Abstract
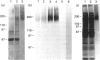
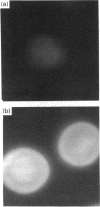
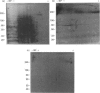
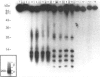
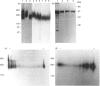
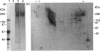
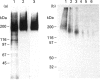
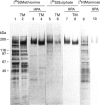
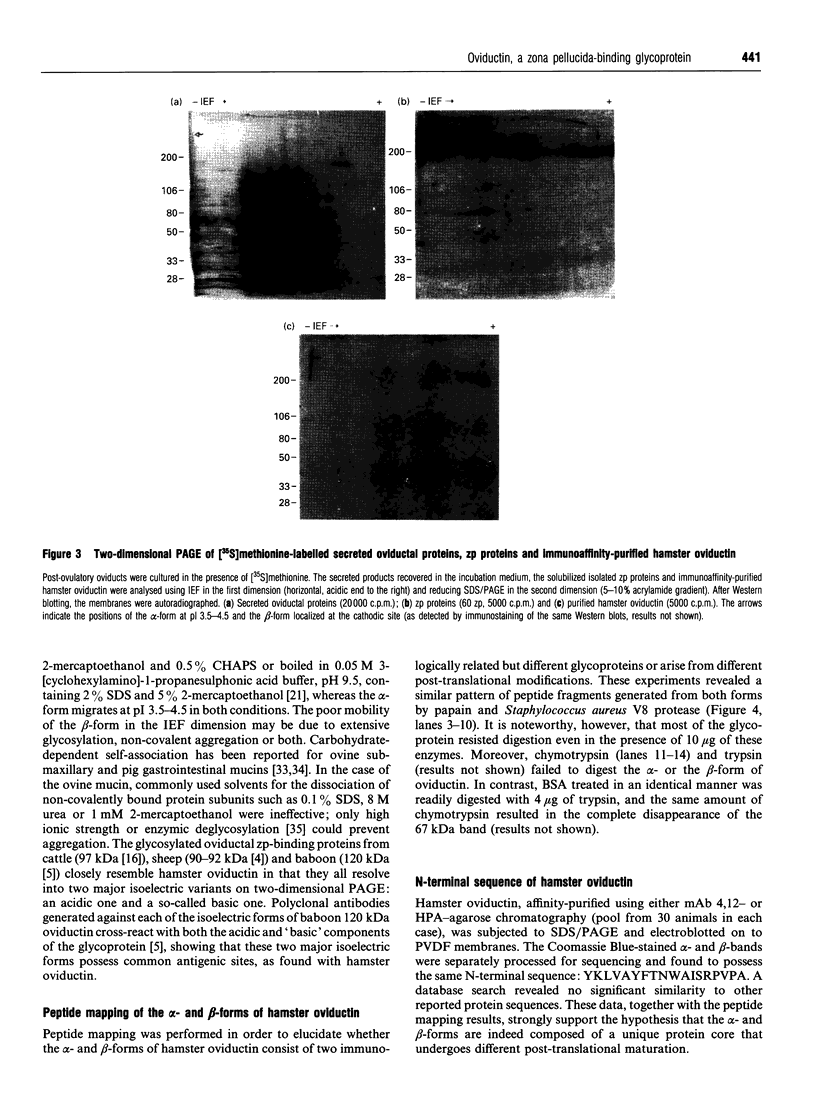
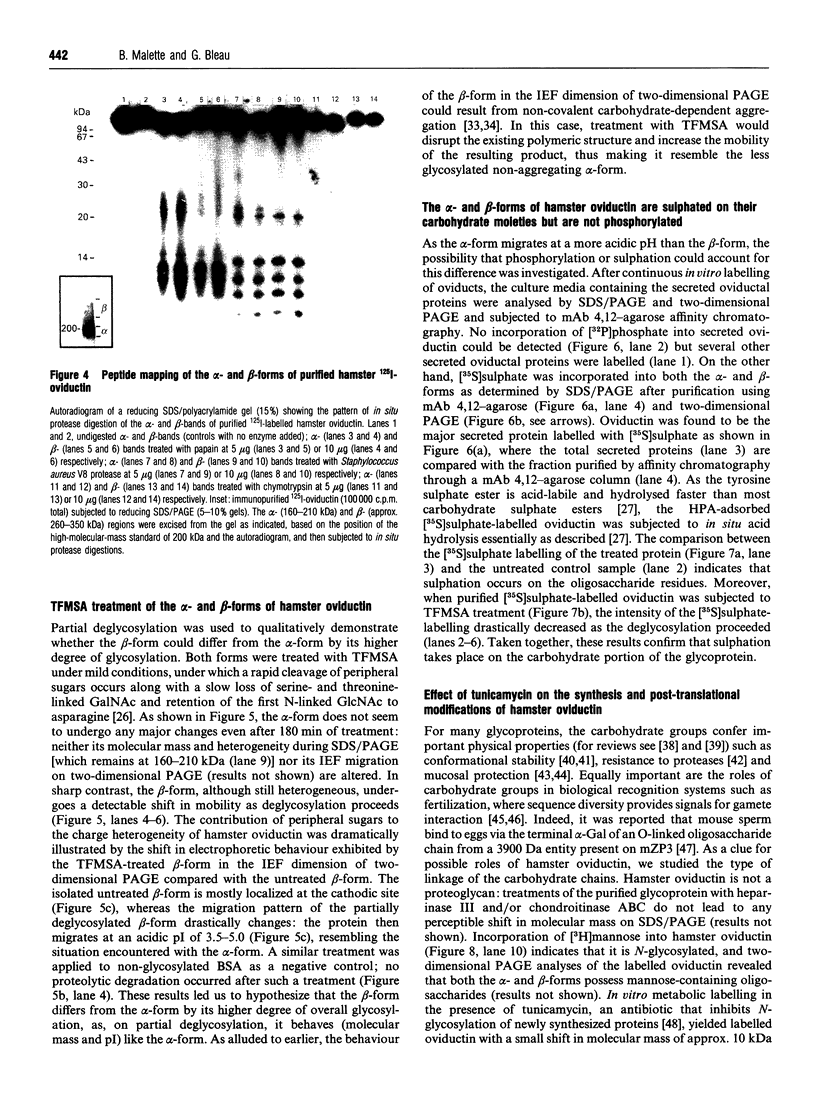
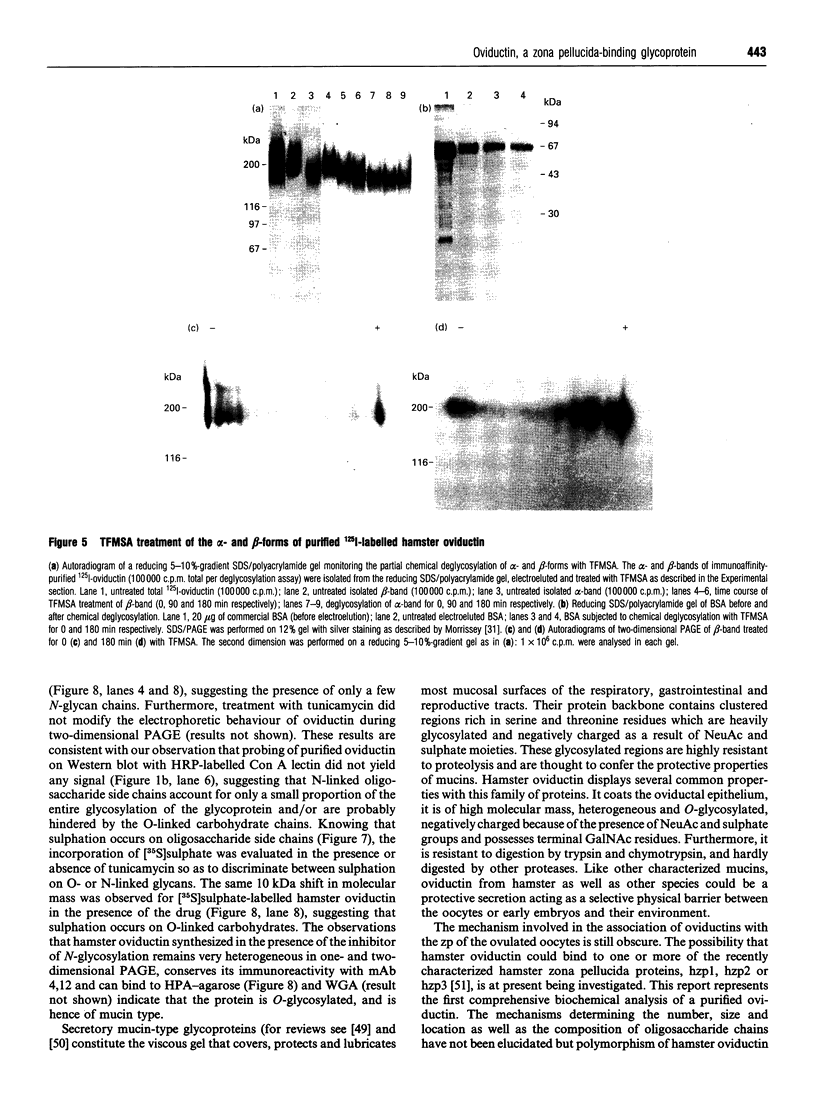
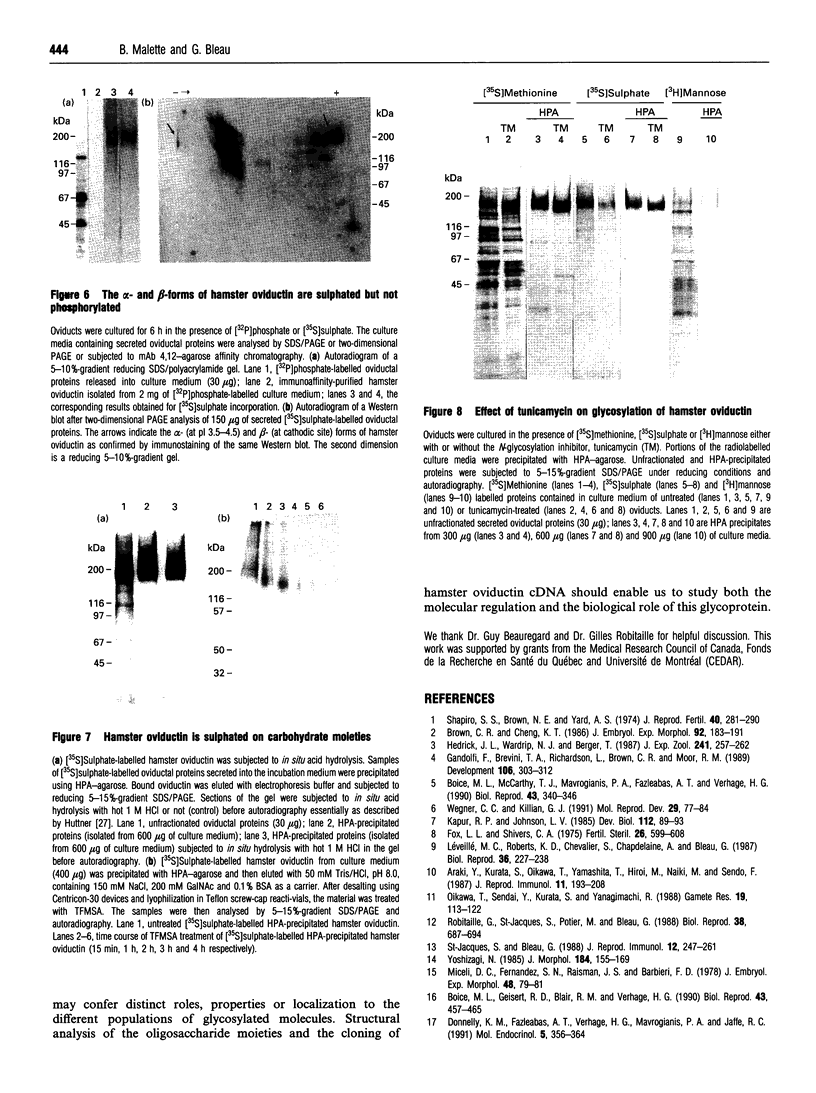
Oviductins are a family of glycoproteins, synthesized and released by oviductal secretory cells, which bind to the zona pellucida of the oocyte after ovulation. Hamster oviductin migrates as diffuse species of 160-350 kDa during SDS/PAGE under reducing as well as non-reducing conditions. In this report, we describe the one-step purification of hamster oviductin using either immuno- or lectin-affinity chromatography. Probing with specific lectins showed that the glycoprotein contains terminal alpha-D-GalNAc, and either terminal alpha-D-NeuAc or non-terminal beta-D-(GlcNAc)2 residues, but fails to react with concanavalin A and Ulex Europeus A-1 lectins which are specific for branched alpha-D-mannose and alpha-L-fucose moieties respectively. Intraovarian oocytes do not contain this glycoprotein and we demonstrate here that the immunoaffinity-purified oviductin readily binds to their zonae pellucidae in vitro, thus mimicking the in vivo phenomenon. Two major immunologically related forms of hamster oviductin (named alpha and beta) were characterized using one- and two-dimensional gel electrophoresis. The alpha-form (160-210 kDa) has an acidic pI of 3.5-4.5 and the beta-form (approx. 