Abstract
Background:
Although most patients with cancer are treated with local therapy (LT), the proportion of late–phase clinical trials investigating local therapeutic interventions is unknown. The purpose of this study was to determine the proportion, characteristics, and trends of phase 3 cancer clinical trials assessing the therapeutic value of LT over time.
Methods:
This was a cross–sectional analysis of interventional randomized controlled trials in oncology published from 2002 through 2020 and registered on ClinicalTrials.gov. Trends and characteristics of LT trials were compared to all other trials.
Results:
Of 1877 trials screened, 794 trials enrolling 584,347 patients met inclusion criteria. A total of 27 trials (3%) included a primary randomization assessing LT compared with 767 trials (97%) investigating systemic therapy or supportive care. Annual increase in the number of LT trials (slope [m] = 0.28; 95% confidence interval [CI], 0.15–0.39; p < .001) was outpaced by the increase of trials testing systemic therapy or supportive care (m = 7.57; 95% CI, 6.03–9.11; p < .001). LT trials were more often sponsored by cooperative groups (22 of 27 [81%] vs. 211 of 767 [28%]; p < .001) and less often sponsored by industry (5 of 27 [19%] vs. 609 of 767 [79%]; p < .001). LT trials were more likely to use overall survival as primary end point compared to other trials (13 of 27 [48%] vs. 199 of 767 [26%]; p = .01).
Conclusions:
In contemporary late–phase oncology research, LT trials are increasingly under–represented, under–funded, and evaluate more challenging end points compared to other modalities. These findings strongly argue for greater resource allocation and funding mechanisms for LT clinical trials.
Keywords: medical oncology, palliative care, phase 3 clinical trials, radiation oncology, randomized controlled trial, surgical oncology, trial design
Plain language summary
• Most people who have cancer receive treatments directed at the site of their cancer, such as surgery or radiation.
• We do not know, however, how many trials test surgery or radiation compared to drug treatments (that go all over the body).
• We reviewed trials testing the most researched strategies (phase 3) completed between 2002 and 2020.
• Only 27 trials tested local treatments like surgery or radiation compared to 767 trials testing other treatments.
• Our study has important implications for funding research and understanding cancer research priorities.
INTRODUCTION
Cancer care often involves a combination of different therapeutic modalities, including local therapies such as surgery or radiotherapy. Local therapies are designed to treat cancer by removing or eliminating cancer cells in a specific location within the body, rather than targeting cancer cells throughout the body.1–5
Despite the widespread use of local therapy (LT) in cancer treatment, concerns have been raised about shifting paradigms in LT in the absence of prospective trials to support such changes.6–10 Phase 3 trials are typically designed to evaluate the effectiveness and safety of a new treatment compared to the standard of care in a large group of patients, and advances and changes in LT should be tested to the greatest extent reasonably feasible.11 Therefore, the characteristics of phase 3 trials hold significant influence on clinical practice. Numerous studies have examined trends of late–phase research such as funding, leadership, impact factor, and outcomes reporting, among other topics.7,12–21 However, although the majority of patients with cancer are treated with LT at some point during the cancer care continuum, the proportion of late–phase clinical trials investigating LT remains unknown.22,23
Understanding the representation of LT in late–phase clinical trials and factors associated with LT trials may inform future resource allocation, research priorities, and strategic initiatives in the cancer research community. The purpose of this study was to determine the representation and trends of oncology trials assessing LT in contemporary (2002–2020) published phase 3 research and to explore factors associated with design, funding, and outcomes for trials of LT compared to systemic therapy and supportive care.
MATERIALS AND METHODS
This study was a cross–sectional analysis of interventional phase 3 randomized controlled trials (RCTs) in oncology registered on ClinicalTrials.gov, a database of clinical trials conducted in the United States and around the world, and published from 2002 through 2020.24,25 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.26 Institutional review board approval was waived because of the public availability of trial–level data. To identify eligible RCTs, the following advanced query was used: terms “cancer,” phase “Phase 3”, study results “With Results,” and status “excluded: Not yet recruiting.” Nonrandomized trials, single–arm studies, phase 1 or 2 studies, cancer prevention trials, unpublished trials, or trials published in abstract form only were excluded. International studies registered on ClinicalTrials.gov were eligible.
