Abstract
Background:
Use of extended pharmacologic thromboprophylaxis after major abdominopelvic cancer surgery should depend on best-available scientific evidence and patients’ informed preferences. We developed a risk-stratified patient decision aid to facilitate shared decision-making and sought to evaluate its effect on decision-making quality regarding use of extended thromboprophylaxis.
Methods:
We enrolled patients undergoing major abdominopelvic cancer surgery at an academic tertiary care centre in this pre–post study. We evaluated change in decisional conflict, readiness to decide, decision-making confidence, and change in patient knowledge. Participants were provided the appropriate risk-stratified decision aid (according to their Caprini score) in either the preoperative or postoperative setting. A sample size calculation determined that we required 17 patients to demonstrate whether the decision aid meaningfully reduced decisional conflict. We used the Wilcoxon matched-pairs signed ranks test for interval scaled measures.
Results:
We included 17 participants. The decision aid significantly reduced decisional conflict (median decisional conflict score 2.37 [range 1.00–3.81] v. 1.3 [range 1.00–3.25], p < 0.01). With the decision aid, participants had high confidence (median 86.4 [range 15.91–100]) and felt highly prepared to make a decision (median 90 [range 55–100]). Median knowledge scores increased from 50% (range 0%–100%) to 75% (range 25%–100%).
Conclusion:
Our risk-stratified, evidence-based decision aid on extended thromboprophylaxis after major abdominopelvic surgery significantly improved decision-making quality. Further research is needed to evaluate the usability and feasibility of this decision aid in the perioperative setting.
Abstract
Contexte:
L’utilisation d’une thromboprophylaxie pharmacologique prolongée après une chirurgie majeure pour cancer abdomino-pelvien doit reposer sur les meilleures données scientifiques existantes et les préférences des malades bien renseignés. Nous avons conçu un outil décisionnel stratifié selon le risque pour faciliter les prises de décision partagées et nous avons voulu en mesurer l’effet sur la qualité des prises de décision relatives à l’utilisation de la thromboprophylaxie prolongée.
Méthodes:
Pour cette étude avant–après, nous avons inscrit des malades soumis à une chirurgie majeure pour cancer abdomino-pelvien dans un centre universitaire de soins tertiaires. Nous avons évalué les différences aux plans du conflit décisionnel, de l’état de préparation à la prise de décision, du degré de confiance envers le processus décisionnel, et du niveau de connaissances des malades. On a remis aux personnes participantes l’outil décisionnel approprié (selon leur score de risque de Caprini) à l’étape pré- ou postopératoire. Un calcul de la taille de l’échantillon a permis de déterminer qu’il nous fallait 17 personnes pour démontrer que l’outil décisionnel réduisait significativement le conflit décisionnel. Nous avons utilisé le test des rangs signés de Wilcoxon pour paires appariées et mesures par échelle d’intervalles.
Résultats:
Nous avons inclus 17 personnes. L’outil décisionnel a significativement réduit le conflit décisionnel (indice médian de conflit décisionnel 2,37 [éventail 1,00–3,81] c. 1,3 [éventail 1,00–3,25], p < 0,01). Avec l’outil décisionnel, les personnes se sentaient très en confiance (médiane 86,4 [éventail 15,91–100]) et se sentaient prêtes à prendre leur décision (médiane 90 [éventail 55–100]). Les scores médians de connaissances sont passés de 50 % (éventail 0 %–100 %) à 75 % (éventail 25 %–100 %).
Conclusion:
Notre outil décisionnel stratifié selon le risque et fondé sur des données probantes concernant l’utilisation de la thromboprophylaxie prolongée après une chirurgie majeure pour cancer abdomino-pelvien a significativement amélioré la qualité des prises de décision. Il faudra approfondir la recherche pour évaluer l’applicabilité de cet outil décisionnel en contexte périopératoire.
Venous thromboembolism (VTE) is an important cause of perioperative morbidity and death among patients with cancer.1,2 Beginning pharmacologic thromboprophylaxis at the time of surgery and continuing until hospital discharge can reduce the risk of symptomatic postoperative VTE by as much as 70%.3,4 However, the risk of VTE is sustained for weeks beyond surgery, especially among patients with hypercoagulability, such as those with cancer.5,6 Extended pharmacologic thromboprophylaxis for up to 4 weeks after surgery is currently recommended by several professional societies for patients undergoing major abdominopelvic cancer surgery.7–9 These recommendations are often based on randomized clinical trials that used screen-detected (via ultrasonography or venography) deep vein thrombosis among asymptomatic patients as an outcome.10,11 A recent systematic review and meta-analysis of trials reporting only symptomatic VTE found the 30-day postoperative rate of VTE was 1.7%.12 Extended thromboprophylaxis was associated with a significant reduction in the incidence of symptomatic VTE from 2.1% to 1.0% (risk ratio 0.48, 95% confidence interval 0.31–0.74), corresponding to a number needed to treat of 91.12 As such, there is no correct, clear clinical option, and the use of extended thromboprophylaxis after major abdominopelvic cancer surgery should depend on scientific evidence and patient preferences.
