Abstract
Objective
Methotrexate (MTX) is widely administered for the treatment of various cancers. However, MTX induces male reproductive toxicity. In the current study, the effect of ozone therapy (OT) on reducing the toxic effects of MTX in the mouse testicles has been investigated.
Methods
Twenty-four mice were divided into four groups: control, OT (4 mg/kg ozone), MTX (20 mg/kg), and MTX + OT. Testosterone levels, histological changes, and oxidative stress biomarkers were assessed to evaluate the protective effects of OT.
Results
The results demonstrated that MTX disrupted germinal epithelium, reduced serum testosterone levels, and enhanced oxidative stress in testicular tissue. However, treatment with OT attenuated these adverse effects. OT effectively restored the levels of antioxidant enzymes, such as catalase (CAT), glutathione (GSH), and superoxide dismutase (SOD). OT reduced lipid peroxidation, as indicated by decreased malondialdehyde (MDA) levels. OT preserved normal spermatogenesis, improved morphometric parameters, and reduced histological changes by MTX. Moreover, OT effectively restored testosterone levels.
Conclusions
OT protects against MTX-induced testicular damage by suppressing oxidative stress.
Keywords: ozone, methotrexate, chemotherapy, oxidative stress, testis
INTRODUCTION
Chemotherapy is a practical choice to treat cancer (Mathan et al., 2022). Methotrexate (MTX) is a chemotherapeutic agent for managing malignancies, such as acute lymphoblastic leukemia, lymphoma, and breast cancer (Yulug et al., 2013). Many studies indicated that MTX has toxic impacts on seminiferous tubules, impairs spermatogenesis, and induces sperm DNA mutation in mice (Padmanabhan et al., 2009) or rats (Daggulli et al., 2014; Kilinc & Uz, 2021). These events are caused by heightened levels of reactive oxygen species (ROS) induced by MTX (Yulug et al., 2013). Since oxidative stress promotes male infertility, antioxidants can improve fertility (De Luca et al., 2021). Previous studies demonstrated that ozone therapy (OT) can potentially decline pathological complications caused by oxidative stress (Al-Gendy & El-Sharkawy, 2016; Tusat et al., 2017).
The mechanism responsible for the activation of antioxidant cascades by OT is the formation of hydrogen peroxide and ROS, as well as malondialdehyde (MDA). Then this slight oxidative stress induced by OT induces catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) generation (Bocci et al., 1998; Inal et al., 2011; Merhi et al., 2018). OT improves sperm quality and reduces oxidative stress in testicular disorders induced by chemotherapy drugs, testicular torsion, and estrogen-induced testicular toxicity (Merhi et al., 2018; Mills et al., 2015; Tusat et al., 2017). This study explores whether OT effectively protects the mouse testis against MTX-induced testicular tissue toxicity by evaluating oxidative stress.
MATERIAL AND METHODS
Animals and Experimental Design
Twenty-eight NMRI mice (6-8 weeks; 25-30 g) were used in this work. This study was done in the animal house of the Cellular and Molecular Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The mice were kept under the same standard conditions, which included 12 hours light/ 12 hours darkness, 25°C with free access to water and food. This work was done with the approval of the Ethics Committee of Jundishapur University of Medical Sciences, Ahvaz (IR.AJUMS.ABHC. REC.1401-016). The study groups were the following groups (seven animals per group).
- Control group: 0.2 ml normal saline (i.p.) for ten days.
- OT Group: 4mg/kg OT intraperitoneally for ten days.
- MTX Group: Normal saline (i.p.) was administered for ten days, and a single dose of MTX (20 mg/kg) was injected on the seventh day
- MTX + OT Group: OT was administered for ten days, and a single dose of MTX (20 mg/kg) was injected on the seventh day (Yulug et al., 2013).
Ozone has been generated by an oxygen-to-ozone converter (Gardina Co.) that produces a mixture containing approximately 3% ozone/oxygen. Ozone concentration has been determined by ultraviolet light with a wavelength of 254 nm.
On the 11th day, blood samples were collected under deep anesthesia, the left testis was fixed in Buin’s solution for histological examination, and the right was frozen to measure the levels of CAT, GSH, SOD, and MDA.
Testosterone measurement
The blood samples were collected directly from the hearts under deep anesthesia. After clotting, the serum was separated by a centrifuge (200 rpm, 15 min). A mouse ELISA testosterone kit (ARG80662, Taiwan) was used to measure testosterone serum levels.
