Abstract
The major macromolecules on the surface of the parasitic protozoan Leishmania major appear to be down-regulated during transformation of the parasite from an insect-dwelling promastigote stage to an intracellular amastigote stage that invades mammalian macrophages. In contrast, the major parasite glycolipids, the glycoinositol phospholipids (GIPLs), are shown here to be expressed at near-constant levels in both developmental stages. The structures of the GIPLs from tissue-derived amastigotes have been determined by h.p.l.c. analysis of the deaminated and reduced glycan head groups, and by chemical and enzymic sequencing. The deduced structures appear to form a complete biosynthetic series, ranging from Man alpha 1-4GlcN-phosphatidylinositol (PI) to Gal alpha 1-3Galf beta 1-3Man alpha 1-3Man alpha 1-4GlcN-PI (GIPL-2). A small proportion of GIPL-2 was further extended by addition of a Gal residue in either alpha 1-6 or beta 1-3 linkage. From g.c.-m.s. analysis and mild base treatment, all the GIPLs were shown to contain either alkylacylglycerol or lyso-alkylglycerol lipid moieties, where the alkyl chains were predominantly C18:0, with lower levels of C20:0, C22:0 and C24:0. L. major amastigotes also contained at least two PI-specific phospholipase C-resistant glycolipids which are absent from promastigotes. These neutral glycolipids were resistant to both mild acid and mild base hydrolysis, contained terminal beta-Gal residues and were not lost during extensive purification of amastigotes from host cell membranes. It is likely that these glycolipids are glycosphingolipids acquired from the mammalian host. The GIPL profile of L. major amastigotes is compared with the profiles found in L. major promastigotes and L. donovani amastigotes.
Full text
PDF


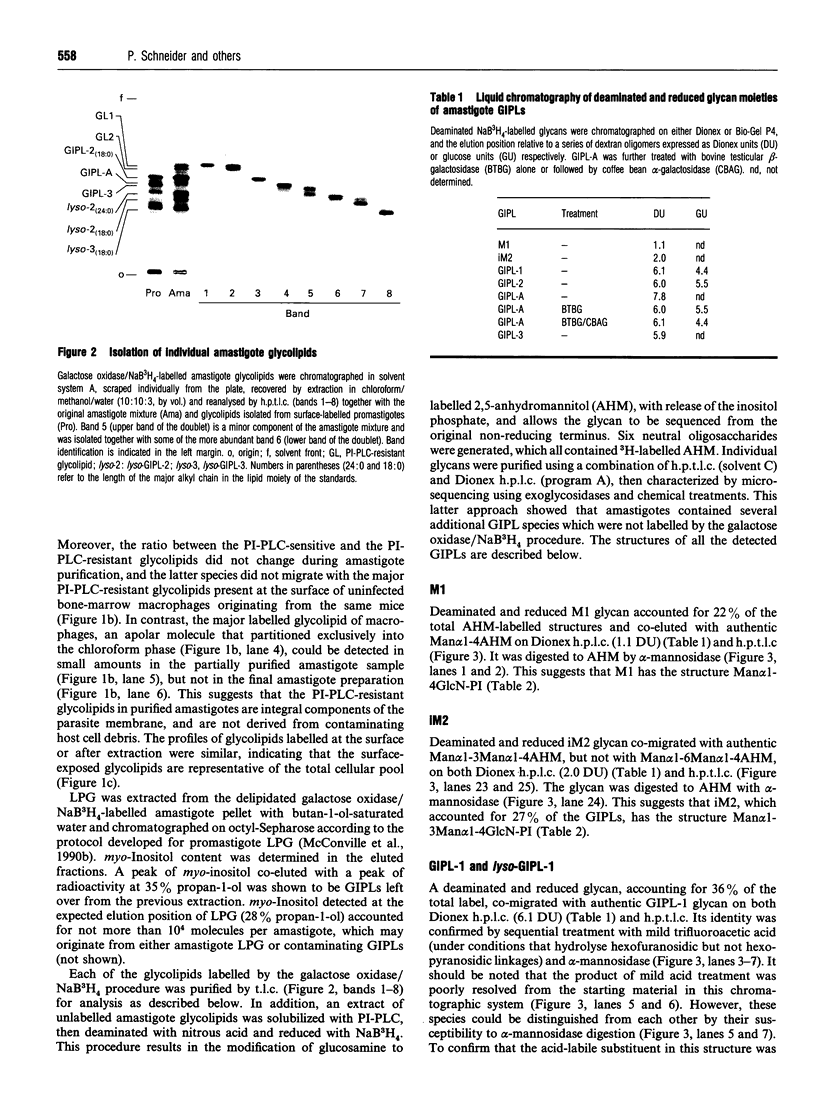






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine J. C., Prina E., Jouanne C., Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990 Mar;58(3):779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Acosta A. Glycoinositol phospholipids from American Leishmania and Trypanosoma spp: partial characterization of the glycan cores and the human humoral immune response to them. J Clin Microbiol. 1991 Oct;29(10):2305–2312. doi: 10.1128/jcm.29.10.2305-2312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr V., Stierhof Y. D., Ilg T., Demar M., Quinten M., Overath P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993 Mar;58(1):107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Chakravarti D., Ibeanu G. C., Tano K., Mitra S. Cloning and expression in Escherichia coli of a human cDNA encoding the DNA repair protein N-methylpurine-DNA glycosylase. J Biol Chem. 1991 Aug 25;266(24):15710–15715. [PubMed] [Google Scholar]
- Descoteaux A., Turco S. J., Sacks D. L., Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991 Apr 15;146(8):2747–2753. [PubMed] [Google Scholar]
- Galili U., Shohet S. B., Kobrin E., Stults C. L., Macher B. A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov 25;263(33):17755–17762. [PubMed] [Google Scholar]
- Glaser T. A., Moody S. F., Handman E., Bacic A., Spithill T. W. An antigenically distinct lipophosphoglycan on amastigotes of Leishmania major. Mol Biochem Parasitol. 1991 Apr;45(2):337–344. doi: 10.1016/0166-6851(91)90102-c. [DOI] [PubMed] [Google Scholar]
