Abstract
The localization of Cu,Zn-superoxide dismutase in the mitochondrial intermembrane space suggests a functional relationship with superoxide anion (O2•−) released into this compartment. The present study was aimed at examining the functionality of Cu,Zn-superoxide dismutase and elucidating the molecular basis for its activation in the intermembrane space. Intact rat liver mitochondria neither scavenged nor dismutated externally generated O2•−, unless the mitochondrial outer membrane was disrupted selectively by digitonin. The activation of the intermembrane space Cu,Zn-superoxide dismutase following the disruption of mitochondrial outer membrane was largely inhibited by bacitracin, an inhibitor of protein disulphide-isomerase. Thiol alkylating agents, such as N-methylmaleimide or iodoacetamide, decreased intermembrane space Cu,Zn-superoxide dismutase activation during, but not after, disruption of the outer membrane. This inhibitory effect was overcome by exposing mitochondria to low micromolar concentrations of H2O2 before disruption of the outer membrane in the presence of the alkylating agents. Moreover, H2O2 treatment alone enabled intact mitochondria to scavenge externally generated O2•−. These findings suggest that intermembrane space Cu,Zn-superoxide dismutase is inactive in intact mitochondria and that an oxidative modification of its critical thiol groups is necessary for its activation.
Keywords: Cu,Zn-superoxide dismutase; hydrogen peroxide; mitochondrial intermembrane space; mitochondrion; superoxide anion
Abbreviations: DMPO, 5,5′-dimethyl-1-pyrroline-N-oxide; IAM, iodoacetamide; NBT, Nitro Blue Tetrazolium; NEM, N-methylmaleimide; XTT, 3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulphonic acid hydrate
INTRODUCTION
Mitochondria are major cellular sources of superoxide anion (O2•−) and hydrogen peroxide (H2O2) [1]. O2•−, generated by autoxidation of ubisemiquinone [2,3], is converted into H2O2 by the Mn-superoxide dismutase in the mitochondrial matrix. O2•− and H2O2 generated in mitochondria can also diffuse into the cytosol [4]. In addition to their potentially damaging effects on cellular constituents, O2•− and H2O2, at low levels, are essential participants in cell signalling events that regulate cell growth, differentiation and death [5]. Therefore pathways that maintain steady-state levels of intracellular O2•− and/or H2O2 have an important impact on the redox regulation of cell signalling and, as a corollary, cell function.
Early studies on the subcellular distribution of superoxide dismutase demonstrated that Mn-superoxide dismutase was localized in the mitochondrial matrix, whereas Cu,Zn-superoxide dismutase was only localized in cytosol [6]. However, the latter was reported to occur in the mitochondrial intermembrane space isolated from rat liver [7,8] and yeast [9]. Subsequent studies in yeast revealed that only an immature form of Cu,Zn-superoxide dismutase, lacking both Cu2+ and Zn2+, could enter mitochondria. Moreover, the conserved disulphide bond in yeast Cu,Zn-superoxide dismutase, which is essential for its activity, was reduced [10].
The mitochondrial localization of Cu,Zn-superoxide dismutase suggests that the enzyme has a functional relationship with the occurrence of O2•− in this compartment. The concentration of O2•− in the intermembrane space would be determined by its vectorial release from the side O of the cytochrome bc1 complex in the inner membrane [11] and by diffusion from cytosol across the mitochondrial outer membrane [12,13]. Hence, intermembrane space Cu,Zn-superoxide dismutase may be implicated not only in the regulation of redox-sensitive pathways within mitochondria, but also in maintaining steady-state levels of O2•− and H2O2 in the cytosol. However, it is not known whether post-translational modifications of Cu,Zn-superoxide dismutase (insertion of Zn2+ and Cu2+, and formation of conserved disulphide bond) take place in mammalian mitochondria in a similar fashion as in cytosol and whether intermembrane space Cu,Zn-superoxide dismutase is functional in intact mitochondria. Early studies reported that Cu,Zn-superoxide dismutase is in a latent state in intact mitochondria and becomes active after disruption of mitochondrial membranes [12–15].
The present study was aimed at assessing the functionality of intermembrane space Cu,Zn-superoxide dismutase and at elucidating the molecular basis for its latent state in intact mitochondria. These aims were addressed by modulating the redox status of Cu,Zn-superoxide dismutase and assessing its activity by spectroscopic methods.
