/FLAIR showing abnormal hyperintense signal with atrophy and associated widening of the temporal horn (right arrowhead) and choroid fissure (left arrowhead). Postoperative axial T2 (C) MRI at the level of the pons demonstrating postoperative changes following left anterior temporal lobectomy and (D) T2/FLAIR at the level of the mesencephalon showing abnormal hyperintense signal in the residual left hippocampal tail.
Pathology
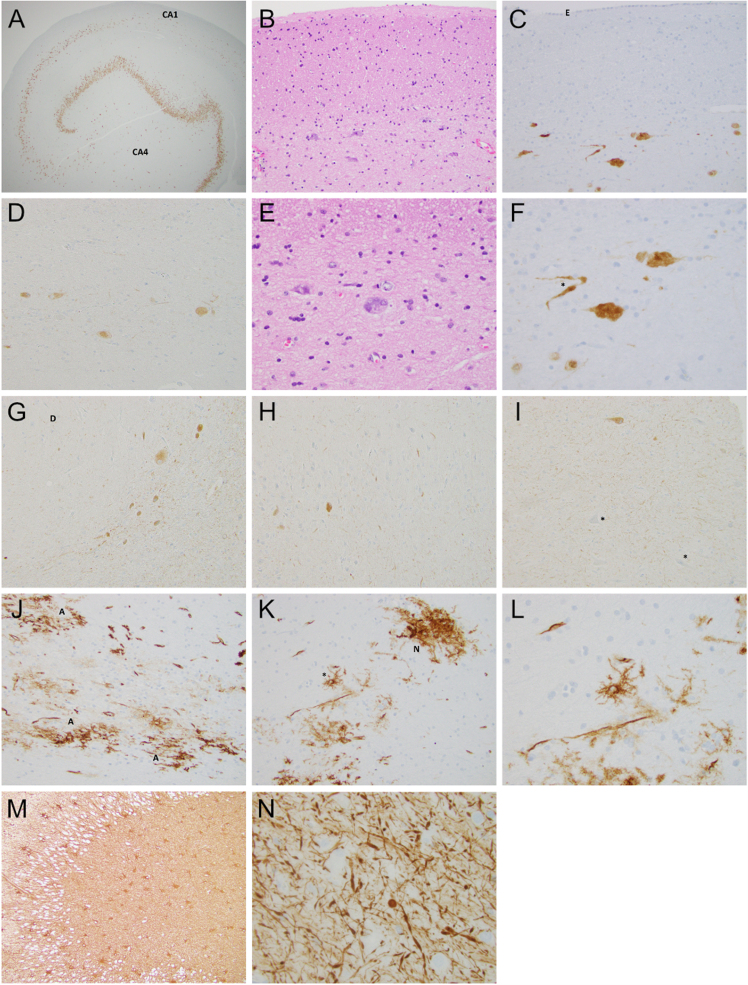
Surgical specimens of the temporal lobe, amygdala, and hippocampus were submitted. Immunostaining for NeuN highlighted the relative paucity of neurons in the CA1 and CA4 regions consistent with HS ILAE Type 1 (Figure 2A). Granule cell dispersion was present. Chromogranin immunostaining showed a similar pattern of regional neuronal loss. In the patient’s specimen, neurons of the CA1 and CA2 regions were often oriented parallel to the ventricular surface (Figure 2B–G) instead of the normally predominant perpendicular orientation, giving a streaming appearance. Occasional scattered BRAFV600E+ cells that have the morphology of neurons were present in the CA, most prominently in the CA4 and CA3 regions but represented less than 1 % of cells. In CA2/CA3, BRAF+ neurons also tended to have a parallel streaming orientation (Figure 2D). There were far more NeuN immunopositive neurons than BRAF mutant neurons. Hypertrophic and dysmorphic neurons were identified (Figure 2E–F). A curvilinear distribution of BRAF mutant neurons mimics the usual neuroanatomic architecture of the CA (Figure 2G). In the amygdala specimen that also had dentate gyrus of the hippocampus, there were a few scattered BRAFV600E+ neurons. A section of the temporal lobe did not show any BRAFV600E immunoreactivity. In the dentate gyrus, the BRAFV600E+ neurons were sparse and retained the perpendicular orientation of their many immunonegative peers (Figure 2H). BRAFV600E+ threads, probably reflecting neurites, were few, but in some areas were abundant and excessive for what might be anticipated relative to the observed neuronal soma (Figure 2G, 2I). CD34 immunostaining of the hippocampus-stained blood vessels was otherwise largely negative except for sparse foci of CD34 immunopositive (CD34+) stellate cells present as loose or nodular aggregates, or single cells (Figure 2J–L). CD34+ cells are less than 1 % of cells. GFAP immunostaining showed extensive reactive astrocytosis (Figure 2M). Neurofilament highlighted occasional pyramidal neurons and abundant axonal injury characterized by variably thickened axons with spindled expansions, torpedoes or spheroids in the hippocampus (Figure 2N). Synaptophysin immunostaining demonstrated strong staining of the brain neuropil with scattered immunopositive neurons. The