Abstract
Background and Hypothesis
Corollary discharge mechanism suppresses the conscious auditory sensory perception of self-generated speech and attenuates electrophysiological markers such as the auditory N1 Event-Related Potential (ERP) during Electroencephalographic (EEG) recordings. This phenomenon contributes to self-identification and seems to be altered in people with schizophrenia. Therefore, its alteration could be related to the anomalous self-experiences (ASEs) frequently found in these patients.
Study Design
To analyze corollary discharge dysfunction as a possible substrate of ASEs, we recorded EEG ERP from 43 participants with schizophrenia and 43 healthy controls and scored ASEs with the ‘Inventory of Psychotic-Like Anomalous Self-Experiences’ (IPASE). Positive and negative symptoms were also scored with the ‘Positive and Negative Syndrome Scale for Schizophrenia’ (PANSS) and with the ‘Brief Negative Symptom Scale’ (BNSS) respectively. The N1 components were elicited by two task conditions: (1) concurrent listening to self-pronounced vowels (talk condition) and (2) subsequent non-concurrent listening to the same previously self-uttered vowels (listen condition).
Study Results
The amplitude of the N1 component elicited by the talk condition was lower compared to the listen condition in people with schizophrenia and healthy controls. However, the difference in N1 amplitude between both conditions was significantly higher in controls than in schizophrenia patients. The values of these differences in patients correlated significantly and negatively with the IPASE, PANSS, and BNSS scores.
Conclusions
These results corroborate previous data relating auditory N1 ERP amplitude with altered corollary discharge mechanisms in schizophrenia and support corollary discharge dysfunction as a possible underpinning of ASEs in this illness.
Keywords: event-related potentials, N1, attenuation, speech, ipseity, symptoms
Introduction
Anomalous Self-Experiences (ASEs) have been replicated as a relevant finding in people with psychosis,1 even in its early stages2 and in at-risk states,3 and remain stable in this syndrome.4 These experiences translate into an impairment or loss of the natural preconscious evidence by which we identify our mental contents as our own, including cognitive and somatic aspects. ASEs are also evidenced in the subject’s relationship with the surrounding world, including dimensions such as personal involvement or time, among others. Also, they are constituted by non-psychotic aberrations of experience in the domain of affect, perception, cognition, acting, and body, which are acknowledged as relevant and early phenotypes of schizophrenia.5 Thus, ASEs can potentially impair the experience of ipseity, ie, the automatic and preconscious experience of individual identity, which can be altered in psychotic states. In this context, schizophrenia has been proposed as a self-disorder.6 According to this proposal, both negative and positive symptoms dimensions may arise from a basic disturbance of self-experience, respectively relating to: (1) a diminished sense of existing as an individual subject, and (2) a decreased subjective self-agency, which could hamper the experience of one’s own thoughts and can result in its attribution to external sources. In addition, ASEs may also influence motor behavior, which is included in the corresponding assessment instruments.7 In this sense, it has been shown that prediction of the sensory consequences of one’s own actions is hampered in people with schizophrenia.8
The gold-standard instrument for scoring ASEs is the “Examination of Anomalous Self-Experiences” (EASE),7 whose scores show a high correlation with those of the self-administered “Inventory of Psychotic-Type Anomalous Self-Experiences” (IPASE).9,10
Given their preconscious nature, it is likely that some neural alteration underlies ASEs. This possibility is reinforced by the findings that neurocognition is significantly related to ASEs as assessed by both the EASE11 and the IPASE12 questionnaires. Indeed, in a resting-state functional Magnetic Resonance Imaging (rs_fMRI) study, functional connectivity between the parahippocampal and cingulate cortexes was positively associated with IPASE scores.13 Similarly, aberrant functional interactions (background/intrinsic connectivity) of the right ventral premotor cortex and bilateral posterior insula with posterior cingulate cortex were directly related to ASEs.14
However, these studies reveal little about the possible neural mechanisms underlying ASEs. In contrast, studies of corollary discharge allow a more direct approach to these phenomena. The corollary discharge is a neural mechanism by which the sensory consequences of self-initiated acts are attenuated or suppressed.15 For example, the auditory N1 Event-Related Potential (ERP) is significantly attenuated when healthy participants listen to their own voice during speech, whereas it is preserved when they passively listen to their previously recorded voice.16–19 Therefore, this mechanism would allow a preconscious identification of the origin of the sources of sensory stimulation, ie, whether it comes from within or from outside oneself, which would probably be involved in self-identification. Interestingly, alterations in corollary discharge have been consistently reported in schizophrenia, with patients showing significantly less attenuation of the N1 potential while speaking.18 Therefore, preconscious identification of mental contents as “self” might be impaired in this illness. Furthermore, corollary discharge mechanisms may also have a role in internal representations not related to external stimuli20 and thus may contribute to the natural, preconscious experience of the self and the outside world whose disturbances are reflected in the ASEs. In this context, core ASEs may be likely contributed by abnormal corollary discharge mechanisms: this can be the case of alterations in self-presence and self-awareness (related to basic identity and connection with the world), demarcation (related to the disintegration of self-world boundaries) or cognition (where memory plays a key role). Thus, neural mechanisms such as the corollary discharge may play a role in both implicit motor behavior (ie, below the threshold of consciousness), and self-experience, whose alterations may arise in case of this mechanism dysfunction.
The possible relationship between ASEs and corollary discharge alterations was proposed in a recent review,21 where the authors hypothesized that early dysfunction of the corollary discharge may lead to a progressive fading of the sense of self-agency about one’s own experiences. This, in turn, may lead to ASEs and even to positive symptoms, such as delusions of passivity or verbal hallucinations. However, to our knowledge, this hypothesis has not been tested to date.
Given the scarce knowledge about neural underpinnings involved in ASEs, we hypothesize here that they would be significantly related to the corollary discharge mechanism alteration. To this end, we (1) compared the corollary discharge effect (assessed using electroencephalographic [EEG] as a suppression of the N1 evoked potential) between people with schizophrenia and Healthy Controls (HC), and (2) assessed its relationship with ASEs (measured as IPASE scores). Considering the possible relations between ASEs and psychotic symptoms,21 and the discordant data supporting22–24 or not25–32 their relation to corollary discharge measures, these associations were additionally tested.