Histological study
The right testes of mice were fixed, and histological slides were prepared. The slides were stained with Hematoxylin and Eosin (H&E), and structural changes were assessed under a light microscope. The number of seminiferous that had vacuoles was divided by the number of healthy tubes in a field and multiplied by 100. At least 20 fields were examined for each testis.
Johnsen’s testicular biopsy score was used to evaluate the maturation of spermatogenesis. In brief, After counting more than 100 cross-sectioned somniferous tubules per/ animal, they were ranked from 1 to 10, as previously described (Johnsen, 1970).
The seminiferous tubule diameter was determined by measuring the distance between two basal membranes at two opposing poles using the Motic Images software program at 400 x magnification.
Measuring MDA, SOD, CAT, and GSH
To measure the oxidative stress biomarkers, the left testicles of mice were kept in a -80 freezer. The tissues had homogenized, and MDA, SOD, CAT, and GSH were measured using ZellBio GmbH (Germany) kits.
Statistical analyses
The sample size calculated by Power and Sample Size Calculation Software (version 3.1.2) was 28 male mice. The statistical power of the study was 85%. Statistical analyses were conducted using SPSS version 21.0 (SPSS, Chicago, IL, USA). ANOVA and the post-hoc least significant difference (LSD) or Tukey’s tests were performed to multiple comparison analyses to assess differences among groups. A p<0.05 was assumed significant.
RESULTS
Testosterone assay
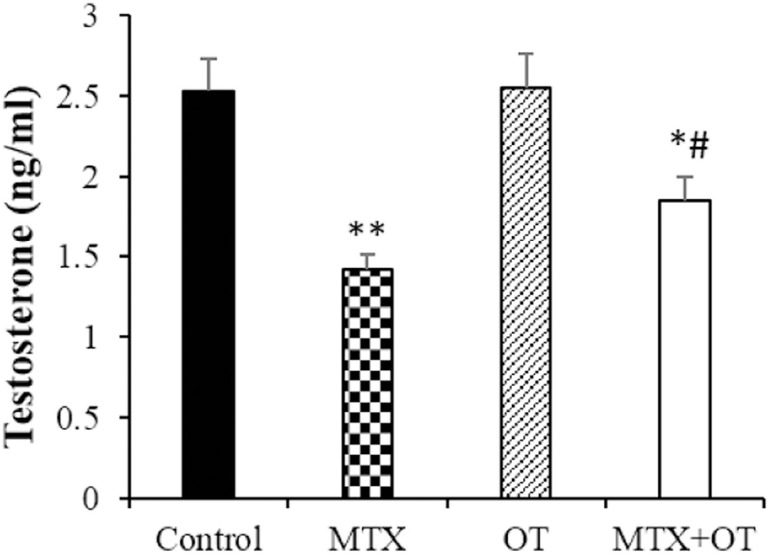
There was no difference in testosterone concentration between the control and OT groups. A significant decrease in this hormone concentration occurred in the MTX group compared to the control (p<0.001). In the MTX+OT group, the testosterone level was increased compared to the MTX-injected mice (p<0.01) (Figure 1).
Figure 1.
Testosterone levels in the different groups (Mean ± SD; n = 7). * & # p<0.05, ** p<0.001. The asterisk and # symbols indicate a comparison to the control and MTX-intoxicated groups, respectively.
