Abstract
Background:
Conflicts of interest (COI) between oncologists and the pharmaceutical industry might considerably influence how the presentation of the research results is delivered, impacting treatment decisions, and policy-making. While there are regulations on reporting COI in high-income countries (HICs), little is known about their reporting in low- and middle-income countries (LMICs). ONCOlogy TRansparency Under Scrutiny and Tracking (ONCOTRUST-1) is a pilot global survey to explore the knowledge and perceptions of oncologists regarding COI.
Methods:
We designed an online 27-question-based survey in English language to explore the perceptions and knowledge of oncologists regarding COI, with an emphasis on LMICs. Illustrative examples of COI were proposed, based on definitions from the American Society of Clinical Oncology (ASCO) and published literature. Descriptive statistics and the CROSS guidelines were used to report the findings.
Results:
ONCOTRUST-1 surveyed 200 oncologists, 70.9% of them practicing in LMICs. Median age of the respondents was 36 (range: 26-84) years; 47.5% of them were women. The median number of years of clinical practice was 9 (range: 1-51). 40.5% of respondents reported weekly visits by pharmaceutical representatives to their institutions. Regarding oncologists’ perceptions of COI that require disclosure, direct financial benefits, such as honoraria ranked highest (58.5%), followed by gifts from pharmaceutical representatives (50%) and support for attending conferences (44.5%). In contrast, personal or institutional research funding, sample drugs, consulting or advisory board, expert testimony, and food and beverage funded by pharmaceutical industry were less frequently considered as COI. Moreover, only 24% of surveyed oncologists could correctly categorize all situations representing a COI. Regarding support received from industry, 51.5% of respondents acknowledged trips to conferences as the most common form of support, followed by sample drugs (20.5%). Despite recognizing these interactions, 15% of respondents admitted feeling pressured to prescribe specific drugs due to their COI. Regarding COI reporting, a notable portion of participants indicated they report COI in their presentations (59%) or when publishing their research (30%). The presence of local regulations to manage COI were reported by 35.5% of respondents. The majority advocated for clearer policies and regulations (65%), training and education (63.5%), and an open COI database (55%) to improve COI reporting in oncology.
Conclusions:
These findings underscore the importance of clear guidelines, education, and transparency in reporting COI in oncology. This hypothesis-generating pilot survey provided the rationale for ONCOTRUST-2 study which will compare perceptions of COI among oncologists in LMICs and HICs.
Keywords: Conflicts of interest, oncologists, pharmaceutical Industry, policy-making, low- and middle-income countries, global Survey
1. Introduction
Historically, pharmaceutical companies have influenced clinical practice, prescriptions volume, and reimbursement policy in the field of oncology through various strategies (Mitchel et al. 2016; Saito et al. 2019; Rubagumya et al. 2023). This includes advertisements, payments to various stakeholders, sample drugs, gifts, and trips to conferences, among other marketing tactics. Numerous studies have highlighted the prevalence of activities and relationships between the pharmaceutical industry and physicians, noting their significant impact on the adoption of new medical products (Fickweiler et al. 2017; Mitchel et al. 2021; Werner et al. 2023; Pokorny et al. 2023). In a recent systematic review, a global correlation between payments from industry to physicians was found to influencechanges in clinical practice (Mitchel et al. 2021). This interaction frequently resulted in a higher volume of prescriptions for the products in question, indicating a direct influence of industry on medical decision-making (Mitchel et al. 2021). While the prevalence and impact of these practices are well-documented in high-income countries (HICs), there is a paucity of data from low- and middle-income countries (LMICs), especially in Africa (Rubagumya et al. 2023).
The healthcare system in LMICs is emerging as a new and appealing market for the pharmaceutical industry (Rubagumya et al. 2023; Fadlallah et al. 2018). Although a significant portion of clinical trials and their participants have traditionally originated from HICs (Ramaswami et al. 2018), the regulatory and research environment for oncology studies in LMICs has recently experienced significant developments, making it more favorable to drug development (Rubagumya et al. 2022). This has undoubtedly enhanced the percentage of physicians in LMICs interacting with pharmaceutical companies (Fadlallah et al. 2018). There is also evidence indicating that offering expensive personal gifts and cash payments to doctors is becoming more frequent in LMICs (Fadlallah et al. 2018; Rubagumya et al. 2023). In LMICs, such as in sub-Saharan Africa, where oncologists earn significantly less than their counterparts in HICs, maintaining continuous medical education (CME) presents a substantial challenge (Rubagumya et al. 2023). Consequently, financial incentives from the pharmaceutical industry, including travel grants to conferences and other forms of support, often become the sole solution for these oncologists. This situation elevates the risk of potential conflicts of interest (COI) in LMICs compared to HICs. Collectively, these factors together may make the oncologists-industry interactions denser in these regions. While these complex relationships might be beneficial for some settings, they also raise concerns about the ethics and integrity of clinical research and practice in LMICs.
In this context, the ONCOlogy TRansparency Under Scrutiny and Tracking (ONCOTRUST-1) was developed as a hypothesis-generating pilot global survey to explore the knowledge and perceptions of oncologists regarding COI, with a focus on LMICs.