Materials and Methods
Sample
Forty-three participants with schizophrenia (18 first-episode [FE] and 25 chronic stable; age range 19–56 years) and 43 HC (age range 18–54 years), all with normal hearing ability, participated in the study. Patients were diagnosed by two expert psychiatrists (VM and JSF) according to the Diagnostic and Statistical Manual of Mental Disorders (5th edition). Exclusion criteria were (i) neurological disease, (2) history of head trauma with loss of consciousness, (3) current substance abuse (except nicotine or caffeine), (4) Intelligence Quotient (IQ) less than 70, and (5) any psychiatric treatment (for controls) or (6) current diagnosis other than schizophrenia (for patients). Sociodemographic, behavioral, cognitive, and clinical data are shown in table 1. All participants gave written informed consent after receiving complete printed information. The ethical committees of the participating hospitals endorsed the study.
Table 1.
Sociodemographic, Clinical Characteristics, and Neurophysiological Values of the Participants
| Healthy Controls n = 43 |
Schizophrenia n = 43 (25 CH/18 FE) |
First-Episode n = 18 |
Chronic Schizophrenia n = 25 |
|
|---|---|---|---|---|
| Sex (M/F) | 22/21 | 28/15 | 8/10 | 20/5* |
| Age (years) | 29.63 (10.94) | 35.93 (11.69) | 29.80 (9.75) | 40.56 (10.89)** |
| Education (years) | 14.51 (2.35) | 13.15 (2.99) | 13.90 (2.90) | 12.65 (3.05)** |
| Illness duration (months) | — | 73.17 (115.02) | 11.05 (17.20) | 132.06 (136.350) |
| CPZ equivalents (mg) | — | 386.10 (253.50) | 292.65 (118.59) | 475.24 (302.78) |
| IPASE-Total ASEs | — | 110.33 (40.09) | 112.07 (38.65) | 112.73 (44.38) |
| IPASE-Cognition | — | 12.28 (4.69) | 12.07 (5.33) | 13.09 (5.23) |
| IPASE-Self Awareness and Presence | — | 41.61 (17.64) | 43.40 (16.07) | 41.77 (19.71) |
| IPASE-Consciousness | — | 13.11 (5.85) | 12.73 (6.30) | 13.77 (5.81) |
| IPASE-Somatization | — | 34.42 (12.17) | 35.33 (11.99) | 34.73 (13.05) |
| IPASE-Demarcation/Transitivism | — | 8.86 (4.07) | 8.53 (3.56) | 9.27 (4.44) |
| PANSS-Positive symptoms | — | 11.53 (4.08) | 13.10 (6.06) | 11.18 (3.16) |
| BNSS-Negative symptoms | — | 19.58 (15.34) | 22.70 (18.00) | 18.50 (13.89) |
| WAIS-Total IQ | 110.84 (9.24) | 95.22 (12.50)*** | 94.47 (12.19)*** | 94.75 (13.70)*** |
| BACS-Verbal memory | 52.96 (8.69) | 45.03 (10.55)** | 49.35 (6.86) | 41.52 (11.81)*** |
| BACS-Working memory | 23.00 (3.12) | 19.13 (4.53)*** | 20.12 (3.44)** | 18.33 (5.20)*** |
| BACS-Motor speed | 72.89 (13.80) | 58.37 (12.03)*** | 58.35 (11.19)*** | 58.38 (12.94)*** |
| BACS-Verbal fluency | 28.10 (4.62) | 22.05 (5.82)*** | 22.29 (3.67)*** | 21.86 (12.94)** |
| BACS-Processing fluency | 70.25 (9.83) | 48.84 (12.14)*** | 53.18 (10.41)*** | 45.33 (12.53)*** |
| BACS-Problem solving | 17.64 (2.84) | 17.05 (3.61) | 17.59 (3.47) | 16.62 (3.75) |
| WCST-Perseverative errors (%) | 8.35 (3.73) | 11.53 (7.23)* | 9.37 (6.56) | 13.26 (7.43)* |
| Amplitude N1 LS (µV) | −3.29 (1.96) | −2.42 (1.69)* | −2.48 (1.77) | −2.38 (1.66)* |
| Amplitude N1 TK (µV) | −0.46 (2.38) | −1.58 (2.09)* | −1.28 (2.37) | −1.79 (1.88)* |
| Amplitude P2 LS (µV) | 0.32 (1.85) | 0.83 (1.94) | 1.10 (1.80) | 0.64 (2.04) |
| Amplitude P2 TK (µV) | 0.73 (3.16) | 0.28 (2.28) | 0.40 (2.61) | 0.20 (2.06) |
Note: Data are given as mean (standard deviation). Neurophysiological values correspond to the average of the electrodes and epochs used in the statistics.
CH, Chronic schizophrenia; FE, First episode of schizophrenia; M/F, Masculine/Feminine; CPZ, Chlorpromazine; IPASE, Inventory of Psychotic-Like Anomalous Self-Experiences; ASEs, Anomalous Self-Experiences; PANSS, Positive and Negative Syndrome Scale; BNSS, Brief Negative Symptom Scale; WAIS, Wechsler Adult Intelligence Scale; IQ, Intelligence Quotient; BACS, Brief Assessment of Cognition in Schizophrenia; WCST, Wisconsin Card Sorting Test; LS, Listen Self; TK, Talk.
*P < .05;
**P < .01;
***P < .001 (Chi square test or Student’s test when corresponding) in comparison to healthy controls.
Abnormal Self-experiences Assessment
Abnormal Self-Experiences (ASEs) were evaluated using the IPASE,10 a 57-item self-report scale with a 5-factor structure in which participants, in the presence of the researcher, indicate the extent to which they agree with statements on a scale from 1 (Strongly Disagree) to 5 (Strongly Agree). The Cognition factor consists of items related to difficulties in thought processes, such as thought interference; the Self-Awareness and Presence factor contains items related to loss of self or basic identity, and loss of connection with the world. The Consciousness factor includes items on alterations in the experience of time, alterations in intentionality, and difficulty distinguishing between imagination and reality. The Somatization factor includes items about disturbances in bodily experiences, such as the feeling that the body changed shape or was difficult to control, and thoughts of not feeling physically or psychically present in one’s own body. Finally, the Demarcation/Transitivism factor consists of items related to the disintegration of boundaries between the self and the world, or a feeling of nonexistence.
Symptoms and Cognitive Assessment
Patients’ positive and negative symptoms were respectively assessed using the positive subscale of the “Positive and Negative Syndrome Scale for Schizophrenia” (PANSS),33 and the “Brief Negative Symptom Scale” (BNSS).34 Cognitive performance was assessed using the Spanish version of the “Brief Assessment in Cognition in Schizophrenia Scale” (BACS),35,36 and the “Wisconsin Card Sorting Test” (WCST: percentage of perseverative errors).37 IQ was estimated using the “Wechsler Adult Intelligence Scale-III” (WAIS-III).38,39 The cognitive assessment was done for descriptive purposes to ensure that patients have equivalent impairment to that of our previous studies.