Histology
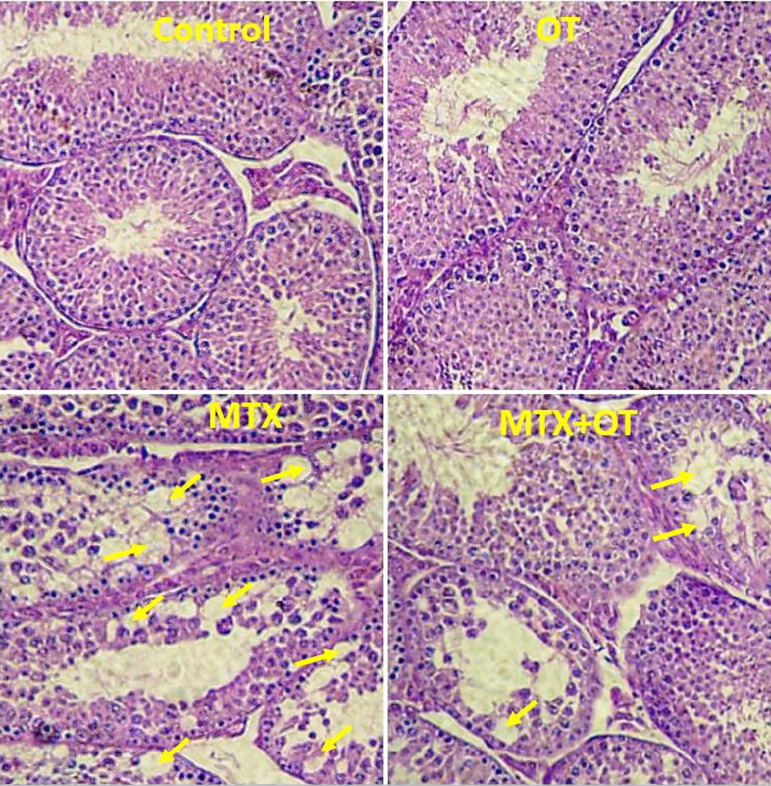
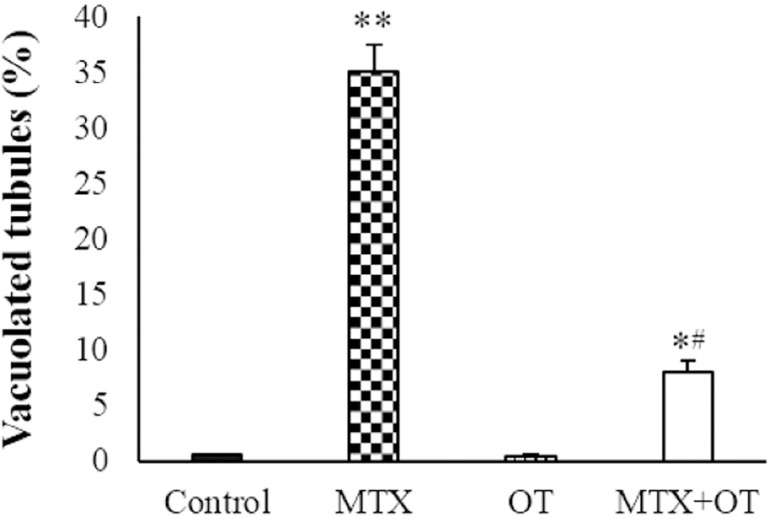
The cross sections of the testicular tissue (Figure 2) showed normal spermatogenesis and intact epithelium of seminiferous tubules in the control and OT groups. In the MTX group, the percentage of vacuolated seminiferous tubules was significantly increased compared to the control (p<0.001). The vacuolization was lesser in the MTX+OT group than in the MTX-treated animals (p<0.01, Figure 3).
Figure 2.
Light microscopy of testicular tissue from the control and experimental groups (Arrows show vacuoles in the germinal epithelium); H&E staining; Magnifications: ×250.
Figure 3.
Testosterone levels in the different groups (Mean ± SD; n = 7). * & # p<0.05, ** p<0.001. The asterisk and # symbols indicate a comparison to the control and MTX-intoxicated groups, respectively.
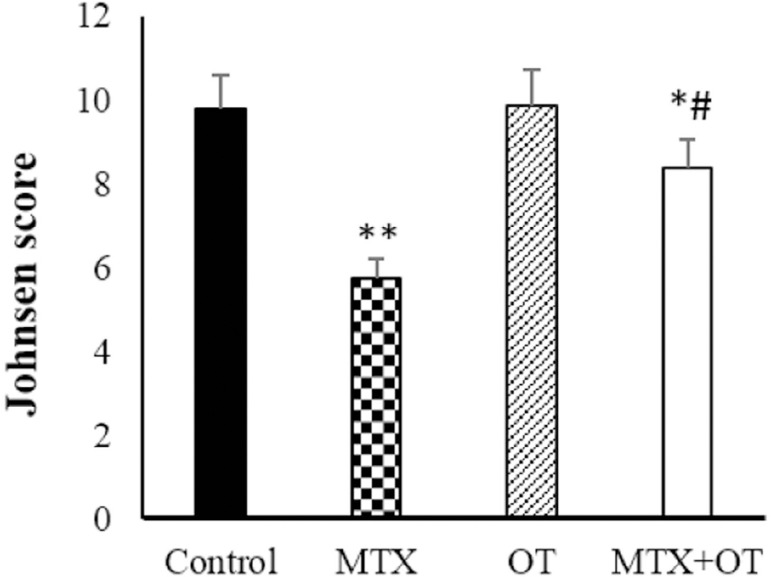
Assessment of spermatogenesis maturation based on the Johnsen score (Figure 4) demonstrated a significant diminish in the MTX group compared to the control (p<0.001). However, Johansen’s scoring in the MTX+OT group increased compared to the MTX alone (p<0.001).
