Abstract
CD19-targeted chimeric antigen receptor (CAR) T cell therapies have driven a paradigm shift in the treatment of relapsed/refractory B-cell malignancies. However, >50% of CD19-CAR-T-treated patients experience progressive disease mainly due to antigen escape and low persistence. Clinical prognosis is heavily influenced by CAR-T cell function and systemic cytokine toxicities. Furthermore, it remains a challenge to efficiently, cost-effectively, and consistently manufacture clinically relevant numbers of virally engineered CAR-T cells. Using a highly efficient piggyBac transposon-based vector, Quantum pBac™ (qPB), we developed a virus-free cell-engineering system for development and production of multiplex CAR-T therapies. Here, we demonstrate in vitro and in vivo that consistent, robust and functional CD20/CD19 dual-targeted CAR-T stem cell memory (CAR-TSCM) cells can be efficiently produced for clinical application using qPB™. In particular, we showed that qPB™-manufactured CAR-T cells from cancer patients expanded efficiently, rapidly eradicated tumors, and can be safely controlled via an iCasp9 suicide gene-inducing drug. Therefore, the simplicity of manufacturing multiplex CAR-T cells using the qPB™ system has the potential to improve efficacy and broaden the accessibility of CAR-T therapies.
Introduction
Over the last decade, chimeric antigen receptor (CAR)-T therapy has become one of the most promising cancer treatments, but the success achieved by CAR-T therapy in B-cell lymphomas has not been replicated in solid tumors [1–3]. Moreover, the heavy reliance on lentiviral and retroviral vectors for CAR-T cell manufacturing makes the therapy expensive and inaccessible to many patients [4]. Frequent cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are associated with conventional CAR-T therapies, raising important safety concerns [5]. Furthermore, virus-based gene therapies have limited gene payload capacity, making them less suitable for engineering multiplex armored CAR-T cells [6, 7].
Transposon systems, such as Sleeping Beauty and piggyBac, are virus-free alternatives for generating CAR-T cells, but these systems often have limitations such as low gene transfer efficiency and limited expansion of engineered cells [6, 8, 9]. While the manufacture of clinically relevant numbers of high-quality CAR-T cells from senescent and exhausted patient T cells is challenging with virus-based cell-engineering, the challenge is even greater when using many virus-free methods. The poor gene delivery rates and severe cell damage associated with electroporation of virus-free vectors typically lead to low CAR-T yield. One approach to expanding the CAR+ T cell population is by culturing the cells with artificial antigen presenting cells (aAPC), but this method often leads to a more differentiated and short-lived TEFF/exhausted phenotypes [10]. Another approach is to include markers within the CAR-T construct for drug selection or antibody-mediated cell isolation during or after CAR-T ex vivo expansion. However, such approaches increase the payload size and introduce therapeutically irrelevant genes into the genome [7, 11, 12].
To overcome the limitations of transposon systems, we recently developed an advanced version of the piggyBac system known as Quantum pBac™ (qPB). This binary piggy-Bac system comprises a minicircle donor vector and a helper plasmid expressing a molecular-engineered piggyBac transposase, Quantum PBase™ (qPBase) [13, 14]. Two versions of this transposase, qPBase v1 and qPBase v2, were respectively derived from wild-type piggyBac and hyperactive transposase (hyPBase) via fusion with GFP [14]. We further showed that qPBase v2 mediates significantly higher transposition activities compared to conventional hyperactive piggyBac transposase (hyPBase) in CAR-T cell production [13]. Furthermore, our previous work showed that qPB-modified CAR-T cells predominantly exhibit an efficacious TSCM phenotype and can eradicate tumors both in vitro and in vivo. TSCM is a class of immunological memory T cells, and enrichment of this class can improve the success of adoptive T cell therapies [15–19]. However, we also observed donor-dependent variability in the percentage of CAR-TSCM cells among total CAR+ cells and in the performance of qPB-modified CAR-T cells. These drawbacks have potential to severely hamper clinical translation of this technology.
For development of CAR-T cell technologies, B-cell malignancies are among the most actively studied cancers. Since Brentjens et al. first demonstrated the eradication of B-cell tumors by CD19-targeted second-generation CAR-T cells, several commercial CAR-T products have been approved by regulatory agencies [20, 21]. However, treatment with CD19-directed CAR-T therapy currently results in only 30–40% long-term progression-free survival in aggressive Non-Hodgkin lymphoma (NHL) patients, and the median event-free survival of adult B-cell ALL patients is only 6.1 months [22, 23]. Evidence suggests that downregulation of CD19 may be in part responsible for patient relapse [24, 25]. In line with this idea, recent clinical studies have shown that CD19 and CD20 dual-targeting CAR-T therapy can improve patient survival compared to CD19-directed CAR-T therapy [26, 27].
In this report, we detail further advancements in our qPB-based system using a multiplex gene of interest that incorporates iCasp9 suicide gene and CD19/CD20 dual targeting CARs for treatment of B-cell malignancy. We first show that qPB-modified CAR-T manufacturing can be improved with Quantum Booster™ (qBT), a supplement containing nutrients that promote robust CAR-T expansion and enrich the CAR+ TSCM population. We further observed enhanced anti-tumor efficacy of qPB-modified CAR-T cells in both in vitro and in vivo xenotransplant immunodeficient mouse models of B-cell malignancy. The superior quality of qPB-modified CAR-T cells produced with qBT was confirmed in a solid tumor xenograft model. Additionally, we found that clinically relevant numbers of highly potent CAR-TSCM cells could be rapidly generated from cancer patients using G-Rex culture vessels. By shortening the developmental timeline of virus-free multiplex CAR-T therapy, the qPB system represents a technological advancement for CAR-T generation with the potential to facilitate widespread patient access.
Materials and methods
Generation/expansion of CAR-T cells
Human blood samples were collected in January-April 2020 and March-April 2021. Frozen peripheral blood mononuclear cells (PBMCs) from adult healthy donors/patients were obtained from Chang Gung Memorial Hospital (IRB#: 201900578A3) and MacKay Memorial Hospital (IRB#: 20MMHIS330e). PBMCs were extracted using Ficoll-Hypaque gradient separation (Cytiva). Primary human CD3+ T cells were isolated from PBMCs by negative selection (EasySep Human T Cell Isolation Kit, StemCell Technologies) or by positive selection (Dynabeads Human T-Expander CD3/CD28, Thermo Fisher), and activated with Dynabeads (3:1 bead to cell ratio). An iCasp9-CD20/CD19-CAR transposon minicircle carrying CD20 scFv[1] and CD19 scFv[2] and a qPBase plasmid were delivered into activated T cells using 4D-Nucleofector device (Lonza) and Quantum Nufect™ (GenomeFrontier). Irradiated CD19-expressing K562 cells (aAPC) were added to electroporated T cells (1:1) on day 3. Cells were cultured in OpTmizer medium (Thermo) supplemented with Quantum Booster (GenomeFrontier) under fed-batch or perfusion culture condition for 10 days. For lentiviral CAR-T production, primary human CD3+ T cells isolated from cryopreserved human PBMCs (human Pan T Cell Isolation Kit, Miltenyi Biotec, 130-096-535) were activated and expanded with Dynabeads (1:1), and transduced with 1 MOI concentrated virus containing 8 μg/mL Polybrene (Merck, TR-1003-G). CAR-T cells were then cultured in IL-7- and IL-15-supplemented media until harvest/use.
Cell culture conditions
Fed-batch
On day 1, 1 × 105 nucleofected cells were seeded into a well of 24-well G-Rex (Wilson Wolf) containing 3 ml of medium. Fresh medium (2 ml) was added on days 3 and 5, followed by replacement of 2 ml medium on day 7.
Perfusion
On day 1, nucleofected cells were seeded into conventional 24-well plates at a cell concentration of 2.5 × 105 cells/ml. Every 2–3 days, the cell numbers were counted, and fresh medium was added to maintain cell concentration at 2.5 × 105 cells/ml. Then, cells were placed back into culture; depending on the volume, a culture dish of up to 100-mm diameter was used [28, 29]. For both of the culture strategies, cells were harvested on day 10 for use in subsequent experiments.
Flow cytometry
CAR expression was assessed as described previously [13]. Briefly, CAR expression on T cells was detected by flow cytometry following staining of cells at 4°C for 30 min with F(ab’)2 fragment-specific, biotin-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch Laboratories) and R-phycoerythrin (PE)-conjugated streptavidin (Jackson ImmunoResearch Laboratories). Surface molecule expression was determined by staining T cells with fluorochrome-conjugated antibodies. The following antibodies were used: CD3-PE-Cy5, CD4-AlexaFluor532, CD4-AlexaFlour700, CD8-BV605, CD8-Pacific Blue, CD27-PE-Cy7, CD28-APC, CD45RO-PE, CD62L-PE-Dazzle594, CD197-BV510, CD279/PD-1-PE/Cy7, CD366/Tim-3-BV650, CD223/LAG-3-BV711, KLRG-1-BV-421, CD57-PerCP-Cy5.5, CD19-PE, CD56-BV711 (BioLegend), CD45RA-BUV737, and CD95-BUV395 (BD Biosciences). Ghost dye Red780 fixable viability dye (Cytek), 7AAD or propidium iodide (PI) was used to determine cell viability. Mouse blood cell samples were stained with anti-hCD45-APC antibody (BioLegend). Flow cytometry data were acquired on an SA3800 Spectral Analyzer (Sony), a BD FACSCantoTM II (BD), or a BD LSRFortessa analyzer (Sony).
