Abstract
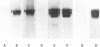
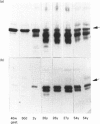
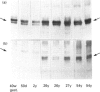
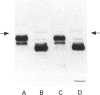
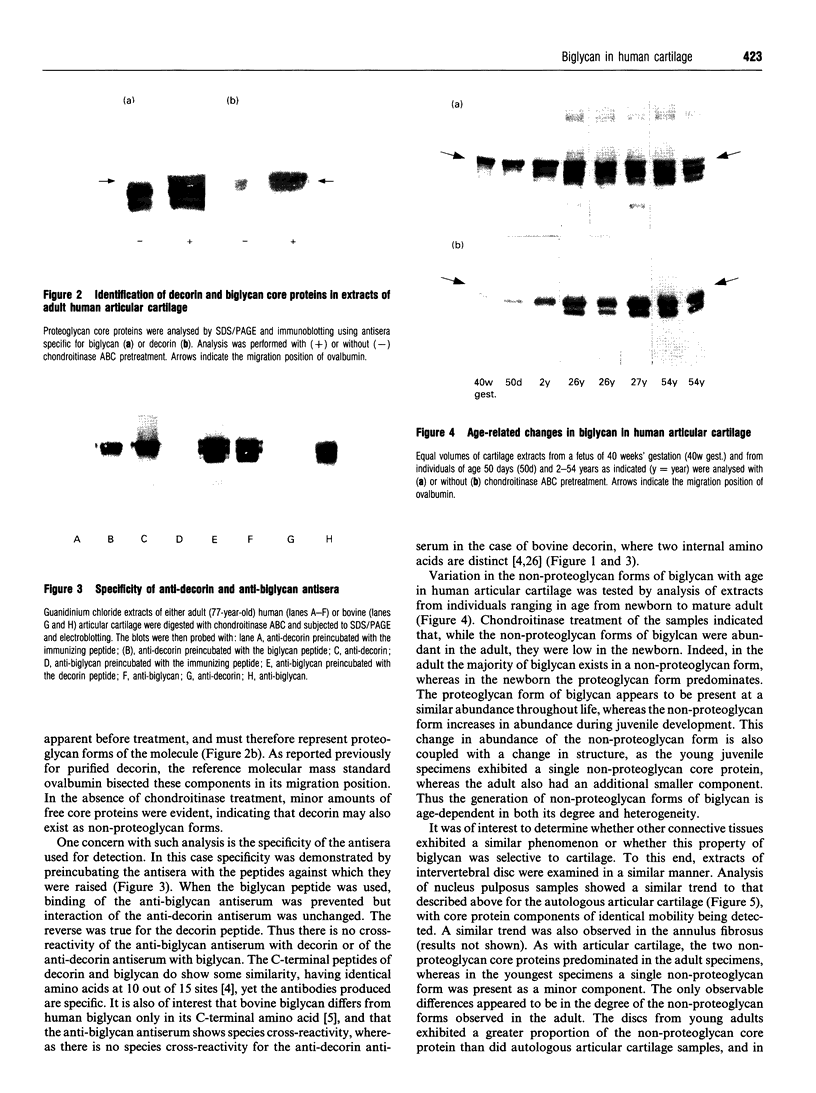
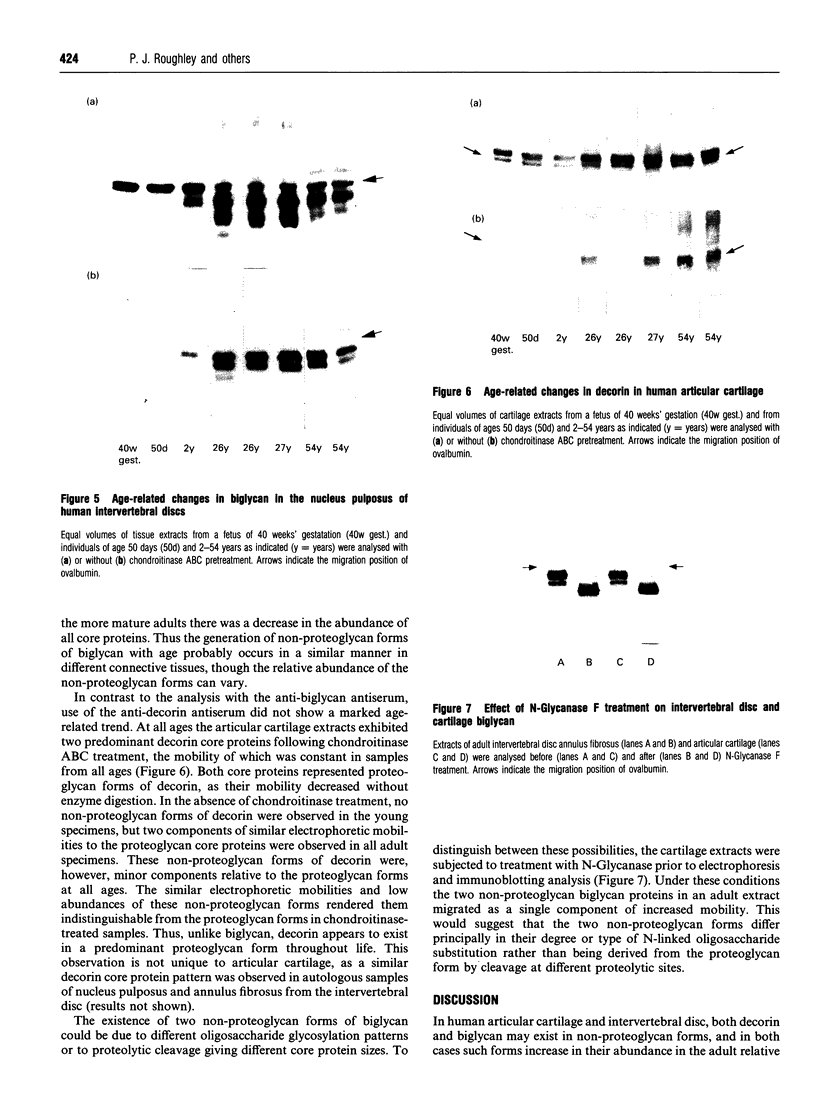
Polyclonal anti-peptide antibodies were raised to the C-terminal regions of human biglycan and decorin. These antibodies were used in immunoblotting to study structural variations with age in the proteoglycan core proteins present in extracts of human articular cartilage and intervertebral disc. Three forms of the biglycan core protein were identified. The largest form was detected only after chondroitinase treatment and represents the proteoglycan form of the molecule from which the glycosaminoglycan chains have been removed. However, chondroitinase treatment did not alter the electrophoretic mobility of the two smaller proteins, which appear to represent non-proteoglycan forms of the molecule, resulting either from a failure to substitute the intact proteoglycan core protein with glycosaminoglycan chains during its synthesis or from proteolytic processing of the intact proteoglycan causing removal of the N-terminal region bearing the glycosaminoglycan chains. The non-proteoglycan forms constituted a minor proportion of biglycan in the newborn, but were the major components in the adult. A similar trend was seen in both articular cartilage and intervertebral disc. In comparison, decorin appears to exist predominantly as a proteoglycan at all ages, with two core protein sizes being present after chondroitinase treatment. Non-proteoglycan forms were detected in the adult, but they were always a minor constituent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatowicz M. S., Matsueda G. R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986 May 15;155(1):95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Breuer B., Schmidt G., Kresse H. Non-uniform influence of transforming growth factor-beta on the biosynthesis of different forms of small chondroitin sulphate/dermatan sulphate proteoglycan. Biochem J. 1990 Jul 15;269(2):551–554. doi: 10.1042/bj2690551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. U., Johnson T. L., Pal S., Tang L. H., Rosenberg L., Neame P. J. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-sepharose chromatography. J Biol Chem. 1989 Feb 15;264(5):2876–2884. [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Hughes C. E., Caterson B., White R. J., Roughley P. J., Mort J. S. Monoclonal antibodies recognizing protease-generated neoepitopes from cartilage proteoglycan degradation. Application to studies of human link protein cleavage by stromelysin. J Biol Chem. 1992 Aug 15;267(23):16011–16014. [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Fisher L. W., Young M. F. Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively. Genomics. 1990 Feb;6(2):219–225. doi: 10.1016/0888-7543(90)90560-h. [DOI] [PubMed] [Google Scholar]