Experimental Procedure for the Evaluation of the Corollary Discharge Mechanism
Participants were seated 60 cm from a computer screen with a white cross in the center of a black background. A microphone (model NT1) was placed 15 cm from the subject’s mouth during the speaking condition. Each participant accomplished two different conditions40:
◦ Talk condition: participants were instructed to vocalize [a:] approximately every 1–2 s for 4 min, with a 30-s rest after the first 2 min. Concurrently with each vocalization, the sound was picked up by a microphone, amplified, and heard by the participant in real-time through headphones (model SE215).
◦ Listen condition: Subsequently, participants were instructed to listen passively to their own vocalizations as recorded in the previous condition, also played through headphones.
Prior to recording the talk condition, each participant was trained to maintain a 15-cm space between their mouth and the microphone, and to vocalize the phoneme [a:] briefly (<300 ms) with a volume between 65 and 75 dB Sound Pressure Level (SPL). During this training, feedback on their performance was given. Participants were also instructed to remain still, open their mouths before uttering the sound, fixate their eyes on the white cross during the entire recording, and maintain a steady tone of voice. Constant volume intensity was monitored with a sound level meter (model PCE-353N-ICA), placed 6 cm in front of the mouth. The volume was the same in all conditions, according to the balance of the headphone audio output, measured with a dB-meter.40 During the recording of both conditions, a signal coincident to each vocalization was sent, via a preamplifier (actiCHamp), to a sound processing software (Audacity) so that it could generate a trigger pulse. Each trigger pulse was produced on the rising edge of the rectified signal and was included in the EEG recording. There were no significant differences in mean speech loudness between the author’s recordings and those of the participants. Participants were trained to get used to emit vowels within the range of 65–75 dB, and the sensitivity level of the amplifier was setup to generate triggers only when a minimum of 65 dB voice volume was generated by the participant. Trials with a vocalization outside the 65–75 dB range were not recorded. To mask the effect of bone conduction during vocalization, the mean speech SPL reproduced through headphones was increased by 15 dB over each subject’s mean speech SPL in both conditions.24,28
The corollary discharge effect was assessed as the suppression of sensory consequences following self-initiated acts compared to those following passive external stimulation (ie, the difference in amplitude of the studied evoked potentials corresponding to the talk condition minus the listen condition).
EEG Data Acquisition and Analysis
A 64-channel EEG system recorded the EEG data (BrainVision, Brain Products GmbH). The active electrodes were placed on an elastic cap using the international 10–10 system (FP1, FP2, F7, F8, F3, F4, Fz, FC5, FC6, FC1, FC2, T7, T8, C3, Cz, C4, CP5, CP6, CP1, CP2, TP9, TP10, P7, P8, P3, P4, Pz, O1, O2, Oz, AF7, AF3, AFz, F1, F5, FT7, FC3, FCz, C1, C5, TP7, CP3, P1, P5, PO7, PO3, POz, PO4, PO8, P6, P2, CPz, CP4, TP8, C6, C2, FC4, FT8, F6, F2, AF4, AF8). The impedance did not exceed 5 kΩ and the sampling frequency was 500 Hz. The in-line reference was the average mastoid ((TP9 + TP10)/2). Data pre-processing was performed using EEGLAB v13.6.5b41 and Matlab R2022b (MathWorks Inc., MA, USA). A low-pass filter of 30 Hz and a high-pass filter of 0.05 Hz were applied. Each continuous EEG recording during the talk condition was visually monitored trial by trial for excessive muscle artifacts at speech onset. Any trial onset whose noise signal was indistinguishable from background EEG activity was excluded from further analysis. Ambiguous speech onsets involving some abnormal activity peaks were also excluded.40 Subsequently, eye movements, blinking and any artifact related to facial muscle activity (especially during the talk condition) were identified and rejected with an Independent Components Analysis (ICA).42 EEG data epochs were established from 100 ms prior to the auditory stimulus onset (used for baseline correction) to 250 ms after the stimulus onset. Trials containing artifacts (voltages greater than ± 90 µV) were rejected and 20 participants with less than 30% of trials on average (13 patients and 7 controls out of the 106 initial participating volunteers) were excluded from the analysis.
Based on previous literature,40,43,44 the amplitudes at the vertex of N1 and P2 ERPs were independently analyzed. Nine electrodes around the vertex (FC1, FCz, FC2, C1, Cz, C2, CP1, CPz, CP2) were selected for statistical analysis to check that possible differences were found over a region and not limited to a single sensor. N1 was identified as a negative fronto-central peak between 60 and 120 ms after the onset of the phoneme [a:]; and P2 was the subsequent fronto-central positivity between 120 and 220 ms.
Statistical Analysis
Sociodemographic, behavioral, cognitive, and ERP amplitude differences between people with schizophrenia and HC were examined using Chi-squared or Student’s t-tests for independent samples when corresponding. The corollary discharge mechanism was analyzed by comparing the average amplitude of the potential trials (N1 and P2) between conditions (talk vs. listen). The following ERP analyses were performed separately for both N1 and P2 potentials.
As a preliminary step, separately for people with schizophrenia and HC, differences between conditions at each of the electrodes in the area corresponding the FC1, FCz, FC2, C1, Cz, C2, CP1, CPz, and CP2 electrodes were analyzed using Student’s t-test for related measures. In case all electrodes showed significant differences in the same direction, it was planned to average their amplitude values (ie, mean voltages in their respective time windows) for subsequent analyses.
Two types of analysis were then performed. First, a within-group analysis to assess the differences between conditions (talk vs listen) and, therefore, the occurrence of the corollary discharge mechanism, for which a repeated measures t-test was used. Secondly, in order to assess the differences between groups in corollary discharge, the amplitude difference between conditions (talk minus listen) was used as a parameter of interest, since this measure reflects the sensory suppression generated by the corollary discharge mechanism. This analysis was performed only for those ERPs that showed significant differences in the preceding within-group comparisons (ie, N1, P2, or both). Thus, a second Student’s t-test was performed (in this case for independent measures) where the patients and HC groups were compared on this measure of corollary discharge.
Finally, we tested the main study hypothesis: the predicted relationship between the magnitude of corollary discharge (ie, significant differences in the sensory suppression measure in the previous step), and the intensity of ASEs (IPASE scores) and symptoms (PANSS and BNSS scores) in people with schizophrenia using linear regression analysis.