210-350 kDa) is localized at the cathodic site in the isoelectric focusing dimension; in between these two major forms lies a smear of minor-charge isomers. Peptide mapping of both major forms with papain and Staphylococcus aureus V8 protease yielded fragments of identical size. Moreover, the two forms share the same N-terminal sequence which display no significant homology with other reported proteins. Treatment with trifluoromethanesulphonic acid showed that a protein with the size and pI of the alpha-form can be generated from the beta-form. Both the alpha- and beta-forms are sulphated on O-linked oligosaccharide side chains but are not phosphorylated. Collectively, these results suggest that the hamster oviductin polymorphism observed in two-dimensional PAGE is a consequence of different glycosylation patterns and not the polypeptide chain itself. Hamster oviductin is mostly O-glycosylated and contains a few N-linked oligosaccharide side chains (approx. 10 kDa). We propose that hamster oviductin is a mucin-type glycoprotein which might act as a protective secretion influencing the first steps of the reproductive process necessary for the normal triggering of fertilization and early embryonic development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980 Mar;21(3):249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Kurata S., Oikawa T., Yamashita T., Hiroi M., Naiki M., Sendo F. A monoclonal antibody reacting with the zona pellucida of the oviductal egg but not with that of the ovarian egg of the golden hamster. J Reprod Immunol. 1987 Jul;11(3):193–208. doi: 10.1016/0165-0378(87)90057-x. [DOI] [PubMed] [Google Scholar]
- Boice M. L., Geisert R. D., Blair R. M., Verhage H. G. Identification and characterization of bovine oviductal glycoproteins synthesized at estrus. Biol Reprod. 1990 Sep;43(3):457–465. doi: 10.1095/biolreprod43.3.457. [DOI] [PubMed] [Google Scholar]