- Glaser T. A., Wells S. J., Spithill T. W., Pettitt J. M., Humphris D. C., Mukkada A. J. Leishmania major and L. donovani: a method for rapid purification of amastigotes. Exp Parasitol. 1990 Oct;71(3):343–345. doi: 10.1016/0014-4894(90)90039-f. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990 Jan 1;38(1):57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- McConville M. J. Glycosylated-phosphatidylinositols as virulence factors in Leishmania. Cell Biol Int Rep. 1991 Sep;15(9):779–798. doi: 10.1016/0309-1651(91)90033-f. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990 Nov 15;265(32):19611–19623. [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol. 1990 Apr 1;144(7):2745–2750. [PubMed] [Google Scholar]
- Medina-Acosta E., Karess R. E., Schwartz H., Russell D. G. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989 Dec;37(2):263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- Meerpohl H. G., Lohmann-Matthes M. L., Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol. 1976 Mar;6(3):213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- Pimenta P. F., Saraiva E. M., Sacks D. L. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp Parasitol. 1991 Feb;72(2):191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- Rosen G., Påhlsson P., Londner M. V., Westerman M. E., Nilsson B. Structural analysis of glycosyl-phosphatidylinositol antigens of Leishmania major. J Biol Chem. 1989 Jun 25;264(18):10457–10463. [PubMed] [Google Scholar]
- Schneider P., Bordier C., Etges R. Membrane proteins and enzymes of Leishmania. Subcell Biochem. 1992;18:39–72. doi: 10.1007/978-1-4899-1651-8_2. [DOI] [PubMed] [Google Scholar]
- Schneider P., Ferguson M. A., McConville M. J., Mehlert A., Homans S. W., Bordier C. Structure of the glycosyl-phosphatidylinositol membrane anchor of the Leishmania major promastigote surface protease. J Biol Chem. 1990 Oct 5;265(28):16955–16964. [PubMed] [Google Scholar]
- Schneider P., Ralton J. E., McConville M. J., Ferguson M. A. Analysis of the neutral glycan fractions of glycosyl-phosphatidylinositols by thin-layer chromatography. Anal Biochem. 1993 Apr;210(1):106–112. doi: 10.1006/abio.1993.1158. [DOI] [PubMed] [Google Scholar]
- Schneider P., Rosat J. P., Bouvier J., Louis J., Bordier C. Leishmania major: differential regulation of the surface metalloprotease in amastigote and promastigote stages. Exp Parasitol. 1992 Sep;75(2):196–206. doi: 10.1016/0014-4894(92)90179-e. [DOI] [PubMed] [Google Scholar]
- Smith R., Braun P. E., Ferguson M. A., Low M. G., Sherman W. R. Direct measurement of inositol in bovine myelin basic protein. Biochem J. 1987 Nov 15;248(1):285–288. doi: 10.1042/bj2480285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Sacks D. L. Expression of a stage-specific lipophosphoglycan in Leishmania major amastigotes. Mol Biochem Parasitol. 1991 Mar;45(1):91–99. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]
- de Lederkremer R. M., Casal O. L., Alves M. J., Colli W. Evidence for the presence of D-galactofuranose in the lipopeptidophosphoglycan from Trypanosome cruzi. Modification and tritium labeling. FEBS Lett. 1980 Jul 11;116(1):25–29. doi: 10.1016/0014-5793(80)80521-7. [DOI] [PubMed] [Google Scholar]