The primary outcome of this study was to compare the proportion of trials investigating locally directed therapies as a primary randomization arm in relation to other oncology trials such as those investigating systemic therapy or supportive care as the primary arms. Secondary outcome measures included differences in design (such as the primary end point), funding source, and outcome characteristics of trials between treatment modalities. The treatment modality was classified as systemic therapy (such as cytotoxic chemotherapy, immunotherapy, targeted therapy, hormone therapy, cellular therapy, radiopharmaceutical therapy, or similar), radiotherapy, surgery, other interventional local treatments (e.g., radiofrequency ablation, radioembolization), or supportive care (i.e., interventions without direct antitumor effects aimed at improving sequelae of cancer or treatment, such as opioids, anti–emetics, or acupuncture). Other strategies, such as those infusing chemotherapy directly into tumor or hyperthermic intraperitoneal chemotherapy, were not tested in the examined trials. LT was defined as radiotherapy, surgery, or ablation. RCTs with a primary research question assessing concurrent systemic therapy or supportive care strategies with LT (e.g., androgen deprivation therapy and radiotherapy in patients with prostate cancer or memantine in whole–brain radiotherapy in patients with brain metastases) were defined as systemic therapy or supportive care trials and additionally in a separate analysis as “concurrent–LT” trials.27,28 Trials studying preoperative or postoperative systemic therapy (e.g., comparing two different neoadjuvant chemotherapy regimens followed by resection for pancreas cancer or evaluating adjuvant endocrine therapies following resection in breast cancer) were not considered concurrent-LT trials. Trial features were obtained from the registry, protocol, and/or primary publication.12 The impact factor of the publication journal was recorded based on its value at the time of data abstraction as previously defined.13 Funding sources were defined as cooperative group trials and/or industry sponsored trials and were considered independent variables as certain trials were both industry–funded and performed through a multi–institutional cooperative group. The primary end point was defined as met if the pre–specified protocol primary end point was statistically achieved in the manuscript, regardless of author conclusions or subsequent impact on practice. Surrogate end points were defined as end points indirectly representative of overall survival (OS) or quality of life (e.g., progression–free survival, overall response rate, or pathologic complete response).29,30
Descriptive statistics were used to summarize the results. Normality was assessed using the Shapiro–Wilk test. Trends in RCT primary treatment modality utilization over time were characterized by ordinary least squares linear regression, with the slope (m) of the regression defining change over time. Design and funding characteristics of LT trials were compared with systemic therapy and supportive care trials using Pearson’s χ2 test or the Mann–Whitney U test depending on whether categorical or continuous variables were being tested, respectively. Categorical trial outcomes were compared using binary logistic regression and odds ratios (OR) were calculated. Analyses were considered exploratory and therefore correction for multiple comparisons was not performed. To reduce bias, subset analysis was performed excluding trials of supportive care and a sensitivity analysis was performed by repeating the analysis among nonmetastatic solid tumor trials. All testing was two–sided with an α of 0.05 and confidence intervals (CIs) at 95%. Analyses were conducted with SAS v9 (Cary, North Carolina) and plotted in GraphPad Prism v9 (La Jolla, California).
RESULTS
A total of 1877 phase 3 RCTs were screened for eligibility; 841 were excluded because they were: not specific for cancer (n = 587), single-arm (n = 103), phase 1 or 2 (n = 41), nonrandomized (n = 49), preventative (n = 31), or noninterventional (n = 30). An additional 242 trials were excluded because the primary end point was not published in a manuscript (Figure S1). A total of 794 trials enrolling a combined total of 584,347 patients were included in the analysis. The median year of publication was 2014 (interquartile range [IQR], 2012–2017), the median year of enrollment completion was 2010 (IQR, 2008–2012), and the median year of enrollment opening was 2009 (IQR, 2006–2014).
Of the 794 published phase 3 trials, 27 (3%) investigated LT, whereas 767 (97%) tested other treatments, including 631 systemic anticancer therapy trials (79%) and 136 supportive care trials (17%) (Figure 1). Local therapies tested in RCTs included radiotherapy (18 [2%]), surgical resection (8 [1%]), and radioembolization (1 [1%]). The number of RCTs increased over time, but the increase in the number of RCTs testing systemic therapy or supportive care (m = 7.57; 95% CI, 6.03–9.11; p < .001) was considerably greater than the increase observed in LT trials (m = 0.28; 95% CI, 0.15–0.39; p < .001) (Figure 2A–C). Excluding supportive care trials, the increase in systemic therapy trials (m = 6.29; 95% CI, 5.18–7.41; p < .001) still significantly outpaced LT trials (Figure 2D). The relative frequency of LT trials did not significantly increase over time (m = 0.20; 95% CI, – 0.10 to 0.50; p = .17), nor did the relative frequency of any specific treatment modality (Figure 2E–G).
FIGURE 1.
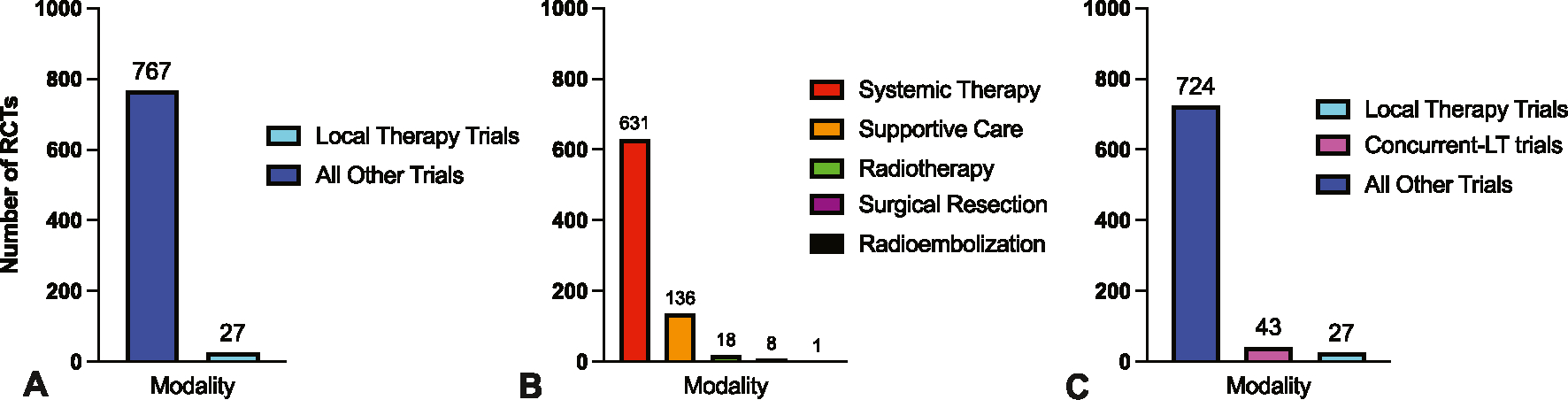
The primary modality tested in modern phase 3 randomized controlled oncology trials. (A) The proportion of randomized control trials (RCTs) studying local therapy (LT) or all other modalities. (B) The proportion of RCTs by study modality. (C) The proportion of RCTs testing LT, concurrent–LT strategies, and all other modalities.