2. Materials and Methods
2.1. Survey Design
A cross-sectional survey study targeting practicing oncologists from different specialties was conceptualized on Microsoft® Forms consisting of 27 items in English (Supplement 1). The questionnaire involved three sections. The first part assessed demographic characteristics of participants and use of Evidence-Based Medicine (EBM) in practice (Questions 2–12). The second focused on interactions with the pharmaceutical industry (Questions 13-22). And the third component addressed potential measures to improve the reporting of COI (Questions 23–28). These three themes were developed based on a combination of both open-ended, multiple-choice, and Likert scale questions after a review of the literature to find research gaps on COI reporting in LMICs (Rubagumya et al. 2022; Decensi et al. 2018). These themes were subsequently structured with emphasis on specificity, neutrality, and clarity to avoid difficult concepts. A number of questions were designed with multiple-choice options to limit acquiescence bias, and it was preplanned to be converted into binary outcomes during data analysis, as previously described (El Bairi et al. 2022). An open answer option was added to some questions to limit the effect of answer order bias by capturing further participants’ perspectives. An internal critical review of the survey content and design was conducted in the initial testing phase. All co-authors discussed the draft questionnaire to address any inconsistencies prior to its online release and distribution. Feedback from the pretesting was used to refine the survey to ensure clarity, relevance, and comprehensiveness.
Eligibility criteria included oncologists who had been in practice or in research for at least one year. The pilot online survey was left open for inclusion for 1 month from 01 January 2024 to 30 January 2024. The distribution of the survey was performed through multiple channels such as professional associations, email lists, and social media platforms using snowball sampling methods, based on previous survey experiences from our research team (Trapani et al. 2021; Fundytus et al. 2021). Measures were adopted to guarantee a diverse representation of oncologists from LMICs and HICs. No incentives were offered to encourage participant responses. To prevent multiple submissions from the same individual, the survey platform was configured to "Allow only one response per user".
2.2. Data handling, ethical approval, and informed consent
Data handling adhered to the EU General Data Protection Regulation (GDPR 2016/679) to maintain privacy, with outcomes aggregated to prevent revealing participants' identities. The study received a waiver from the need for ethical committee clearance, classified as minimal risk by an ethics board review (Ref: IRB00012973- Research Ethics Committee of the Polydisciplinary Faculty of Taroudant, Morocco). The survey started with an introductory letter, outlining the terms under which the authors consent to the use of their responses for research purposes, including the expected dissemination of findings. Moreover, respondents were informed about the study's objectives, the voluntary nature of their participation, and the confidentiality of their responses. Participation was subject to respondents' affirmative electronic consent and information was collected ensuring total anonymity and privacy.
2.3. Data analysis
Excel (Microsoft® Office) and IBM SPSS Statistics 25 (SPSS, Chicago, IL, USA) were used for data extraction, coding, and analysis. For this hypothesis-generating pilot survey, descriptive statistics were employed to report categorical and quantitative data as appropriate. Data were analyzed based on the two World Bank (WB) economy groups (https://www.worldbank.org (2022 data); HICs, high-income countries and LMICs, low- and middle-income countries).
3. Results
3.1. Demographic characteristics and general features of survey participants
The demographic characteristics and general features of 200 surveyed oncologists are summarized in Table 1. The median age of respondents was 36 years (IQR= 7, range: 26 to 84). The gender distribution among participants was balanced (gender ratio= 1.05). Regarding academic qualifications, most participants (71%, n=142) held a medical doctorate (MD), followed by 18.5% (n=37) who had dual degrees (MD/PhD or MD/MSc). Those with a PhD constituted 7% (n=14), and a small fraction had either a doctorate of pharmacy or a master of pharmacy (2%, n=4), with 1.5% (n=3) reporting other academic qualifications. A significant majority of participants were engaged in clinical oncology disciplines (91.5%, n=183), with medical oncology being the most common specialty (54%, n=108). Surgical oncology and oncology pharmacy were less represented, alongside a few participants from other specialties. Most respondents were specialist doctors or attending physicians (59%, n=118), followed by professors (23.5%, n=47) and resident physicians or physicians in training (14.5%, n=29). The primary affiliation for the majority was the public sector (68%, n=130), followed by the private sector (15.5%, n=31), and a group reporting practicing in both (15%, n=30). Regarding the economic backgrounds of the participants' countries and based on the World Bank classification, 70.5% (n=141) were from LMICs, whereas 29% (n=58) were from HICs.
Table 1.