- Boice M. L., McCarthy T. J., Mavrogianis P. A., Fazlebas A. T., Verhage H. G. Localization of oviductal glycoproteins within the zona pellucida and perivitelline space of ovulated ova and early embryos in baboons (Papio anubis). Biol Reprod. 1990 Aug;43(2):340–346. doi: 10.1095/biolreprod43.2.340. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Cheng W. K. Changes in composition of the porcine zona pellucida during development of the oocyte to the 2- to 4-cell embryo. J Embryol Exp Morphol. 1986 Mar;92:183–191. [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Donnelly K. M., Fazleabas A. T., Verhage H. G., Mavrogianis P. A., Jaffe R. C. Cloning of a recombinant complementary DNA to a baboon (Papio anubis) estradiol-dependent oviduct-specific glycoprotein. Mol Endocrinol. 1991 Mar;5(3):356–364. doi: 10.1210/mend-5-3-356. [DOI] [PubMed] [Google Scholar]
- Dunbar B. S., Kimura H., Timmons T. M. Protein analysis using high-resolution two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 1990;182:441–459. doi: 10.1016/0076-6879(90)82036-2. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. L., Shivers C. A. Immunologic evidence for addition of oviductal components to the hamster zona pellucida. Fertil Steril. 1975 Jun;26(6):599–608. doi: 10.1016/s0015-0282(16)41179-9. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Ploegh H. Inhibitors of oligosaccharide processing. Biochim Biophys Acta. 1985 Jun 24;825(2):95–110. doi: 10.1016/0167-4781(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Gandolfi F., Brevini T. A., Richardson L., Brown C. R., Moor R. M. Characterization of proteins secreted by sheep oviduct epithelial cells and their function in embryonic development. Development. 1989 Jun;106(2):303–312. doi: 10.1242/dev.106.2.303. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Wardrip N. J., Berger T. Differences in the macromolecular composition of the zona pellucida isolated from pig oocytes, eggs, and zygotes. J Exp Zool. 1987 Feb;241(2):257–262. doi: 10.1002/jez.1402410213. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Kan F. W., St-Jacques S., Bleau G. Immunocytochemical evidence for the transfer of an oviductal antigen to the zona pellucida of hamster ova after ovulation. Biol Reprod. 1989 Mar;40(3):585–598. doi: 10.1095/biolreprod40.3.585. [DOI] [PubMed] [Google Scholar]
- Kapur R. P., Johnson L. V. An oviductal fluid glycoprotein associated with ovulated mouse ova and early embryos. Dev Biol. 1985 Nov;112(1):89–93. doi: 10.1016/0012-1606(85)90122-8. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kozarsky K., Kingsley D., Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leese H. J. The formation and function of oviduct fluid. J Reprod Fertil. 1988 Mar;82(2):843–856. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- Léveillé M. C., Roberts K. D., Chevalier S., Chapdelaine A., Bleau G. Uptake of an oviductal antigen by the hamster zona pellucida. Biol Reprod. 1987 Feb;36(1):227–238. doi: 10.1095/biolreprod36.1.227. [DOI] [PubMed] [Google Scholar]
- Macart M., Gerbaut L. An improvement of the Coomassie Blue dye binding method allowing an equal sensitivity to various proteins: application to cerebrospinal fluid. Clin Chim Acta. 1982 Jun 16;122(1):93–101. doi: 10.1016/0009-8981(82)90100-0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Miceli D. C., Fernández S. N., Raisman J. S., Barbieri F. D. A trypsin-like oviducal proteinase involved in Bufo arenarum fertilization. J Embryol Exp Morphol. 1978 Dec;48:79–91. [PubMed] [Google Scholar]