Figure 4.
Johnsen scored assessments in the different groups. Values expressed as Mean ± SD for six mice. * & # p<0.05, ** p<0.001. The asterisk and # symbols indicate a comparison to the control and MTX-intoxicated groups, respectively.
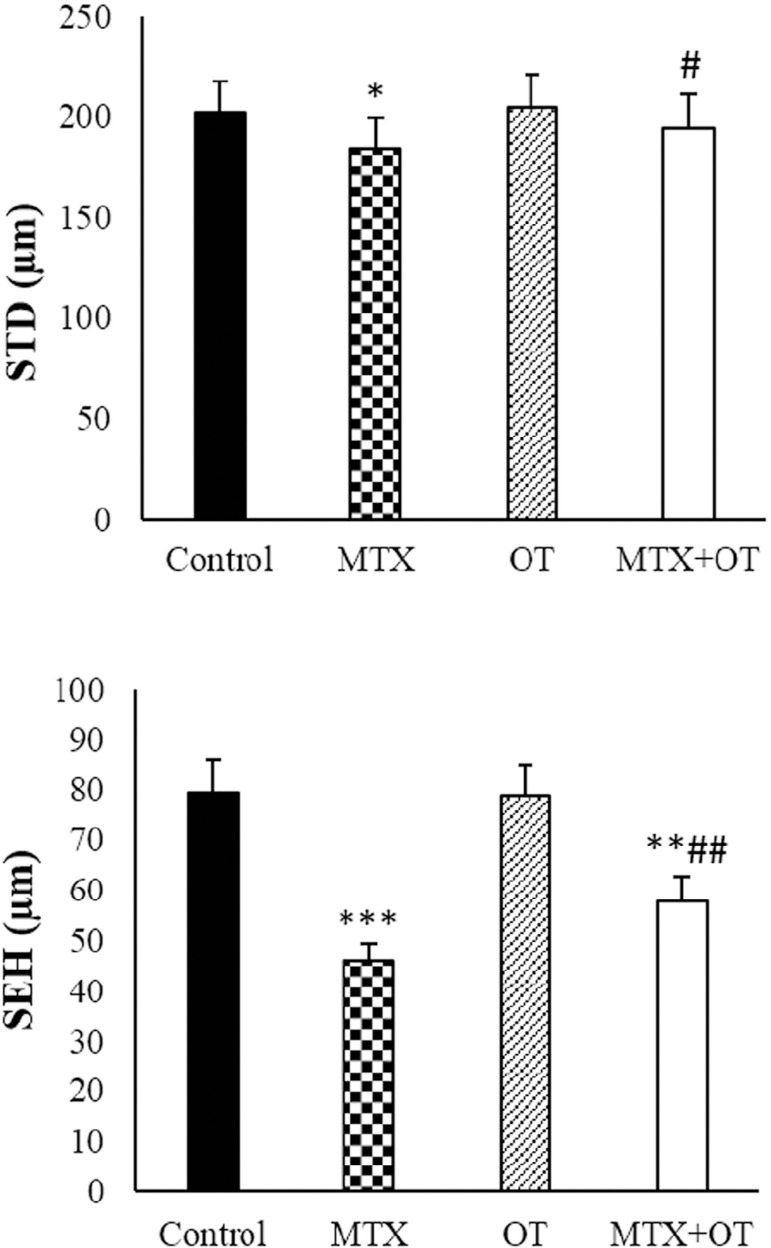
Morphometry parameters
As depicted in (Figure 5), the OT and control groups had similar seminiferous epithelium height and seminiferous tubule diameter. Morphometric parameters significantly diminished in the MTX-injected mics. In the MTX+OT group, morphometric parameters were significantly elevated compared with MTX-injected animals (p<0.01).
Figure 5.
Seminiferous tubule diameter (STD) and seminiferous epithelium height (SEH) in the different groups. Values expressed as Mean ± SD for six mice. *& # p<0.05, **& ## p<0.01, ***p<0.001. The asterisk and # symbols indicate a comparison to the control and MTX-intoxicated groups, respectively.