FIGURE 2.
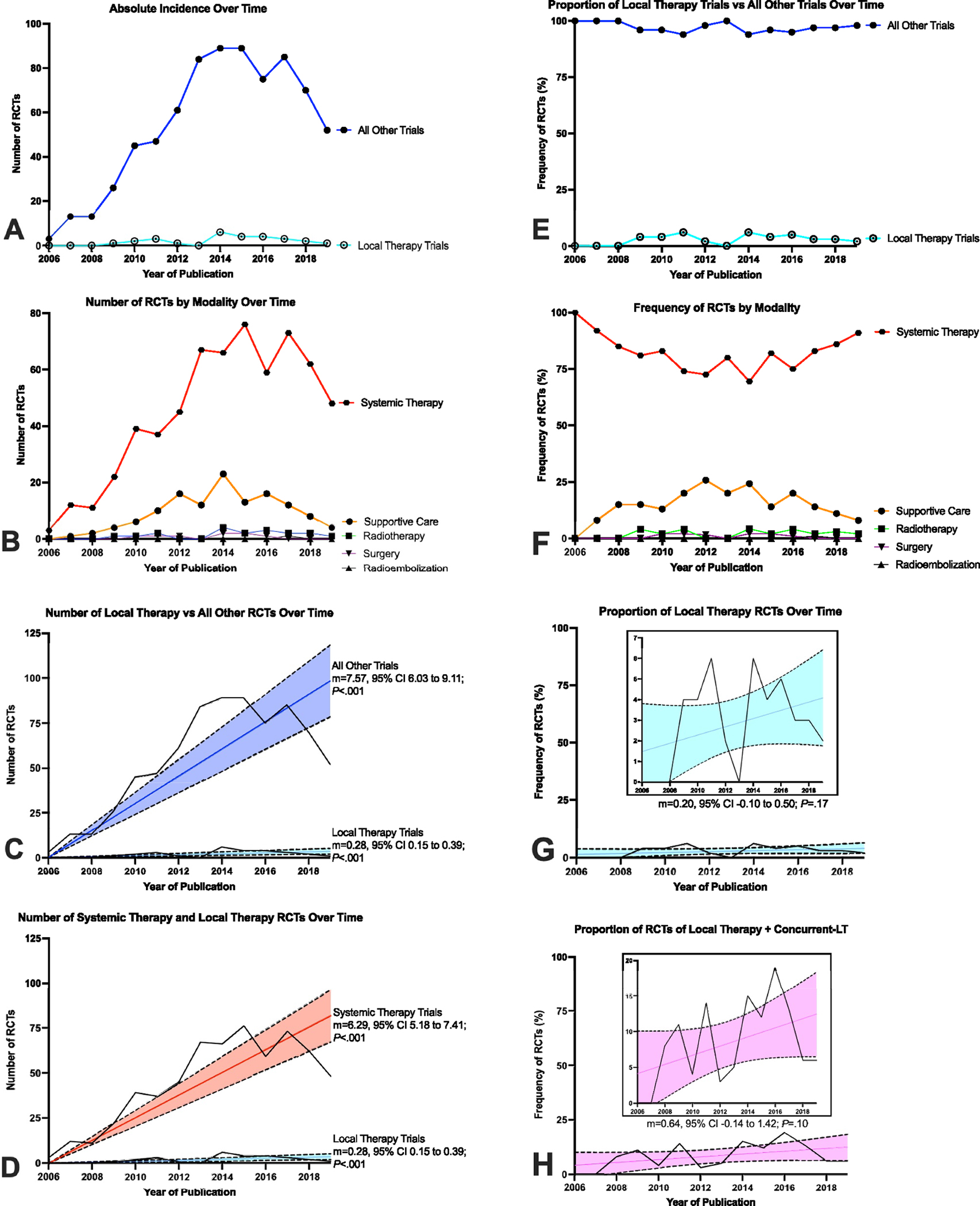
Trends of modality use in modern oncology phase 3 randomized controlled trials. (A) Number of local therapy (LT) randomized control trials (RCTs) over time versus all other RCTs. (B) Number of RCTs by study modality over time. (C) Growth of RCTs testing LT versus all other strategies. (D) Growth of RCTs testing systemic therapy versus RCTs testing LT. (E) Proportion of LT RCTs compared to all other RCTs over time. (F) Proportion of RCTs by study modality over time. (G) Constant proportion of LT RCTs over time. (H) Constant proportion of RCTs testing either LT or concurrent–LT strategies. In (C), (D), (G), and (H), the colored lines represent the linear regression over time and shaded regions represent the 95% confidence interval.
Of 767 RCTs studying systemic therapy or supportive care, 43 (6%) were defined as concurrent–LT trials. Radiotherapy was the LT used in 42 concurrent–LT trials (98%). Concurrent–LT trials sought to improve efficacy of LT through the addition of radiosensitizing systemic therapy to radiotherapy (31 [72%]) or to ameliorate toxicity (12 [28%]). The annual incidence of concurrent–LT trials grew over time (m = 0.45; 95% CI, 0.27–0.63; p < .001) at a rate similar to that of RCTs testing LT. When combining trials of LT with concurrent–LT trials, a total of 70 RCTs (9%) were published, but there was no change in their relative frequency among all cancer trials over time (m = 0.64; 95% CI, −0.14 to 1.42; p = .10) (Figure 2H). Most RCTs of LT focused on central nervous system, pediatric, and gynecologic cancers (Figure S2). Sarcoma, skin, and hematologic cancers were not investigated in phase 3 trials of LT.