Student’s t-test analyses (within and between group comparisons) were conducted for all schizophrenia patients first, and then for FE and chronic patients alone. To study possible differences generated by chronicity and illness treatment, FE patients were also analyzed separately with linear regression models. Additionally, to rule out a possible major effect of the treatment, the relationships with treatment dose (chlorpromazine equivalents in mg/day) were also studied using regression models.
Results
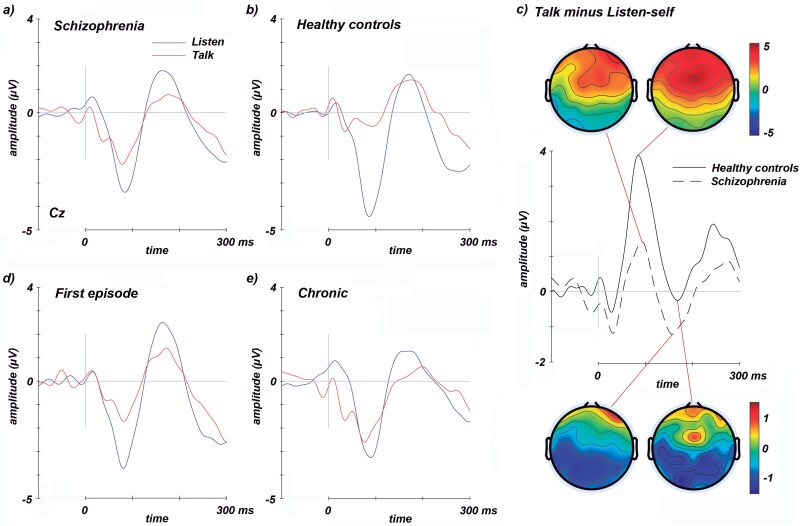
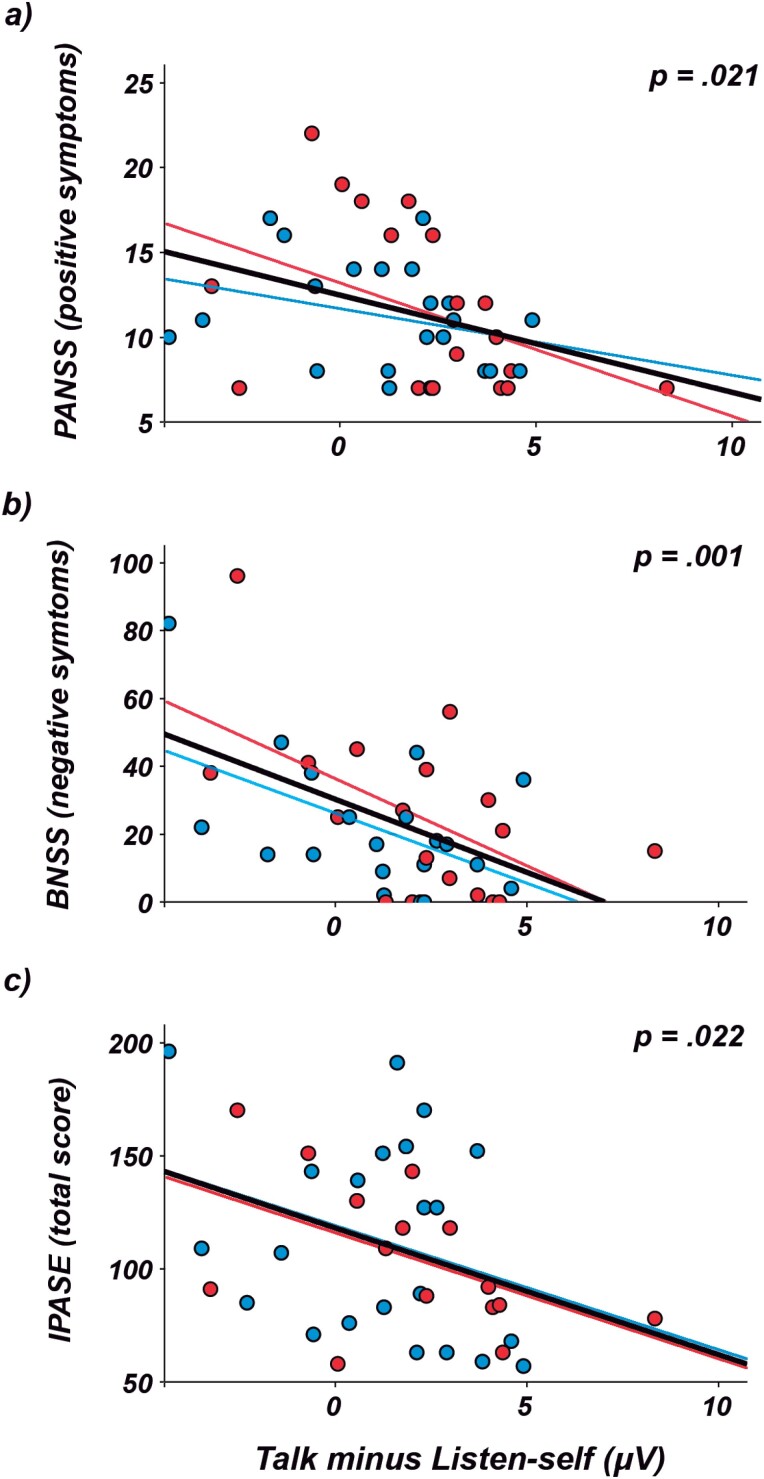
There were no significant differences between groups (schizophrenia patients vs HC) in age, sex, or years of education. Schizophrenia patients showed generalized deficits in cognitive scores compared to HC (table 1). Figure 1 shows the waveforms of the two ERPs analyzed (N1 and P2) in schizophrenia patients and HC. Supplementary figure 1 depicts the mean suppression in N1 amplitude (ie, talk minus listen condition, our corollary discharge measure) averaged over the nine electrodes around the vertex in schizophrenia patients and HC. Figure 2 shows the significant results found in the linear regression analyses conducted between measures of evoked response suppression and ASEs and symptoms scores. Supplementary figure 2 shows the correlations between the evoked response suppression and the specific IPASE factors and BNSS symptoms.
Fig. 1.