Ki-67 proliferation index was very low with only rare scattered immunoreactive nuclei. An IDH1 R132H immunostain was negative. We attempted two BRAFV600E polymerase chain reaction (PCR) assays which did not detect a BRAF alteration. We then attempted next-generation sequencing that also did not detect a BRAF alteration but did detect a Fanconi Anemia Complementation Group L (FANCL) deletion in 48.5 % of alleles. The frequency of the BRAF V600E+ neurons in our case is very low estimated at less than 1 % and our PCR testing can only detect reliably at the 20–40 % or above mutant allele level, while our next-generation sequencing detects down to ~5 % mutant alleles. The BRAFV600E immunohistochemistry is robust as routine use produces clean staining and, in the setting of tumors with abundant BRAFV600E+ tumor cells, the immunostaining is corroborated by positive PCR findings.
Figure 2.
A. NeuN immunostaining highlights the loss of neurons in the CA1 and CA4 regions of the hippocampus. (20X). B. In the CA1 and subiculum, residual pyramidal neurons often stream in parallel to the ventricular surface rather than being mostly oriented in the typical perpendicular orientation. (H&E 200X). C. NeuN immunostained CA1 neurons are oriented parallel to the ventricular surface lined by ependymal cells. E = Ependymal lining of ventricle. (200X). D. BRAFV600E+ neurons in the stratum radiatum of the CA3 region. (200X). E. Occasional neurons may be large and, in this case, dysmorphic with an ovoid or swollen appearing soma and abnormal distribution of basophilic Nissl substance. (H&E 400X). F. Two pyramidal neurons show lumpy cytoplasmic contours, reminiscent of popcorn. A variably thickened neuritic process (asterisk) is also present. (NeuN 400X). G. BRAFV600E+ neurons in the CA3 region stream in a curvilinear fashion around a terminus of the dentate (D) gyrus. Many slender, delicate immunopositive “threads” – presumably neuritic processes – are in the background, including areas without obvious neuronal soma. (200X). H. Only sparse BRAFV600E+ neurons are identified in the dentate gyrus. (200X). I. Numerous BRAF immunopositive “threads” are seen in this area of CA4. An immunopositive neuron is in the upper image. Immunonegative neurons are marked by an asterisk (200X). J. Aggregates of CD34+ stellate cells (A) form irregular plaques (200X). K. CD34+ stellate cells may have a “nodular” pattern (N) or may be single (asterisk). (200X). L. The CD34+ stellate cell at higher magnification. (400X). M. GFAP immunopositive hypertrophic astrocytes in the dentate gyrus and CA4 region are illustrated. (100X). N. Neurofilament stain shows axonal injury characterized by thickened axons including spheroidal, spindled, or torpedo-shaped expansions. (400X).