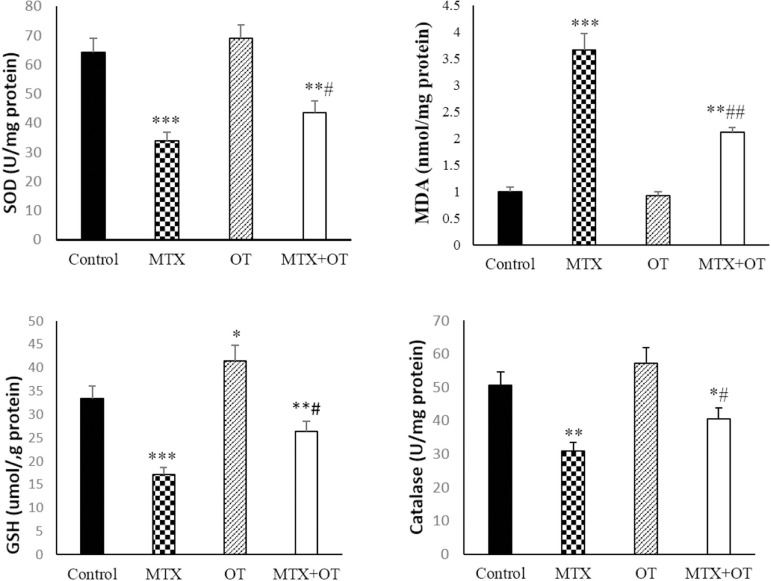
Oxidative stress biomarkers
In the MTX group, the amount of MDA increased compared to the control (p<0.001). MDA level in the MTX+OT group significantly decreased compared to MTX (p<0.001). SOD and CAT levels decreased in the MTX group compared to the control (p<0.001). In the MTX+OT group, the amount of CAT enhanced compared to MTX alone (p<0.05), however, it was not significant for SOD. The amount of GSH was elevated in the OT group compared to the control group (p<0.001). GSH decreased significantly in the MTX group compared to the control (p<0.001). GSH levels were significantly elevated in the MTX+OT group compared to the MTX-treated animals (p<0.01). These results are available in the (Figure 6).
Figure 6.
Oxidative stress biomarkers in different groups (Mean ± SD; n = 7). *&#p<0.05, **&##p<0.01, ***p<0.001. The asterisk and # symbols indicate a comparison to the control and MTX-intoxicated groups, respectively.
DISCUSSION
In this study, MTX disrupted the germinal epithelium, reduced serum testosterone, and induced oxidative stress in the testicular tissue of the mice. Many studies have been implemented about chemotherapy drugs and their destructive effects on body tissues. One of the most widely used chemotherapy drugs is MTX, which causes oxidative stress and induces reproductive toxicity (Daggulli et al., 2014; Yüncü et al., 2015).
The decreased activity of SOD and CAT enzymes caused by the MTX is in line with other studies (Belhan et al., 2019; Maremanda & Jena, 2017; Vardi et al., 2009). SOD is one of the primary antioxidants in spermatogenesis, which protects the testicular tissue against oxidative stress (Fujii et al., 2003; Yaman et al., 2018). Cellular exposure to MTX enhances susceptibility to oxidative stress, attributed to a reduction in the quantity of free NADPH, a crucial cofactor utilized by GSH. Thus, cells become vulnerable to ROS-related damage when the antioxidant defense system is markedly diminished in the MTX exposure (Vardi et al., 2009). OT could partially decrease SOD levels, significantly increase CAT activity and GSH contents in the MTX-intoxicated mouse testicles. These findings indicate that OT activates both enzymatic and non-enzymatic antioxidant systems.
In the current study, MTX could also significantly increase the amount of MDA in the testicular tissue, consistent with previous studies (Pinar et al., 2018; Yalcin et al., 2020). MDA, a product of lipid peroxidation, indicates tissue damage induced by MTX (Belhan et al., 2019; Yaman et al., 2018). MDA levels in the testicular tissue of the MTX+OT treatment have reversed, which indicates that OT can attenuate lipid peroxidation in the mouse testicular tissue. In line with our results, OT reduced lipid peroxidation in the testicular tissue of Busulfan-treated mice (Moghadam et al., 2021).
Ozone may induce moderate oxidative stress due to interaction with intracellular elements. Moderate oxidative stress enhances Nrf2 (nuclear factor-erythroid 2-related factor 2) transcription. Nrf2 activates antioxidant response elements. Activating these elements results in the generation of several antioxidant enzymes, such as SOD, GPx, CAT, and heme-oxygenase-1 (HO-1). Based on these facts, OT may activate Nrf2 via moderate oxidative stress (Sagai & Bocci, 2011).