ERP waveforms show N1 and P2 components during the talk (red) and listen (blue) conditions recorded at Cz. The N1 amplitude during the talk condition is reduced relative to listen in schizophrenia patients (first-episode and chronic patients averaged; a) and healthy controls (b). This effect is attenuated in schizophrenia patients and, therefore, the waves and topographies show that the N1 amplitude suppression (talk minus listen condition) is higher in healthy controls compared to schizophrenia patients (c). There were no significant amplitude differences for first-episode (d) and chronic patients (e).
Fig. 2.
Significant linear correlations in the regression analyses between N1 suppression measures (talk minus listen condition), clinical symptoms and anomalous self-experiences in schizophrenia patients (first-episode [red] and chronic patients [blue]). Notice the higher scores in positive symptoms (PANSS; a), negative symptoms (BNSS; b), and anomalous self-experiences (IPASE; c) as the difference between the talk and listen condition is smaller.
In our preliminary analysis, in both the schizophrenia patients and HC, all electrodes showed significant differences (P < .005) in the same direction between talk and listen conditions. Therefore, the averaged amplitude for these electrodes was used in the corresponding windows of the N1 and P2 potentials for subsequent comparisons.
ERP Amplitude: Within-Group Comparisons
The within-group Student’s t-tests resulted in significant differences due to a lower N1 amplitude in the talk condition compared to the listen condition, in both schizophrenia patients (t = −3.13, P = .003; figure 1a) and HC (t = −7.24, P < .001; figure 1b). The same results were found for the FE patients’ group (t = −2.69, P < .015) and for chronic patients (t = −1.36, P < .018). No differences were found between conditions for the P2 amplitude measure in any group. Thus, further analyses were limited to the N1 ERP.
ERP Amplitude: Between-Groups Comparisons
Student’s t-test between schizophrenia patients and HC showed significant differences in the measure of suppression in the N1 component (t = 3.81, P < .001; figure 1c), with greater attenuation in HC than in patients. When studying patients separately, the same results were found in chronic patients compared to HC (t = 3.62, P < .001) and a trend to statistical significance in FE patients when compared to HC (t = −1.79, P = .077).
ERP Correlates of ASEs and Clinical Symptoms
Regression analyses in schizophrenia patients showed that lower measures of N1 suppression correlated significantly with higher scores in ASEs (IPASE; R2 = 0.14, P = .022), positive symptoms (PANSS; R2 = 0.13, P = .021), and negative symptoms (BNSS; R2 = 0.25, P = .001) (figure 2). The FE patients, when studied separately, showed the same relationships with a trend toward statistical significance on measures of ASEs (IPASE; R2 = 0.25, P = .057), positive symptoms (PANSS; R2 = 0.20, P = .058), and negative symptoms (BNSS; R2 = 0.31, P = .016).
No significant relation was found between the N1 amplitude attenuation and antipsychotic doses in schizophrenia patients.
Discussion
The main objective of this study was to evaluate the suppression of speech-related potentials, as possible altered substrates of the corollary discharge mechanism in people with schizophrenia, and its relationship with the severity of the ASEs presented by these patients.
In our sample, the auditory N1 potential was, as expected,29,31,45 suppressed in both HC and people with schizophrenia while speaking compared to the passive listening to one’s own voice, although this attenuation was significantly lower in the schizophrenia group (figure 1). Additionally, the amount of N1 suppression was associated with the severity of ASEs as well as with positive and negative symptoms in schizophrenia patients (figure 2). Both FE and chronic patients showed similar attenuation, and there was no significant relationship between antipsychotic doses and attenuation of N1 or the severity of ASEs, thus ruling out chronicity and treatment as major determinants. On the other hand, in comparison to HC, schizophrenia patients showed no significant differences in the P2 component, indicating that listening to one’s own voice during the talk condition is indeed processed by the cortex, thus supporting that the lack of N1 suppression when speaking is not a peripheral sensory problem, but is likely caused by central mechanisms as other previous studies have reported.46
The paradigm in our study relies on the attenuation of the sensory consequences of the self-generated motor acts, such as talking, via corollary discharge. The relation between the deficit in this sensorimotor attenuation and ASEs in our patients seems coherent with the broader functions that have been attributed to corollary discharge, including the disengagement of the brain from its environment.20 According to this idea, starting from navigation without explicit external clues, corollary discharge contributes to the representation of current and past experiences and thus to memory and planning. ASEs may thus be contributed by difficulties in self-positioning in the world (past or present), projecting itself into the future and/or integrating memories, among other possibilities.
Despite the altered suppression of N1 during the talk condition, schizophrenia patients showed a normal N1 potential while listening to their own pre-recorded voice (listen condition). This also indicates that the sensory recognition of their own voice is not impaired in these patients. Thus, the dissimilar attenuation shown in both groups (schizophrenia patients and HC) during the talk condition would be likely secondary to the effects of the corollary discharge, ie, a signal sent from the motor areas (where the action is initiated) to the receiving sensory areas which attenuates the sensorial consequences of self-initiated acts.
In addition to the likely alteration of this corollary discharge mechanism in people with schizophrenia, the novel finding in this report is that the amount of such suppression is inversely related to ASEs in our patients. In other words, ASEs are likely associated with a deficit in corollary discharge which, as previously stated, is a neural mechanism that may be key in identifying the source of action as one’s own, ie, playing a role in self-identification and thus making its dysfunction a likely neural signature of altered ipseity.
The smaller N1 suppression in people with schizophrenia indicates that the neural processing for mental (or internal) contents linked to the subject’s actions is similar to that of sensory stimulation with an external source or, at least, more similar than in HC. Consequently, this would make it more difficult for schizophrenia patients to discriminate the source of mental contents, which is very consistent with the characteristics of the ASEs and may contribute to the diminished ipseity reported in schizophrenia. Additionally, given the automatic, early, and preconscious nature of the corollary discharge, as well as the normal N1 under the passive listening condition and the unaltered P2, it seems highly unlikely that lack of collaboration could play a role in the disturbance of this mechanism in people with schizophrenia.
