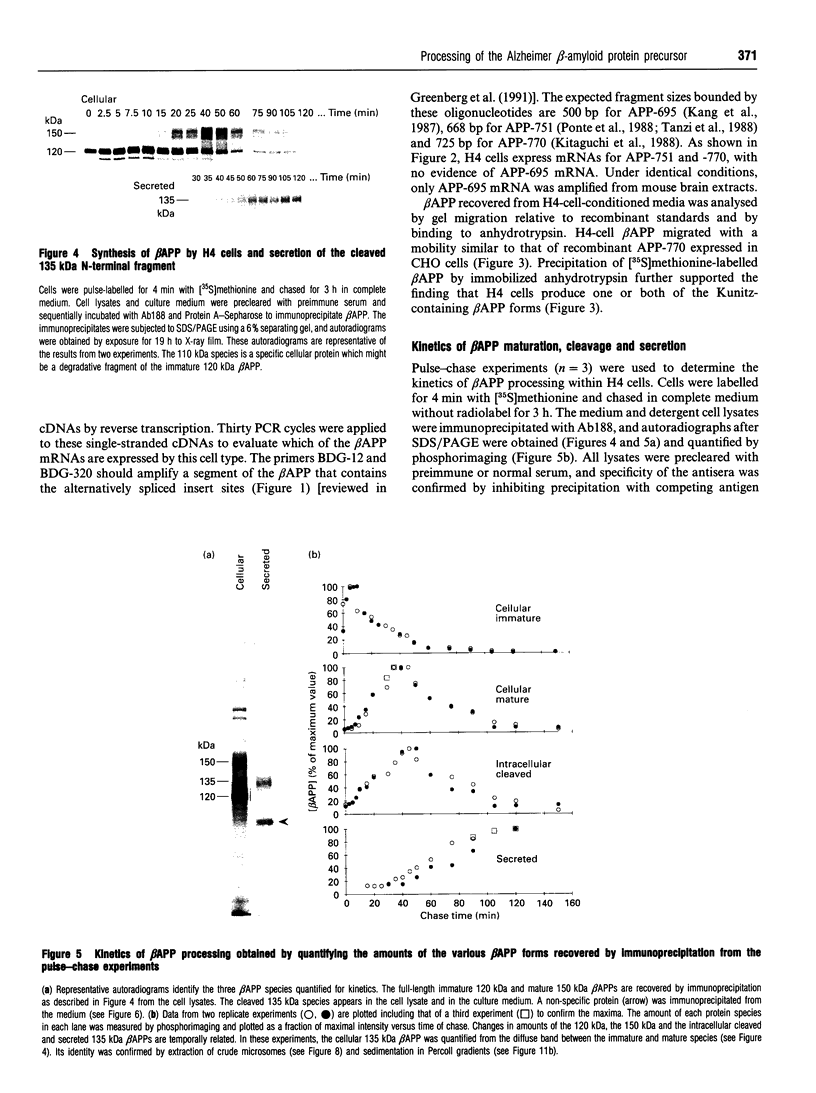
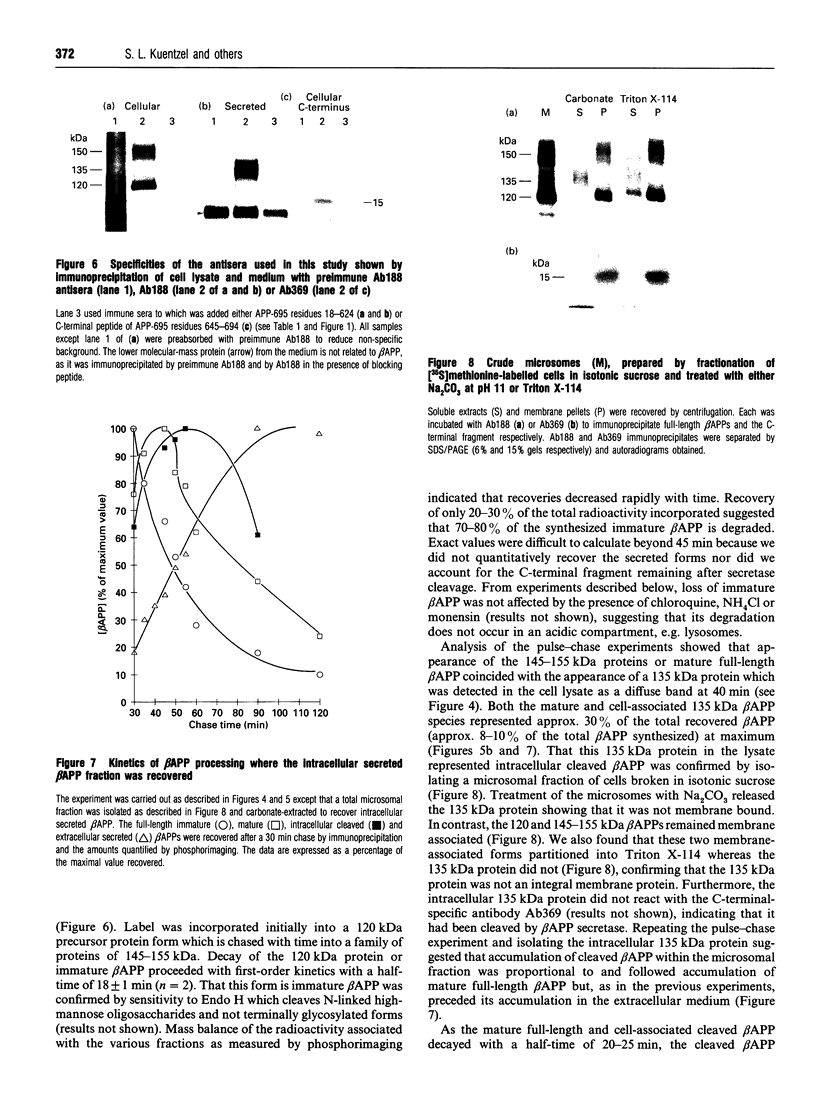
Abstract
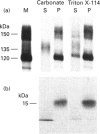
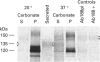
Alzheimer beta-amyloid protein precursor (beta APP) is expressed endogenously and abundantly by human neuroglioma (H4) cells. Its secretory processing has been shown to involve discrete proteolysis within the beta A4 region, thus preventing beta-amyloid formation, by an enzyme which has been referred to as 'beta APP secretase'. This cleavage results in secretion of a soluble N-terminal 135 kDa protein and retention of an integral membrane C-terminal fragment within the cell. The membrane-associated C-terminal fragment is sorted to lysosomes where it undergoes limited degradation. We show here that most newly synthesized beta APP is degraded via a non-lysosomal pathway before maturation in H4 cells, and most mature beta APP is processed predominantly by the so-called secretase. The rapid kinetics of appearance/disappearance of a cleaved 135 kDa protein within a microsomal fraction and the slow accumulation of this form in the extracellular medium indicated that secretase cleaves beta APP in an intracellular compartment. Low-temperature block (20 degrees C) was used to demonstrate that beta APP is cleaved within a late Golgi compartment after sulphation which occurs in the trans-Golgi network (TGN). This is consistent with (1) the immunolocalization of most of the beta APP within a Golgi compartment that reacts with wheat germ agglutinin, (2) the fact that less than 1.5% of the total mature full-length beta APP is present at the plasma membrane and (3) subcellular fractionation studies which showed that the mature full-length and intracellular cleaved beta APPs co-sediment with a membrane fraction that is slightly more dense than the plasma membrane. This study provides evidence that most of the beta APP secretase in H4 cells is intracellular, and confirms that the resulting C-terminal fragment is delivered to lysosomes immediately after cleavage. These results are discussed with regard to the possibility that mature full-length beta APP escapes secretase cleavage and is delivered directly from the TGN to the lysosome without passing through the plasma membrane. Either pathway will result in the generation of amyloidogenic fragments.