By increasing the oxidation process, MTX can disrupt the maturation process of sperms, increase the number of immature germ cells, and, as a result, reduce the thickness of the epithelium (Yulug et al., 2013). OT could reverse Johnsen scoring and morphometric parameters, which reflects improving spermatogenesis by the OT in the MTX-treated animals. In another study, ozone alone or in combination with other antioxidants reduced testis damage and preserved spermatid and spermatogonia cells (Salem et al., 2017).
The mice treated with MTX exhibited a reduction in serum testosterone levels. MTX significantly reduces the size of Leydig cells and testosterone production in the testicular tissue (Gutierrez & Hwang, 2017). Leydig cells are very vulnerable to oxidative stress (Awny et al., 2021). Previous studies confirm that oxidative stress induces apoptosis in the Leydig cells (Sun et al., 2017). Hence, the decreased testosterone level by the MTX may result from Leydig cell damage.
Reduced testosterone concentration causes histological changes in the seminiferous tubules (Zeng et al., 2018). Furthermore, a link between declining testosterone levels and inducing apoptosis in germ cells has been established (Rivas et al., 2022). The vacuoles in the germinal epithelium indicate germ cell apoptosis induced by MTX. Previous studies have demonstrated that these vacuoles indicate germ-cell apoptosis (Sönmez et al., 2016; Sultana et al., 2022).
Numerous reports suggest that oxidative stress induces apoptosis, and antioxidants protect against apoptosis in noncancerous cells (Moradi et al., 2023; Sengul et al., 2023; Ijaz et al., 2023). OT could enhance testosterone secretion and decrease vacuolization in the germinal epithelium. Therefore, OT may protect testicular tissue from MTX damage by suppressing germ cell apoptosis. Previous research has demonstrated that OT inhibits apoptosis in response to oxidative stress in diverse tissues, including the kidney, liver, and testis (Mete et al., 2017; Oztosun et al., 2012; Safwat et al., 2014).
In a previous study, OT showed a protective role against ischemia/reperfusion-induced testicular injury by decreasing apoptosis (Aydos et al., 2014).
A limitation of this study was that the relationship between oxidative stress, inflammation, and apoptosis in the experimental groups was not examined. Future studies are needed to evaluate OT impacts on apoptosis and inflammation induced by MTX in the mouse testicles. However, we showed that OT effectively reverses the toxic impacts of MTX on the mouse testicular tissue via enhancing antioxidant activity. Therefore, OT, as an antioxidant, can improve fertility during chemotherapy.
CONCLUSION
This study has shown that OT reduces the destructive effects of MTX on testicular tissue by activating antioxidant production. In addition, the reversed testosterone level by OT may be due to the androgenic function of Ozone through the preservation of Leydig cells in the MTX-treated mice.
ACKNOWLEDGMENT
This work was supported by the Cellular and Molecular Research Center at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
REFERENCES
- Al-Gendy A, El-Sharkawy A. Effect of ozone therapy on spermatogenesis in experimentally gentamycin-induced testicular lesion in adult male albino rat. Al-Azhar Med J. 2016;45:403–416. doi: 10.12816/0029138. [DOI] [Google Scholar]
- Awny MM, Al-Mokaddem AK, Ali BM. Mangiferin mitigates di-(2-ethylhexyl) phthalate-induced testicular injury in rats by modulating oxidative stress-mediated signals, inflammatory cascades, apoptotic pathways, and steroidogenesis. Arch Biochem Biophys. 2021;711:108982–108982. doi: 10.1016/j.abb.2021.108982. [DOI] [PubMed] [Google Scholar]
- Aydos TR, Basar MM, Kul O, Atmaca HT, Uzunalıoğlu T, Kisa Ü, Efe OE. Effects of ozone therapy and taurine on ischemia/reperfusion-induced testicular injury in a rat testicular torsion model. Turk J Med Sci. 2014;44:749–755. doi: 10.3906/sag-1308-20. [DOI] [PubMed] [Google Scholar]