Person-centred care has been recognized as increasingly important, given the number of treatment options available with similar benefits, increasing health care costs, and concerns about the sustainability of health care systems. Person-centred care is a concept that involves eliciting individuals’ preferences to guide aspects of their health care to the extent that the individual desires.13 Several strategies exist to achieve this, including building health literacy skills, supporting self-care, encouraging co-creation of services, and promoting shared decision-making.14,15 Shared decision-making is a collaborative process (sometimes aided by decision-making tools) by which health care providers explain the “pros and cons and costs and benefits of treatment options and help patients choose the treatment option that best aligns with their preferences, values, beliefs, emotional state, and perceived capabilities.”16 Patient decision aids are clinical tools designed to improve patients’ understanding of options, elicit their expectations of benefits and harms, and facilitate active involvement in decision-making.17 Patient decision aids have been shown to improve decision quality and reduce decisional needs when compared with usual care.18
Shared decision-making has been recognized as an important factor in improving the quality of surgical care.19,20 Collaborating with patients and engaging them in decision-making is central in helping them appreciate the relevance of their own values and preferences, particularly for preference-sensitive decisions.21 However, time constraints in the perioperative setting pose a challenge for clinicians to communicate important decisional information with patients.21 There are substantial deficits in the structures, processes, and results of perioperative decision-making, and existing processes do not consistently meet patient decisional needs.19 Patient decision aids have the potential to improve health outcomes by presenting complex medical information in a patient-centred manner, and they can help patients weigh different options to clarify their personal preferences and values.22 We sought to perform beta-testing of a previously developed patient decision aid, designed to help decision-making about extended-duration thromboprophylaxis after major abdominopelvic surgery, and to determine the effect of the decision aid on the quality of the decision-making process.
Methods
We developed and alpha-tested a patient decision aid for adults aged 18 years or older regarding extended-duration thromboprophylaxis for 4 weeks after major abdominopelvic surgery, which demonstrated acceptability with patients and clinicians.23 The decision aid was developed in accordance with International Patient Decision Aids Standards (IPDAS) and the Ottawa Decision Support Framework (ODSF).23–25
We conducted this pre–post study according to the IPDAS development process and the ODSF.23–25 The results of this study are reported according to the Standards for Universal Reporting of Patient Decision Aid Evaluation checklist.26
Setting
We conducted the study at a single academic tertiary care centre that serves a population of 1.3 million.27 We recruited patients based on research team availability between Oct. 26, 2021, and Mar. 21, 2022. Two health care professionals on the research team consecutively recruited patients from preoperative ambulatory care clinics and postoperative inpatient wards.
Participant eligibility
Patients were eligible for inclusion if they were admitted to hospital after major abdominopelvic surgery for cancer or had signed consent for an upcoming major abdominal surgery for cancer in a preoperative clinic. We excluded patients if they had contraindications to extended-duration thromboprophylaxis, an indication for therapeutic anticoagulation, or an anticipated postoperative length of stay in hospital exceeding 14 days. We also excluded patients who did not speak English, as study instruments were validated only in English.
Intervention
The patient decision aid examined in this study makes explicit the decision and 2 options, namely whether to proceed with or decline extended-duration thromboprophylaxis after major abdominopelvic surgery (Appendix 1, available at www.canjsurg.ca/lookup/doi/10.1503/cjs.014722/tab-related-content). Information on VTE and prophylaxis is written at an eighth-grade reading level, and the benefits and harms of taking or declining extended-duration thromboprophylaxis using low-molecular-weight heparin (LMWH) are stratified according to the Caprini risk assessment model.28 The patient decision aid contains a 4-item exercise to clarify patient values for each option, a 4-item knowledge test, and the SURE (sure of myself, understand information, risk–benefit ratio, encouragement) test to screen for clinically important decisional conflict.29
Data collection, measurement instruments, and outcomes
We collected baseline sociodemographic and clinical data for all participants. We measured their health literacy using the Newest Vital Sign, a validated 6-question tool designed to identify patients at risk for low health literacy.30
The primary outcome was change in decisional conflict, measured using the 16-item Decisional Conflict Scale (DCS).31 The DCS measures personal perceptions of decisional conflict and related factors, and has been validated in more than 250 studies, demonstrating good reliability.32,33 It is divided into 5 subscales. The uncertainty subscale assesses patients’ perception of uncertainty; the informed subscale assesses knowledge of options, risks, and benefits; the values clarity subscale measures clarity of what matters most to patients for this decision; the support subscale determines perceived support in decision-making; and the effective decision subscale measures consistency between patients’ informed values and decisions.