Full text
PDF



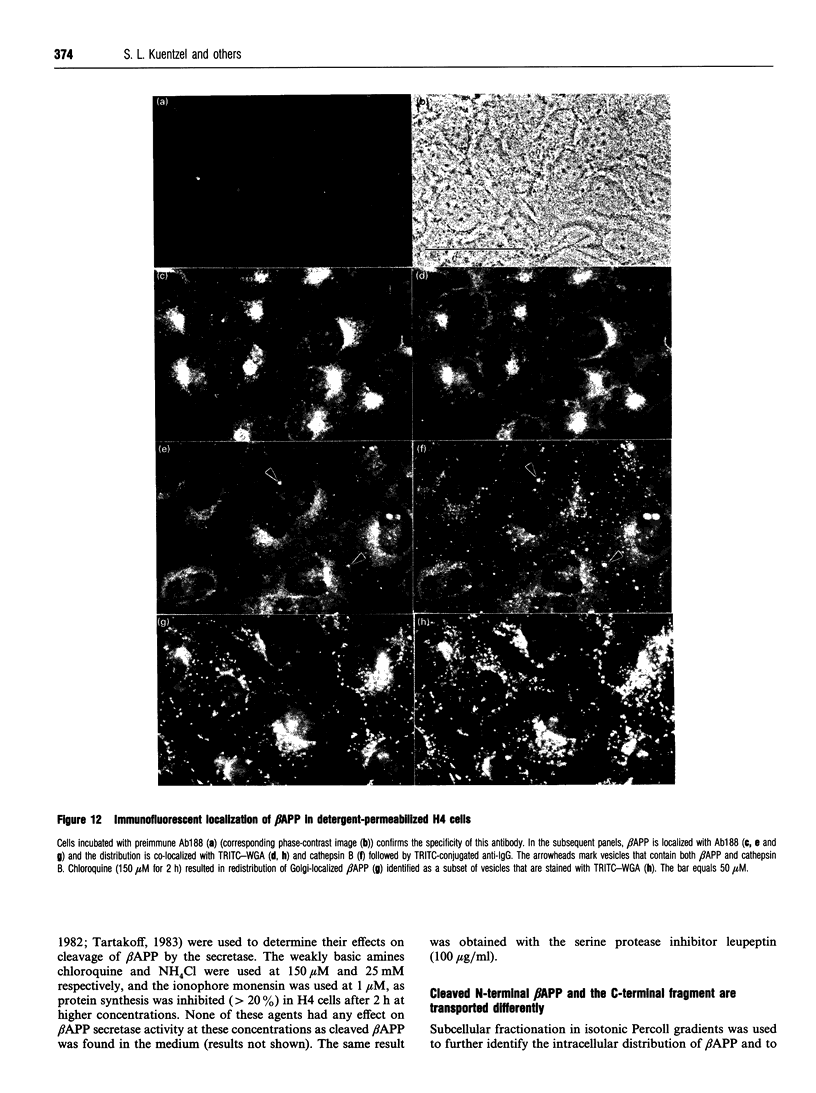




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcaraz G., Kinet J. P., Kumar N., Wank S. A., Metzger H. Phase separation of the receptor for immunoglobulin E and its subunits in Triton X-114. J Biol Chem. 1984 Dec 10;259(23):14922–14927. [PubMed] [Google Scholar]
- Anderson J. P., Esch F. S., Keim P. S., Sambamurti K., Lieberburg I., Robakis N. K. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991 Jul 8;128(1):126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L. I., Rodriguez W., Paskevich P., Mufson E. J., Schenk D., Neve R. L. The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol. 1989 Dec;106(3):237–250. doi: 10.1016/0014-4886(89)90156-8. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F., Refolo L. M., Friedrich V. L., Jr, Casper D., Blum M., Robakis N. K. The Alzheimer's amyloid precursor protein is produced by type I astrocytes in primary cultures of rat neuroglia. J Neurosci Res. 1990 Mar;25(3):431–440. doi: 10.1002/jnr.490250321. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch Biochem Biophys. 1992 Aug 1;296(2):630–639. doi: 10.1016/0003-9861(92)90619-8. [DOI] [PubMed] [Google Scholar]
- Catteruccia N., Willingale-Theune J., Bunke D., Prior R., Masters C. L., Crisanti A., Beyreuther K. Ultrastructural localization of the putative precursors of the A4 amyloid protein associated with Alzheimer's disease. Am J Pathol. 1990 Jul;137(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Van Leuven F., Van den Berghe H. Alpha 2-macroglobulin and other proteinase inhibitors do not interfere with the secretion of amyloid precursor protein in mouse neuroblastoma cells. FEBS Lett. 1992 Aug 10;308(1):50–53. doi: 10.1016/0014-5793(92)81048-q. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Kamino K., Deeb S. S., Smith A. C., Dang T., Martin G. M. Overexpression of amyloid precursor protein alters its normal processing and is associated with neurotoxicity. Biochem Biophys Res Commun. 1992 Jan 15;182(1):165–173. doi: 10.1016/s0006-291x(05)80126-3. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S. E., Bhasin R., Ramabhadran T. V., Koo E. H., Price D. L., Goldgaber D., Greengard P. Alzheimer beta/A4-amyloid precursor protein: evidence for putative amyloidogenic fragment. J Neurochem. 1992 Jan;58(1):383–386. doi: 10.1111/j.1471-4159.1992.tb09322.x. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Green S. A., Zimmer K. P., Griffiths G., Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987 Sep;105(3):1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Selkoe D. J. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991 Dec;11(12):3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Hall C. W., Liebaers I., Di Natale P., Neufeld E. F. Enzymic diagnosis of the genetic mucopolysaccharide storage disorders. Methods Enzymol. 1978;50:439–456. doi: 10.1016/0076-6879(78)50048-7. [DOI] [PubMed] [Google Scholar]