Demographic characteristics and general features of survey participants (n=200)
| Features | % (n) |
|---|---|
| Age | |
| Median (IQR)= 36 (7) (range: 26-84) | |
| Mean (±SD) = 38.87 (9.5) | |
| Gender | |
| Man | 51.5 (103) |
| Woman | 47.5 (95) |
| I prefer not to answer | 1 (2) |
| Academic degree | |
| MD | 71 (142) |
| MD/PhD or MD/MSc | 18.5 (37) |
| PhD | 7 (14) |
| PharmD/MPharm | 2 (4) |
| Other‡ | 1.5 (3) |
| Current oncology specialty | |
| Clinical Oncology disciplines | 91.5 (183) |
| Medical Oncology | 54 (108) |
| Radiation Oncology | 17 (34) |
| Clinical Oncology | 18.5 (37) |
| Haematology/Oncology | 2 (4) |
| Surgical Oncology | 2.5 (5) |
| Oncology Pharmacy | 4.5 (8) |
| Other specialties¥ | 1.5 (4) |
| Current position | |
| Specialist doctor/Attending physician | 59 (118) |
| Resident physician/Physician in training | 14.5 (29) |
| Professor (assistant, associate, full) | 23.5 (47) |
| Other† | 3 (6) |
| Primary affiliation type | |
| Public sector | 68 (130) |
| Private sector | 15.5 (31) |
| Public and private | 15 (30) |
| Other‖ | 1.5 (3) |
| Country of participant | |
| Morocco | 25.5 (51) |
| Turkey | 7 (14) |
| United Kingdom | 5.5 (11) |
| United States of America | 5.5 (11) |
| Bangladesh | 5 (10) |
| Belgium | 5 (10) |
| Kenya | 4.5 (9) |
| Pakistan | 4 (8) |
| Egypt | 4 (8) |
| Ethiopia | 3.5 (7) |
| Kigndom of Saudi Arabia | 2.5 (5) |
| Italy | 2.5 (5) |
| Algeria | 2.5 (5) |
| All other countries₱ | 22.5 (155) |
| Missing data | 0.5 (1) |
| Income group of participants’ country α | |
| Low- and middle-income country | 70.5 (141) |
| High-income country | 29 (58) |
| Missing data | 0.5 (1) |
Abbreviations: MD: medical doctorate, PhD:philosophy doctorate, SD: standard deviation, IQR: interquartile range, MS: master of science, PharmD: Doctorate of pharmacy, MPharm: master of pharmacy. ¥Gynecologic oncology (n=1), pediatric oncology (n=1), digestive oncology (n=1), and one unknown specialty (n=1, the participant indicated physician scientist). ‡PharmD/PhD (n=2), MPhil (n=1). †Research fellow (n=2), PhD candidate (n=1), hospital director (n=1), chief pharmacist (n=1). ‖Academia (n=2), academic private (n=1). ₱details on all countries can be found in Supplement 2 (Table 1). αBased on the World Bank classification (2022 data); available here: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022. Venezuela, previously classified as a an upper-middle income country, was reclassified as a lower-middle income country (https://publications.iadb.org/en/venezuela-still-upper-middle-income-country-estimating-gni-capita-2015-2021).
3.2. Experience, source of continuous education, and confidence of practicing EBM in daily practice of participants
Experience in oncology varied, with a median of 9 years (IQR=8, range: 1 to 51 years), suggesting a mixture of early-career to highly experienced professionals. Regarding continuous medical education (CME) of respondents (Table 2 in Supplement 2), a notable preference was given to internationally recognized guidelines and their educational materials, with 75.5% (n=151) acknowledging these as their main source of CME. Academic institutions and hospitals also played a significant role in providing CME, as indicated by 61% (n=122) of the respondents. Additionally, medical websites such as UptoDate, OncoAlert, and MedScape were favored by 68% (n=136) of the professionals. Oncology conferences emerged as another crucial CME source (73%, n=146) in addition to scientific journals and books (62%, n=124). Conversely, key oncologists on social media platforms, the national board of medicine and pharmaceutical companies, through their representatives, were less frequently cited as primary CME sources (25.5%, n=51; 24%, n=48; and 23.5%, n=47, respectively). Social media platforms were identified by a smaller group of respondents (25.5%, n=51) as a primary source of CME. Regarding confidence in applying EBM within their clinical practice, a substantial proportion of participants (33.5%, n=67) indicated high confidence in their EBM skills, and 50% (n=100) felt confident. 12.5% (n=25) were somewhat confident and 4% (n=8) had difficulties in implementing EBM principles effectively.
3.3. Perception of participants on conflicts of interest and their interactions with pharmaceutical industry
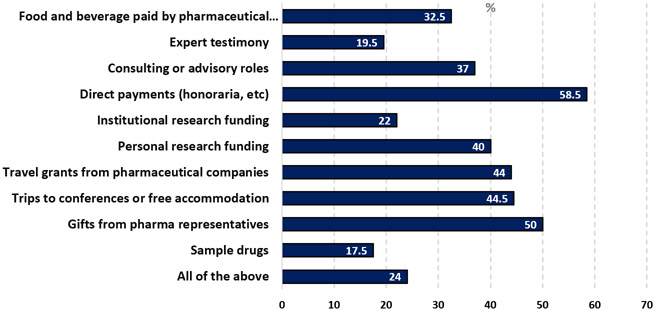
The survey results showed various scenarios considered as COI that require disclosure (Table 2). A significant portion of participants (82.5%) did not view receiving sample drugs as a COI requiring disclosure, whereas 50% of surveyed oncologists considered gifts from pharmaceutical representatives as COI. Similarly, trips to conferences or free accommodation and travel grants from pharmaceutical companies were considered COI by 44.5% and 44% of respondents, respectively. Personal research funding was seen as a COI by 40%, while institutional funding was less frequently viewed as such (22%). Direct payments, such as honoraria, was considered COI that need to be disclosed by the majority of participants (58.5%). 37% of respondents considered consulting or advisory roles as COI. Expert testimony, food, and beverages paid by the pharmaceutical industry were seen as COI by 19.5% and 32.5% of respondents, respectively. Interestingly, only 24% of participants indicated that all the listed items were considered COI (Figure 1).