Table 1 compares trial–related factors, including end point selection, sponsorship, and outcomes, between LT trials and all other trials. There were no significant differences in the number of enrolled patients (median 412 vs. 500; p = .38), impact factor of the publication journal (median 26 vs. 26; p = .49), odds of completing target accrual (19 of 26 [73%] vs. 489 of 644 [76%], OR, 0.86; 95% CI, 0.36–2.09; p = .74), use of a superiority primary end point (21 of 27 [78%] vs. 637 of 767 [83%]; p = .47), or odds of meeting the primary end point (10 of 25 [40%] vs. 396 of 756 [52%], OR, 0.61; 95% CI, 0.27–1.37; p = .23). Cooperative group sponsorship was more common among LT trials (22 of 27 [81%] vs. 211 of 767 [28%]; p < .001), whereas biopharmaceutical industry support/sponsorship was more common for RCTs assessing systemic therapy or supportive care (5 of 27 [19%] vs. 609 of 767 [79%]; p < .001). OS was more likely to be the primary end point in trials of LT compared to all other trials (13 of 27 [48%] vs. 199 of 767 [26%]; p = .01), and RCTs of LT were less likely to use surrogate primary end points (7 of 27 [26%] vs. 406 of 766 [53%]; p = .006) (Figure 3A). Pooling trials of LT with concurrent–LT trials revealed a similar correlation with OS as the primary end point (32 of 70 [46%] vs. 180 of 724 [25%]; p < .001) rather than a surrogate primary end point (16 of 70 [23%] vs. 397 of 723 [55%]; p < .001) compared to trials of systemic therapy or supportive care (Figure 3B). This relationship remained when comparing LT plus concurrent–LT trials directly to systemic therapy trials with exclusion of supportive care trials (OS primary end point: 32 of 70 [46%] vs. 177 of 600 [30%]; p = .006; surrogate primary end point: 16 of 70 [23%] vs. 373 of 599 [62%]; p < .001). Trials of LT or concurrent–LT were more likely to be sponsored by cooperative groups (51 of 70 [73%] vs. 182 of 724 [25%]; p < .001) and less likely to be sponsored by industry sources (23 of 70 [33%] vs. 591 of 724 [82%]; p < .001).
TABLE 1.
Design, funding, and outcome characteristics of randomized controlled trials evaluating LT versus all other modalities by Pearson’s χ2 test, the Mann-Whitney U test, or binary logistic regression.
| Characteristics | LT trials (n = 27) | All other trials (n = 767) | p |
|---|---|---|---|
|
| |||
| Primary end point design | |||
| Overall survival, No. (%) | 13 (48) | 199 (26) | .01 |
| Surrogate, No. (%) | 7 (26) | 406 (53) | .006 |
| Superiority, No. (%) | 21 (78) | 637 (83) | .47 |
| Sponsorship | |||
| Cooperative group, No. (%) | 22 (81) | 211 (28) | <.001 |
| Industry, No. (%) | 5 (19) | 609 (79) | <.001 |
| Outcomes | |||
| Enrolled patients, median (IQR) | 412 (213–813) | 500 (281–803) | .38 |
| Accrual completion, No. (%) | 19 (73) | 489 (76) | .74 |
| Meeting primary end point, No. (%) | 10 (40) | 396 (52) | .23 |
| Impact factor, median (IQR) | 26 (7–36) | 26 (14–48) | .49 |
Abbreviations: IQR, interquartile range; LT, local therapy.
FIGURE 3.
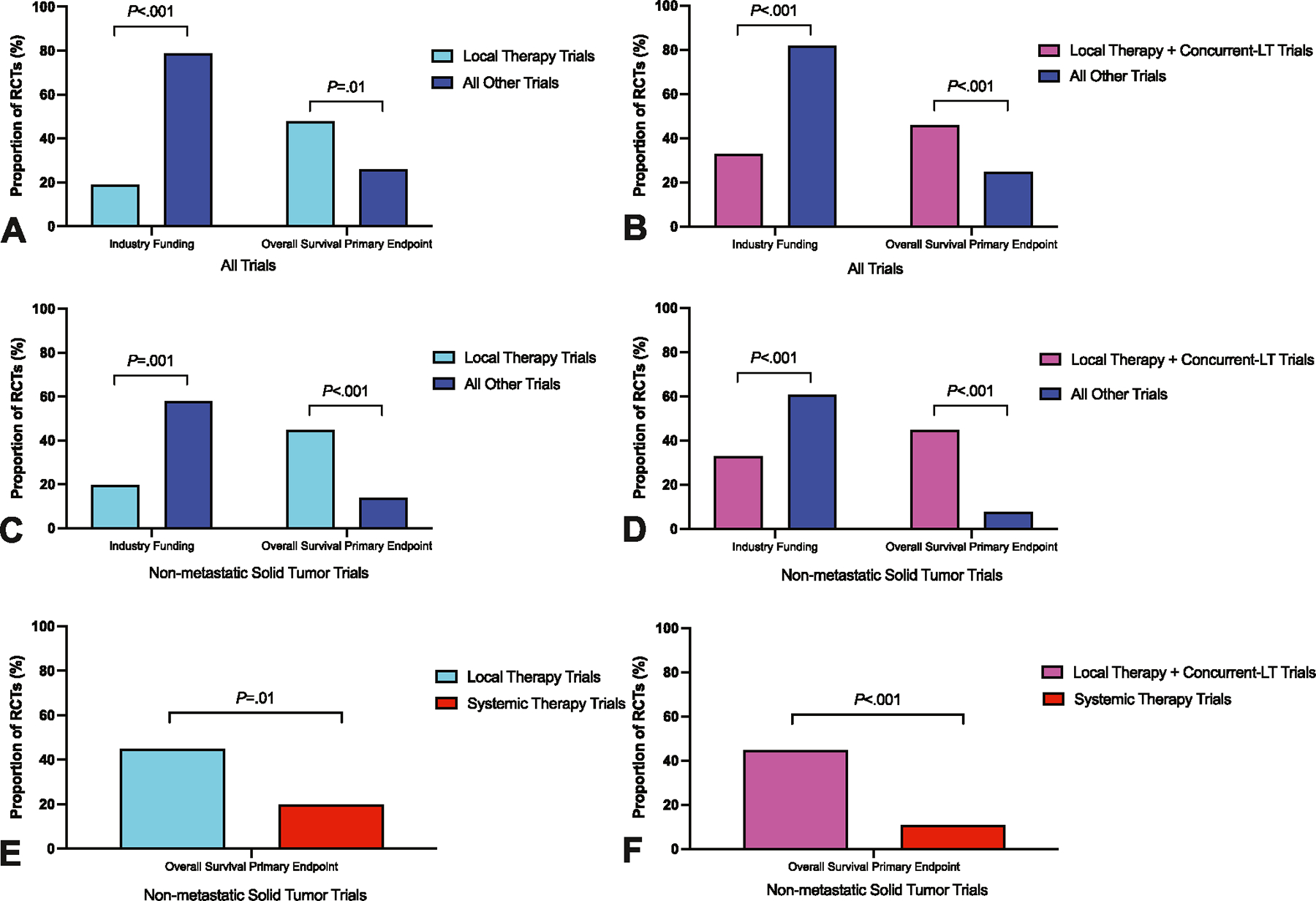