- Melching L. I., Roughley P. J. The synthesis of dermatan sulphate proteoglycans by fetal and adult human articular cartilage. Biochem J. 1989 Jul 15;261(2):501–508. doi: 10.1042/bj2610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame P. J., Choi H. U., Rosenberg L. C. The primary structure of the core protein of the small, leucine-rich proteoglycan (PG I) from bovine articular cartilage. J Biol Chem. 1989 May 25;264(15):8653–8661. [PubMed] [Google Scholar]
- Nguyen Q., Liu J., Roughley P. J., Mort J. S. Link protein as a monitor in situ of endogenous proteolysis in adult human articular cartilage. Biochem J. 1991 Aug 15;278(Pt 1):143–147. doi: 10.1042/bj2780143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R. H., Mathieson J. M., Mort J. S., Roughley P. J. Effect of age on the abundance and fragmentation of link protein of the human intervertebral disc. J Orthop Res. 1989;7(6):861–867. doi: 10.1002/jor.1100070612. [DOI] [PubMed] [Google Scholar]
- Plaas A. H., Neame P. J., Nivens C. M., Reiss L. Identification of the keratan sulfate attachment sites on bovine fibromodulin. J Biol Chem. 1990 Nov 25;265(33):20634–20640. [PubMed] [Google Scholar]
- Ronzière M. C., Ricard-Blum S., Tiollier J., Hartmann D. J., Garrone R., Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990 Apr 19;1038(2):222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 1989 Sep 15;262(3):823–827. doi: 10.1042/bj2620823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. The dermatan sulfate proteoglycans of the adult human meniscus. J Orthop Res. 1992 Sep;10(5):631–637. doi: 10.1002/jor.1100100505. [DOI] [PubMed] [Google Scholar]
- Sampaio L. de O., Bayliss M. T., Hardingham T. E., Muir H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J. 1988 Sep 15;254(3):757–764. doi: 10.1042/bj2540757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J., Fisher L. W. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987 Oct 29;148(2):658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- Witsch-Prehm P., Miehlke R., Kresse H. Presence of small proteoglycan fragments in normal and arthritic human cartilage. Arthritis Rheum. 1992 Sep;35(9):1042–1052. doi: 10.1002/art.1780350909. [DOI] [PubMed] [Google Scholar]