Table 2.
Perception of participants on conflicts of interest and their interactions with pharmaceutical industry
| Survey items/variables | n (%) |
|---|---|
| Frequency of pharmaceutical industry visits cancer care facilities | |
| Daily | 14 (7) |
| Weekly | 81 (40.5) |
| Monthly | 48 (24) |
| Occasionally | 37 (18.5) |
| Rarely | 11 (5.5) |
| Never | 9 (4.5) |
| Types of support received from the pharmaceutical industry (last 5 years) β | |
| Sample drugs | 159 (79.5) |
| Trips to conferences | 103 (51.5) |
| I have not received any support | 47 (23.5) |
| I prefer not to declare | 20 (10) |
| Gifts | 18 (9) |
| Other | 19 (9.5) |
| Amount of honorarium (direct payment) received from pharmaceutical industry | |
| <500 US dollars | 13 (6.5) |
| 500-1000 US dollars | 6 (3) |
| 1000-2000 US dollars | 11 (5.5) |
| 2000-5000 US dollars | 7 (3.5) |
| >5000 US dollars | 5 (2.5) |
| I prefer not to declare | 25 (12.5) |
| I have not received any amount of money | 132 (66) |
| Missing data | 1 (0.5) |
| Pressure from pharmaceutical industry to support the prescriptions of their drugs when having conflicts of interest with them | |
| Yes | 22 (11) |
| No | 145 (72.5) |
| Not sure | 33 (16.5) |
| Influence of prescriptions and treatment recommendations during multidisciplinary team meetings when being a speaker in industry sponsored events | |
| Yes | 20 (10) |
| No | 150 (75) |
| Not sure | 30 (15) |
| Objective appraisal of clinical trials when having conflicts of interest with pharmaceutical industry | |
| Yes | 120 (60) |
| No | 33 (16.5) |
| Not sure | 47 (23.5) |
| Declin or remodulation to use a recommendation from an international oncology society due to conflicts of interest with industry | |
| Yes | 22 (11) |
| No | 148 (74) |
| Not sure | 30 (15) |
| Acceptance to get involved with pharmaceutical industry regarding a new drug when the evidence from their related clinical trials is weak or clinically irrelevant | |
| Yes | 17 (8.5) |
| No | 148 (74) |
| Not sure | 35 (17.5) |
| Oncologists’ approaches to disclosing conflicts of interest β | |
| I don’t report my conflicts of interest | 30 (15) |
| I report them before starting lectures | 118 (59) |
| I acknowledge them when publishing research | 60 (30) |
| I prefer not to answer | 28 (14) |
| I don’t have any conflicts of interest | 5 (2.5) |
binary outcomes retrieved from multiple choice questionnaire
Figure 1.
Cases that constitute conflicts of interest according to participants
Regarding the frequency of pharmaceutical industry visits to cancer care facilities of surveyed oncologists, 40.5% of the participants reported weekly visits, followed by 24% reporting monthly visits. The types of support received from the pharmaceutical industry in the last 5 years showed that 79.5% had received sample drugs, 51.5% had been offered trips to conferences, and 9% received gifts. 23.5% stated they had not received any form of support. Concerning honorariums received from the pharmaceutical industry, there was varied landscapes of financial interactions (Table 2) but the majority of respondents (66%) reported not having received any form of direct payment. Additionally, 12.5% of the participants preferred not to declare the amount they received. The pressure to support the prescription of drugs from companies with which participants had COI was reported by 11%, while 72.5% did not feel pressured, and 16.5% were unsure. The influence of being a speaker at industry-sponsored events on prescription behavior and treatment recommendations during multidisciplinary team meetings was acknowledged by 10% of the participants, with 75% reporting no influence. When it came to objectively appraising clinical trials in the context of COI with the pharmaceutical industry, 60% of the respondents believed they could still do so, while 16.5% disagreed, and 23.5% were unsure. Additionally, 11% of participants admitted to declining or remodulating the use of a recommendation from an international oncology society due to their COI. Lastly, the willingness to get involved with the pharmaceutical industry regarding a new drug, when the evidence from related clinical trials was weak or clinically irrelevant, was admitted by 8.5% of the respondents, while 74% were not willing, and 17.5% remained unsure. Most of participants (59%) reported disclose of their COI before starting lectures, while 30% acknowledged them when publishing research. Conversely, 15% of the respondents admitted they do not report their COI at all. Another 14% chose not to answer the question, indicating a possible sensitivity or reluctance about the topic.