Differences in funding and the use of overall survival as the primary end point between randomized trials investigating local therapy (LT) compared with other modalities. (A) Primary analysis. (B) Pooled analysis of LT trials together with concurrent–LT trials versus all other trials. (C) Sensitivity analysis of nonmetastatic solid tumor trials. (D) Sensitivity analysis of nonmetastatic solid tumor trials comparing pooled analysis of LT trials together with concurrent–LT trials versus all other trials. (E) Sensitivity analysis of nonmetastatic solid tumor trials after the exclusion of supportive care trials comparing LT trials versus systemic therapy trials. (F) Sensitivity analysis of nonmetastatic solid tumor trials after the exclusion of supportive care trials comparing LT trials pooled together with concurrent–LT trials versus systemic therapy trials. p indicates the results of Pearson’s χ2 test.
In this cross–sectional analysis, most trials evaluated conditions for which LT is generally not the predominant standard of care (i.e., metastatic solid tumors and hematologic malignancies). Therefore, a sensitivity analysis for nonmetastatic solid tumor trials was performed. This subset included 210 RCTs, of which 20 trials (10%) investigated LT (Table S1). Similar to the primary analysis, trials of LT were more likely to be sponsored by cooperative groups (16 of 20 [80%] vs. 95 of 190 [50%]; p = .01) and less likely to be funded by industry (4 of 20 [20%] vs. 110 of 190 [58%]; p = .001). The use of OS as the primary end point was more common in trials of LT compared to all other trials (9 of 20 [45%] vs. 26 of 190 [14%]; p < .001), and this association remained when comparing LT trials exclusively to systemic therapy trials with exclusion of supportive care trials (9 of 20 [45%] vs. 26 of 133 [20%]; p = .01) (Figure 3C,E). Of the 51 trials (24%) that investigated LT or concurrent–LT, a higher proportion were supported by cooperative groups (38 of 51 [75%] vs. 73 of 159 [46%]; p < .001) and a lower proportion sponsored by industry (17 of 51 [33%] vs. 97 of 159 [61%]; p < .001) versus all other trials. In addition, the primary end point was more likely to be OS (23 of 51 [45%] vs. 12 of 159 [8%]; p < .001) and less likely to be a surrogate (13 of 51 [25%] vs. 75 of 159 [47%]; p = .006) compared to all other trials (Figure 3D). Excluding supportive care trials, the primary end point was more likely to be OS in LT or concurrent–LT trials compared to systemic therapy trials (23 of 51 [45%] vs. 12 of 109 [11%]; p < .001) (Figure 3F).
DISCUSSION
This comprehensive cross–sectional analysis of published phase 3 cancer clinical trials registered on ClinicalTrials.gov identified marked underrepresentation of studies addressing LT including those with localized disease. Even though LT is the predominant curative modality for patients with cancer and the majority of patients with cancer are treated with LT, systemic therapy trials, which are more often industry–sponsored, have far outpaced LT trials, which are less often industry–sponsored. LT trials also disproportionally use overall survival as the primary end point, a considerably more difficult end point to achieve compared to surrogate end points, impacting the likelihood of positive findings and dissemination into clinical practice. These findings strongly argue for greater resource allocation and funding mechanisms specifically for LT clinical trials.