Enzyme-linked immunosorbent assay (ELISA)
For the in vitro assay, supernatant was collected after 24 hours of CAR-T and Raji-GFP/Luc cell (Creative Biogene, CSC-RR0320) co-culture (E:T = 1:1). For the in vivo assay, mouse plasma samples were collected on days 2, 5, 8, and 14 after T cell injection. IFN-γ (Thermo), TNF-α (Thermo), IL-6 (Thermo), and IL-2 (BioLegend) were quantified by ELISA according to the manufacturer’s instructions.
Genomic DNA extraction and quantitative PCR (qPCR)
Genomic DNA from mouse blood was extracted using DNeasy Blood & Tissue Kit (Qiagen). For qPCR analysis, CAR-T cells and Raji-GFP/Luc cells were assessed using the following primer sequences: CAR: fwd-5’-ACGTCGTACTCTTCCCGTCT-3’, rev-5’-GATCACCCTGTACTGCAACCA-3’; Luciferase: fwd-5’-GGACTTGGACACCGGTAAGA-3’, rev-5’-GGTCCACGATGAAGAAGTGC-3’. qPCR assays were performed using a 7500 fast real-time PCR system (Applied Biosystems). Absolute transgene levels were calculated using the standard curve method. The threshold cycle (CT) value of each target was converted to a concentration using the appropriate standard curve. The percentage of cells expressing CAR was calculated according to copy number per cell.
In vitro cytotoxicity assay
Cytotoxicity was assessed using Celigo image cytometry (Nexcelom) as previously described [13]. CAR-T cells were added to Raji-GFP/Luc cells at 5:1, 1:1, and 1:5 E:T ratios.
Anti-tumor efficacy in mouse tumor models
Studies on in vivo mouse xenograft models were conducted as previously described [13] at the Development Center for Biotechnology (Taiwan) using Taiwan Mouse Clinic IACUC (2020-R501-035) approved protocols. Six- to eight-week-old male immunodeficient (NOD.Cg-PrkdcscidIl2rgtm1Wjl/YckNarl) mice (National Laboratory Animal Center, Taiwan) were engrafted by intravenous (i.v.) injection of 1.5 × 105 Raji-GFP/Luc tumor cells or by subcutaneous (s.c.) injection of Matrigel (1:1, BD Bioscience) containing 2 × 106 NCI-N87-Luc cells on the right flank. For the Raji tumor model in Fig 1, mice were infused one week after engraftment with 3 × 106 CAR-T cells or control Pan-T cells (non-transfected T cells). The duration of the experiment was 90 days. Twenty-four mice were used in the experiment; all 24 mice survived the study and were euthanized at the conclusion of the experiment. For the Raji tumor model in Fig 4, mice were infused one week after engraftment with 7.5 × 105 (low dose), 3 × 106 (medium dose), or 1 × 107 (high dose) CAR-T cells. After 35 days, 1.5 × 105 Raji-GFP/Luc tumor cells were injected (i.v.) to half of the mice in each group. The duration of the experiment was 98 days. Twenty-eight mice were used in the experiment; 28 mice were euthanized, and none were found dead. Raji-GFP/Luc tumor cells were quantified using the Xenogen-IVIS Imaging System (Caliper Life Sciences). For the NCI-N87 tumor model in Fig 5, mice were injected i.v. 11 days after engraftment with 4 × 106 control or CAR-T cells. The duration of the experiment was 48 days. Fifteen mice were used in the experiment; 15 mice were euthanized, and none were found dead. Mice were imaged on an Ami-HT optical imaging system (Spectral Instruments Imaging), following intraperitoneal (i.p.) injection of D-Luciferin (BIOSYNTH, L-8220). In all mouse studies, animal health and behavior were monitored daily. A warm pad was provided, and animal feed was replaced with nutritionally fortified water gel when animals were observed to be weak/lethargic. An animal was euthanized if one of the following conditions occurred: 1) the appearance of abnormal movement or paralysis, or 2) body weight loss exceeded 20% of the weight at initial treatment. Animals were euthanized within one day after reaching endpoint criteria.
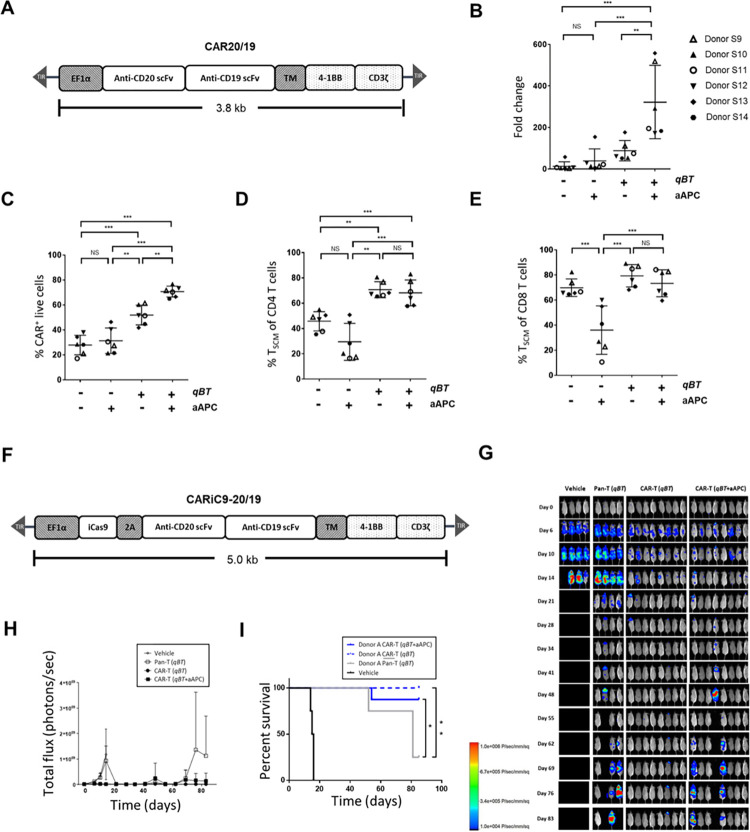
Fig 1. Effect of qBT and aAPC on CAR-T cells.
(A) Schematic diagram of the CAR20/19 CAR construct. (B-E) Human peripheral blood mononuclear cells (PBMC) electroporated with Quantum pBac™-expressing CAR and cultured for 10 days in the presence or absence of aAPC and/or Quantum Booster™ (qBT) were harvested and assessed for (B) cell expansion fold change, (C) percentage CAR+ of live cells (CD3+ PI-, >90%), and percentage of TSCM cell subsets in (D) CD4+ and (E) CD8+ cells. Data shown are from six healthy donors. Horizontal lines represent the mean and s.e.m. fold change (B), mean and s.e.m. percentage of CAR+ cells (C), and mean and s.e.m. percentage of TSCM cell subsets (D and E). * p < 0.05, ** p < 0.01, *** p < 0.001. (F) Schematic diagram of the CARiC9-20/19 CAR construct. A T2A sequence enables co-expression of iCasp9 and CD20/CD19-targeted scFvs under the EF1α promoter. (G-I) In vivo functional characterization of CAR-T cells produced by perfusion culture system. (G) In vivo cytotoxicity of CAR-T cells with or without pre-incubation with aAPC in Raji-GFP/Luc-bearing immunodeficient mice. Fluorescence intensity values (H) and survival curves (I) of mice from (G) plotted against time. Results shown are from 4 to 8 mice/group. T cells were obtained from a representative donor. Vehicle and Pan-T cells (non-engineered T cells) were used as controls. qBT was present in all cell culture conditions. Groups were compared by log-rank (Mantel-Cox) test, *p < 0.05, **p < 0.01.
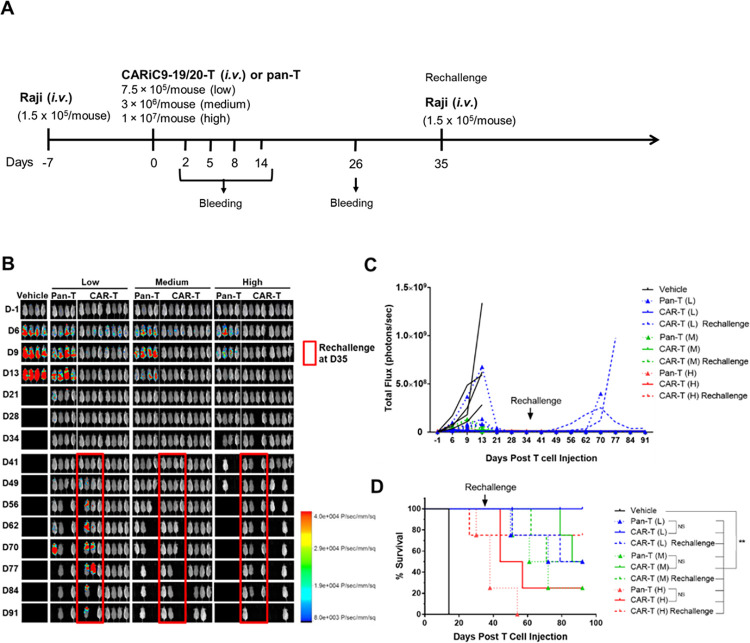
Fig 4. Anti-tumor activity of CARiC9-20/19 CAR-T cells in a B-cell lymphoma immunodeficient xenograft mouse model.