EXPERIMENTAL
Chemicals
DMPO (5,5′-dimethyl-1-pyrroline-N-oxide), p-hydroxyphenylacetate, Cu,Zn-superoxide, bovine heart cytochrome c, partially acetylated bovine heart cytochrome c, dextran sulphate (molecular mass 8000 Da), BSA, XTT (3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulphonic acid hydrate), digitonin, IAM (iodoacetamide), NEM (N-methylmaleimide) and uric acid were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). p-Nitrophenolate was from Aldrich Chemicals (Milwaukee, WI, U.S.A.).
Isolation of liver mitochondria
Liver mitochondria were isolated from adult male Wistar rats by differential centrifugation as described previously [11]. Rat livers were excised, chopped into fine pieces, washed with 0.25 M sucrose and homogenized in isolation buffer containing 210 mM mannitol, 70 mM sucrose and 2 mM Hepes, pH 7.4, plus 0.05% (w/v) BSA. The homogenate was centrifuged at 800 g for 8 min, the pellet was removed, and the centrifugation process was repeated. The supernatant was centrifuged at 8000 g for 10 min, the pellet was washed with the isolation buffer, and the centrifugation was repeated. The pellet containing a mixture of organelles was further fractionated by centrifugation at 8500 g for 10 min in a Percoll gradient [consisting of three layers of 18, 30 and 60% (w/v) Percoll in sucrose/Tris buffer (0.25 M sucrose, 1 mM EDTA and 50 mM Tris/HCl), pH 7.4] [16]. Mitochondria were collected from the interface of 30% and 60% Percoll and washed with the sucrose/Tris buffer. Mitochondrial proteins were determined as described in [17].
Isolation of mitochondrial intermembrane space contents
The mitochondrial outer membrane was disrupted selectively by treating mitochondria (40 mg/ml) with 0.11 mg of digitonin/mg of protein [18]. Mitoplasts were removed from the intermembrane space preparation by centrifugation at 10000 g for 15 min.
Assays of marker enzymes
The purity of isolated mitochondria was characterized enzymatically by measuring, in both granule- and Percoll-purified mitochondrial fractions, the activities of acid phosphatase (a lysosomal marker), urate oxidase (a peroxisomal marker), and fumarase and sulphite oxidase (mitochondrial markers), as described previously [19–22]. The activities of the marker enzymes in the mitochondrial fraction were expressed as percentages of total activities observed in the granule fraction.
Measurement of superoxide dismutase activity
Superoxide dismutase activity was assayed by generating O2•− with the xanthine/xanthine oxidase system in the presence of DMPO [11], acetylated cytochrome c [23] or sulphonated tetrazolium salt (XTT) [24]. Cyanide (5 mM) was used to inhibit selectively the activity of intermembrane space Cu,Zn-superoxide dismutase. Superoxide dismutase activity was also assayed in native gels following electrophoresis by NBT (Nitro Blue Tetrazolium) staining [25].
EPR spectroscopy
EPR spectra were obtained with a Bruker ECS 106 spectrometer (operating at X-band) equipped with a cylindrical cavity operating in TM110 mode. Samples were transferred to bottom-sealed Pasteur pipettes (volume, 150 μl), and EPR spectra were obtained at room temperature (22 °C) with the following settings: microwave frequency, 9.77 GHz; microwave power, 20 mW; field modulation frequency, 100 kHz; field modulation amplitude, 2 G; receiver gain, 1×106; time constant, 164 ms; scan rate, 1.9 G·s−1. The spectra shown were the result of four accumulations starting at 5 min after mixing the reaction components. Computer simulations of the spectra were obtained using the WinSIM program. DMPO, used in EPR experiments, was dissolved in water containing 0.1 mM DETAPAC (diethylenetriaminepenta-acetic acid) and was purified several times with activated charcoal; DMPO concentration was calculated spectrophotometrically (ε232=7700 M−1·cm−1); the stock solution was kept under helium at −20 °C.
Fluorescence spectroscopy
Fluorescence measurements were performed on a PerkinElmer LS-5 spectrofluorometer. H2O2 formation was measured by monitoring horseradish-peroxidase-catalysed H2O2-dependent oxidation of p-hydroxyphenylacetate (λexcitation=320 nm; λemission=400 nm) [26].