The value of LT in achieving curative outcomes cannot be over-emphasized, and there are several examples of modern late–phase LT trials yielding hypothesis–reversing results that immediately impacted patient care. For example, the minimally invasive versus abdominal radical hysterectomy trial in patients with cervical cancer found that newer laparoscopic or robot–assisted surgical techniques were associated with shorter disease–free survival and overall survival compared with traditional open surgery.11 The Radiation Therapy Oncology Group 0617 trial showed that dose–escalation with conformal radiotherapy in patients with locally advanced non–small cell lung cancer resulted in shorter overall survival.31 These and other trials highlight the need for better representation of high–quality late–phase research in LT and their potential impact on patient care and outcomes and raise questions about the disparities between LT and pharmacologic therapy funding by nonprofit governmental sources.32
The reasons for underrepresentation of LT trials in late–phase clinical cancer research are likely complex and multifactorial, but the significant differences in trial sponsorship for LT trials compared with systemic therapy trials suggest that funding sources may play a role. This finding was consistent across different subset and sensitivity analyses and has been supported by other studies.6,7 It is important to note that this study does not suggest that there are inherent differences between the research of for–profit and nonprofit funding sources, and for–profit sources are important vehicles for innovation. The above findings do, however, raise questions about the influence of funding sources in oncology research and how anticipated revenue streams may impact the types of trials conducted as well as their design, accrual, reporting, and outcomes.14–20,33–40 In recent years, the majority of phase 3 trials have been funded by industry.14,41,42 It is worth noting that the process for approving new pharmaceuticals in the United States is different from the process of approving new surgical equipment or adopting new radiotherapy techniques.43 There may also be differences in the characteristics of lead principal investigators for trials of LT compared to systemic therapy that lead to fewer LT late–phase trials, as well as perceived lack of equipoise among patients and physicians for trials randomizing between a LT intervention and no LT intervention.44,45 Of further significant consequence is the fact that National Institutes of Health–sponsored clinical trials have declined in the past 2 decades in the setting of reduced funding from budgetary cuts, sequestration, and inflationary losses, with an increase in industry–sponsored trials.41,42,44 Although there are limitations and challenges to cooperative group studies, this study’s analysis reinforces the importance of nonprofit funding mechanisms and cooperative groups in conducting quality–assured late–phase randomized research in LT.24,46
Trials evaluating LT were more likely to use overall survival, a clinically meaningful and easily interpretable measure, as the primary end point compared to other trials, which were more likely to use surrogate end points. This finding remained consistent after pooling LT trials with concurrent–LT trials, excluding trials of supportive care, and restricting the analysis to nonmetastatic solid tumors. One contributing factor for this end point difference may be the inherent challenge of using local failure as a primary end point, as evaluation of cumulative local control requires more longitudinal testing (with more cost) than measuring progression–free survival to first failure or overall survival. On the other hand, most often, improving overall survival is considerably more challenging than improving a surrogate end point, such as progression–free survival, which may be more reflective of radiologic changes rather than patient longevity or quality of life. The optimal design of phase 3 trials and role for surrogate end points versus overall survival are controversial topics that are beyond the scope of this study.29,30 However, these data highlight the potential value proposition of LT trials in the late–phase investigative landscape.
There are several limitations to consider when interpreting the results of this study. Some of these analyses are correlative and should not be interpreted as causal. Additionally, the requirement for registration on ClinicalTrials.gov has changed over time since its inception in 2000, and as such older RCTs may be underrepresented or not included in this data set. Notably, the study aim was to evaluate the trends of completed, published late–phase trials that are currently impacting contemporary clinical practice. As such, this study does not directly address the interesting, but separate, question of how therapeutic modality relates to current phase 3 trial design, as the timeline from conception of a phase 3 trial to publication is heterogenous and may systematically differ based on the trial sponsor and trial modality; moreover, recent data demonstrate that one in 15 completed oncology randomized controlled trials are not published.17,47 Trials published after the data collection date were not included in the study, and the study findings must be interpreted with this in mind. Trials conducted without affiliation or enrollment in the United States that do not register on ClinicalTrials.gov, including perhaps those performed in low–middle income countries or Europe, were also not included in the analysis. As such, more research is needed to determine if the findings of this study are applicable to a broader global oncology clinical research landscape.21,48–50
Despite these limitations, this study highlights the poor representation of LT in modern late–phase oncology research and suggests systematic differences in design and funding. These findings strongly argue for greater funding support of LT trials. By understanding the modern representation of LT in late–phase trials and the traits associated with LT trials, researchers, oncologists, and policymakers may be better informed in developing research priorities and resource allocation strategies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Christine F. Wogan, MS, EL, from the Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, for editing the manuscript and Carrie L. Sherry, BS, for editing the figures. This work was supported in part by National Cancer Institute, National Institutes of Health (P30CA016672), the Sabin Family Fellowship Foundation (to Ethan Ludmir), and the Fund for Innovation in Cancer Informatics (to Ethan Ludmir). Institutional review board approval was waived for this study because of the public availability of trial–level data.