- Harter C., Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992 Apr;117(2):311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kashiwagi K., Yoshikawa K. Protease inhibitors generate cytotoxic fragments from Alzheimer amyloid protein precursor in cDNA-transfected glioma cells. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1249–1255. doi: 10.1016/0006-291x(92)90437-p. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim C. L., Selkoe D. J. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992 Aug 25;267(24):17110–17115. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Knops J., Lieberburg I., Sinha S. Evidence for a nonsecretory, acidic degradation pathway for amyloid precursor protein in 293 cells. Identification of a novel, 22-kDa, beta-peptide-containing intermediate. J Biol Chem. 1992 Aug 15;267(23):16022–16024. [PubMed] [Google Scholar]
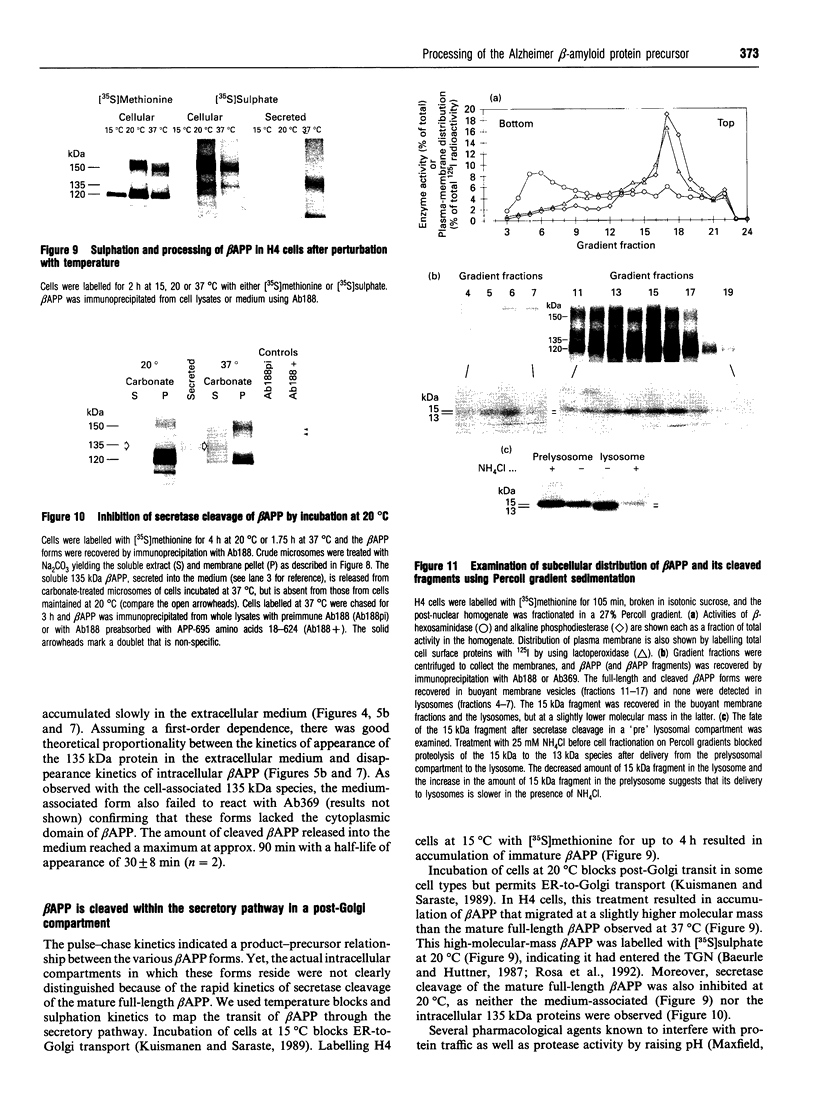
- Kuismanen E., Saraste J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol. 1989;32:257–274. doi: 10.1016/s0091-679x(08)61174-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992 Jun 26;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery D. E., Pasternack J. M., Gonzalez-DeWhitt P. A., Zürcher-Neely H., Tomich C. C., Altman R. A., Fairbanks M. B., Heinrikson R. L., Younkin S. G., Greenberg B. D. Alzheimer's amyloid precursor protein produced by recombinant baculovirus expression. Proteolytic processing and protease inhibitory properties. J Biol Chem. 1991 Oct 15;266(29):19842–19850. [PubMed] [Google Scholar]
- Luo L. Q., Martin-Morris L. E., White K. Identification, secretion, and neural expression of APPL, a Drosophila protein similar to human amyloid protein precursor. J Neurosci. 1990 Dec;10(12):3849–3861. doi: 10.1523/JNEUROSCI.10-12-03849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstedt C., Caporaso G. L., Thyberg J., Gandy S. E., Greengard P. Identification of the Alzheimer beta/A4 amyloid precursor protein in clathrin-coated vesicles purified from PC12 cells. J Biol Chem. 1993 Jan 5;268(1):608–612. [PubMed] [Google Scholar]
- Nordstedt C., Gandy S. E., Alafuzoff I., Caporaso G. L., Iverfeldt K., Grebb J. A., Winblad B., Greengard P. Alzheimer beta/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8910–8914. doi: 10.1073/pnas.88.20.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Overly C. C., Fritz L. C., Lieberburg I., McConlogue L. The beta-amyloid precursor protein is not processed by the regulated secretory pathway. Biochem Biophys Res Commun. 1991 Dec 16;181(2):513–519. doi: 10.1016/0006-291x(91)91218-2. [DOI] [PubMed] [Google Scholar]