(A) Schematic diagram of in vivo Raji xenograft model experimental design. (B-C) Bioluminescence imaging was performed to monitor tumor cell persistence (n = 4/group) and tumor growth were quantified by total flux (photon/s). (D) Kaplan-Meier survival curves for the different treatment groups are shown. **p < 0.01, *p < 0.05. NS, not statistically significant.
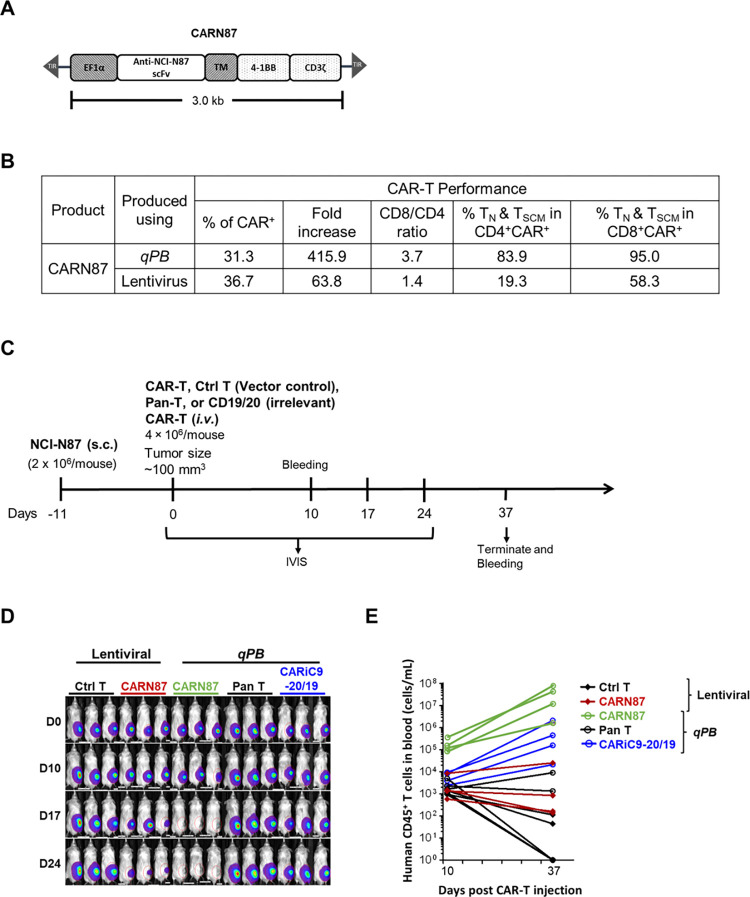
Fig 5. Anti-tumor activity of CARN87 cells in a gastric cancer immunodeficient xenograft mouse model.
(A) Schematic diagram of the CARN87 CAR construct. (B) Performance of CAR-T cells produced using lentiviral and non-viral qPB cell production. (C) Schematic diagram of in vivo NCI-N87 xenograft model experimental design. (D) Bioluminescent imaging was performed to monitor tumor cell persistence (n = 3/group). (E) Human T cell counts of blood samples taken from the indicated group of mice at day 10 and day 37 post-CAR-T injection.
In vitro iCasp9 safety assay
Activation of inducible caspase 9 (iCasp9) was performed in vitro with 2.5, 5, or 10 nM of dimerizer (AP1903), and CAR-T cell elimination was assessed by flow cytometry 24 hours later.
ddPCR copy number assays
Following genomic DNA (gDNA) extraction from CARiC9-20/19 CAR-T cells, minicircle DNA (positive control) was manufactured by Aldevron. Before ddPCR, minicircle DNA was digested for 1 min at 37°C with BsrGI (Thermo). Each reaction for ddPCR Copy Number Variation Assay was set up in 20 μl sample volume containing 10 μl of 2X ddPCR Supermix (no dUTP, BioRad), 1 μl of FAM-labeled target primers/probe (BioRad), 1 μl of HEX-labeled reference primers/probe (BioRad), 0.1 μl of BsrGI, and 50 ng of gDNA or minicircle DNA (volume 2 μl). RPP30 was used as a housekeeping gene. BsrGI completes restriction digests within the ddPCR reaction mixture. Droplets were generated in a QX200 droplet generator (BioRad). After DNA amplification, droplets were analyzed in a QX200 droplet reader (BioRad). Data analysis was performed with QuantaSoft (BioRad).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7. Two-tailed unpaired t-tests were used to compare between two groups. One-way ANOVA was used for comparing three or more groups in a single condition. P ≤ 0.05 was considered statistically significant; significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001. The data generated for this study will be made available by the corresponding author upon reasonable request.
Results
Quantum Booster™ (qBT) enriches the percentage of CAR+ TSCM and enhances anti-tumor efficacy of CAR-T cells
We previously demonstrated that qPB may be used for clinical application in CAR-T therapy [13]. However, even in the presence of artificial antigen presenting cells (aAPC), T cells from some donors still failed to sufficiently expand. To address the difficulty of manufacturing sufficient quantities of high-quality CAR-T cells for clinical application, we developed a serum-free CAR-T cell culture supplement named Quantum Booster™ (qBT), which contains cellular proteins and a set of cytokines at defined concentrations. The components of qBT promote proliferation of the CAR-T cells while maintaining stemness, limiting senescence and preventing exhaustion. We analyzed the effects of qBT, aAPC, and the combination (aAPC+qBT) on characteristics and performance of qPB-produced CAR-T cells engineered with CAR20/19 DNA, a second-generation bicistronic CAR containing binding domains that recognize CD20 and CD19 driven by the EF1α promoter (Fig 1A). The results showed that qBT increased the production and expansion of CAR+ T cells (Fig 1B and 1C). While culture with aAPC alone did not significantly improve production and expansion of CAR+ T cells, aAPC+qBT significantly enhanced both of these parameters. More importantly, qBT-cultured CAR-T cells were characterized by high percentages of CD45RA+CD62L+CD95+ TSCM cells in both the CD4+ and CD8+ populations (Fig 1D and 1E). In contrast, cells cultured with aAPC alone had markedly lower percentages of TSCM cells, suggesting that aAPC may promote T cell maturation. Furthermore, cells cultured with aAPC+qBT did not show reduced percentages of TSCM cells, suggesting that qBT maintains cells in a less differentiated TSCM state (Fig 1D and 1E). These results demonstrated that qBT markedly enhances the expansion capacity of CAR-T cells and preserves CAR-T quality.
We next compared the anti-tumor activities of CAR-T cells cultured with qBT alone and those cultured with qBT in combination with aAPC (qBT+aAPC) using an in vivo xenotransplant Raji tumor model. For this purpose, we engineered CAR-T cells with CARiC9-20/19, a second-generation bicistronic CAR containing binding domains that recognize CD20 and CD19 as well as an iCasp9 suicide gene driven by the EF1α promoter (Fig 1F). Xenografted mice were treated with vehicle, Pan-T, or CAR-T cells cultured with qBT or qBT+aAPC. The results confirmed that CAR-T cells cultured in either qBT alone or qBT in combination with aAPC (aAPC+qBT) both exhibited enhanced anti-tumor efficacy and prolonged survival of treated mice compared to the control (Fig 1G–1I). Notably, there was a slightly greater anti-tumor efficacy seen in qBT cultured CAR-T cells than in qBT+aAPC cultured CAR-T cells. Given that qBT markedly enhanced the expansion and quality of CAR-T cells, all subsequent experiments were conducted using qPB-engineered CAR-T cells cultured with qBT.
Comparison of fed-batch (G-Rex) and perfusion (conventional)-cultured CAR-T cells
Maintaining sterility and controlling mycoplasma and endotoxin contamination in the CAR-T product are of utmost importance for safe manufacturing. G-Rex is a good manufacturing practice (GMP)-compliant cell culture vessel used for cell expansion [28, 29]. The minimal manual operation of this culture vessel reduces contamination risks compared to perfusion-cultured flasks. Using CARiC9-20/19, we assessed qPB-engineered CAR-T cells expanded in G-Rex (fed-batch) and conventional (perfusion) plates (S1A Fig). We observed comparable percentages of CAR+ and TSCM cells with greater cytotoxicity among the G-Rex CAR-T cells (S1B Fig). As G-Rex will be adopted for GMP CAR-T manufacturing, all subsequent experiments were conducted using cells expanded with G-Rex.
Safety assessment of CARiC9-20/19 CAR-T cells
As a means to better manage CAR-T therapy-associated adverse events, the iCasp9 suicide gene was included in the CARiC9-20/19 construct. We demonstrated that AP1903 treatment, which activates iCasp9, eradicated CAR-T cells (S2A Fig). To comply with GMP safety standards for CAR-T manufacturing, we modified CAR-T cells using different amounts of CARiC9-20/19 DNA and determined that the CAR copy number remained low (<5 copies per cell) when 15 μg of total DNA was used for nucleofection (S2B Fig). All subsequent experiments were conducted using 15 μg of DNA per 5 × 106 cells in order to maximize CAR+ T cell production while maintaining safe levels of CAR DNA copy numbers.