Footnotes
CONFLICT OF INTEREST STATEMENT
Prajnan Das reports honoraria from the American Society of Clinical Oncology, the American Society for Radiation Oncology, the National Cancer Institute, Physicians Education Resource, Conveners, Imedex, and Bayer. C. David Fuller reports unrelated funding and salary support from National Institutes of Health National Institute of Biomedical Imaging and Bioengineering Research Education Programs for Residents and Clinical Fellows, a National Institute of Dental and Craniofacial Research Academic Industrial Partnership Grant, a NCI Parent Research Project Grant, an National Institutes of Health/National Cancer Institute Cancer Center Support Grant, and an National Science Foundation Division of Civil, Mechanical, and Manufacturing Innovation grant; direct industry grant support, honoraria, and travel funding from Elekta AB unrelated to this project; and direct infrastructure support from the multidisciplinary Radiation Oncology/Cancer Imaging Program of The MD Anderson Cancer Center and The MD Anderson Program in Image–Guided Cancer Therapy. Cullen Taniguchi reports consulting fees from Phebry and Xerient and holds a patent related to radioprotection of the upper gastrointestinal tract. The other authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Research data are stored in an institutional repository and will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Citrin DE. Recent developments in radiotherapy. N Engl J Med. 2017;377(11):1065–1075. doi: 10.1056/nejmra1608986 [DOI] [PubMed] [Google Scholar]
- 2.Lane T A short history of robotic surgery. Ann R Coll Surg Engl. 2018;100(supp 6):5–7. doi: 10.1308/rcsann.supp1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holsti LR. Development of clinical radiotherapy since 1896. Acta Oncol.1995;34(8):995–1003. doi: 10.3109/02841869509127225 [DOI] [PubMed] [Google Scholar]
- 4.Lederman M The early history of radiotherapy: 1895–1939. Int J Radiat Oncol Biol Phys. 1981;7(5):639–648. doi: 10.1016/0360-3016(81)90379-5 [DOI] [PubMed] [Google Scholar]
- 5.Gawande A Two hundred years of surgery. N Engl J Med. 2012;366(18):1716–1723. doi: 10.1056/nejmra1202392 [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Zhang Y, Tang LL, et al. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol.2018;4(8):1073–1079. doi: 10.1001/jamaoncol.2018.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jairam V, Yu JB, Aneja S, Wilson LD, Lloyd S. Differences in funding sources of phase III oncology clinical trials by treatment modality and cancer type. Am J Clin Oncol. 2017;40(3):312–317. doi: 10.1097/coc.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 8.Wong BO, Perera ND, Shen JZ, et al. Analysis of registered clinical trials in surgical oncology, 2008–2020. JAMA Netw Open. 2022;5(1): e2145511. doi: 10.1001/jamanetworkopen.2021.45511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menezes AS, Barnes A, Scheer AS, et al. Clinical research in surgical oncology: an analysis of ClinicalTrials.gov. Ann Surg Oncol. 2013; 20(12):3725–3731. doi: 10.1245/s10434-013-3054-y [DOI] [PubMed] [Google Scholar]
- 10.Naredi P, La Quaglia MP. The future of trials in surgical oncology. Nat Rev Clin Oncol. 2015;12(7):425–431. doi: 10.1038/nrclinonc.2015.72 [DOI] [PubMed] [Google Scholar]
- 11.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–1904. doi: 10.1056/nejmoa1806395 [DOI] [PubMed] [Google Scholar]
- 12.Abi Jaoude J, Kouzy R, Ghabach M, et al. Food and Drug Administration approvals in phase 3 cancer clinical trials. BMC Cancer. 2021;21(1):695. doi: 10.1186/s12885-021-08457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abi Jaoude J, Kouzy R, Rooney M, et al. Impact factor and citation metrics in phase III cancer trials. Oncotarget. 2021;12(18):1780–1786. doi: 10.18632/oncotarget.28044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26(33):5458–5464. doi: 10.1200/jco.2008.16.5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Als–Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290(7):921–928. doi: 10.1001/jama.290.7.921 [DOI] [PubMed] [Google Scholar]
- 16.Patel RR, Verma V, Fuller CD, McCaw ZR, Ludmir EB. Transparency in reporting of phase 3 cancer clinical trial results. Acta Oncol.2021; 60(2):191–194. doi: 10.1080/0284186x.2020.1856410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin TA, Fuller CD, Verma V, et al. Trial sponsorship and time to reporting for phase 3 randomized cancer clinical trials. Cancers (Basel). 2020;12(9):2636. doi: 10.3390/cancers12092636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abi Jaoude J, Kouzy R, Minsky BD, et al. Sponsor–involved statistical analyses in Phase III cancer clinical trials. Int J Cancer. 2020;147(12):3579–3581. doi: 10.1002/ijc.33180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouzy R, Abi Jaoude J, Mainwaring W, et al. Professional medical writer assistance in oncology clinical trials. Oncologist. 2020;25(11):e1812–e1815. doi: 10.1634/theoncologist.2020-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger AL, Kusi Appiah A, Wright CM, et al. Financial relationships between industry and principal investigators of US cooperative group randomized cancer clinical trials. Int J Cancer. 2021;149(9):1683–1690. doi: 10.1002/ijc.33719 [DOI] [PubMed] [Google Scholar]
- 21.Ludmir EB, Mainwaring W, Miller AB, et al. Women’s representation among lead investigators of clinical trials in oncology. JAMA Oncol. 2019;5(10):1501–1502. doi: 10.1001/jamaoncol.2019.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence–based clinical guidelines. Cancer. 2005;104(6):1129–1137. doi: 10.1002/cncr.21324 [DOI] [PubMed] [Google Scholar]
