Abstract
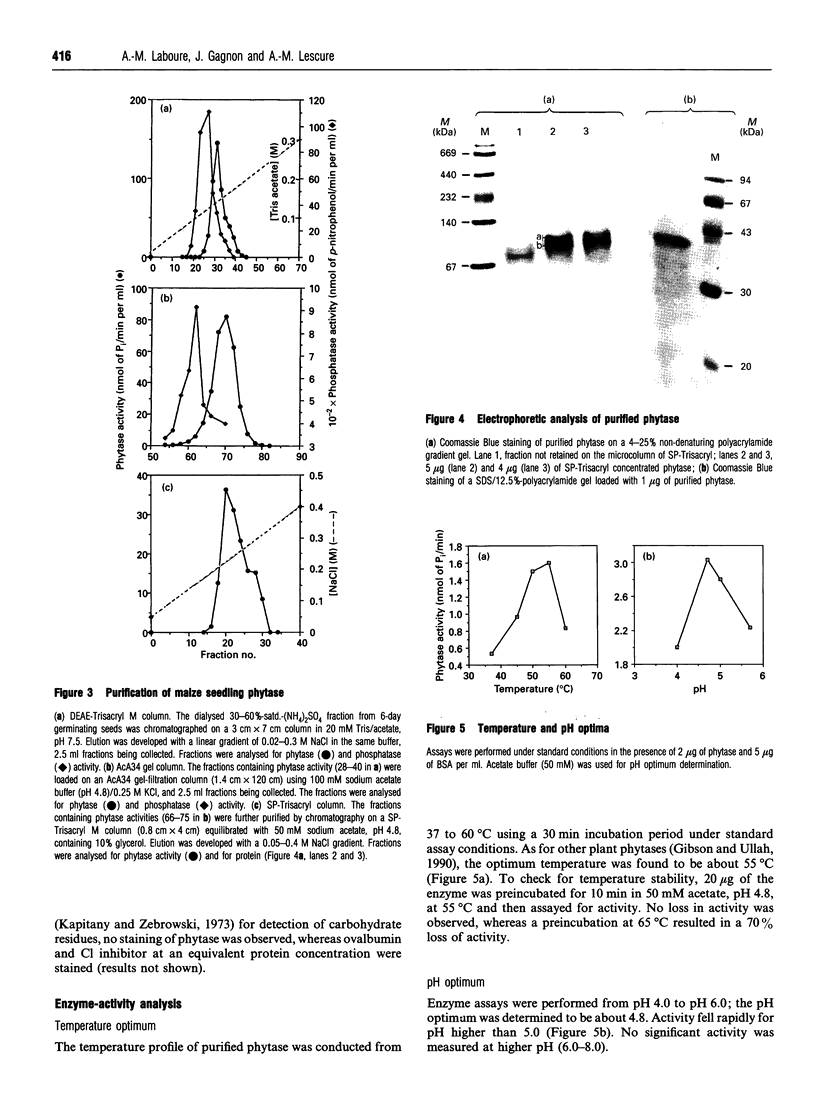
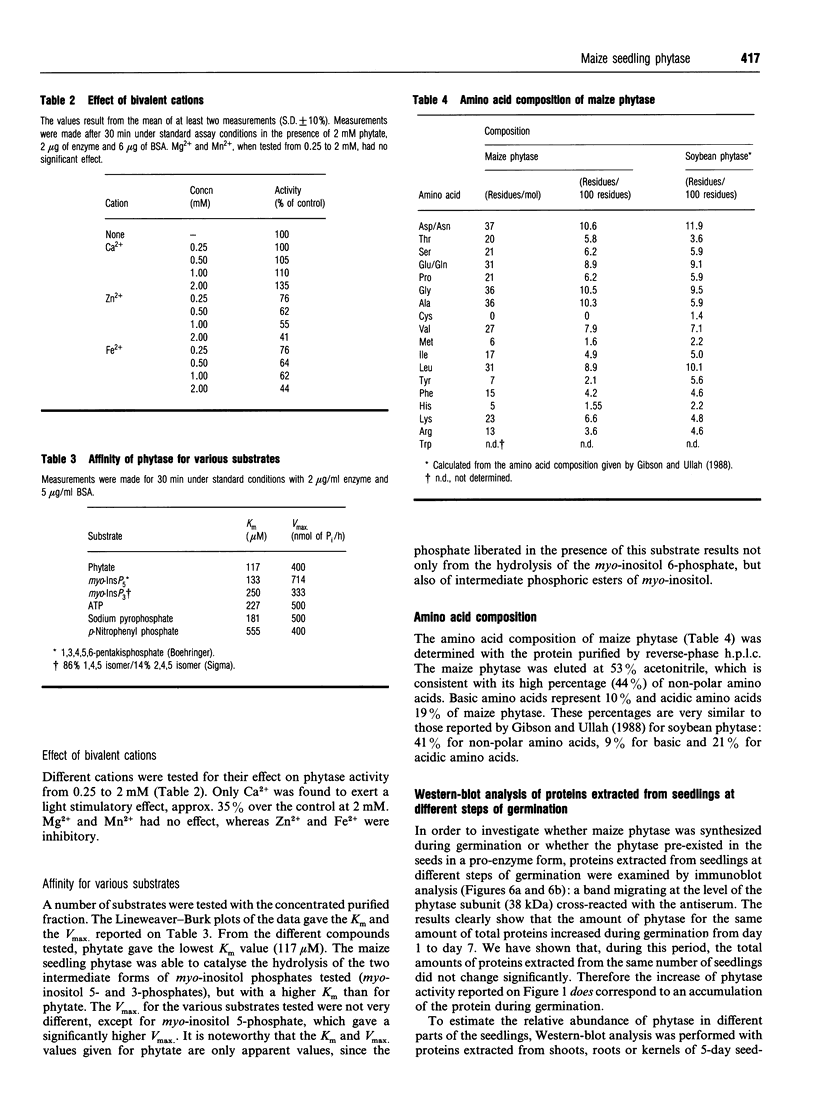
Phytase (myo-inositol-hexakisphosphate phosphohydrolase, EC 3.1.3.8) has been purified from 5-7-day-old maize (Zea mays) seedlings, using a four-step purification procedure. The native protein has a molecular mass of about 76 kDa and is built up from two 38 kDa subunits. The pH and temperature optima of the purified enzyme were respectively 4.8 and 55 degrees C. The apparent Km for phytate was estimated to be 117 microM. Like other acidic phytases, the maize seedling enzyme exhibited a broad affinity for various phosphorylated substrates and especially for penta- and tri-phosphate esters of myo-inositol. The amino acid composition of the h.p.l.c.-purified protein indicated a high hydrophobicity (44% non-polar amino acids). Rabbit antibodies were produced in response to maize seedling phytase. Western-blot analyses clearly demonstrate that the increase of phytase activity observed during the first 7 days of germination corresponded to an accumulation of the protein in maize seedlings. Phytase accumulated essentially in the shoots (mesocotyl plus coleoptiles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Ullah A. H. Purification and characterization of phytase from cotyledons of germinating soybean seeds. Arch Biochem Biophys. 1988 Feb 1;260(2):503–513. doi: 10.1016/0003-9861(88)90475-4. [DOI] [PubMed] [Google Scholar]