Concerning the correlations between corollary discharge alterations and symptoms, our data support that such alterations may increase the likelihood and/or severity of both positive and negative symptoms. The association between positive symptoms and corollary discharge deficits seems easier to explain since a loss of subjective self-agency could lead to experiencing one’s own thoughts as external stimuli, in the form of auditory hallucinations, or delusions of passivity, for example. In particular, Auditory Verbal Hallucinations (AVH) may be underpinned by a decreased sense of agency related to inner speech, that may progressively lead to a speech misattribution to an external source, thus resulting in the experiencing of at least some own thoughts as a result of other addressing the subject (ie, AVHs). Since the corollary discharge alterations may underpin the decreased self-agency experience, it may be proposed that corollary discharge alterations might progressively lead to AVHs development. The relations with negative symptoms would be less evident. However, Sass and Parnas47 propose in their model of ipseity disturbance a central role for diminished “self-affection” (ie, a diminished experienced sense of existing as a living and unified subject of awareness). In this context, self-agency alterations underpinned by corollary discharge dysfunction may also contribute to negative symptoms. Moreover, corollary discharge also contributes to sensorimotor learning and planning, according to findings in different mammals: while low-order corollary discharge would have a major role in sensory filtration, high-order corollary discharge would enable predictive control for perceptual cohesion and action sequencing.48 These authors propose that “the corollary discharge signal impinges on higher-level structures that are highly sensory and/or executive in nature. As a result, appropriate behaviors can be prepared for the future (planning) and modified based on the lessons of the past (learning).” As a consequence, it could be explored the role that corollary discharge disturbance might have in the neuromotor alterations described in schizophrenia, even at premorbid states, where ASEs can be also found.2
The lack of association between corollary discharge mechanisms and symptoms in other studies may speculatively relate to the heterogeneity of substrates for these symptoms, being the corollary discharge alterations just one of its possible underpinnings. For instance, dopaminergic over-reactivity is associated with positive symptoms, and may not be necessarily related to corollary discharge alterations.49 Moreover, differences in sample size and clinical assessments performed by personnel not involved in the clinical care of the patients may also play a role in those discrepancies.
During vocalization, corollary discharge mechanisms would be activated through an inhibitory feed-forward process in which interneurons located in the auditory cortex inhibit pyramidal neurons.50–53 The neural underpinnings of corollary discharge could be related in this way to synchronization meditated by inhibitory transmission between the efferent motor area and the sensory region receiving the corresponding signal.22,54,55 In this context, the expected deficit in corollary discharge mechanism in our patients seems compatible with the consequences of an overactive cortex, in turn, consistent with GABA deficits in the cortex reported in schizophrenia.56 According to some previous data using a P300 paradigm, task-related modulation of brain activity is significantly decreased in schizophrenia, which has been proved in three different samples,57–59 and this hypomodulation was predicted by a hyperactive basal state.60 This allows us to speculate that basal hyperactivity might hamper corollary discharge and thus interfere with self-identification in people with schizophrenia.
The spatiotemporal model of psychopathology61 proposes a relationship between the functional characteristics of the brain at rest and self-experience. Thus, self-experience may be rooted in spontaneous functional networks of the brain. If these functional networks are hypomodulated, the neural operations that allow discriminating the origin of experiences may be impaired. For instance, the hypomodulation reflected in the smaller N1 change in schizophrenia patients, speculatively related to GABA hypofunction, could hamper the discrimination of the source of mental experiences. The spatiotemporal model proposes a link between subjective-experiential and objective-neuronal measures that can be directly related to subjective experience, as well as a distinction between “deeper” and “superficial” symptoms linking both to different forms of neural activity. Thus, the deeper level refers to the subjective experience of the self, where preconscious operations such as corollary discharge would play a relevant role in the experience of ipseity.
The correlative association between ASEs and corollary discharge deficits would imply that corollary discharge might be preserved in a subset of schizophrenia patients without ASEs. This would be consistent with the likelihood of multiple biotypes coexisting within the schizophrenic syndrome. In this line, a schizophrenia biotype, characterized by a decrease in task-induced modulation of bioelectrical activity has been described.62 Similarly, we have reported in a biotype defined based on cognitive deficit a hyperactive cortical activity associated with a deficit of P300-induced modulation.63
Among the limitations of our study, we did not include a treatment-naïve sample, but the similar profiles of FE and chronic patients, as well as the absence of differences in the P2 waveform, make it unlikely that treatment explains our findings. We used the IPASE to assess ASEs, rather than the gold-standard EASE. However, scores using both instruments show a high correlation,9 and a researcher was present to assist the participant in case of misunderstanding item phrasing. The correlation between ASEs and corollary discharge does not indicate causation, but corollary discharge is a neural mechanism likely underlying the ipseity experience. A larger sample of FE patients is required to statistically confirm the trend of the results. We did not include patients with other functional psychoses, such as bipolar disorder, where dissimilar findings could be found.
Conclusions
Our brain recognizes its own voice through a process that occurs before the perception of the emitted sound. Through the activation of the corollary discharge, we can predict the consequences of our actions based on pre-existing connectivity and dynamics. People with schizophrenia present alterations in this mechanism, which could be the origin of the so-called abnormal self-experiences that are identified in this disorder. The present study shows that alterations in corollary discharge may underpin psychotic symptoms and ASEs in people with schizophrenia.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Funding
This work was supported by the following grants: “Instituto de Salud Carlos III” (ID PI22/00465), “Gerencia Regional de Salud de Castilla y León” (IDs GRS 2368A/21 and GRS 2487/A/22), ‘Fundació La Marató’ (ID 571/C/2022) and by predoctoral grants “Consejería de Educación, Junta de Castilla y León” (Spain) and European Social Fund (IDs VA-223-19 to RMBR and VA-183-18 to IFL). Funding sources had no other role than financial support providers. We appreciate the collaboration of all the participants in our research.
Contributor Information
Rosa M Beño-Ruiz-de-la-Sierra, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain.
Antonio Arjona-Valladares, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain.
Marta Hernández-García, Psychiatry Service, University Clinical Hospital of Valladolid, Valladolid, Spain.
Inés Fernández-Linsenbarth, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain.
Álvaro Díez, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain.
Sabela Fondevila Estevez, UCM-ISCIII Center for Human Evolution and Behaviour, Madrid, Spain.
Carolina Castaño, Psychiatry Service, Doce de Octubre Hospital, Madrid, Spain.
Francisco Muñoz, UCM-ISCIII Center for Human Evolution and Behaviour, Madrid, Spain; Psychobiology and Behavioural Sciences Methods Department, Complutense University of Madrid, Madrid, Spain.
Javier Sanz-Fuentenebro, Psychiatry Service, Doce de Octubre Hospital, Madrid, Spain.
Alejandro Roig-Herrero, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain; Imaging Processing Laboratory, University of Valladolid, Valladolid, Spain.