3.4. Motivation regarding engagements with pharmaceutical industry and strategies to improve transparency and regulation
The motivations behind oncologists' engagements with the pharmaceutical industry and their perspectives on managing COI are summarized in Table 3. This survey section revealed that 35.5% of participants reported the existence of local regulations or policies in their home countries designed to manage COI with the pharmaceutical industry. However, a significant proportion of respondents were either unaware of such policies (33%) or confirmed their absence (31.5%). To enhance COI reporting in oncology, the participants suggested several measures. The most favored proposals were the establishment of an open COI database (55%) and clear policies and local regulations (65%). Furthermore, 63.5% advocated for regular training and education, underscoring the need for continuous professional development in ethical practices. Patient and public involvement was also supported by 31% of respondents for better transparency.
Table 3.
Local regulations, motivations of engagements with pharmaceutical companies, and strategies to manage conflicts of interest
| Survey items/variables | n (%) |
|---|---|
| Local regulations or policies in home country of participants to manage conflicts of interest with pharmaceutical industry | |
| Yes | 71 (35.5) |
| No | 63 (31.5) |
| I don’t know | 66 (33) |
| Participants’ proposed improvements for conflict of interest reporting in oncology β | |
| Open conflict of interest database | 110 (55) |
| Patient and public involvement | 62 (31) |
| Regular training and education | 127 (63.5) |
| Clear policy and local regulations | 130 (65) |
| Reasons for not reporting conflicts of interest by oncologists β | |
| Lack of awareness | 110 (55) |
| Complex relationships with pharmaceutical industry | 101 (50.5) |
| Fear of losing financial incentives | 75 (37.5) |
| Fear of negative impact on credibility or reputation | 88 (44) |
| Lack of policy in home countries of practice | 112 (56) |
| Minimizing impact of conflicts in oncology in practice | 39 (19.5) |
| Do you think that income of oncologists is an important factor of having these relationships with pharmaceutical industry? | |
| Strongly agree | 37 (18.5) |
| Agree | 73 (36.5) |
| Neutral | 57 (28.5) |
| Disagree | 23 (11.5) |
| Strongly disagree | 10 (5) |
binary outcomes retrieved from multiple choice questionnaire
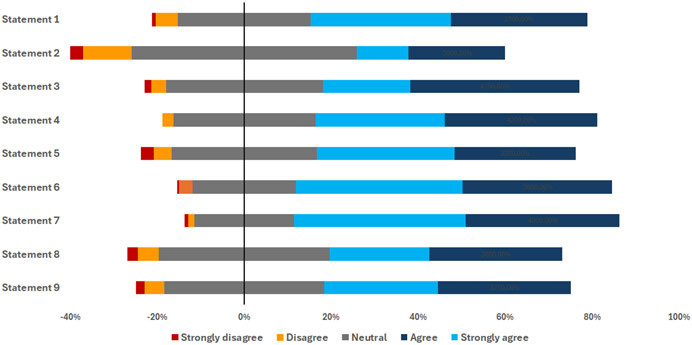
The reasons for not reporting COI suggested by surveyed oncologists were diverse. A majority cited a lack of awareness (55%) and complex relationships with the pharmaceutical industry (50.5%) as primary factors. Concerns over losing financial incentives (37.5%) and negative impacts on credibility or reputation (44%) were also important. Interestingly, 56% of participants identified the absence of policies in their home countries as a critical barrier for implementing improved transparency and regulation. Regarding the influence of income on the establishment of relationships with the pharmaceutical industry, opinions were varied. A majority of respondents (55%) strongly agreed or agreed that income is an important factor, whereas 45% were neutral, disagreed, or strongly disagreed. The mahority of participants agreed with various statements on strategies to manage COI (Figure 2).
Figure 2.
Participants’ agreement with statements on strategies to manage conflicts of interest. Statement 1: Complete transparency in reporting conflicts of interest is necessary for patients and their advocates; Statement 2: Restricting physician– industry interactions could be one potentially effective option to consider, particularly in light of the evidence that restriction policies may improve prescribing behaviors; Statement 3: Encouraging multiple pharmaceutical companies to collaborate on supporting various access initiatives could mitigate bias favoring a specific product and potentially establish a sustainable framework for these activities; Statement 4: Research on industry-oncologist relationships should be supported and promoted by hospitals and academic institutions; Statement 5 Cancer societies should independently select scientific committees, content during their meetings and speakers should be forbidden from promoting any specific cancer medicine; Statement 6: Medical schools should incorporate programs to increase awareness of potential conflicts of interest with the pharmaceutical industry; Statement 7: Oncology societies and academic institutions should develop and use clear conflict of interest declaration policies for all speakers of their events; Statement 8: Countries should build programs to publicly report payments to doctors from the pharmaceutical industry; Statement 9: A third-party or intermediary entity (such as an oncology society) could collect donations from various pharmaceutical companies and allocate the funds towards supporting research and educational activities, rather than directly providing them to individual oncologists.
Discussion
In our study, we aimed to examine industry-oncologist’s interactions and identify associated factors. Our goal was to establish a rationale for recommendations and educational interventions to increase global awareness on this issue. Most importantly, our findings may help formulate hypotheses for ONCOTRUST-2, which will compare these interactions between LMICs and HICs.