Secondary outcomes were confidence in decision-making, readiness to make a decision, change in relevant knowledge, and change in participant preference. The Decisional Self-Efficacy scale (DSES) is an 11-item measure of belief in one’s decision-making abilities.34 The Preparation for Decision-Making Scale (PDMS) is a 10-item scale that assesses patients’ readiness to make a decision based on their perception of how useful the patient decision aid was in helping them to identify the need for a decision, preparing them to communicate with their surgical team, and involving them in the decision-making process.35 The DSES and PDMS are psychometrically validated for this purpose.34,36 Knowledge questions were written at an eighth grade reading level, in accordance with IPDAS, and validated in the alpha-testing.23, 37
Procedures
Before using the patient decision aid, participants completed the DCS, DSES, and the 4 knowledge questions, and were asked to state a preference on use of extended-duration thromboprophylaxis. A clinical research assistant calculated participants’ VTE risk using the Caprini risk assessment model,28 and provided them with their individualized, risk-stratified patient decision aid. After reviewing the decision aid, participants answered the same 4 knowledge questions and re-recorded their preference of whether they wanted to take extended-duration thromboprophylaxis. Participants then repeated the DCS and completed the PDMS. Narrative comments from participants were collected on the decision aid and extracted verbatim.
Statistical analysis
We performed all analyses with StataMP (version 17.0).38 We estimated the sample size based on the paired t test for comparing means of decisional conflict outcome before and after using the patient decision aid. For an α of 0.05, power (1 – β) of 0.80, a standard deviation of 0.6, and a correlation between pre- and post-test scores of 0.80, the sample size was estimated at 17 to detect a difference of 0.3 in the decisional conflict score, out of a possible score of 1–5. The effect size was therefore 0.5, which is defined as a medium effect.39 In decisional conflict, this effect size is clinically important as it is commonly observed between those who make versus delay decisions.40 To detect changes between pre- and post-intervention measures, we used Wilcoxon matched-pairs signed-ranks test for interval scaled measures.
We conducted descriptive analyses to report results of all secondary outcomes. We performed a post hoc exploratory analysis to compare the proportion of eligible participants that completed the study in the preoperative versus postoperative settings, as well as changes in decisional conflict according to Caprini risk assessment score and health literacy.
Ethics approval
This study was approved by the University of Ottawa Research Ethics Board (OHSN-REB 20200570–01H).
Results
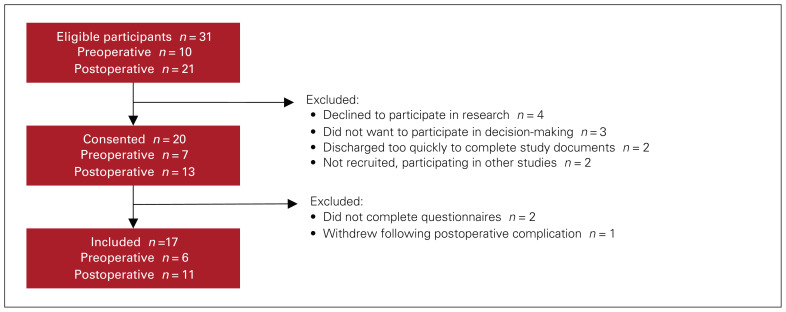
Of 31 eligible patients, 17 completed the study (Figure 1). The median age was 69 (range 28–82) years, and most participants were male (n = 14) (Table 1). In terms of risk of developing VTE, based on the Caprini risk assessment model, 1 participant was low risk, 6 were moderate risk, 5 were high risk, and 5 were very high risk.
Fig. 1.
Flow diagram showing participant inclusion process. See Related Content tab for an accessible version.
Table 1.
Patient sociodemographic and clinical characteristics
| Characteristic | No. of patients* n = 17 |
|---|---|
| Age, median (range), yr | 68 (28–82) |
| Sex | |
| Female | 4 |
| Male | 13 |
| Race/ethnicity | |
| White | 15 |
| South Asian | 2 |
| Education | |
| High school | 4 |
| College or university degree | 7 |
| Graduate degree or other | 6 |
| Marital status | |
| Single | 2 |
| Married or partnered | 11 |
| Divorced or widowed | 4 |
| No. of children, median (range) | 2 (0–5) |
| Occupation | |
| Working | 7 |
| Paid sick leave or unemployed | 4 |
| Retired | 6 |
| Health insurance | |
| Government | 9 |
| Private | 6 |
| Other | 2 |
| Country of birth | |
| Canada | 14 |
| Other | 3 |
| Health literacy | |
| Low | 5 |
| Intermediate | 3 |
| Adequate | 9 |
| Caprini score | |
| 3–4 (low risk) | 1 |
| 5–6 (moderate risk) | 6 |
| 7–8 (high risk) | 5 |
| ≥ 9 (very high risk) | 5 |
| Personal history VTE | 1 |
| Family history VTE | 3 |
| Grade 3 complication (30 d) | 1 |
| Extended-duration LMWH | |
| Routinely prescribed | 4 |
| Not routinely prescribed | 13 |
| Procedure† | |
| Laparoscopic | 5 |
| Open | 12 |
| Segmental colectomy | 5 |
| Proctectomy | 5 |
| Hepatectomy | 3 |
| Distal pancreatectomy | 1 |
| Small bowel resection | 3 |
| Subtotal gastrectomy | 1 |
Note: LMWH = low-molecular-weight heparin; VTE = venous thromboembolism.