Vicente Molina, Psychiatry Department, School of Medicine, University of Valladolid, Valladolid, Spain; Psychiatry Service, University Clinical Hospital of Valladolid, Valladolid, Spain.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Raballo A, Sæbye D, Parnas J.. Looking at the schizophrenia spectrum through the prism of self-disorders: an empirical study. Schizophr Bull. 2011;37(2):344–351. doi: 10.1093/schbul/sbp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haug E, Oie M, Andreassen OA, et al. Anomalous self-experiences contribute independently to social dysfunction in the early phases of schizophrenia and psychotic bipolar disorder. Compr Psychiatry. 2014;55(3):475–482. doi: 10.1016/j.comppsych.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 3. Madeira L, Bonoldi I, Rocchetti M, et al. An initial investigation of abnormal bodily phenomena in subjects at ultra high risk for psychosis: their prevalence and clinical implications. Compr Psychiatry. 2016;66:39–45. doi: 10.1016/j.comppsych.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 4. Nordgaard J, Nilsson LS, Sæbye D, Parnas J.. Self-disorders in schizophrenia-spectrum disorders: a 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2018;268(7):713–718. doi: 10.1007/s00406-017-0837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klosterkotter J, Ebel H, Schultze-Lutter F, Steinmeyer EM.. Diagnostic validity of basic symptoms. Eur Arch Psychiatry Clin Neurosci. 1996;246(3):147–154. doi: 10.1007/BF02189116 [DOI] [PubMed] [Google Scholar]
- 6. Sass LA, Parnas J.. Schizophrenia, Consciousness, and the Self. Schizophrenia Bulletin 2003;29(3):427–444. doi: 10.1093/oxfordjournals.schbul.a007017 [DOI] [PubMed] [Google Scholar]
- 7. Parnas J, Møller P, Kircher T, et al. EASE: Examination of anomalous self-experience. Psychopathology 2005;38(5):236–258. doi: 10.1159/000088441 [DOI] [PubMed] [Google Scholar]
- 8. Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD.. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry 2014;71(1):28–35. doi: 10.1001/jamapsychiatry.2013.2974 [DOI] [PubMed] [Google Scholar]
- 9. Nelson B, Li E, Cicero DC, et al. The construct validity of the Inventory of Psychotic-Like Anomalous Self-Experiences (IPASE) as a measure of minimal self-disturbance: preliminary data. Early Interv Psychiatry 2019;13(3):686–691. doi: 10.1111/eip.12711 [DOI] [PubMed] [Google Scholar]
- 10. Cicero DC, Neis AM, Klaunig MJ, Trask CL.. The inventory of psychotic-like anomalous self-experiences (IPASE): development and validation. Psychol Assess. 2017;29(1):13–25. doi: 10.1037/pas0000304 [DOI] [PubMed] [Google Scholar]
- 11. Nelson B, Lavoie S, Gawęda Ł, et al. The neurophenomenology of early psychosis: an integrative empirical study. Conscious Cogn. 2020;77:102845. doi: 10.1016/j.concog.2019.102845 [DOI] [PubMed] [Google Scholar]
- 12. Hernández-García M, Gómez-García M, Sotelo E, et al. Anomalous self-experiences are related to general cognition deficits in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271(4):707–712. doi: 10.1007/s00406-020-01213-z [DOI] [PubMed] [Google Scholar]
- 13. Roig-Herrero A, Planchuelo-Gómez A, Hernández-García M, et al. Default mode network components and its relationship with anomalous self-experiences in schizophrenia: a rs-fMRI exploratory study. Psychiatry Res Neuroimaging. 2022;324:111495. doi: 10.1016/j.pscychresns.2022.111495 [DOI] [PubMed] [Google Scholar]
- 14. Ebisch SJH, Mantini D, Northoff G, et al. Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophr Bull. 2014;40(5):1072–1082. doi: 10.1093/schbul/sbt153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43(6):482–489. doi: 10.1037/h0055479 [DOI] [PubMed] [Google Scholar]
- 16. Hubl D, Schneider RC, Kottlow M, et al. Agency and ownership are independent components of “sensing the self” in the auditory-verbal domain. Brain Topogr. 2014;27:672–682. doi: 10.1007/s10548-014-0351-0 [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Mathalon DH, Roach BJ, et al. Action planning and predictive coding when speaking. Neuroimage 2014;91:91–98. doi: 10.1016/j.neuroimage.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitford TJ. Speaking-induced suppression of the auditory cortex in humans and its relevance to schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(9):791–804. doi: 10.1016/j.bpsc.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 19. Beño-ruiz-de-la-sierra RM, Arjona-valladares A.. Corollary discharge function in healthy controls: evidence about self-speech and external speech processing. Eur J Neurosci 2023;58:3705–3713. doi: 10.1111/ejn.16125 [DOI] [PubMed] [Google Scholar]
- 20. Buzsáki G. Internalization of experience: cognition from action. In: Buzsáki G ed.. The Brain From Inside Out. NY: Oxford University Press; 2012:101–140. [Google Scholar]
- 21. Poletti M, Tortorella A, Raballo A.. Review impaired corollary discharge in psychosis and at-risk states: integrating neurodevelopmental, phenomenological, and clinical perspectives. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(9):832–841. doi: 10.1016/j.bpsc.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 22. Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT.. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51(6):485–492. doi: 10.1016/S0006-3223(01)01335-X [DOI] [PubMed] [Google Scholar]
- 23. Ford J. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164(3):458. [DOI] [PubMed] [Google Scholar]
- 24. Heinks-maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM.. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64(3):286–296. doi: 10.1001/archpsyc.64.3.286 [DOI] [PubMed] [Google Scholar]
- 25. Bühler T, Kindler J, Schneider RC, et al. Disturbances of agency and ownership in schizophrenia: an auditory verbal event related potentials study. Brain Topogr. 2016;29(5):716–727. doi: 10.1007/s10548-016-0495-1 [DOI] [PubMed] [Google Scholar]
- 26. Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT.. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50(7):540–549. doi: 10.1016/S0006-3223(01)01166-0 [DOI] [PubMed] [Google Scholar]
- 27. Ford JM, Mathalon DH.. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 2005;58(2–3):179–189. doi: 10.1016/j.ijpsycho.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 28. Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH.. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology 2007;44(4):522–529. doi: 10.1111/j.1469-8986.2007.00533.x [DOI] [PubMed] [Google Scholar]
- 29. Ford JM, Mathalon DH, Roach BJ, et al. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr Bull. 2013;39(6):1272–1280. doi: 10.1093/schbul/sbs129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kort NS, Ford JM, Roach BJ, et al. Role of N-methyl-d-aspartate receptors in action-based predictive coding deficits in schizophrenia. Biol Psychiatry. 2017;81(6):514–524. doi: 10.1016/j.biopsych.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathalon DH, Roach BJ, Ferri JM, et al. Deficient auditory predictive coding during vocalization in the psychosis risk syndrome and in early illness schizophrenia: the final expanded sample. Psychol Med. 2019;49(11):1897–1904. doi: 10.1017/S0033291718002659 [DOI] [PubMed] [Google Scholar]