- Belhan S, Çomakli S, Küçükler S, Gülyüz F, Yıldırım S, Yener Z. Effect of chrysin on methotrexate-induced testicular damage in rats. Andrologia. 2019;51:e13145–e13145. doi: 10.1111/and.13145. [DOI] [PubMed] [Google Scholar]
- Bocci V, Valacchi G, Corradeschi F, Fanetti G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediators Inflamm. 1998;7:313–317. doi: 10.1080/09629359890820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggulli M, Dede O, Utangac MM, Bodakci MN, Hatipoglu NK, Penbegul N, Sancaktutar AA, Bozkurt Y, Türkçü G, Yüksel H. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med. 2014;7:5511–5516. [PMC free article] [PubMed] [Google Scholar]
- De Luca MN, Colone M, Gambioli R, Stringaro A, Unfer V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants (Basel) 2021;10:1283–1283. doi: 10.3390/antiox10081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl. 2003;5:231–242. [PubMed] [Google Scholar]
- Gutierrez JC, Hwang K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin Drug Metab Toxicol. 2017;13:51–58. doi: 10.1080/17425255.2017. [DOI] [PubMed] [Google Scholar]
- Ijaz MU, Mustafa S, Ain QU, Hamza A, Ahmed H, Abdel-Daim MM, Albadrani GM, Najda A, Ali S. Eriodictyol attenuates Furan induced testicular toxicity in Rats: Role of oxidative stress, steroidogenic enzymes and apoptosis. Ecotoxicol Environ Saf. 2023;259:115003–115003. doi: 10.1016/j.ecoenv.2023.115003. [DOI] [PubMed] [Google Scholar]
- Inal M, Dokumacioglu A, Özcelik E, Ucar O. The effects of ozone therapy and coenzyme Q10 combination on oxidative stress markers in healthy subjects. Ir J Med Sci. 2011;180:703–707. doi: 10.1007/s11845-011-0675-7. [DOI] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Kilinc L, Uz YH. Protective effects of curcumin against methotrexate-induced testicular damage in rats by suppression of the p38-MAPK and nuclear factor-kappa B pathways. Clin Exp Reprod Med. 2021;48:211–220. doi: 10.5653/cerm.2020.04105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremanda KP, Jena GB. Methotrexate-induced germ cell toxicity and the important role of zinc and SOD1: Investigation of molecular mechanisms. Biochem Biophys Res Commun. 2017;483:596–601. doi: 10.1016/j.bbrc.2016.12.098. [DOI] [PubMed] [Google Scholar]
- Mathan SV, Rajput M, Singh RP. In: Understanding Cancer. Jain B, Pandey S, editors. New York: Academic Press; 2022. Chemotherapy and radiation therapy for cancer; pp. 217–236. [DOI] [Google Scholar]
- Merhi Z, Bazzi A, Moseley-LaRue R, Moseley A, Smith A, Zhang J, Ruggiero M. Ozone therapy: overview of its potential utility in male reproduction. Am J Immunol. 2018;14:15–25. doi: 10.3844/ajisp.2018.15.25. [DOI] [Google Scholar]
- Mete F, Tarhan H, Celik O, Akarken I, Vural K, Ekin RG, Aydemir I, Ilbey YO. Comparison of intraperitoneal and intratesticular ozone therapy for the treatment of testicular ischemia-reperfusion injury in rats. Asian J Androl. 2017;19:43–46. doi: 10.4103/1008-682X.171570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills MR, Arias-Salazar K, Baynes A, Shen LQ, Churchley J, Beresford N, Gayathri C, Gil RR, Kanda R, Jobling S, Collins TJ. Removal of ecotoxicity of 17a-ethinylestradiol using TAML/peroxide water treatment. Sci Rep. 2015;5:10511–10511. doi: 10.1038/srep1051126068117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam MT, Dadfar R, Khorsandi L. The effects of ozone and melatonin on busulfan-induced testicular damage in mice. JBRA Assist Reprod. 2021;25:176–184. doi: 10.5935/1518-0557.20200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Hashemian MA, Douhandeh E, Peysokhan M, Hashemian AH, Faramarzi A. The protective role of melatonin in citalopram-induced reproductive toxicity via modulating nitro-oxidative stress and apoptosis in male mice. Reprod Toxicol. 2023;118:108368–108368. doi: 10.1016/j.reprotox.2023.108368. [DOI] [PubMed] [Google Scholar]