Discussion
MTLE-HS is a heterogeneous syndrome characterized by intractable epilepsy, progressive neurological changes, and variable histopathology. Although MTLE-HS is thought to have a genetic component, it classically arises after an IPI, such as trauma, hypoxia, or febrile seizures, followed by a latent period and eventual seizure onset.11 When refractory to medical management, surgical resection of the seizure focus can yield favorable short- and long-term outcomes, including seizure control, seizure freedom, and improved quality of life.12,13 Nonetheless, positive response to surgery is not uniform, and prior studies have reported association of febrile seizures, hippocampal sclerosis, and certain histopathological patterns with surgical outcomes.14
The ILAE classification system defines types of HS in temporal lobe epilepsy, according to regions of hippocampal neuronal loss and gliosis. Type 1 HS is the most common, features the most severe neuronal loss, and is frequently associated with an IPI before the age of 5. Histologically, neuronal loss occurs mostly in the CA1 and CA4 regions.7,8 Types 2 and 3 are less common and involve predominantly CA1 and CA4 neuronal loss (with gliosis), respectively. Of all types, Type 2 is most closely associated with an IPI.6,15 Lastly, a no-HS pattern involves gliosis only with no neuronal loss.8 Significant interest and research have been dedicated to describing the clinical correlates and surgical outcomes of different ILAE subtypes.16-18 A retrospective cohort study of 213 patients found that those with Type 1 HS had a longer duration of epilepsy and older age at the time of surgical treatment, compared to patients with Type 2 HS.16 Independent of HS type, greater than 80 % of patients had Engel class I (free of disabling seizures) outcomes in the short- and long-term. However, the Type 2 HS patients had better Engel class Ia (complete seizure freedom) outcomes than the Type 1 HS patients.16 In another cohort of 389 patients, there was no detectable correlation between ILAE HS type and surgical outcome.19 A more recent series of 247 patients reported that Type 1 HS with hypertrophic neurons in the CA4 was associated with worse outcomes in both Engel I (freedom from disabling seizures) and Ia (complete seizure freedom) scores.6 While our case is Type 1 HS and also contains hypertrophic neurons, our patient remains seizure-free at the latest follow-up.
In addition to the pattern of HS, expression of CD34, a stem-cell marker of hematopoietic cells involved in early neurulation and cell function,20 has recently been recognized as a marker that varies among histological subtypes of MTLE-HS.6 CD34 was previously shown to be useful in the prognostication of a range of low-grade epilepsy-associated tumors (LEATs), including gangliogliomas, dysembryoplastic neuroepithelial tumors, and multinodular and vacuolating neuronal tumors.15,21 A retrospective series of 187 patients identified CD34 expression in up to half of patients with LEATs.22 In those patients, CD34 positivity was associated with longer duration of epilepsy, older age at the time of surgical treatment, and a higher likelihood of achieving complete tumor resection. The authors also noted that 89 % of patients with CD34+ histology had drug-resistant epilepsy and that a slightly higher proportion of patients with unfavorable seizure outcomes (Engel class II–IV) were CD34+.22
CD34 immunoreactivity is not restricted to glioneuronal neoplasms. A study by Calderon-Garciduenas et al.6 described the distribution and clinical correlates of CD34 in a large series of surgically treated MTLE-HS patients. They reported that 17 % (40/236) of their MTLE-HS cases had CD34+ stellate cells. These CD34+ cells could form a “nodule” or exist as scattered single cells. Type 1 HS was least likely to express CD34 compared to other types. CD34+ stellate cells were more frequently found in Type 2 HS and non-HS cases (i.e., gliosis only), while a CD34+ nodular pattern was more frequently found in Type 3 HS and non-HS cases and was associated with dysmnesic auras. Nodular aggregates were noted in our case and the patient does have dysmnesic auras. The authors found that CD34+ stellate cells were associated with a trend towards better postsurgical outcomes and speculate that CD34+ cells may represent epileptogenic seizure foci curable by surgery.6 Interestingly, our patient presented with the less common combination of Type 1 MTLE-HS with a few nodules of CD34+ stellate cells. He responded favorably to surgical treatment and, while he still has occasional auras, he remains seizure-free after 2 years.