Unless indicated otherwise.
One patient had combined hepatectomy and segmental colectomy.
Primary outcome
The median pre-test total decisional conflict score was 2.38 (range 1.00–3.81), compared with a post-test score of 1.31 (range 1.00–3.25) (p < 0.01). All subscales of the DCS were reduced following use of the patient decision aid (Table 2). Before using the decision aid, 6 participants presented with high total decisional conflict (DCS score > 2.5), compared with 2 participants after using the decision aid.
Table 2.
Change in decisional conflict
| Scale | Median score* | Change | p value | z score† | |
|---|---|---|---|---|---|
| Pre-test | Post-test | ||||
| Total | 2.38 | 1.31 | −1.06 | < 0.01 | 3.53 |
| Uncertainty | 2.67 | 1.33 | −1.33 | ||
| Informed | 2.67 | 1.33 | −1.33 | ||
| Values clarity | 2.67 | 1.00 | −1.67 | ||
| Support | 2.00 | 1.33 | −0.67 | ||
| Effective decision | 2.25 | 1.50 | −0.75 | ||
Range 1.0 to 5.0. Lower scores indicate more effective decisions and reduced decisional conflict. Those who make choices have average decisional conflict scores of 2 or less.40
Obtained from Wilcoxon matched-pairs signed-ranks test.
Secondary outcomes
The median DSES score was 86.36 out of 100 (range 15.90–95.45), indicating high confidence in participants’ ability to make a decision. After using the decision aid, the median PDMS score was 90 out of 100 (range 55–100), indicating that participants felt ready to decide following review of the patient decision aid; the decision aid supported communication with their health care team and their ability to decide. Median knowledge scores increased from 50% (range 0%–100%) of questions answered correctly before reviewing the patient decision aid, to 75% (range 25%–100%) after review. Before the use of the decision aid, 7 participants were unsure if they wanted to accept or decline extended-duration LMWH, 7 declined, and 3 accepted. After use of the decision aid, 2 participants were unsure, 6 declined, and 9 accepted (Table 3). The cost of LMWH was not funded for this study. Of the 6 participants who declined LMWH, only 1 did not have private insurance or access to a government-funded program to cover the cost of the medication. Of the 9 who accepted LMWH, 1 participant did not have private insurance or access to a government-funded program to cover the cost of the medication. The participant who was unsure ultimately went home with no prescription as this was the standard of care at the study institution. Only 1 participant reported a narrative comment on the decision aid, which stated, “I don’t want to take injections.”
Table 3.
Change in preferred option
| Caprini score category | Decision | Change in decision | |
|---|---|---|---|
| Pre-test | Post-test | ||
| Low risk | Unsure | Decline | Yes |
| Moderate risk | Unsure | Accept | Yes |
| Unsure | Accept | Yes | |
| Decline | Accept | Yes | |
| Decline | Decline | No | |
| Decline | Unsure | Yes | |
| Decline | Decline | No | |
| High risk | Unsure | Accept | Yes |
| Unsure | Unsure | No | |
| Decline | Decline | No | |
| Decline | Decline | No | |
| Accept | Accept | No | |
| Very high risk | Unsure | Accept | Yes |
| Unsure | Accept | Yes | |
| Decline | Decline | No | |
| Accept | Accept | No | |
| Accept | Accept | No | |
Exploratory analyses
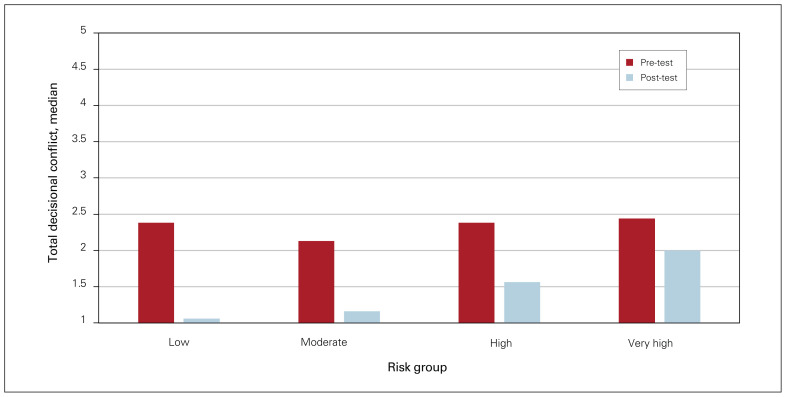
A larger proportion of eligible patients were recruited from the preoperative clinics (n = 7 of 10) than postoperatively (n = 10 of 21). Participants who were calculated to be at lower risk of developing VTE according to the Caprini risk assessment model demonstrated larger reduction in total decisional conflict (Figure 2). The change in median total scores was similar across participants with low, moderate, and high health literacy scores at −0.94, −0.81, and −0.94, respectively.