ONCOTRUST-1 provided important insights that need to be discussed with the global oncology community. First, the representation of oncologists mainly from LMICs (70%) presented a context for understanding the perceptions and practices regarding COI. Indeed, few studies have historically investigated COI in these regions (Fadlallah et al. 2018), mainly in non-oncological specialties. In the past decade, there has been significant progress in industry-sponsored cancer drug developments, which has notably improved patient outcomes. While these advances have mostly occurred in HICs, LMICs are increasingly attractive to pharmaceutical companies. This interest is driven by the evolving economic landscape of these countries, the global growth ambitions of these companies, and the unmet need for real-world data to inform further developments. Promoted by various factors mentioned earlier (and elsewhere see: Rubagumya et al. 2023), as well as the fact that the regulatory and policy environment in these regions is fragile and not yet mature enough to enforce transparent reporting of COI, relationships between the industry and oncologists may be intensified. Indeed, this was confirmed by our survey results. About 40% of respondents reported frequent weekly visits of industry representatives to cancer care facilities. Notably, surveyed oncologists were relatively of early-career experience (median: 9 years) and relied on international society guidelines, medical websites (UptoDate, OncoAlert, and MedScape, etc.), and major oncology conferences as their main source of CME. In fact, this could potentially increase COI among oncologists in several ways. Early-career oncologists may be more susceptible to being influenced by pharmaceutical companies. These physicians, in the path of building their knowledge, might not yet have critical skills needed to objectively appraise industry-sponsored clinical trials. Moreover, over reliance on medical websites, international society guidelines, and major oncology conferences as primary sources of CME can be problematic as these platforms may be sponsored or influenced by pharmaceutical companies (Desai et al. 2020; Mitchel et al. 2021; Saleh et al. 2019; Powel et al. 2022; Chopra et al. 2020, VanDeMark et al. 2022; Boytchev, 2024).
Regarding perceptions of oncologists on what constitutes COI, the vast majority of respondents did not consider receiving sample drugs to be a COI requiring disclosure. Additionally, there were varied perceptions regarding COI, indicating that not all mentioned scenarios were recognized as potential COI by oncologists, even though these cases are generally considered COI worldwide. Only direct payments from the industry to oncologists and gifts were viewed as COI by nearly half of the participants. Unexpectedly, only a small percentage of oncologists (24%) considered all presented scenarios to be COI. This variation in perceptions may stem from a lack of awareness about unified definitions of COI among physicians and health researchers worldwide (Rohwer et al. 2017). The absence of comprehensive training and inconsistent policies across institutions and countries could also contribute to the heterogeneity and variability in understanding COI across different healthcare systems and cultural contexts. Thus, standardizing COI definitions and ensuring they are culturally and contextually relevant could help reduce these disparities. Additionally, failing to recognize certain activities widely considered as COI can lead to ethical and integrity issues (Solyum, 2004; Grinnell, 2014; Nahai et al. 2011). Implementing clear guidelines and robust disclosure mechanisms could mitigate these risks. The relationships between oncologists and pharmaceutical companies, particularly in attitudes towards sample drugs, further underscore the complexity of these perceptions (Rubagumya et al. 2023). Barriers to reporting COI related to concerns over losing financial incentives by oncologists, further underscores the challenges in establishing a culture of transparency in these settings, as indicated by the results of our survey. This is further supported by the responses from a group of respondents who fail to consistently disclose COI in their professional activities, including presentations and research publications. A noteworthy finding from our survey is that the income of oncologists may be a crucial factor encouraging them to accept support from pharmaceutical companies. This is supported by a majority of respondents who agreed that the income of oncologists plays a significant role, in line with previous observations of pivotal works in this field (Rubagumya et al. 2023). Further to this, the lack of awareness among oncologists regarding existing policies and regulations on COI disclosure in their home countries may also contribute to this behavior. Alarmingly, the acknowledgment of feeling pressured to prescribe specific drugs due to COI by some respondents underline the potential impact of these interactions on patient care and treatments. In their systematic review of 49 included studies, Fickweiler et al. demonstrated that physician-pharmaceutical industry interactions contributed to irrational prescribing of the company's drugs (Fickweiler et al. 2017). There is also evidence suggesting that these interactions may be more harmful when oncologists receive direct payments from industry. In fact, this was shown in a recent cohort study that found that industry payments were associated with prescriptions of non-recommended and low value anticancer drugs (Mitchel et al. 2023). This raises serious concerns regarding oncologist’s integrity in practice, and thus, the urgent need to build actionable policy by healthcare authorities for surveillance actions. In our survey, a small group of respondents reported that they declined or remodulated guidelines from an international oncology society due to their COI with the industry. It is hypothesized that additional participants may have had the same response but did not disclose it.