CARiC9-20/19 CAR-T cells are dominated by TSCM cells with high proliferative capacity, low exhaustion and senescence, and enhanced cytotoxic function
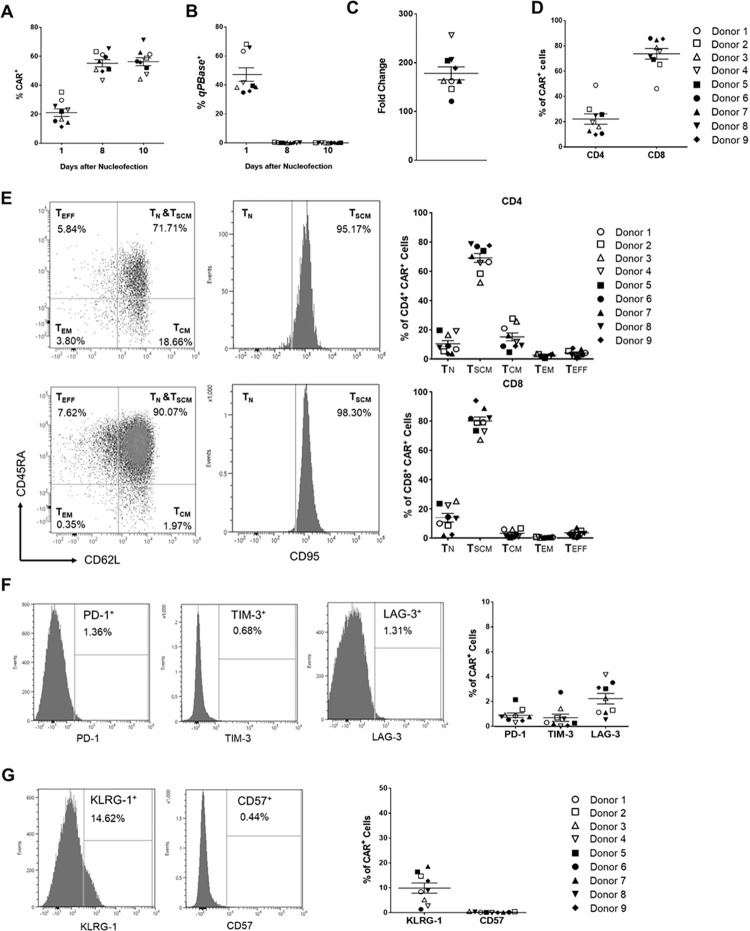
We next examined the performance of healthy donor-derived CAR-T cells. We observed 21.49% (±7.87%, n = 9) CAR+ T cell population on day 1, which subsequently increased and stabilized at 55.7% (±7.33%, n = 9) and 56.75% (±8.20%, n = 9) on days 8 and 10, respectively (Fig 2A). As the long-term presence of transposase (Quantum PBase, or qPBase) in modified T cells may lead to genotoxicity, we also monitored the qPBase levels following nucleofection. While the qPBase levels were high on day 1 (47.31% ± 14.02%, n = 9), insignificant levels (<0.2%) of qPBase+ cells remained on days 8 and 10, suggesting there is minimal concern regarding qPBase-induced integrant remobilization (Fig 2B). We also observed an average of 178.86-fold (±14.02-fold, n = 9) CARiC9-20/19 CAR-T cell expansion (Fig 2C).
Fig 2. Characteristics of CARiC9-20/19 CAR-T cells.
Percentages of (A) CAR+ and (B) transposase+ (qPBase+) cells were analyzed by flow cytometry on days 1, 8 and 10 after nucleofection. (C) Fold change of CARiC9-20/19 CAR-T cells after 10 days of culture in G-Rex are shown. (D) Percentage of CD4 and CD8 T cells in the CAR+ population on day 10 after nucleofection. (E) Distributions of TN, TSCM, TCM, TEM, and TEFF subsets in the CD4 (upper panel) and CD8 populations (lower panel). (F) Expression of exhaustion markers PD-1, TIM-3, and LAG-3 in CARiC9-20/19 CAR-T cells at 10 days post-nucleofection. (G) Expression of senescence markers KLRG-1 and CD57 in CARiC9-20/19 CAR-T cells. (A-G) Data represent mean ± SD for 9 healthy donors, n = 9. Each set of histogram plots shown in (C), (D), (E), and (F) represents one donor.
Among the healthy donors, we consistently observed a higher proportion of CD8+ cells (73.58% ± 12.71%, n = 9) compared to CD4+ cells (21.98% ± 12.31%, n = 9) among the CAR+ population (Fig 2D). To further assess the phenotypic compositions of CARiC9-20/19 CAR-T cells, we determined the percentages of CD45RA+CD62L+CD95- naïve T cell (TN), CD45RA+CD62L+CD95+ stem cell memory T cell (TSCM), CD45RA-CD62L+CD95+ central memory T cell (TCM), CD45RA-CD62L-CD95+ effector memory T cell (TEM), and CD45RA-CD62L-CD95- effector T cell (TEFF) subsets in the CD4+ and CD8+ CAR+ populations. Compared to other T cell subsets, high percentages of CAR-TSCM cells were observed in both the CD4+ (68.94% ± 9.13%, n = 9) and CD8+ (79.84% ± 8.19%, n = 9) populations (Fig 2E). Furthermore, these CAR-T cells displayed low expression of exhaustion markers (PD-1, TIM-3, and LAG-3) and a senescence marker (CD57) at 10 days post-nucleofection (Fig 2F and 2G). Interestingly, a small but significant population (9.92% ± 6.11%, n = 9) expressed the senescence marker KLRG-1.
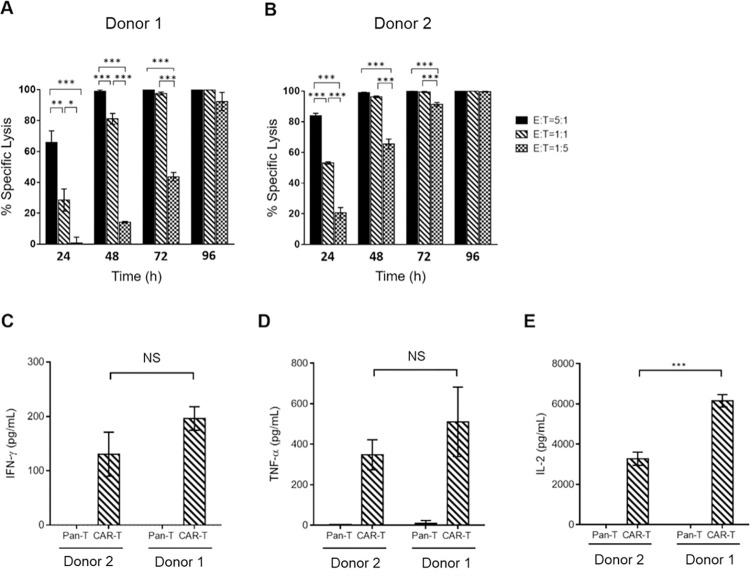
We next assessed the efficacy of CARiC9-20/19 CAR-T cells in Raji-GFP/Luc tumor and CARiC9-20/19 CAR-T cell co-cultures. While CARiC9-20/19 CAR-T cells lysed Raji-GFP/Luc tumors in a dose-dependent manner at early time points (24–48 h), complete tumor lysis was seen at 96 h (Fig 3A and 3B). The CARiC9-20/19 CAR-T cells also lysed NALM-6/Luc tumors with similar kinetics (S3 Fig). Furthermore, CARiC9-20/19 CAR-T cells secreted significant levels of pro-inflammatory cytokines IFN-γ, TNF-α and IL-2, as compared to pan-T cells (Fig 3C and 3E). Collectively, these data suggest that functional CARiC9-20/19 CAR-T cells can be generated using qPB.
Fig 3. In vitro functional analysis of CARiC9-20/19 CAR-T cells.
(A-B) CAR-T cells derived from two healthy donors were assessed for cytotoxicity against Raji-GFP/Luc cells by Celigo image cytometry. Groups were compared by One-way ANOVA with Tukey multiple comparison, ***p < 0.001, **p < 0.01, *p < 0.05. (C) IFN-γ, (D) TNF-α, and (E) IL-2 secretion by CARiC9-20/19 CAR-T cells following antigen stimulation was determined by ELISA. Pan-T cells (non-modified cells) served as a control group. Data represent mean ± SD, n = 3. Groups were compared by Student’s t-test, ***p < 0.001. NS, not statistically significant.
CARiC9-20/19 CAR-T cells display enhanced and persistent anti-tumor function in vivo
We next evaluated the anti-tumor efficacy of G-Rex cultured CARiC9-20/19 CAR-T cells in an in vivo xenotransplant immunodeficient mouse model. We injected 1.5 × 105 Raji-GFP/Luc tumor cells i.v. followed by i.v. injection of 7.5 × 105 (low dose), 3.0 × 106 (medium dose), or 1 × 107 (high dose) CARiC9-20/19 CAR-T cells 7 days later (Fig 4A). As assessed by bioluminescence imaging, all mice in the vehicle group succumbed to tumors by day 21 (Fig 4B and 4C). In contrast, Raji-GFP/Luc tumors were completely eradicated by day 13 in all three treatment groups. Anti-Raji activity was also observed in mice injected with non-transfected pan-T cells that were cultured under the same conditions. This graft-versus-tumor effect was observed beginning approximately two weeks following injection, which suggests it likely resulted from in vivo expansion of cells that recognize and kill the tumor cells via a CAR-independent, non-specific TCR-mediated mechanism. Graft-versus-tumor effect has also been observed by others in similar CAR-T xenograft tumor models [30–32]. While non-specific TCR-mediated killing may occur as a result of host-donor MHC mismatch, we would not expect such an anti-tumor effect in clinical autologous T-cell therapy. Cytokine release syndrome (CRS), associated with high levels of serum cytokines such as IL-6, is an important adverse effect in CAR-T therapy [5, 33]. In our study, the production of IL-6, IFN-γ, IL-2, and TNF-α remained at low or basal levels on days 2, 8 and 14 (S4A Fig). In a clinical study on CD19-41BBz CAR, a similar lack of serum cytokine induction has been reported, suggesting low likelihood of severe CRS [34]. On day 26, no detectable levels of Raji-GFP/Luc tumors were observed in all three treatment groups (S4B Fig). Moreover, residual CAR gene was observed in the blood of the medium and high dose groups (S4C Fig).