Fig. 2.
Median change in total decisional conflict according to risk of venous thromboembolism.
Discussion
The decision to take extended thromboprophylaxis after major abdominopelvic surgery for cancer is complex, and may depend on the importance the informed patient places on benefits, harms, and scientific uncertainties. We developed our patient decision aid using a proven, systematic approach to development and testing.23 It informs patients about treatment options and possible outcomes of options using the latest quality-rated scientific evidence. We found that our novel, risk-stratified, evidence-based patient decision aid improved the quality of the decision-making process. It significantly reduced decisional conflict among participants, improved patients’ readiness to decide, and enhanced relevant knowledge.
The high DSES scores before using the patient decision aid indicated that patients were confident in their abilities to participate in decision-making. Participant DCS scores indicated that, after the decision aid, patients were aware a decision needed to be made, and had improved perception of their understanding of the benefits and risks associated with both options. In addition, the decision aid was effective in helping patients clarify their values, facilitating communication between patients and their surgical team, and providing a sense of effective, shared decision-making. Participants with high decisional conflict are more likely to delay their decision, change their mind, or express decisional regret.41 Our study demonstrates the proportion of patients with high DCS scores decreased after using the patient decision aid. The results of this study are comparable with previous work investigating the effectiveness of decision aids through beta-testing,42–44 and are consistent with trials of decision aids.17,45
We saw an overall improvement in knowledge following use of the patient decision aid, indicating that most participants were able to synthesize and understand the information presented. Patients with the highest health literacy demonstrated the greatest improvement in knowledge after using the decision aid. Patients with intermediate health literacy answered fewer questions correctly after reviewing the decision aid. This may suggest that people with higher health literacy were better able to synthesize the information presented in the patient decision aid. Although shared decision-making is often advantageous, not all patients consenting to surgery use this method to obtain the knowledge necessary to make their decision.46 Identifying which patients may want to participate in shared decision-making is important when considering using a patient decision aid.
The clinical equipoise regarding the net clinical benefit of extended-duration thromboprophylaxis and the need to trade off the potential harms of anticoagulation in the postoperative setting make the decision to receive extended-duration thromboprophylaxis a preference-sensitive decision.17 The cost of LMWH was not funded specifically for this study. Although this may have influenced some participant decisions, only 2 of 17 participants did not have insurance to cover the cost of the medication, including 1 of 6 participants who declined the medication, and 1 of 11 who accepted it. This makes it less likely that cost influenced the results of this study. Health care providers need to account for the strength and probabilistic nature of available evidence for these decisions as it relates to individual patients to help them reach a decision based on their informed values and preferences. Patient decision aids are a tool that can help in this process, but they have had limited uptake in the perioperative setting, where information is rarely personalized to individual characteristics. A unique feature of our patient decision aid is the risk-stratification of data by patients’ Caprini risk scores. This allows patients to identify and interpret their personal risk of developing VTE. Our results demonstrated that patients at higher risk were more likely to prefer extended-duration thromboprophylaxis, suggesting that risk-stratified decision aids may allow for more personalized shared decision-making. However, some patients in this risk category declined LMWH. The decision to take or decline LMWH depends on the value each patient places on the benefits and harms of each option. Some patients may have favoured the risk profile of taking LMWH, while others may have been comfortable with the quoted risk of VTE. Only 1 participant provided a narrative comment on their decision aid. They belonged to the high-risk category and declined extended-duration LMWH, citing not wanting to take injections as part of the reason for their decision. Patients in the high-risk category who declined extended-duration LMWH highlight an important area for further investigation, as this group may derive the most benefit from extended-duration thromboprophylaxis and it is important to understand how to better align their values with the benefit of extended prophylaxis. Personalizing risks and benefits of patient decision aids should be considered, when appropriate.