Complex industry-oncologists’ relationships can be advantageous in certain contexts. One can argue that capacity building in LMICs can’t be achieved without industry support through fellowships, sponsorship to attend meetings of oncology societies, and research grants; particularly given the shortage of funding sources available to oncologists in these regions (Rubagumya et al. 2023). Our survey findings also highlighted these educational opportunities provided by pharmaceutical companies to attend conferences for CME. However, collective engagement of oncologists to develop an ethical framework of these interactions in LMICs is urgently needed (Rubagumya et al. 2023). In this perspective, various short-term solutions and suggestions were made by expert panels from Sub-Saharan Africa on how to mitigate the risks of industry–oncologist interactions (Rubagumya et al. 2023). This encompasses a) integrating courses on COI during early and postgraduate trainings in medical schools, b) developing research projects to understand industry-oncologists’ interactions, c) ensuring that content of oncology meetings is selected by independent committees and prohibiting promotion of specific drugs by oncologists, d) adopting and implementing clear COI policies, such as open payments databases, and e) using a third-party intermediary to collect pharmaceutical donations for CME and research.
Our study has various strengths and limitations. To our knowledge, this is the first survey to explore perceptions and interactions of oncologists with pharmaceutical industry in LMICs, where data on COI are generally scarce. This may provide the rationale to build regulatory frameworks and targeted educational programs as well as advocacy efforts for enhanced transparency. Promisingly, ONCOTRUST-1 also provided the foundation for ONCOTRUST-2, which will be methodologically improved and powered to compare these interactions between LMICs and HICs. Regarding limitations, our cross-sectional survey was a pilot study that collected data in a short period of time and had limited geographic representation in terms of respondents. In addition, the reliance on English as a language of the survey may also cause barriers to non-English speaking oncologists which may potentially skew our data and not fully capture diversity of practices. Due to the sensitive nature of this topic, self-reported data may be under-reported and/overestimated. The lack of qualitative insights that could explain some participants’ responses is also a limitation of our findings. Therefore, careful interpretation of these results is essential. Nevertheless, this will be addressed by improving the survey design of ONCOTRUST-2.
Conclusion
The ONCOTRUST-1 study findings serve as a pivotal step towards understanding and addressing the challenges posed by COI in oncology in LMICs. There is a significant gap in the awareness and management of COI among oncologists, especially in LMICs. While most respondents recognize direct financial benefits as COI, there is less agreement on other forms such as sample drugs. Few oncologists could correctly identify all COI scenarios and despite frequent doctors-industry interactions. Accordingly, advancing the dialogue on COI in oncology will be essential for a better use of available evidence and ethical standards when manipulating anticancer drugs. Lessons from this pilot study should inform future efforts to develop guidelines adapted to LMICs on how to promote COI reporting and advocate for awareness among health professionals and authorities in these settings. The problem with COI in oncology is that the relationship between cancer physicians and industry is too complex to be investigated accurately in one survey. Thus, more research on this issue of practice integrity is awaited.
Supplementary Material
Acknowledgements
We would like to thank all institutions of this research’ authors for their significant participation in sharing the survey during data collection. The first author of this research would like to thank Conquer Cancer, the ASCO Foundation® for providing travel support as a part of the IDEA Award to present this paper’s data at its annual congress (Chicago, USA, 2024).
Footnotes
Disclosure
Khalid El Bairi reports receiving fees and honorariums from NCODA, Techspert, Elsevier and Springer and funding from the Cancer Research Institute (Morocco). Details on other authors’ conflicts of interest can be found at: https://coi.asco.org/
Data Sharing Statement
The dataset of this research is available upon reasonable requests.
References
- Boytchev H. Exclusive: Outcry as Philip Morris International funds smoking cessation courses on Medscape. BMJ. 2024. Apr 9;385:q830. doi: 10.1136/bmj.q830. [DOI] [PubMed] [Google Scholar]
- Chopra AC, Tilberry SS, Sternat KE, et al. Quantification of Conflicts of Interest in an Online Point-of-Care Clinical Support Website. Sci Eng Ethics. 2020. Apr;26(2):921–930. doi: 10.1007/s11948-019-00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCensi A, Numico G, Ballatori E, et al. Conflict of interest among Italian medical oncologists: a national survey. BMJ Open. 2018. Jun 30;8(6):e020912. doi: 10.1136/bmjopen-2017-020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AP, Chengappa M, Go RS, et al. Financial conflicts of interest among National Comprehensive Cancer Network clinical practice guideline panelists in 2019. Cancer. 2020. Aug 15;126(16):3742–3749. doi: 10.1002/cncr.32997. [DOI] [PubMed] [Google Scholar]