While residual CARiC9-20/19 CAR-T cells could be detected up to day 26, it remained unclear whether these residual cells retained anti-tumor function. To address this question, we rechallenged half of the mice in each treatment group with 1.5 × 105 Raji-GFP/Luc cells on day 35. Due to the lack of CAR-T cell persistence, most of the rechallenged mice in the low dose group eventually succumbed to tumors (Fig 4B and 4C). In contrast, the rechallenged mice in the medium and high dose groups remained tumor-free throughout the experiment. Some mice in the medium and high dose groups were euthanized after meeting the criteria for euthanasia. The major symptoms of graft-versus-host disease (GvHD) after bone marrow transplantation are weight loss, ruffling of the fur, and hunched posture [35]. The euthanized mice from the medium and high dose groups likely suffered from GvHD, as severe fur ruffling and weight loss were observed. Of note, GvHD in the context of xenograft mouse models is a known major disadvantage of this experimental strategy [36, 37]. Most importantly, however, CARiC9-20/19 CAR-T treatment significantly improved the overall survival of tumor-bearing mice compared to vehicle control (Fig 4D).
We next directly compared qPB-engineered CAR-T cells with CAR-T cells manufactured using a conventional viral vector. Given the large transgene size used to generate CARiC9-20/19 CAR-T cells (>5 kb), it would be difficult to express the same transgene in a viral vector system. Since this difficulty in transgene introduction would introduce additional variables that can potentially influence the results, we designed a construct that only carries a single CAR gene targeting a pan-cancer glycan antigen, designated as CARN87 (Fig 5A). In an in vivo xenotransplant immunodeficient mouse model, we injected 2 × 106 NCI-N87 tumor cells subcutaneously (s.c.) followed by 4 × 106 lentivirus- or qPB-produced CARN87 CAR-T cells (i.v.) 11 days later. Mice injected with control T cells or pan-T cells served as negative controls. In addition, mice injected with qPB-produced CARiC9-20/19 CAR-T cells (do not target the NCI-N87 tumor cells) served as an additional control. We demonstrated that qBT-cultured qPB-produced CARN87 CAR-T cells were superior in performance compared to lentivirus-engineered counterparts, as indicated by their greater in vitro expansion capacity, higher CD8/CD4 ratio, and enrichment of CAR+ TSCM and CAR+ TN cells (Fig 5B). We further compared the anti-tumor efficacy of CAR-T cells carrying CARN87 delivered using either qPB or a lentivirus approach in a NCI-N87 gastric carcinoma xenotransplant model (Fig 5C). Since solid tumors are more challenging to eradicate, the differences in quality of the CAR-T cells produced using qPB and the lentivirus system in the NCI-N87 tumor model may be easier to differentiate. Complete tumor clearance was observed in qPB-produced CARN87 CAR-T cell-treated mice on day 17 but not in those treated with lentivirus-engineered CAR-T cells (Fig 5D). Furthermore, unlike the lentivirus-engineered counterparts, circulating qPB-produced CAR-T cells proliferated robustly (10- to 100-fold) between days 10 and 37 (Fig 5E). This difference may reflect the higher percentage of TN and TSCM cells present in qPB-produced CAR-T CD4 and CD8 populations (Fig 5B).
Functional CARiC9-20/19 CAR-TSCM cells can be generated in sufficient numbers from cancer patients
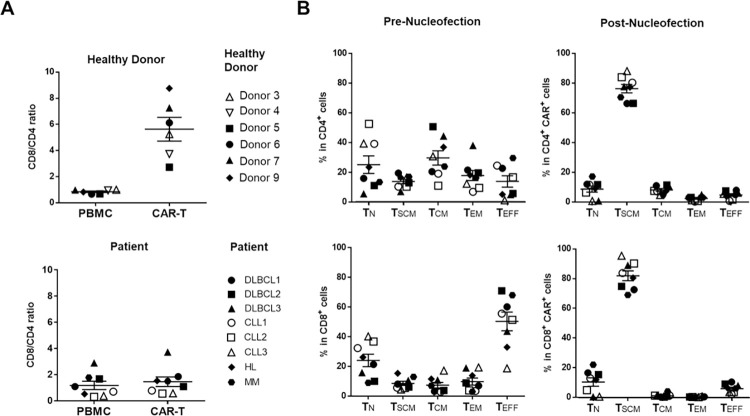
We next assessed whether sufficient numbers of high-quality CARiC9-20/19 CAR-T cells can be generated for clinical applications. In our experiments to this point, we had expanded all CARiC9-20/19 CAR-T cells with CD19-expressing aAPC, unless otherwise indicated. To streamline the manufacturing process, we tested whether we could still generate highly potent patient-derived CARiC9-20/19 CAR-TSCM cells in the absence of aAPC selective expansion. We isolated PBMCs from six healthy donors, three diffuse large B-cell lymphoma (DLBCL) patients, three chronic lymphocytic leukemia (CLL) patients, one Hodgkin lymphoma (HL) patient, and one multiple myeloma (MM) patient to generate CARiC9-20/19 CAR-T cells. Fig 6 and Table 1 show summaries of the characteristics of the donor/patient-derived CAR-T cells.
Fig 6. Assessment of cancer-patient-derived CARiC9-20/19 CAR-T cells.
T cells derived from healthy donors and cancer patients were nucleofected with CARiC9-20/19. (A) CD8/CD4 ratios were assessed before (PBMC) and after (CAR-T) nucleofection. (B) Distribution of TN, TSCM, TCM, TEM, and TEFF subsets in CD4+ (upper panel) and CD8+ (lower panel) CAR-T cell populations among the patient samples in (A). DLBCL, diffuse large B-cell lymphoma; CLL, chronic lymphocytic leukemia; HL, Hodgkin’s lymphoma; MM, multiple myeloma.
Table 1. Comparison of CARiC9-20/19 CAR-T cells generated from healthy donors and cancer patients.
| Disease | Healthy Donor | Patients | DLBCL1 | DLBCL2 | DLBCL3 | CLL1 | CLL2 | CLL3 | HL | MM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Copy Number | 1.85–3.47 | 1.82–5.46 | 2.51 | 5.46 | 2.21 | 3.36 | 2.81 | 3.76 | 2.04 | 1.82 | |
| % of CAR + | 32.5–49.6 | 19.93–53.22 | 26.33 | 50.01 | 34.23 | 40.64 | 33.41 | 53.22 | 26.24 | 19.93 | |
| Fold Expansion | 43.54–157.55 | 57.95–198.96 | 67.22 | 67.30 | 60.38 | 104.57 | 57.95 | 198.96 | 149.75 | 139.35 | |
| Cell Number (x10 6 ) | 17.34–50.60 | 4.58–27.54 | 7.29 | 4.95 | 5.80 | 15.76 | 7.71 | 4.58 | 24.08 | 27.54 | |
| % of B Cells (CD19 + ) | 0.01–0.36 | 0–1.18 | 0.77 | 1.18 | 0.35 | 0 | 0 | 0.61 | 0 | 0 | |
| % of NK Cells (CD3 - , CD56 + ) | 0.04–0.27 | 0.02–2.69 | 0.06 | 2.69 | 0.63 | 0.03 | 0.02 | 0.02 | 0.08 | 0.05 | |
| % of Exhaustion Marker+ Cells | PD-1 | 0.40–1.96 | 0.96–7.80 | 5.35 | 4.15 | 4.16 | 0.96 | 2.42 | 6.01 | 1.70 | 7.80 |
| TIM-3 | 0.10–5.34 | 1.07–2.96 | 1.77 | 2.96 | 1.98 | 1.40 | 2.32 | 2.37 | 1.35 | 1.07 | |
| LAG-3 | 0.90–7.00 | 2.36–22.82 | 9.88 | 12.38 | 22.82 | 3.24 | 4.03 | 2.55 | 2.36 | 2.56 | |
| % of Senescence Marker+ Cells | KLRG-1 | 2.24–14.61 | 2.83–14.70 | 11.80 | 14.70 | 8.20 | 2.83 | 4.15 | 6.06 | 4.05 | 5.33 |
| CD57 | 0.03–0.68 | 0.63–5.82 | 5.82 | 1.46 | 2.54 | 1.11 | 1.78 | 0.63 | 1.13 | 1.28 | |
|
Potency (% of Raji cell lysis)
(fresh cells, E/T = 5, 48 h) |
89.74–95.38 | 98.96–99.88 | 99.66 | 99.7 | 99.7 | 99.88 | 99.45 | 99.07 | 98.96 | 99.42 | |
|
Potency (% of Raji cell lysis)
(frozen cells, E/T = 5, 48 h) |
44.99–61.96 | 45.46–84.31 | 73.35 | 74.21 | 75.21 | 84.31 | 75.70 | 45.46 | 77.49 | 70.31 | |
CAR-T cells generated from PBMCs of 6 healthy donors and 8 cancer patients were cultured in 24-well G-Rex for 10 days. The cell number of 24-well G-Rex culture is 50-times 50 less than a 1 L G-Rex culture (2.29×108–1.38×109 cells in 1 L G-Rex). DLBCL, diffuse large B-cell lymphoma; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; MM, multiple myeloma.