- Palacios G., Palacios J. M., Mengod G., Frey P. Beta-amyloid precursor protein localization in the Golgi apparatus in neurons and oligodendrocytes. An immunocytochemical structural and ultrastructural study in normal and axotomized neurons. Brain Res Mol Brain Res. 1992 Oct;15(3-4):195–206. doi: 10.1016/0169-328x(92)90109-o. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Refolo L. M., Wittenberg I. S., Friedrich V. L., Jr, Robakis N. K. The Alzheimer amyloid precursor is associated with the detergent-insoluble cytoskeleton. J Neurosci. 1991 Dec;11(12):3888–3897. doi: 10.1523/JNEUROSCI.11-12-03888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Mantovani S., Rosboch R., Huttner W. B. Monensin and brefeldin A differentially affect the phosphorylation and sulfation of secretory proteins. J Biol Chem. 1992 Jun 15;267(17):12227–12232. [PubMed] [Google Scholar]
- Sahasrabudhe S. R., Spruyt M. A., Muenkel H. A., Blume A. J., Vitek M. P., Jacobsen J. S. Release of amino-terminal fragments from amyloid precursor protein reporter and mutated derivatives in cultured cells. J Biol Chem. 1992 Dec 15;267(35):25602–25608. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sambamurti K., Shioi J., Anderson J. P., Pappolla M. A., Robakis N. K. Evidence for intracellular cleavage of the Alzheimer's amyloid precursor in PC12 cells. J Neurosci Res. 1992 Oct;33(2):319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989 Aug 25;58(4):611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton E. R., Cohn R., Fish L., Obernolte R., Tahilramani R., Nestor J. J., Chan H. W. Characterization of beta-amyloid precursor proteins with or without the protease-inhibitor domain using anti-peptide antibodies. J Neurochem. 1990 Jul;55(1):60–69. doi: 10.1111/j.1471-4159.1990.tb08821.x. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoka A., Kalaria R. N., Lieberburg I., Selkoe D. J. Identification of a stable fragment of the Alzheimer amyloid precursor containing the beta-protein in brain microvessels. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1345–1349. doi: 10.1073/pnas.89.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Lectin-binding sites as markers of Golgi subcompartments: proximal-to-distal maturation of oligosaccharides. J Cell Biol. 1983 Oct;97(4):1243–1248. doi: 10.1083/jcb.97.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991 Aug;3(4):634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Van Nostrand W. E., Schmaier A. H., Farrow J. S., Cunningham D. D. Protease nexin-II (amyloid beta-protein precursor): a platelet alpha-granule protein. Science. 1990 May 11;248(4956):745–748. doi: 10.1126/science.2110384. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Bacic M., Merrill M. J., Lesch K. P., Chen C., Lebovics R. S., Sunderland T. Differential expression of carboxyl terminal derivatives of amyloid precursor protein among cell lines. J Neurosci Res. 1992 Sep;33(1):163–169. doi: 10.1002/jnr.490330121. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Howell K. E., Simons K. Isolation of a fraction enriched in the trans-Golgi network from baby hamster kidney cells. Exp Cell Res. 1988 Apr;175(2):248–265. doi: 10.1016/0014-4827(88)90190-5. [DOI] [PubMed] [Google Scholar]