Health care providers have been encouraged to integrate patients more actively as partners in making decisions, where both patients and providers contribute to reaching a shared decision.47–49 Despite this, previous studies have demonstrated that more than 50% of patients may not be aware a treatment decision needs to be made.50 A shared decision-making process has the potential to improve outcomes across several levels, ranging from individual patients and providers to organizations and health care systems. The effects of this process can be measured in both the short and long term.51 We found that the use of a patient decision aid improved the quality of decision-making regarding the use of extended-duration thromboprophylaxis, which represents a short-term outcome at the individual level. The effect of decision aids on health care systems cost and savings remain largely unknown and understudied.52
Surgical and perioperative settings present unique challenges for shared decision-making. Limited time for discussion is a widely cited barrier to implementation of patient decision aids, as well as identifying the extent to which a given patient prefers to be involved in decisionmaking.53,54 We administered the decision aid in both the pre- and postoperative setting. Preoperatively, patients who were consented for major abdominopelvic surgery were presented with the decision aid and made their decision before their hospital admission. A potential benefit in the preoperative setting is patients having more time to consider their options and discuss with their support network before making their decision. However, we observed that patients receiving new cancer diagnoses in the clinic are often overwhelmed. Considering extended duration thromboprophylaxis after receiving a new diagnosis of cancer and being consented for surgery may create distress for some patients, and the patient decision aid may become too difficult to complete. Offering the decision aid postoperatively may be of benefit as there is more immediate impact of the decision as patients proceed with LMWH injections shortly thereafter.
The rate of participation differed when patients were recruited in the preoperative versus postoperative period. In the preoperative period, 10 patients were eligible to participate, 7 consented, and 6 completed all study activities. In the postoperative period, 21 patients were eligible to participate, 13 consented, and 11 completed all study activities. Those contacted postoperatively cited not wanting to participate in research as their reason for declining. This could be because they felt fatigued or overwhelmed with their recovery from major abdominopelvic surgery. In addition, patients who choose to take extended-duration thromboprophylaxis using LMWH require patient education on self-injections and a prescription for the medication, which may be logistically challenging on short admissions. These observations are consistent with growing recognition of the need to develop strategies to optimize shared decision-making that are tailored to the needs of patients, providers, and contexts.16 It is important to determine how best to fit shared decision-making within the patient care pathway for those undergoing major abdominopelvic surgery for cancer. The constraints of time and the challenges in the pre- and postoperative settings created difficulties in recruiting patients and clinicians to use the patient decision aid in the clinical setting; only 17 of 31 eligible patients were able to complete the study. Strategies to improve the usability of the patient decision aid should be explored to improve its feasibility in clinical settings.
The ideal timing and method of engaging patients in shared decision-making is yet to be determined.21 Broad implementation of patient decision aids into clinical practice has not yet occurred, and there is an intention–behaviour gap when decision aids are used in routine clinical settings.55 A review of 23 implementation studies of patient decision aids identified implementation strategies, including co-producing decision aid content and processes, training the entire clinical team, preparing and promoting patients to engage in decision-making, senior-level buy-in, and linking outcomes of patient decision aids with measures of organization values.56 These mechanisms were identified as strategies to promote successful and sustainable implementation of decision aids into routine clinical practice. Many strategies can be implemented to support shared decision-making. Alternative examples include decision coaching, training or education programs for clinicians, and social prescribing.57,58 There is limited evidence comparing the effectiveness and utility of patient decision aids, both at the individual and system level, with alternative shared decisionmaking strategies.
Limitations
Most participants had higher education and health literacy levels, which may not represent the general population. Our sample size was too small to compare secondary outcomes by sociodemographic and clinical factors. For example, future studies should assess patient preference for accepting or declining extended-duration thromboprophylaxis with LMWH according to VTE risk. We did not conduct exit interviews with participants who elected not to participate in the study. These data may have yielded important information on some of the barriers to implementation of patient decision aids in clinical practice. Finally, our data are based on a systematic review completed in August 2021. This process can result in a delay between dissemination of new evidence and integration into patient decision aids. Efforts are underway to automate the retrieval and critical appraisal of new evidence using machine learning.59 This can be combined with Web-based, living patient decision aids to allow for real-time updates and ensure the most accurate, up-to-date information is presented to patients.60 Decision aids could be integrated with electronic health records in the future to increase the data points available to personalize the risks and benefits further.
Conclusion
Our risk-stratified, evidence-based patient decision aid on extended-duration thromboprophylaxis after major abdominopelvic surgery significantly reduced decisional conflict and improved quality parameters of participant decision-making. Participants’ confidence in their decisionmaking, knowledge of options, and decision readiness improved after using the decision aid. Further research is needed to evaluate the usability and feasibility of this patient decision aid in the perioperative setting.
Footnotes
This work was previously presented at the 2022 Canadian Surgical Forum, Toronto, Ont.