In 10 days, we expanded the CAR-T cells to 4.58 × 106–2.76 × 107 cells in a 24-well G-Rex culture, which is equivalent to a clinically relevant number of 2.29 × 108–1.38 × 109 cells in a 1 L G-Rex culture vessel. The measured values of CAR expression (19.9–53.22%, n = 8) and fold expansion (58.0–198.96-fold, n = 8) for patient cells were comparable to those from healthy donors (32.5–49.6% and 43.54–157.55-fold, n = 6) (Table 1). CAR-T purity was confirmed by the low percentage of CD19+ B cells and CD3-CD56+ NK cells in both healthy donors (0.01–0.36% B cells; 0.04–0.27% NK cells, n = 6) and patients (0–1.18% B cells; 0.02–2.69% NK cells, n = 8) (Table 1). The proportions of CD8+ and CD4+ cells were balanced in both the healthy donors (CD8/CD4 = 0.85 ± 0.15, n = 6) and patients (CD8/CD4 = 1.17 ± 0.9, n = 8) prior to nucleofection (Fig 6A). After nucleofection, donor-derived CAR-T cells had higher CD8/CD4 ratios (5.64 ± 2.23, n = 6). However, only some patients had increase CAR+ CD8 T cell population (Fig 6A). Notably, compared to pre-nucleofection (PBMC) levels, the percentages of TSCM subsets at 10 days post-nucleofection increased in all patients among CD4 (from 13.75% ± 4.25% to 76.25% ± 7.99%, n = 6) and CD8 (from 8.62% ± 3.96% to 81.90% ± 9.35%, n = 6) populations, confirming the ability of our qPB-based CAR-T production system to support TSCM differentiation (Fig 6B, see S5 Fig for gating strategy). Low percentages of CARiC9-20/19 CAR-T cells expressed the exhaustion markers PD-1 and TIM-3 and senescence marker CD57 at 10 days post-nucleofection (Table 1). In addition, the percentage of cells expressing the exhaustion marker LAG-3 at 10 days post-nucleofection was low in CAR-T cultures derived from CLL, HL, and MM patients (2.4–4%, n = 5), but it was slightly elevated in cells derived from DLBCL patients (9.9–22.8%, n = 3). While KLRG-1 expression (7.28% ± 4.83%, n = 6) was detectable in CARiC9-20/19 CAR-T patient cells, it was significantly lower than KLRG-1 expression before nucleofection (21.27% ± 9.37%, n = 6; p = 0.0087) (S6A Fig). The decrease in KLRG-1 expression was especially prominent in the CD8+ population (52.50% ± 14.51% vs. 14.59% ± 10.15%, n = 6; p = 0.0004) (S6B Fig). In a 48-h cytotoxicity assay, we further confirmed that CARiC9-20/19 CAR-T cells derived from both healthy donors and patients could eradicate Raji tumors (Table 1). Overall, these results suggest that qPB-based CAR-T engineering system is a promising technology for generating functional CAR-TSCM cells for cancer immunotherapy applications.
Discussion
One strategy to overcome challenges faced by solid cancers is to engineer T cells to simultaneously express multiple therapeutic genes for enhanced functionality. qPB can produce CAR-T cells with transgene size >7.6 kb in a single cargo, which is not achievable by conventional lentiviral/retroviral CAR-T engineering. The limited cargo capacity of lentiviral/retroviral vectors may account for the lack of current CAR-T clinical trials targeting B-cell malignancies with both dual-targeting CARs and incorporating an iCasp9 safety switch gene (total 5.2 kb transgene size). In this report, we present evidence supporting the feasibility of manufacturing potent iCasp9-regulatable CD20/CD19-targeted CAR-TSCM cells using qPB and culture with qBT for clinical application. We have demonstrated the purity, potency and consistent profiles of CAR-T cells manufactured using the qPB system.
Random gene integration in CAR-T therapy is a major safety concern, as some patients in a recent clinical study developed CAR-T-cell lymphoma following piggyBac-modified CAR19 therapy [38]. A mechanistic study on this topic revealed a high number of piggyBac integrants, but none were inserted into or near typical oncogenes [39]. Nevertheless, this study highlights the importance of keeping a low integrant copy number per genome for gene therapy products. By adjusting the total DNA and the qPB donor to helper DNA ratio, we can further lower the copy number to <5 copies per CAR+ cell without compromising yield quality and quantity (data not shown).
Acute toxicities such as CRS and ICANS have also been major concerns in CAR-T therapy-treated patients [11, 12, 40]. To safely manage toxicities, CAR-T cells can be eradicated by triggering death of CAR-T cells (e.g., anti-EGFRt antibody) via antibody-dependent cellular cytotoxicity (ADCC) or by using a small molecule that activates a suicide gene introduced in the CAR design (e.g., iCasp9) [40]. Triggering ADCC has a slower onset to killing than activation of a suicide gene. We included an iCasp9 safety switch in our CARiC9-20/19 CAR-T design so that we would be able to mitigate adverse effects of CRS and ICANS within hours. Indeed, Foster et al. has recently demonstrated that acute ICANS can be effectively controlled by treating CAR-T therapy patients with the iCasp9-activating agent, rimiducid (AP1903) [41].
In this study, we also addressed challenges associated with manufacture of CAR-T cells by testing the relative contributions of qBT and aAPC. While we used aAPC in our initial experiments, we could generate sufficient quantities of patient-derived CARiC9-20/19 CAR-T cells without aAPC-induced enrichment by culturing cells with qBT alone. The use of qBT highly enriched CD4+ TSCM populations, which may be critical for maintaining CD8+ CAR-T cells at the TSCM differentiation stage. We also confirmed that qPB-engineered CAR-T cells could effectively control tumors without aAPC enrichment. Furthermore, we demonstrated that 2.29 × 108–1.38 × 109 T cells could be generated in a 1-L G-Rex culture within a short time-frame of 10 days using cancer patient-derived T cells. Even at a low CAR+ percentage of 20%, sufficient numbers of CAR+ CAR-T cells (4.58 × 106–2.76 × 107) can still be produced for treatment, and CAR+ T cells could easily be enriched with aAPC should the need arise.
The high potency of CARiC9-20/19 CAR-T cells can be attributed to the balanced CD8/CD4 ratio among CAR-T cells and the high enrichment of TSCM in the engineered cells. It has been reported that a balanced CD8/CD4 CAR-T ratio results in high remission rates [42, 43]. To achieve this balance, others have engineered CD4+ and CD8+ CAR-T cells separately and combined the cultures for adoptive transfer [42]. While qPB-engineered CAR-T cells derived from healthy donors generally exhibited CD8 enrichment, cancer patient-derived CAR-T cells had a balanced CD8/CD4 CAR-T ratio (Fig 6). Thus, qPB naturally achieves the desired CD8/CD4 balance without the need to engineer CD4 and CD8 CAR-T cells separately, as in SB100X-modified CAR-T clinical trials [44]. In a solid tumor NCI-N87 xenograft model, we further demonstrated superior performance, anti-tumor efficacy, and in vivo expansion of qPB-produced CAR-T cells over lentivirus-produced CAR-T cells. Overall, these observations suggest that qPB-produced CAR-T cells are potent.
One approach to harness the potency of less-differentiated T cells such as TSCM is to shorten the ex vivo expansion time of CAR-T product. Ghassemi et al. demonstrated that shortening the lentiviral CAR-T manufacturing process to 24-hours resulted in a higher percentage of CAR-T cells with a memory phenotype [45]. Although these CAR-T cells displayed stronger in vivo anti-tumor activity than conventional counterparts, successful clinical translation of this process remains to be demonstrated. Another approach is to culture CAR-T cells with specific cytokines or chemical reagents [18, 19, 46–48]. Additionally, CAR-T cell production from defined T cell subsets has been shown to be beneficial in enriching the TSCM population [49]. However, none of these approaches can enrich TSCM populations to a level similar to that achieved by our qPB-based system. While TSCM cells make up a small percentage of T cells derived from cancer patients, genetically modifying these cells using qPB followed by CAR-T cell expansion with qBT resulted in enrichment of TSCM populations as high as >80% in both the CD4+ and CD8+ CAR+ T cell populations. Furthermore, we observed low expression of exhaustion (PD-1, TIM-3 and LAG-3) and senescence (CD57) biomarkers in CARiC9-20/19 CAR-T cells at 10 days post-nucleofection. While a significant population of CARiC9-20/19 CAR-T cells expressed the exhaustion marker KLRG-1, this proportion was significantly lower than that seen in freshly isolated PBMCs. Using a conditional knockout mouse model to map the fate of T cells, Herndler-Brandstetter et al. described a class of memory T cell population developed from KLRG-1+ effector CD8 T cells that have lost KLRG-1 expression [15]. These “exKLRG-1 memory cells” have been demonstrated to inhibit tumor growth more efficiently than KLRG-1+ cells in an OT-I melanoma mouse model. TSCM cells generally lack KLRG-1 expression, and KLRG-1+ TSCM cells have been associated with cancer patients undergoing relapse [16, 17]. Given the significant decrease in KLRG-1 expression in CARiC9-20/19 CAR-T cells, it is possible that a significant proportion of CAR-TSCM cells in our model were actually exKLRG-1 memory cells. Thus, one potential mechanism that may contribute to the ability of qPB to generate highly potent CAR-TSCM cells may be the conversion of KLRG-1+ effector CD8+ T cells to exKLRG-1 memory cells.