- Miller D. J., Macek M. B., Shur B. D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature. 1992 Jun 18;357(6379):589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Moller C. C., Bleil J. D., Kinloch R. A., Wassarman P. M. Structural and functional relationships between mouse and hamster zona pellucida glycoproteins. Dev Biol. 1990 Feb;137(2):276–286. doi: 10.1016/0012-1606(90)90254-g. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parker C. W. Radiolabeling of proteins. Methods Enzymol. 1990;182:721–737. doi: 10.1016/0076-6879(90)82056-8. [DOI] [PubMed] [Google Scholar]
- Paulson J. C. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989 Jul;14(7):272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Pulido R., Sánchez-Madrid F. Glycosylation of CD45: carbohydrate processing through Golgi apparatus is required for cell surface expression and protein stability. Eur J Immunol. 1992 Feb;22(2):463–468. doi: 10.1002/eji.1830220226. [DOI] [PubMed] [Google Scholar]
- Robitaille G., St-Jacques S., Potier M., Bleau G. Characterization of an oviductal glycoprotein associated with the ovulated hamster oocyte. Biol Reprod. 1988 Apr;38(3):687–694. doi: 10.1095/biolreprod38.3.687. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydr Res. 1988 Jul 15;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- Shapiro S. S., Brown N. E., Yard A. S. Isolation of an acidic glycoprotein from rabbit oviducal fluid and its association with the egg coating. J Reprod Fertil. 1974 Oct;40(2):281–290. doi: 10.1530/jrf.0.0400281. [DOI] [PubMed] [Google Scholar]
- St-Jacques S., Bleau G. Monoclonal antibodies specific for an oviductal component associated with the hamster zona pellucida. J Reprod Immunol. 1988 Mar;12(4):247–261. doi: 10.1016/0165-0378(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner C. C., Killian G. J. In vitro and in vivo association of an oviduct estrus-associated protein with bovine zona pellucida. Mol Reprod Dev. 1991 May;29(1):77–84. doi: 10.1002/mrd.1080290112. [DOI] [PubMed] [Google Scholar]