Contributors: Megan Delisle, Dawn Stacey, Jad Abou-Khalil, Fady Balaa, and Rebecca Auer contributed to the conception and design of the work. Kimberly A. Bertens, Shaheer Tadros, and Marc Carrier contributed to data acquisition. Victoria Ivankovic, Guillaume Martel, Kristen McAlpine, and Carolyn Nessim contributed to data analysis and interpretation. Victoria Ivankovic and Marc Carrier drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Competing interests: Dawn Stacey reports funding from the Canadian Institutes of Health Research and the Canadian Cancer Society, as well as meeting support from the Washington State Health Care Authority, the Centre for Shared Decision Making Advisory Board, and the Beijing University of Chinese Medicine. She is co-lead of the steering committee for the International Patient Decision Aid Standards Collaboration and vice-dean of research at the Faculty of Health Sciences, University of Ottawa. Brittany Dingley reports honoraria from Sanofi. Kristen McAlpine reports honoraria from or advisory board participation with Tolmar, Tersera, Bayer, Knight, and Verity. Marc Carrier reports funding from BMS, LEO Pharma, and Pfizer, as well as consulting fees from BMS, Bayer, LEO Pharma, Sanofi, Pfizer, Servier, and Valeo. No other competing interests were declared.
Funding: This study was supported by the Canadian Institute of Health Research, The Ottawa Hospital Academic Medical Organization, and The Ottawa Hospital Foundation.
References
- 1.Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: a review. Oncologist 2017;22:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyman GH, Culakova E, Poniewierski MS, et al. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res 2018;164:S112–8. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. N Engl J Med 1988;318: 1162–73. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson M, Chan N, Bhagirath V, et al. Prevention of venous thromboembolism in 2020 and beyond. J Clin Med 2020;9:2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS Project. Ann Surg 2006;243:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrych J, Kvasnicka T, Fryba V, et al. 28 day post-operative persisted hypercoagulability after surgery for benign diseases: a prospective cohort study. BMC Surg 2016;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum 2018;61:14–20. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;38:496–520. [DOI] [PubMed] [Google Scholar]
- 9.Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 2021; 5:927–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heijkoop B, Nadi S, Spernat D, et al. Extended versus inpatient thromboprophylaxis with heparins following major open abdominopelvic surgery for malignancy: a systematic review of efficacy and safety. Perioper Med (Lond) 2020;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagarasanu A, Alotaibi GS, Hrimiuc R, et al. Role of extended thromboprophylaxis after abdominal and pelvic surgery in cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2016;23:1422–30. [DOI] [PubMed] [Google Scholar]
- 12.Knoll W, Fergusson N, Ivankovic V, et al. Extended thromboprophylaxis following major abdominal/pelvic cancer-related surgery: a systematic review and meta-analysis of the literature. Thromb Res 2021;204:114–22. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin C. Person-centered care: a definition and essential elements. J Am Geriatr Soc 2016;64:15–8. [DOI] [PubMed] [Google Scholar]
- 14.Coulter A, Oldham J. Person-centred care: What is it and how do we get there? Future Hosp J 2016;3:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nardini C, Osmani V, Cormio PG, et al. The evolution of personalized healthcare and the pivotal role of European regions in its implementation. Per Med 2021;18:283–94. [DOI] [PubMed] [Google Scholar]
- 16.Resnicow K, Catley D, Goggin K, et al. Shared decision making in health care: theoretical perspectives for why it works and for whom. Med Decis Making 2022;42:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa decision support framework: part 3 overview of systematic reviews and updated framework. Med Decis Making 2020;40:379–98. [DOI] [PubMed] [Google Scholar]
- 19.Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns 2014;94:328–33. [DOI] [PubMed] [Google Scholar]
- 20.de Mik SML, Stubenrouch FE, Balm R, et al. Systematic review of shared decision-making in surgery. Br J Surg 2018;105:1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper Z, Sayal P, Abbett SK, et al. A conceptual framework for appropriateness in surgical care: reviewing past approaches and looking ahead to patient-centered shared decision making. Anesthesiology 2015;123:1450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankolekar A, Dekker A, Fijten R, et al. The benefits and challenges of using patient decision aids to support shared decision making in health care. JCO Clin Cancer Inform 2018;2:1–10. [DOI] [PubMed] [Google Scholar]
- 23.Ivankovic V, McAlpine K, Delic E, et al. Extended duration thromboprophylaxis for abdominopelvic surgery: development and evaluation of a risk stratified patient decision aid to facilitate shared decision making. Res Pract Thromb Haemost 2022;6:e12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Patient Decision Aids Standards C IPDAS. 2005. International Patient Decision Aid Standards (IPDAS) Collaboration background document. [Google Scholar]