- Oztosun M, Akgul EO, Cakir E, Cayci T, Uysal B, Ogur R, Ozcan A, Ozgurtas T, Guven A, Korkmaz A. The effects of medical ozone therapy on renal ischemia/reperfusion injury. Ren Fail. 2012;34:921–925. doi: 10.3109/0886022X.2012.692752. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Methotrexate-induced cytotoxicity and genotoxicity in germ cells of mice: intervention of folic and folinic acid. Mutat Res. 2009;673:43–52. doi: 10.1016/j.mrgentox.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Pinar N, Çakirca G, Özgür T, Kaplan M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed Pharmacother. 2018;97:1486–1492. doi: 10.1016/j.biopha.2017. [DOI] [PubMed] [Google Scholar]
- Rivas C, Flores M, Pérez J, Gallegos E, Cardenas M, Ayala ME, Aragón A. Acute effects of para-chloroamphetamine on testosterone and markers of apoptosis in seminiferous epithelium of prepubertal male rats. Syst Biol Reprod Med. 2022;68:396–406. doi: 10.1080/19396368.2022.2116369. [DOI] [PubMed] [Google Scholar]
- Safwat MH, El-Sawalhi MM, Mausouf MN, Shaheen AA. Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: effects of pre- and post-ageing administration. Biochemistry. 2014;79:450–458. doi: 10.1134/S0006297914050095. [DOI] [PubMed] [Google Scholar]
- Sagai M, Bocci V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med Gas Res. 2011;1:29–29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem EA, Salem NA, Hellstrom WJ. Therapeutic effect of ozone and rutin on adriamycin-induced testicular toxicity in an experimental rat model. Andrologia. 2017;49:e12603–e12603. doi: 10.1111/and.12603. [DOI] [PubMed] [Google Scholar]
- Sengul E, Gelen V, Yildirim S, Cinar I, Aksu EH. Effects of naringin on oxidative stress, inflammation, some reproductive parameters, and apoptosis in acrylamide-induced testis toxicity in rat. Environ Toxicol. 2023;38:798–808. doi: 10.1002/tox.23728. [DOI] [PubMed] [Google Scholar]
- Sönmez MF, Çilenk KT, Karabulut D, Ünalmış S, Deligönül E, Öztürk İ, Kaymak E. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed Pharmacother. 2016;79:44–51. doi: 10.1016/j.biopha.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Sultana S, Haris M, Naz F, Iftikhar S, Rehman Z. Effect of salicylidene salicylhydrazide on testes of albino mice: A histomorphological study. Prof Med J. 2022;29:101–109. doi: 10.29309/TPMJ/2022.29.01.6396. [DOI] [Google Scholar]
- Sun J, Wang H, Liu B, Shi W, Shi J, Zhang Z, Xing J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed Pharmacother. 2017;88:500–506. doi: 10.1016/j.biopha.2017.01.066. [DOI] [PubMed] [Google Scholar]
- Tusat M, Mentese A, Demir S, Alver A, Imamoglu M. Medical ozone therapy reduces oxidative stress and testicular damage in an experimental model of testicular torsion in rats. Int Braz J Urol. 2017;43:1160–1166. doi: 10.1590/s1677-5538.ibju.2016.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil Steril. 2009;92:2028–2033. doi: 10.1016/j.fertnstert.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Yalcin A, Aydin H, Turk A, Dogukan M, Eser N, Onderci M, Uckardes F, Yoldas A, Yilmaz E, Keles10 H. Vitamin D: an effective way to combat methotrexate-induced testis injury. Med Sci. 2020;9:998–1003. doi: 10.5455/med-science.2020.10.222. [DOI] [Google Scholar]
- Yaman T, Uyar A, Kaya MS, Keles ÖF, Uslu BA, Yener Z. Protective effects of silymarin on methotrexate-induced damages in rat testes. Braz J Pharm Sci. 2018;54:e17529–e17529. doi: 10.1590/s2175-97902018000117529. [DOI] [Google Scholar]
- Yulug E, Türedi S, Alver A, Türedi S, Kahraman C. Effects of resveratrol on methotrexate-induced testicular damage in rats. ScientificWorldJournal. 2013;2013:489659–489659. doi: 10.1155/2013/489659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüncü M, Bükücü N, Bayat N, Sencar L, Tarakçioğlu M. The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. Turk J Med Sci. 2015;45:517–525. doi: 10.3906/sag-1409-39. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Yi H, Huang L, An Q, Wang H. Reduced testosterone and Ddx3y expression caused by long-term exposure to arsenic and its effect on spermatogenesis in mice. Environ Toxicol Pharmacol. 2018;63:84–91. doi: 10.1016/j.etap.2018.08.012. [DOI] [PubMed] [Google Scholar]