Of particular interest in our patient is the detection of phenotypic neurons with BRAFV600E immunopositivity. BRAF is a serine/threonine kinase involved in the Ras/MAPK signaling pathway, necessary for cellular division and differentiation. BRAFV600E mutations are among the most common Ras/MAPK pathway abnormalities driving human neoplasms.23 BRAFV600E mutations have been identified in the histopathology of many tumors, including LEATs,24,25 but have also been reported in a significant subset of MTLE-HS.6 Determination of BRAF mutation status has been made easier by the advent of mutation-specific monoclonal antibodies, replacing the time- and resource-intensive genetic sequencing.26 Neither PCR nor next-generation sequencing detected BRAFV600E alterations in our case, but the frequency of BRAF mutant neurons was below the threshold of detection by these modalities. With next-generation sequencing of our case, a FANCL deletion was detected in almost half of the alleles. FANCL mutations can lead to cytogenetic instability, increased chromosomal breakage and defective DNA repair among other effects. This high percentage of mutant allele frequency suggests that many of the histologically normal/BRAF-wildtype cells in the hippocampus have the deletion. We speculate that a FANCL alteration during development may have contributed to acquiring a later BRAF alteration given the very low percentage (less than 1 %) of BRAF immunopositive cells. GWAS studies have previously shown a linkage between the FANCL locus and epilepsy.27
BRAFV600E is associated with a variety of tumors including brain tumors, but it also has been reported in pre-neoplastic or dysplastic processes such as cutaneous nevi, serrated polyps, and cortical dysplasia.23,28,29 A variety of BRAF alterations, including several in the kinase domain region that codon 600 lies within, are associated with epilepsy and cortical dysplasia in patients with Cardiofaciocutaneous syndrome.30 CD34+ cells are well described in glioneuronal tumors but also in cortical dysplasia,20,23 and in about 17 % of hippocampal sclerosis cases.6 Accordingly, the BRAFV600E and CD34 immunopositivity in our case should not be considered specific for tumor, but can also be seen in dysplastic or pre-neoplastic processes.
In their cohort, Calderon-Garciduenas et al. identified 5 out of 236 cases (2 %) of MTLE-HS with CD34+ cells and BRAFV600E+ neurons.6 Three of these cases exhibited the nodular CD34+ pattern, and 2 were CD34+ scarce. There were no neoplasms seen in these 5 patients, suggesting that BRAFV600E mutations may predispose to epilepsy in the absence of overt tumorigenesis.6 In that report, BRAF+ HS was the proposed nomenclature. In a follow-up study from the same group, Lerond et al.9 used the designation of hippocampal BRAF-mutant non-expansive lesion (HBNL) and described similar findings in the neocortex. In 28 CD34 negative cases of HS, no BRAF mutant neurons were identified. In contrast, 20 % of 25 CD34+ MTLE-HS cases were BRAF mutant and therefore designated as HBNL. Lerond et al.9 raised the question of other MAPK alterations besides BRAF in the CD34+ BRAF wildtype cases.
Research in mouse models has implicated BRAFV600E mutations in neuronal hyperexcitability31 and epileptogenic activity.32 In 2018, Koh et al.32 showed that somatic BRAFV600E mutations led to intrinsic epileptogenic activity in mice neurons and concluded that BRAF mutations in progenitor neurons during embryonic development predispose to epilepsy-associated diseases. Moreover, a case series of 20 patients with LEATs found that BRAF mutations were not only present in tumor cells but also in the dysplastic neurons associated with these tumors, further supporting the hypothesis that common BRAF-mutated progenitors may produce dysplastic neurons that accompany the neoplastic dysplasia.23 Lerond et al showed that interictal-like discharges can be detected in HBNL hippocampal slices but that the spatial and discharge patterns differed from BRAF wildtype MTLE-HS cases.9 In our patient with ILAE Type 1 MTLE-HS and immune expression of CD34 and BRAFV600E, it is reasonably likely that these histopathological findings correlate with hyperexcitable neurons promoting epileptogenesis, and their resection may be associated with long-term seizure freedom.