- 25.O’Connor A. Ottawa Decision Support Framework. Framework. 2006. [Google Scholar]
- 26.Sepucha KR, Abhyankar P, Hoffman AS, et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: the development of SUNDAE Checklist. BMJ Qual Saf 2018;27:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivankovic V, Auer RC, Carrier M, et al. Should I take a blood thinner for one (1) month following my major abdominal surgery or not? 2022. [Google Scholar]
- 28.Fuentes H, Paz L, Al-Ogaili A, et al. Validation of a patient-completed Caprini risk score for venous thromboembolism risk assessment. TH Open 2017;1:e106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Légaré F, Kearing S, Clay K, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician 2010;56:e308–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 32.Garvelink MM, Boland L, Klein K, et al. Decisional Conflict Scale use over 20 years: the anniversary review. Med Decis Making 2019;39:301–14. [DOI] [PubMed] [Google Scholar]
- 33.Garvelink MM, Boland L, Klein K, et al. Decisional Conflict Scale findings among patients and surrogates making health decisions: part II of an anniversary review. Med Decis Making 2019;39:315–26. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor AM. User manual - Decisional Self-Efficacy Scale. 1995. [Google Scholar]
- 35.Graham I, O’Connor AM. User manual - Preparation for Decision Making Scale. 1995. [DOI] [PubMed] [Google Scholar]
- 36.Bennett C, Graham ID, Kristjansson E, et al. Validation of a preparation for decision making scale. Patient Educ Couns 2010;78:130–3. [DOI] [PubMed] [Google Scholar]
- 37.Joseph-Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making 2014;34:699–710. [DOI] [PubMed] [Google Scholar]
- 38.StataCorp. Stata Statistical Software: Release 17. TX: StataCorp LLC.; 2021. [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 40.O’Connor AM, Tugwell P, Wells GA, et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy. Med Decis Making 1998;18:295–303. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor AM. User manual - Decisional Conflict Scale (16 item statement format). 1995. [Google Scholar]
- 42.Fiset V, O’Connor AM, Evans W, et al. Development and evaluation of a decision aid for patients with stage IV non-small cell lung cancer. Health Expect 2000;3:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stacey D, O’Connor AM, DeGrasse C, et al. Development and evaluation of a breast cancer prevention decision aid for higher-risk women. Health Expect 2003;6:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood B, Taljaard M, El-Khatib Z, et al. Development and field testing of a tool to elicit women’s preferences among cervical cancer screening modalities. J Eval Clin Pract 2019;25:1169–81. [DOI] [PubMed] [Google Scholar]
- 45.Hoefel L, O’Connor AM, Lewis KB, et al. 20th anniversary update of the Ottawa Decision Support Framework Part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making 2020;40:555–81. [DOI] [PubMed] [Google Scholar]
- 46.Lindsay SE, Alokozai A, Eppler SL, et al. Patient preferences for shared decision making: Not all decisions should be shared. J Am Acad Orthop Surg 2020;28:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns 2012; 86:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: Revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651–61. [DOI] [PubMed] [Google Scholar]
- 49.Blenkinsopp A. From compliance to concordance: How are we doing? Int J Pharm Pract 2001;9:65–6. [Google Scholar]
- 50.Haesebaert J, Adekpedjou R, Croteau J, et al. Shared decision-making experienced by Canadians facing health care decisions: a web-based survey. CMAJ Open 2019;7:E210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elwyn G, Frosch DL, Kobrin S. Implementing shared decisionmaking: consider all the consequences. Implement Sci 2016; 11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh T, Barr PJ, Thompson R, et al. Undetermined impact of patient decision support interventions on healthcare costs and savings: systematic review. BMJ 2014;348:g188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns 2008;73:526–35. [DOI] [PubMed] [Google Scholar]
- 54.Sturgess J, Clapp JT, Fleisher LA. Shared decision-making in peri-operative medicine: a narrative review. Anaesthesia 2019; 74:13–9. [DOI] [PubMed] [Google Scholar]
- 55.Stacey D, Suwalska V, Boland L, et al. Are patient decision aids used in clinical practice after rigorous evaluation? A survey of trial authors. Med Decis Making 2019;39:805–15. [DOI] [PubMed] [Google Scholar]
- 56.Joseph-Williams N, Abhyankar P, Boland L, et al. What works in implementing patient decision aids in routine clinical settings? A rapid realist review and update from the international patient decision aid standards collaboration. Med Decis Making 2021; 41:907–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stiggelbout AM, Van Der Weijden T, De Wit MPT, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 58.Social prescribing. London: NHS. Available: https://www.england.nhs.uk/personalisedcare/social-prescribing/ (accessed 2022 Oct. 1). [Google Scholar]
- 59.Marshall IJ, Noel-Storr A, Kuiper J, et al. Machine learning for identifying Randomized Controlled Trials: an evaluation and practitioner’s guide. In: Research Synthesis Methods. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.GRADEPro. GRADE working group; 2021. Available: https://www.gradepro.org/product (accessed 2022 Nov. 1). [Google Scholar]