One major challenge of CAR-T manufacturing is the variation and inconsistency of CAR-T cell products [18]. In one study of ten CLL and ALL patients, CAR-T cells expanded 23.6-385-fold with retroviral CAR transduction of 4–70% [19]. Lentiviral transduction rates of 5.5–45.3% were reported by other studies of CLL and ALL patients [45, 46]. In our CARiC9-20/19 CAR-T study, we observed a tighter range of CAR+ percentages (19.9–53.22%) and expansion (58.0- to 198.96-fold) among the cells derived from eight cancer patients (Table 1). Most importantly, our data showed that CARiC9-20/19 CAR-T cells derived from both healthy donors and patients could effectively eradicate tumor cells.
Allogeneic CAR-T therapy has been expected to be an inevitable replacement of autologous CAR-T products since the first allogenic CAR-T clinical study in 2016 [50]. However, clinical data suggest allogeneic CAR-T products are less potent and have limited CAR-T cell persistence. Induced pluripotent stem cells (iPSC)-derived CAR-T cells may be a promising alternative for allogeneic CAR-T therapy, but the heavy mutational burden in iPSCs also poses safety concerns for clinical applications. Thus, autologous CAR-T therapy will likely remain the mainstay of CAR-T-based treatment in the short to medium term. In this regard, we have demonstrated that our qPB-based CAR-T production system can address many of the current challenges of autologous CAR-T therapy.
In summary, qPB is an efficient system for the manufacturing of safe, potent and cost-effective CAR-T products. We demonstrated that qPB could be used to produce patient-derived CARiC9-20/19 CAR-T cells with many desirable attributes, including a high proportion of CAR+ TSCM, balanced CD8/CD4 ratio, low exhaustion and senescence marker expression, high expansion capacity, and high anti-tumor efficacy. The simplicity and robustness of our strategy to create multiplex CAR-T cells using qPB may help to improve accessibility of CAR-T therapy and also has potential to allow for the generation of armored CAR-T therapies to treat solid tumors in the future.
Supporting information
(A) Characterization of CARiC9-20/19 CAR-T cells cultured in conventional plates (perfusion) or G-Rex (fed batch) at day 10 after nucleofection. (B) Cytolytic activities of CAR-T cells after 48 or 72 h of co-culture with Raji-GFP/Luc target cells (E:T ratio of 5:1) were assessed by Celigo image cytometry. (A-B) Data represent mean ± SD for 6 healthy donors, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001.
(TIF)
(A) Elimination of CARiC9-20/19 CAR-T cells after iCasp9 activation. Percentage of CAR+ T cells was assessed by flow cytometry 24 h after AP1903 treatment. Data represent mean ± SD mean, n = 3 (One-way ANOVA with Tukey multiple comparison). *p < 0.05, **p < 0.01, ***p < 0.001. (B) Average CAR copy numbers were assessed in CARiC9-20/19 CAR-T cell products following electroporation with different amounts of DNA using primers against the scFv CAR region by ddPCR, n = 2 or 3.
(TIF)
CAR-T cells derived from one healthy donor was assessed for cytotoxicity against Raji-GFP/Luc cells or NALM-6-GFP/Luc by Celigo image cytometry. Groups were compared by One-way ANOVA with Tukey multiple comparison, ***p < 0.001, **p < 0.01, *p < 0.05.
(TIF)
(A) Plasma from mice were collected on days 2, 5, 8, and 14 after CAR-T cell administration and analyzed for IFN-γ, TNF-α, IL-2, and IL-6 by ELISA. Copy numbers of (B) luciferase and (C) CAR were determined in mouse blood samples on day 26 after CAR-T cell administration to monitor the presence of CAR+ T cells and Raji-GFP/Luc cells, respectively, in the mouse circulation (n = 8 for CAR-T (L) and n = 7 for CAR-T (M) and CAR-T (H)). Dotted lines represent the detection limits of qPCR.
(TIF)
CD4 or CD8 TSCM phenotyping and quantification strategy for analysis. (A) PBMCs were performed by sequential gating on (1) lymphocytes without debris, (2) PI- or 7AAD- (live) cells, (3) CD3+ cells, (4) either CD4+ or CD8+ cells, (5) CD45RA+ and CD62L+ cells, and (6) CD95+ cells. (B) CAR-T cells were analyzed as in (A), except in step (2) PI- or 7AAD- (live) and CAR+ cells are gated. Shown are representative data plots of patient DLBCL3.
(TIF)
(A) Expression of exhaustion (PD-1, TIM-3, and LAG-3) and senescence (KLRG-1 and CD57) markers in cells from cancer patients in Table 1 before (PBMC) and after (CAR-T) nucleofection. (B) Expression of KLRG-1 within the CD8 population of the cancer patients. * p < 0.05, ** p < 0.01, *** p < 0.001. NS, not statistically significant.
(TIF)
(DOCX)
Acknowledgments
We thank Ms. Jin-hwa Yang for her assistance with IRB preparation and approval process. We thank Dr. Pei-Yi Tsai for assistance with the animal experiments. We are grateful to Marcus J. Calkins, PhD for providing language editing and comments on the manuscript.
List of abbreviations
- aAPC
artificial antigen presenting cells
- CAR
chimeric antigen receptor
- CLL
chronic lymphocytic leukemia
- CRS
cytokine release syndrome
- CT
threshold cycle
- DLBCL
diffuse large B-cell lymphoma
- EFS
event-free survival
- CARiC9-20/19
bicistronic CD20/CD19-targeted iCasp9 regulatable CAR-T cells
- GMP
good manufacturing practice
- GVHD
graft-versus-host disease
- HL
Hodgkin lymphoma
- ICANS
immune effector cell-associated neurotoxicity syndrome
- iCasp9
inducible caspase 9
- i.v.
intravenously
- MM
multiple myeloma
- NHL
Non-Hodgkin lymphoma
- PBMCs
peripheral blood mononuclear cells
- PE
R-phycoerythrin
- PFS
progression-free survival
- qPB
Quantum
- pBac; TCM
central memory T cell
- TEFF
effector T cell
- TN
naïve T cell
- TSCM
T stem cell memory
Data Availability
All relevant data are available from the figshare database (https://doi.org/10.6084/m9.figshare.26202377).
Funding Statement
GenomeFrontier Therapeutics TW Co., Ltd. funded the study and played a crucial role in the study design, data collection and analysis, decision to publish, and preparation of the manuscripts. The coauthors – Peter S. Chang, Yi-Chun Chen, Wei-Kai Hua, Jeff C. Hsu, Jui-Cheng Tsai, Yi-Wun Huang, Yi-Hsin Kao, Pei-Hua Wu, Kuo-Lan Karen Wen, and Sareina Chiung-Yuan Wu – are affiliated with GenomeFrontier Therapeutics TW Co., Ltd.