In addition to its utility as a predictor of disease pathogenesis and clinical presentation, BRAFV600E may represent a potential target for emerging pharmacologic agents.23,33 BRAF inhibitors, such as vemurafenib and dabrafenib, are commonly and successfully used to treat BRAFV600E+ melanoma.34 Remarkably, emerging rodent data has applied BRAF inhibitors to BRAFV600E+ epileptic disease with promising results. In their study, Koh et al.32 noted that intraventricular injections of vemurafenib in mice with BRAFV600E mutations alleviated epileptic seizures. Oral administration of the drug, however, yielded no benefit, perhaps due to its poor blood-brain barrier penetrance. Trials are currently underway to explore the efficacy of these drugs in treating BRAFV600E-associated gliomas in human subjects.
There remains the unsettled question of whether the BRAF mutant cells in MTLE-HS reflect a benign dysplastic state that has the potential to be converted by additional alterations into a neoplasm, an early stage of a developing neoplasm that would have continued to progress without surgical intervention or a forme fruste wherein a developing neoplasm is effectively frozen before forming a radiologically detectable mass, for example by oncogenic senescence. Lerond et al.32 suggested the presence of somatic mosaicism given the absence of detectable BRAFV600E alterations in the temporal lobe of some of their hippocampus mutant cases. A BRAFV600E immunostaining of the temporal lobe in our cases is negative but this is a limited assessment of the temporal lobe specimen, which consisted of multiple blocks of tissue. At a practical level, we were initially uncertain as to whether classify the lesion as a BRAF mutant HS or as HS associated with a BRAF mutant neoplasm. The lack of a radiological mass, mild hippocampal volume loss by MRI, localization of BRAF mutant cells in a curvilinear distribution that approximated the usual CA cytoarchitecture, and the low Ki-67 index led us to favor the designation of a BRAFV600E+ HS consonant with the preliminary nomenclature at that time.6 The parallel streaming pattern raises the question of aberrant neuronal migration akin to classical cortical dysplasia. Given the unclear nature of our case, the most recently proposed nosology of HBNL, which is a descriptive, neutral term that does not ascribe a non-neoplastic or neoplastic etiology may be appropriate.9 Our case could reflect dual pathology (hippocampal sclerosis and a ganglion cell neoplasm) though that may require postulating migration of mutant neoplastic neurons along the curvature of the CA. We did not identify diagnostic features of a variety of tumor entities that may be CD34+ and BRAF+ such as polymorphous low-grade neuroepithelial tumor of the young (PLNTY), pleomorphic xanthoastrocytoma, multinodular and vacuolated neuronal tumor, and dysembryoplastic neuroepithelial tumor. The major concern for our case was to exclude a gangliocytoma or a ganglioglioma. Based on a limited number of cases, it has been proposed that diffuse sheets of CD34+ cells are more typical of PLNTY and ganglioglioma than for HBNL wherein single scattered CD34+ cells are common9. The nodular CD34+ pattern was not included as a distinguishing feature.9 In addition to the aforementioned radiologic, cytoarchitectural and other histopathologic features, our case only had sparse CD34+ nodules and scattered cells and did not have sheets of such cells, overall favoring an HBNL by the published preliminary criteria.
Conclusion
We provide details of a case of ILAE Type 1 MTLE-HS with BRAFV600E+ neurons and CD34+ stellate cells. We describe a general parallel orientation of many CA neurons relative to the ventricular surface including BRAF mutant ones. BRAF mutant neurons are not restricted to the stratum pyramidale but may be more prominent in the stratum radiatum and strata lucidum. Whether this parallel streaming pattern is typical of HBNL, or an inconstant coincidental dysplastic feature or migration of neoplastic cells requires further research. Additional study is required to better understand the nature of this finding as to whether it is a pre-neoplastic or early neoplastic state or even a forme fruste of a glioneuronal tumor. Nevertheless, these HBNL cases are important as they likely provide a very important glimpse of the early genesis of glioneuronal tumors. Such cases may be missed as BRAFV600E studies are not routinely obtained on HS cases and tumor may be radiologically and histologically inapparent as in our case. Since only approximately 2 % of MTLE-HS cases will have this alteration, it may not be cost-effective to routinely test for BRAFV600E alterations in the community. However, major epilepsy centers that perform relevant MTLE research as well as clinical trials involving MTLE-HS patents should consider doing so routinely.