- El Bairi K, El Kadmiri N, Fourtassi M. Exploring scientific misconduct in Morocco based on an analysis of plagiarism perception in a cohort of 1,220 researchers and students. Account Res. 2022. Aug 18:1–20. doi: 10.1080/08989621.2022.2110866. [DOI] [PubMed] [Google Scholar]
- Fadlallah R, Alkhaled L, Brax H, et al. Extent of physician-pharmaceutical industry interactions in low- and middle-income countries: a systematic review. Eur J Public Health. 2018. Apr 1;28(2):224–230. doi: 10.1093/eurpub/ckx204. [DOI] [PubMed] [Google Scholar]
- Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians' attitudes and prescribing habits: a systematic review. BMJ Open. 2017. Sep 27;7(9):e016408. doi: 10.1136/bmjopen-2017-016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus A, Sengar M, Lombe D, et al. Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey. Lancet Oncol. 2021. Oct;22(10):1367–1377. doi: 10.1016/S1470-2045(21)00463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AP, Basch EM, Dusetzina SB. Financial Relationships With Industry Among National Comprehensive Cancer Network Guideline Authors. JAMA Oncol. 2016. Dec 1;2(12):1628–1631. doi: 10.1001/jamaoncol.2016.2710. [DOI] [PubMed] [Google Scholar]
- Mitchell AP, Mishra A, Dey P, et al. Personal Payments from Pharmaceutical Companies to Authors of Oncology Clinical Practice Guidelines. Oncologist. 2021. Sep;26(9):771–778. doi: 10.1002/onco.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AP, Trivedi NU, Gennarelli RL, et al. Are Financial Payments From the Pharmaceutical Industry Associated With Physician Prescribing? : A Systematic Review. Ann Intern Med. 2021. Mar;174(3):353–361. doi: 10.7326/M20-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. The Interrelationship between Research Integrity, Conflict of Interest, and the Research Environment. J Microbiol Biol Educ. 2014. Dec 15;15(2):162–4. doi: 10.1128/jmbe.v15i2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahai F. Disclosing conflicts of interest to maintain ethical integrity. Aesthet Surg J. 2011. Jul;31(5):591–3. doi: 10.1177/1090820X11412525. [DOI] [PubMed] [Google Scholar]
- Solyom AE. Ethical challenges to the integrity of physicians: financial conflicts of interest in clinical research. Account Res. 2004. Apr-Jun;11(2):119–39. doi: 10.1080/03050620490512313. [DOI] [PubMed] [Google Scholar]
- Pokorny AMJ, Bero LA, Fox P, et al. Interactions between Australian cancer physicians and the pharmaceutical industry: a qualitative study. BMJ Open. 2023. May 26;13(5):e065719. doi: 10.1136/bmjopen-2022-065719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K, Kakkilaya A, Haslam A, et al. Financial conflicts of interest of OncoAlert: An informal oncology professional network. J Cancer Policy. 2022. Dec;34:100369. doi: 10.1016/j.jcpo.2022.100369. [DOI] [PubMed] [Google Scholar]
- Ramaswami R, Paulino E, Barrichello A, et al. Disparities in Breast, Lung, and Cervical Cancer Trials Worldwide. J Glob Oncol. 2018. Sep;4:1–11. doi: 10.1200/JGO.17.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer A, Young T, Wager E, et al. Authorship, plagiarism and conflict of interest: views and practices from low/middle-income country health researchers. BMJ Open. 2017. Nov 22;7(11):e018467. doi: 10.1136/bmjopen-2017-018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubagumya F, Hopman WM, Gyawali B, et al. Participation of Lower and Upper Middle-Income Countries in Clinical Trials Led by High-Income Countries. JAMA Netw Open. 2022;5(8):e2227252. doi: 10.1001/jamanetworkopen.2022.27252. Erratum in: JAMA Netw Open. 2022 Sep 1;5(9):e2235554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AP, Dusetzina SB, Mishra Meza A, et al. Pharmaceutical industry payments and delivery of non-recommended and low value cancer drugs: population based cohort study. BMJ. 2023. Oct 25;383:e075512. doi: 10.1136/bmj-2023-075512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubagumya F, Mutebi M, Manirakiza A, et al. Pharmaceutical industry relationships with oncologists in sub-Saharan Africa. Lancet Oncol. 2023;24(2):e96–e101. doi: 10.1016/S1470-2045(22)00639-8. [DOI] [PubMed] [Google Scholar]
- Saito H, Ozaki A, Sawano T, et al. Evaluation of Pharmaceutical Company Payments and Conflict of Interest Disclosures Among Oncology Clinical Practice Guideline Authors in Japan. JAMA Netw Open. 2019. Apr 5;2(4):e192834. doi: 10.1001/jamanetworkopen.2019.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh RR, Majeed H, Tibau A, et al. Undisclosed financial conflicts of interest among authors of American Society of Clinical Oncology clinical practice guidelines. Cancer. 2019. Nov 15;125(22):4069–4075. doi: 10.1002/cncr.32408. [DOI] [PubMed] [Google Scholar]
- Trapani D, Lengyel CG, Habeeb BS, et al. The global landscape of availability, accessibility and affordability of essential diagnostics and therapeutics for the management of HER2-positive breast cancer: The ONCOLLEGE-001 survey. J Cancer Policy. 2021;28:100285. doi: 10.1016/j.jcpo.2021.100285. [DOI] [PubMed] [Google Scholar]
- VanDeMark SH, Woloszyn MR, Christman LA, et al. Examination of Potential Industry Conflicts of Interest and Disclosures by Contributors to Online Medical Resource Databases. JAMA Netw Open. 2022. Jul 1;5(7):e2220155. doi: 10.1001/jamanetworkopen.2022.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KM, Mercurio MR, Shabanova V, et al. Pediatricians' Reports of Interaction with Infant Formula Companies. Breastfeed Med. 2023. Mar;18(3):219–225. doi: 10.1089/bfm.2022.0217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset of this research is available upon reasonable requests.