References
- 1.Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66: 1425–1436. doi: 10.1007/s00262-017-2034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamers CHJ, Sleijfer S, Van Steenbergen S, Van Elzakker P, Van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21: 904–912. doi: 10.1038/mt.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang W-C, et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2015;21: 4062–4072. doi: 10.1158/1078-0432.CCR-15-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson RC, Maus M V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021. doi: 10.1038/s41568-020-00323-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56: 552–566. doi: 10.1038/s41409-020-01134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meir YJJ, Wu SCY. Transposon-based vector systems for gene therapy clinical Trials: Challenges and considerations. Chang Gung Med J. 2011;34: 565–579. [PubMed] [Google Scholar]
- 7.Singh H, Moyes JSE, Huls MH, Cooper LJN. Manufacture of T cells using the Sleeping Beauty system to enforce expression of a CD19-specific chimeric antigen receptor. Cancer Gene Ther. 2015;22: 95–100. doi: 10.1038/cgt.2014.69 [DOI] [PubMed] [Google Scholar]
- 8.Wu SCY, Meir YJJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, et al. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci U S A. 2006;103: 15008–15013. doi: 10.1073/pnas.0606979103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querques I, Mades A, Zuliani C, Miskey C, Alb M, Grueso E, et al. A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nat Biotechnol. 2019;37: 1502–1512. doi: 10.1038/s41587-019-0291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nukala U, Rodriguez Messan M, Yogurtcu ON, Wang X, Yang H. A Systematic Review of the Efforts and Hindrances of Modeling and Simulation of CAR T-cell Therapy. AAPS J. 2021;23: 1–33. doi: 10.1208/s12248-021-00579-9 [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Chang WC, Wong CLW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118: 1255–1263. doi: 10.1182/blood-2011-02-337360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, He F, He W, Huang Y, Zeng J, Zi F, et al. A transgene-encoded truncated human epidermal growth factor receptor for depletion of anti- B-cell maturation antigen CAR-T cells. Cell Immunol. 2021;363: 104342. doi: 10.1016/j.cellimm.2021.104342 [DOI] [PubMed] [Google Scholar]
- 13.Hua W, Hsu JC, Chen Y, Chang PS, Wen KK, Wang P, et al. Quantum pBac: An effective, high‐capacity piggyBac ‐ based gene integration vector system for unlocking gene therapy potential. FASEB J. 2023;37. doi: 10.1096/fj.202201654R [DOI] [PubMed] [Google Scholar]
- 14.Meir YJJ, Lin A, Huang MF, Lin JR, Weirauch MT, Chou HC, et al. A versatile, highly efficient, and potentially safer piggyBac transposon system for mammalian genome manipulations. FASEB J. 2013;27: 4429–4443. doi: 10.1096/fj.12-223586 [DOI] [PubMed] [Google Scholar]
- 15.Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1+ Effector CD8+ T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity. 2018;48: 716–729.e8. doi: 10.1016/j.immuni.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Sun Z, Chen L. Memory T cells: strategies for optimizing tumor immunotherapy. Protein Cell. 2020;11: 549–564. doi: 10.1007/s13238-020-00707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noviello M, Manfredi F, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019;10: 1–15. doi: 10.1038/s41467-019-08871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 2018;53: 164–181. doi: 10.1016/j.copbio.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 19.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118: 4817–4828. doi: 10.1182/blood-2011-04-348540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9: 279–286. doi: 10.1038/nm827 [DOI] [PubMed] [Google Scholar]
- 21.Cronk RJ, Zurko J, Shah NN. Bispecific chimeric antigen receptor T cell therapy for b cell malignancies and multiple myeloma. Cancers (Basel). 2020;12: 1–15. doi: 10.3390/cancers12092523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin A, Shah NN. Chimeric antigen receptor modified T cell therapy in B cell non-Hodgkin lymphomas. Am J Hematol. 2019;94: S18–S23. doi: 10.1002/ajh.25403 [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378: 449–459. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegel JY, Dahiya S, Jain MD, Nastoupil LJ, Ghobadi A, Lin Y, et al. Outcomes in large B-cell lymphoma progressing after axicabtagene ciloleucel (Axi-cel): Results from the U.S. Lymphoma CAR-T Consortium. J Clin Oncol. 2019;37: 7517–7517. doi: 10.1200/JCO.2019.37.15_suppl.7517 [DOI] [Google Scholar]
- 25.Neelapu SS, Rossi JM, Jacobson CA, Locke FL, Miklos DB, Reagan PM, et al. CD19-Loss with Preservation of Other B Cell Lineage Features in Patients with Large B Cell Lymphoma Who Relapsed Post-Axi-Cel. Blood. 2019;134: 203–203. doi: 10.1182/blood-2019-126218 [DOI] [Google Scholar]
- 26.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26: 1569–1575. doi: 10.1038/s41591-020-1081-3 [DOI] [PubMed] [Google Scholar]
- 27.Tong C, Zhang Y, Liu Y, Ji X, Zhang WW, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B cell lymphoma. Blood. 2020;136: 1632–1644. doi: 10.1182/blood.2020005278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotti E, Tettamanti S, Zaninelli S, Cuofano C, Cattaneo I, Rotiroti MC, et al. Optimization of therapeutic T cell expansion in G-Rex device and applicability to large-scale production for clinical use: GMP-compliant polyclonal T cell expansion in G-Rex. Cytotherapy. 2022;24: 334–343. doi: 10.1016/j.jcyt.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Gagliardi C, Khalil M, Foster AE. Streamlined production of genetically modified T cells with activation, transduction and expansion in closed-system G-Rex bioreactors. Cytotherapy. 2019;21: 1246–1257. doi: 10.1016/j.jcyt.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 30.Wang CQ, Choy FC, Sanny A, Murakami T, Tan AHM, Lam KP. An Inhibitory Role for Human CD96 Endodomain in T Cell Anti-Tumor Responses. Cells. 2023;12: 1–14. doi: 10.3390/cells12020309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landgraf KE, Williams SR, Steiger D, Gebhart D, Lok S, Martin DW, et al. convertibleCARs: A chimeric antigen receptor system for flexible control of activity and antigen targeting. Commun Biol. 2020;3: 1–13. doi: 10.1038/s42003-020-1021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng L, Hu P, Wolfe B, Gonsalves C, Ren L, Khawli LA, et al. Lym-1 chimeric antigen receptor T cells exhibit potent anti-tumor effects against B-cell lymphoma. Int J Mol Sci. 2017;18. doi: 10.3390/ijms18122773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giavridis T, Van Der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade letter. Nat Med. 2018;24: 731–738. doi: 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25: 947–953. doi: 10.1038/s41591-019-0421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai HY, Chou TY, Tzeng CH, Lee OKS. Cytokine profiles in various graft-versus-host disease target organs following hematopoietic stem cell transplantation. Cell Transplant. 2012;21: 2033–2045. doi: 10.3727/096368912X653110 [DOI] [PubMed] [Google Scholar]
- 36.Duncan BB, Dunbar CE, Ishii K. Applying a clinical lens to animal models of CAR-T cell therapies. Mol Ther Methods Clin Dev. 2022;27: 17–31. doi: 10.1016/j.omtm.2022.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebrec H, Maier CC, Maki K, Ponce R, Shenton J, Green S. Nonclinical safety assessment of engineered T cell therapies. Regul Toxicol Pharmacol. 2021;127: 105064. doi: 10.1016/j.yrtph.2021.105064 [DOI] [PubMed] [Google Scholar]
- 38.Bishop DC, Clancy LE, Simms R, Burgess J, Mathew G, Moezzi L, et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood. 2021;138: 1504–1509. doi: 10.1182/blood.2021010813 [DOI] [PubMed] [Google Scholar]
- 39.Micklethwaite KP, Gowrishankar K, Gloss BS, Li Z, Street JA, Moezzi L, et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood. 2021;138: 1391–1405. doi: 10.1182/blood.2021010858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17: 147–167. doi: 10.1038/s41571-019-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster MC, Savoldo B, Lau W, Rubinos C, Grover N, Armistead P, et al. Utility of a safety switch to abrogate CD19.CAR T-cell–associated neurotoxicity. Blood. 2021;137: 3306–3309. doi: 10.1182/blood.2021010784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126: 2123–2138. doi: 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30: 492–500. doi: 10.1038/leu.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prommersberger S, Reiser M, Beckmann J, Danhof S, Amberger M, Quade-Lyssy P, et al. CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 2021;28: 560–571. doi: 10.1038/s41434-021-00254-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter DL, Hwang WT, Frey N V., Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4. doi: 10.1080/2162402X.2015.1027469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funk CR, Wang S, Chen KZ, Waller A, Sharma A, Edgar CL, et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139: 523–537. doi: 10.1182/blood.2021011597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan C, Chang J, Song X, Yan F, Yu W, An Y, et al. Memory stem T cells generated by Wnt signaling from blood of human renal clear cell carcinoma patients. Cancer Biol Med. 2019;16: 109–120. doi: 10.20892/j.issn.2095-3941.2018.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joedicke JJ, Großkinsky U, Gerlach K, Künkele A, Höpken UE, Rehm A. Accelerating clinical-scale production of BCMA CAR T cells with defined maturation stages. Mol Ther—Methods Clin Dev. 2022;24: 181–198. doi: 10.1016/j.omtm.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brudno JN, Somerville RPT, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34: 1112–1121. doi: 10.1200/JCO.2015.64.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Characterization of CARiC9-20/19 CAR-T cells cultured in conventional plates (perfusion) or G-Rex (fed batch) at day 10 after nucleofection. (B) Cytolytic activities of CAR-T cells after 48 or 72 h of co-culture with Raji-GFP/Luc target cells (E:T ratio of 5:1) were assessed by Celigo image cytometry. (A-B) Data represent mean ± SD for 6 healthy donors, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001.
(TIF)
(A) Elimination of CARiC9-20/19 CAR-T cells after iCasp9 activation. Percentage of CAR+ T cells was assessed by flow cytometry 24 h after AP1903 treatment. Data represent mean ± SD mean, n = 3 (One-way ANOVA with Tukey multiple comparison). *p < 0.05, **p < 0.01, ***p < 0.001. (B) Average CAR copy numbers were assessed in CARiC9-20/19 CAR-T cell products following electroporation with different amounts of DNA using primers against the scFv CAR region by ddPCR, n = 2 or 3.
(TIF)
CAR-T cells derived from one healthy donor was assessed for cytotoxicity against Raji-GFP/Luc cells or NALM-6-GFP/Luc by Celigo image cytometry. Groups were compared by One-way ANOVA with Tukey multiple comparison, ***p < 0.001, **p < 0.01, *p < 0.05.
(TIF)
(A) Plasma from mice were collected on days 2, 5, 8, and 14 after CAR-T cell administration and analyzed for IFN-γ, TNF-α, IL-2, and IL-6 by ELISA. Copy numbers of (B) luciferase and (C) CAR were determined in mouse blood samples on day 26 after CAR-T cell administration to monitor the presence of CAR+ T cells and Raji-GFP/Luc cells, respectively, in the mouse circulation (n = 8 for CAR-T (L) and n = 7 for CAR-T (M) and CAR-T (H)). Dotted lines represent the detection limits of qPCR.
(TIF)
CD4 or CD8 TSCM phenotyping and quantification strategy for analysis. (A) PBMCs were performed by sequential gating on (1) lymphocytes without debris, (2) PI- or 7AAD- (live) cells, (3) CD3+ cells, (4) either CD4+ or CD8+ cells, (5) CD45RA+ and CD62L+ cells, and (6) CD95+ cells. (B) CAR-T cells were analyzed as in (A), except in step (2) PI- or 7AAD- (live) and CAR+ cells are gated. Shown are representative data plots of patient DLBCL3.
(TIF)
(A) Expression of exhaustion (PD-1, TIM-3, and LAG-3) and senescence (KLRG-1 and CD57) markers in cells from cancer patients in Table 1 before (PBMC) and after (CAR-T) nucleofection. (B) Expression of KLRG-1 within the CD8 population of the cancer patients. * p < 0.05, ** p < 0.01, *** p < 0.001. NS, not statistically significant.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are available from the figshare database (https://doi.org/10.6084/m9.figshare.26202377).