Acknowledgments
We thank Dr. Jefferson Chan of UC Irvine for assistance with PCR and sequencing of the tissue samples.
Conflicts of Interest Statement
The authors have no conflicts of interest to report.
Funding Statement
The authors acknowledge that they received no funding in support for this work.
Supplementary Material
References
- Palleria C, Coppola A, Citraro R, et al. Perspectives on treatment options for mesial temporal lobe epilepsy with hippocampal sclerosis. Expert Opin Pharmacother. 2015;16(15):2355-2371. 10.1517/14656566.2015.1084504. [DOI] [PubMed]
- Berg AT. The natural history of mesial temporal lobe epilepsy. Curr Opin Neurol. 2008;21(2):173-178. 10.1097/WCO.0b013e3282f36ccd. [DOI] [PubMed]
- Semah F, Lamy C, Demeret S. Hippocampal sclerosis and other hippocampal abnormalities in the early identification of candidates for epilepsy surgery. Arch Neurol. 2002;59(6):1042-1043; author reply 1043. 10.1001/archneur.59.6.1042-a. [DOI] [PubMed]
- Malmgren K, Thom M. Hippocampal sclerosis--origins and imaging. Epilepsia. 2012;53 Suppl 4:19-33. 10.1111/j.1528-1167.2012.03610.x. [DOI] [PubMed]
- Varoglu AO, Saygi S, Acemoglu H, Ciger A. Prognosis of patients with mesial temporal lobe epilepsy due to hippocampal sclerosis. Epilepsy Res. 2009;85(2-3):206-211. 10.1016/j.eplepsyres.2009.03.001. [DOI] [PubMed]
- Calderon-Garcidueñas AL, Mathon B, Lévy P, et al. New clinicopathological associations and histoprognostic markers in ILAE types of hippocampal sclerosis. Brain pathology. 2018;28(5):644-655. 10.1111/BPA.12596. [DOI] [PMC free article] [PubMed]
- Blümcke I, Pauli E, Clusmann H, et al. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113(3):235-244. 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed]
- Blümcke I, Thom M, Aronica E, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54(7):1315-1329. 10.1111/epi.12220. [DOI] [PubMed]
- Lerond J, Mathon B, Scopin M, et al. Hippocampal and neocortical BRAF mutant non-expansive lesions in focal epilepsies. Neuropathol Appl Neurobiol. 2023;49(5):e12937. 10.1111/nan.12937. [DOI] [PubMed]
- Hermann BP, Wyler AR, Somes G, Berry AD, Dohan FC. Pathological status of the mesial temporal lobe predicts memory outcome from left anterior temporal lobectomy. Neurosurgery. 1992;31(4):652-656; discussion 656-657. 10.1227/00006123-199210000-00006. [DOI] [PubMed]
- Wieser HG, ILAE Commission on Neurosurgery of Epilepsy. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45(6):695-714. 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. 10.1056/NEJM200108023450501. [DOI] [PubMed]
- Pereira Dalio MTR, Velasco TR, Feitosa IDF, et al. Long-Term Outcome of Temporal Lobe Epilepsy Surgery in 621 Patients With Hippocampal Sclerosis: Clinical and Surgical Prognostic Factors. Front Neurol. 2022;13:833293. 10.3389/fneur.2022.833293. [DOI] [PMC free article] [PubMed]
- Wieshmann UC, Larkin D, Varma T, Eldridge P. Predictors of outcome after temporal lobectomy for refractory temporal lobe epilepsy. Acta Neurol Scand. 2008;118(5):306-312. 10.1111/j.1600-0404.2008.01043.x. [DOI] [PubMed]
- Pasquier B, Péoc’h M, Fabre-Bocquentin B, et al. Surgical pathology of drug-resistant partial epilepsy. A 10-year-experience with a series of 327 consecutive resections. [Published with videosequences]. Epileptic Disorders. 2002;4(2):99-119. 10.1684/j.1950-6945.2002.tb00480.x. [DOI] [PubMed]
- Deleo F, Garbelli R, Milesi G, et al. Short- and long-term surgical outcomes of temporal lobe epilepsy associated with hippocampal sclerosis: Relationships with neuropathology. Epilepsia. 2016;57(2):306-315. 10.1111/epi.13277. [DOI] [PubMed]
- Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2005;128(Pt 2):395-404. 10.1093/brain/awh358. [DOI] [PubMed]
- Jardim AP, Neves RS da C, Caboclo LOSF, et al. Temporal lobe epilepsy with mesial temporal sclerosis: hippocampal neuronal loss as a predictor of surgical outcome. Arq Neuropsiquiatr. 2012;70(5):319-324. 10.1590/s0004-282x2012000500003. [DOI] [PubMed]
- Mathon B, Bielle F, Samson S, et al. Predictive factors of long-term outcomes of surgery for mesial temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsia. 2017;58(8):1473-1485. 10.1111/epi.13831. [DOI] [PubMed]
- Deb P, Sharma MC, Tripathi M, Sarat Chandra P, Gupta A, Sarkar C. Expression of CD34 as a novel marker for glioneuronal lesions associated with chronic intractable epilepsy. Neuropathol Appl Neurobiol. 2006;32(5):461-468. 10.1111/j.1365-2990.2006.00734.x. [DOI] [PubMed]
- Blümcke I, Giencke K, Wardelmann E, et al. The CD34 epitope is expressed in neoplastic and malformative lesions associated with chronic, focal epilepsies. Acta Neuropathol. 1999;97(5):481-490. 10.1007/s004010051017. [DOI] [PubMed]
- Giulioni M, Marucci G, Cossu M, et al. CD34 Expression in Low-Grade Epilepsy-Associated Tumors: Relationships with Clinicopathologic Features. World Neurosurg. 2019;121:e761-e768. 10.1016/j.wneu.2018.09.212. [DOI] [PubMed]
- Marucci G, de Biase D, Visani M, et al. Mutant BRAF in low-grade epilepsy-associated tumors and focal cortical dysplasia. Annals of Clinical and Translational Neurology. 2014;1(2):130. 10.1002/ACN3.31. [DOI] [PMC free article] [PubMed]
- Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397-405. 10.1007/s00401-011-0802-6. [DOI] [PubMed]
- Koelsche C, Wöhrer A, Jeibmann A, et al. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125(6):891-900. 10.1007/s00401-013-1100-2. [DOI] [PubMed]
- Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122(1):11-19. 10.1007/s00401-011-0841-z. [DOI] [PubMed]
- International League Against Epilepsy Consortium on Complex Epilepsies. GWAS meta-analysis of over 29,000 people with epilepsy identifies 26 risk loci and subtype-specific genetic architecture. Nat Genet. 2023;55(9):1471-1482. 10.1038/s41588-023-01485-w. [DOI] [PMC free article] [PubMed]
- Kiuru M, Tartar DM, Qi L, et al. Improving classification of melanocytic nevi: Association of BRAF V600E expression with distinct histomorphologic features. J Am Acad Dermatol. 2018;79(2):221-229. 10.1016/j.jaad.2018.03.052. [DOI] [PMC free article] [PubMed]
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131(5):1400-1407. 10.1053/j.gastro.2006.08.038. [DOI] [PubMed]
- Battaglia DI, Gambardella ML, Veltri S, et al. Epilepsy and BRAF Mutations: Phenotypes, Natural History and Genotype-Phenotype Correlations. Genes. 2021;12(9):1316. 10.3390/genes12091316. [DOI] [PMC free article] [PubMed]
- Goz RU, Akgül G, LoTurco JJ. BRAFV600E expression in neural progenitors results in a hyperexcitable phenotype in neocortical pyramidal neurons. J Neurophysiol. 2020;123(6):2449-2464. 10.1152/jn.00523.2019. [DOI] [PMC free article] [PubMed]
- Koh HY, Kim SH, Jang J, et al. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat Med. 2018;24(11):1662-1668. 10.1038/s41591-018-0172-x. [DOI] [PubMed]
- Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596-599. 10.1038/nature09454. [DOI] [PMC free article] [PubMed]
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835-1844. 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.