- 23.UK Chemotherapy, Radiotherapy and Tumour Resections in England: 2013–2014. National Cancer Registration and Analysis Service and Cancer Research UK; 2017. [Google Scholar]
- 24.Corrigan KL, Kry S, Howell RM, et al. The radiotherapy quality assurance gap among phase III cancer clinical trials. Radiother Oncol. 2022;166:51–57. doi: 10.1016/j.radonc.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol.2019;5(12):1769–1773. doi: 10.1001/jamaoncol.2019.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/s0140-6736(07)61602-x [DOI] [PubMed] [Google Scholar]
- 27.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole–brain radiotherapy: a randomized, double–blind, placebo–controlled trial. Neuro Oncol. 2013;15(10):1429–1437. doi: 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. doi: 10.1056/nejmoa1012348 [DOI] [PubMed] [Google Scholar]
- 29.Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15(1):134. doi: 10.1186/s12916-017-0902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasalic D, McGinnis GJ, Fuller CD, et al. Progression–free survival is a suboptimal predictor for overall survival among metastatic solid tumour clinical trials. Eur J Cancer. 2020;136:176–185. doi: 10.1016/j.ejca.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley JD, Paulus R, Komaki R, et al. Standard–dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small–cell lung cancer (RTOG 0617): a randomised, two–by–two factorial phase 3 study. Lancet Oncol.2015;16(2):187–199. doi: 10.1016/s1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg M, McBride WH, Vlashi E, Pajonk F. National Institutes of Health funding in radiation oncology: a snapshot. Int J Radiat Oncol Biol Phys. 2013;86(2):234–240. doi: 10.1016/j.ijrobp.2013.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for–profit and not–for–profit organizations: 2000–2005. JAMA. 2006;295(19):2270–2274. doi: 10.1001/jama.295.19.2270 [DOI] [PubMed] [Google Scholar]
- 34.Borzak S Funding of clinical trials. JAMA. 2006;296(16):1969. doi: 10.1001/jama.296.16.1969-a [DOI] [PubMed] [Google Scholar]
- 35.Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316–1320. doi: 10.1200/jco.2011.38.6078 [DOI] [PubMed] [Google Scholar]
- 36.Peppercorn J, Blood E, Winer E, Partridge A. Association between pharmaceutical involvement and outcomes in breast cancer clinical trials. Cancer. 2007;109(7):1239–1246. doi: 10.1002/cncr.22528 [DOI] [PubMed] [Google Scholar]
- 37.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–465. doi: 10.1001/jama.289.4.454 [DOI] [PubMed] [Google Scholar]
- 38.Jagsi R, Sheets N, Jankovic A, Motomura AR, Amarnath S, Ubel PA. Frequency, nature, effects, and correlates of conflicts of interest in published clinical cancer research. Cancer. 2009;115(12):2783–2791. doi: 10.1002/cncr.24315 [DOI] [PubMed] [Google Scholar]
- 39.Pasalic D, Tang C, Jagsi R, Fuller CD, Koong AC, Ludmir EB. Association of industry sponsorship with cancer clinical trial accrual. JAMA Oncol.2020;6(10):1625–1627. doi: 10.1001/jamaoncol.2020.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chopra SS. MSJAMA: industry funding of clinical trials: benefit or bias? JAMA. 2003;290(1):113–114. doi: 10.1001/jama.290.1.113 [DOI] [PubMed] [Google Scholar]
- 41.Ehrhardt S, Appel LJ, Meinert CL. Trends in National Institutes of Health funding for clinical trials registered in ClinicalTrials.gov. JAMA. 2015;314(23):2566–2567. doi: 10.1001/jama.2015.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gresham GK, Ehrhardt S, Meinert JL, Appel LJ, Meinert CL. Characteristics and trends of clinical trials funded by the National Institutes of Health between 2005 and 2015. Clin Trials. 2018;15(1):65–74. doi: 10.1177/1740774517727742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keng MK, Wenzell CM, Sekeres MA. A drug’s life: the pathway to drug approval. Clin Adv Hematol Oncol. 2013;11(10):646–655. [PubMed] [Google Scholar]
- 44.Giacalone NJ, Milani N, Rawal B, et al. Funding support and principal investigator leadership of oncology clinical trials using radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(1):34–43. doi: 10.1016/j.ijrobp.2018.05.037 [DOI] [PubMed] [Google Scholar]
- 45.Davies L, Beard D, Cook JA, Price A, Osbeck I, Toye F. The challenge of equipoise in trials with a surgical and non–surgical comparison: a qualitative synthesis using meta–ethnography. Trials. 2021;22(1):678. doi: 10.1186/s13063-021-05403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schilsky RL. The National Clinical Trials Network and the cooperative groups: the road not taken. Cancer. 2020;126(23):5008–5013. doi: 10.1002/cncr.33210 [DOI] [PubMed] [Google Scholar]
- 47.Pasalic D, Fuller CD, Mainwaring W, et al. Detecting the dark matter of unpublished clinical cancer studies: an analysis of phase 3 randomized controlled trials. Mayo Clin Proc. 2021;96(2):420–426. doi: 10.1016/j.mayocp.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodkins J, Hopman WM, Wells JC, et al. Is clinical research serving the needs of the global cancer burden? An analysis of contemporary global radiation therapy randomized controlled trials. Int J Radiat Oncol Biol Phys. 2022;113(3):500–508. doi: 10.1016/j.ijrobp.2022.01.053 [DOI] [PubMed] [Google Scholar]
- 49.Wells JC, Sharma S, Del Paggio JC, et al. An analysis of contemporary oncology randomized clinical trials from low/middle–income vs high–income countries. JAMA Oncol.2021;7(3):379–385. doi: 10.1001/jamaoncol.2020.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forrester JA, Forrester JD, Wren SM. Trends in country–specific surgical randomized clinical trial publications. JAMA Surg.2018;153(4):386–388. doi: 10.1001/jamasurg.2017.4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared on reasonable request to the corresponding author.