- Graf E., Empson K. L., Eaton J. W. Phytic acid. A natural antioxidant. J Biol Chem. 1987 Aug 25;262(24):11647–11650. [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim P. E., Tate M. E. The phytases. I. Lysolecithin-activated phytase from wheat bran. Biochim Biophys Acta. 1971 Oct;250(1):155–164. doi: 10.1016/0005-2744(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Lim P. E., Tate M. E. The phytases. II. Properties of phytase fractions F 1 and F 2 from wheat bran and the myoinositol phosphates produced by fraction F 2 . Biochim Biophys Acta. 1973 Apr 12;302(2):316–328. doi: 10.1016/0005-2744(73)90160-5. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Dickinson D. B., Ho T. H. Phytic Acid Metabolism in Lily (Lilium longiflorum Thunb.) Pollen. Plant Physiol. 1987 Feb;83(2):408–413. doi: 10.1104/pp.83.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. I. Purification and physical and immunological characterization. Arch Biochem Biophys. 1989 Feb 15;269(1):219–227. doi: 10.1016/0003-9861(89)90103-3. [DOI] [PubMed] [Google Scholar]
- Morris E. R., Ellis R. Isolation of monoferric phytate from wheat bran and its biological value as an iron source to the rat. J Nutr. 1976 Jun;106(6):753–760. doi: 10.1093/jn/106.6.753. [DOI] [PubMed] [Google Scholar]