- 32. Perez VB, Ford JM, Roach BJ, et al. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophr Bull. 2012;38(6):1216–1224. doi: 10.1093/schbul/sbr124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 34. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Segarra N, Bernardo M, Gutierrez F, et al. Spanish validation of the Brief Assessment in Cognition in Schizophrenia (BACS) in patients with schizophrenia and healthy controls. Eur Psychiatry. 2011;26(2):69–73. doi: 10.1016/j.eurpsy.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 36. Keefe RSE, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102(1–3):108–115. doi: 10.1016/j.schres.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 37. Chelune GJ, Baer RA.. Journal of clinical and experimental neuropsychology developmental norms for the Wisconsin card sorting test. J Clin Exp Neuropsychol. 1986;8(3):219–228. doi: 10.1080/01688638608401314 [DOI] [PubMed] [Google Scholar]
- 38. Durá IF, Peris MR, Dasí C, Carlos J, Ruiz R.. Versión abreviada del WAIS-III para su uso en la evaluación de pacientes con diagnóstico de esquizofrenia. Psicothema. 2010;22:202–207. [PubMed] [Google Scholar]
- 39. Wechsler D. Wechsler Adult Intelligence, and Third Edition Administration and Scoring Manual [Published online]. Vol 217. San Antonio: TX Psychol Corp; 1997. [Google Scholar]
- 40. Ford JM, Roach BJ, Mathalon DH.. Assessing corollary discharge in humans using noninvasive neurophysiological methods. Nat Protoc. 2010;5(6):1160–1168. doi: 10.1038/nprot.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delorme A, Makeig S.. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 42. Delorme A, Sejnowski T, Makeig S.. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford JM, Palzes VA, Roach BJ, Mathalon DH.. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40(4):804–812. doi: 10.1093/schbul/sbt072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathias B, Zamm A, Gianferrara PG, Ross B, Palmer C.. Rhythm complexity modulates behavioral and neural dynamics during auditory–motor synchronization. J Cogn Neurosci. 2020;32(10):1864–1880. doi: 10.1162/jocn_a_01601 [DOI] [PubMed] [Google Scholar]
- 45. Whitford TJ, Oestreich LKL, Ford JM, et al. Deficits in cortical suppression during vocalization are associated with structural abnormalities in the arcuate fasciculus in early illness schizophrenia and clinical high risk for psychosis. Schizophr Bull. 2018;44(6):1312–1322. doi: 10.1093/schbul/sbx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Horváth J, Burgyán A.. No evidence for peripheral mechanism attenuating auditory ERPs to self-induced tones. Psychophysiology 2013;50:563–569. doi: 10.1111/psyp.12041 [DOI] [PubMed] [Google Scholar]
- 47. Sass LA, Parnas J.. Explaining schizophrenia: the relevance of phenomenology. In: Cheung Chung M, Fulford KWM, Graham G, eds. Reconceiving Schizophrenia. NY: Oxford University Press; 2007:63–95. [Google Scholar]
- 48. Crapse TB, Sommer MA.. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9(8):587–600. doi: 10.1038/nrn2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Howes OD, Fusar-Poli P, Bloomfield M, Selvaraj S, McGuire P.. From the prodrome to chronic schizophrenia: the neurobiology underlying psychotic symptoms and cognitive impairments. Curr Pharm Des. 2012;18(4):459–465. doi: 10.2174/138161212799316217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eliades SJ, Wang X.. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature 2008;453(7198):1102–1106. doi: 10.1038/nature06910 [DOI] [PubMed] [Google Scholar]
- 51. Nelson A, Schneider DM, Takatoh J, Sakurai K, Wang F, Mooney R.. A circuit for motor cortical modulation of auditory cortical activity. J Neurosci. 2013;33(36):14342–14353. doi: 10.1523/jneurosci.2275-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schneider DM, Nelson A, Mooney R.. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 2014;513(7517):189–194. doi: 10.1038/nature13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reznik D, Mukamel R.. Motor output, neural states and auditory perception. Neurosci Biobehav Rev. 2019;96:116–126. doi: 10.1016/j.neubiorev.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 54. Chen C-MA, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM.. The corollary discharge in humans is related to synchronous neural oscillations. J Cogn Neurosci. 2011;23(10):2892–2904. doi: 10.1162/jocn.2010.21589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ford JM, Gray M, Faustman WO, Heinks TH, Mathalon DH.. Reduced gamma-band coherence to distorted feedback during speech when what you say is not what you hear. Int J Psychophysiol. 2005;57(2):143–150. doi: 10.1016/j.ijpsycho.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 56. Lewis DA, Hashimoto T, Volk DW.. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648 [DOI] [PubMed] [Google Scholar]
- 57. Bachiller A, Díez A, Suazo V, et al. Decreased spectral entropy modulation in patients with schizophrenia during a P300 task. Eur Arch Psychiatry Clin Neurosci. 2014;264(6):533–543. doi: 10.1007/s00406-014-0488-6 [DOI] [PubMed] [Google Scholar]
- 58. Molina V, Bachiller A, Gomez-Pilar J, et al. Deficit of entropy modulation of the EEG in schizophrenia associated to cognitive performance and symptoms: a replication study. Schizophr Res. 2018;195:334–342. doi: 10.1016/j.schres.2017.08.057 [DOI] [PubMed] [Google Scholar]
- 59. Molina V, Lubeiro A, de Luis Garcia R, et al. Deficits of entropy modulation of the EEG: a biomarker for altered function in schizophrenia and bipolar disorder? J Psychiatry Neurosci. 2020;45(5):322–333. doi: 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gomez-Pilar J, de Luis-García R, Lubeiro A, et al. Deficits of entropy modulation in schizophrenia are predicted by functional connectivity strength in the theta band and structural clustering. NeuroImage Clin. 2018;18:382–389. doi: 10.1016/j.nicl.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Northoff G. Spatiotemporal psychopathology II: how does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. doi: 10.1016/j.jad.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 62. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. doi: 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fernández-Linsenbarth I, Planchuelo-Gómez A, Díez A, et al. Neurobiological underpinnings of cognitive subtypes in psychoses: a cross-diagnostic cluster analysis. Schizophr Res. 2021;229:102–111. doi: 10.1016/j.schres.2020.11.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.