Modulation of redox state of mitochondrial thiol groups
Thiol groups of mitochondria were alkylated with either IAM [27] or NEM [28]. Mitochondria were incubated with 100 mM IAM in the presence of digitonin for 15 min. Intermembrane space content was then isolated by centrifugation, its pH was adjusted to 8.9, and incubated further at room temperature for 24 h. Alternatively, mitochondria (10 mg/ml) were incubated with 20 mM NEM for 1 h at room temperature in the presence of digitonin. In order to oxidize thiol groups, mitochondria (2 mg/ml) were treated with various concentrations of H2O2 for 5 min at 37 °C; the organelles were then washed and incubated with IAM (100 mM) in the presence of digitonin.
Data
Data are reported as means±S.D. of at least three independent experiments.
RESULTS
Isolation and purity of mitochondrial preparations
The activities of marker enzymes for lysosomes, peroxisomes, and mitochondria were measured both in the granule- and Percoll-purified mitochondrial preparations. The activities of selected marker enzymes in mitochondrial preparations were expressed as percentages of total activity observed in the granule fraction before centrifugation in Percoll gradients (Table 1). The identity of isolated mitochondrial fractions was confirmed by the presence of high levels of fumarase and sulphite oxidase activities, located in mitochondrial matrix and intermembrane space respectively. The high purity of the mitochondria preparation was confirmed by almost no detectable lysosomal or peroxisomal enzyme activities (Table 1).
Table 1. Marker proteins and their activity in isolated rat liver mitochondria.
Assay conditions are described in the Experimental section. IMS, intermembrane space preparation; n.d., not detected.
| Activity (%) | |||
|---|---|---|---|
| Enzyme | Location | Granule fraction | Mitochondrial fraction |
| Acid phosphatase | Lysosomes | 100 | 0.5±0.1 |
| Urate oxidase | Peroxisomes | 100 | n.d. |
| Fumarase | Mitochondrial matrix | 100 | 95±4 |
| Sulphite oxidase | Mitochondrial IMS | 100 | 80±5 |
Intermembrane space Cu,Zn-superoxide dismutase is inactive in intact mitochondria
Competitive kinetic studies were conducted to assess whether or not intact mitochondria scavenged O2•− generated externally by the xanthine/xanthine oxidase system in the presence of DMPO, XTT or acetylated cytochrome c.
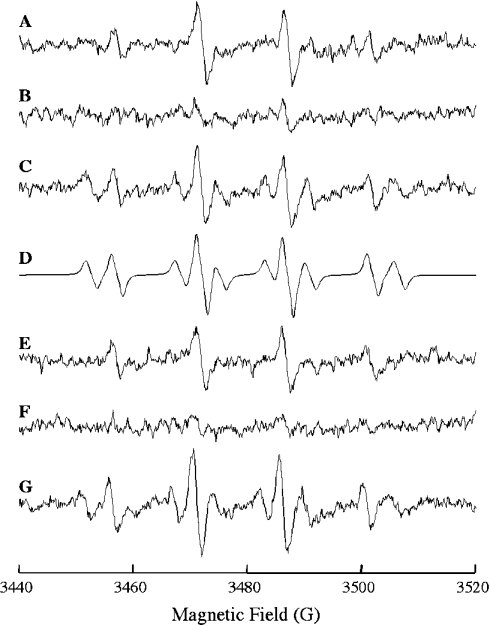
EPR experiments were performed using a concentration of xanthine that was directly proportional to the EPR signal intensity [29]. An EPR signal characteristic of the DMPO–OH spin adduct (quartet signal with intensity proportions of 1:2:2:1; aN=aHβ=14.9 G) was observed when DMPO was added to the xanthine/xanthine oxidase mixture (Figure 1A). This signal was abolished following addition of bovine Cu,Zn-superoxide dismutase (Figure 1B), and was recovered upon inhibition of the dismutase with cyanide (Figure 1C), thus establishing O2•− as the source of the DMPO–OH signal [30]. Hence, the DMPO–OH adduct observed corresponds largely to the spontaneous decay of the superoxide anion adduct (DMPO–OOH). Figure 1(D) presents the computer simulation of spectrum in Figure 1(C), assuming a mixture containing a 0.56 molar ratio of DMPO–OH (aN=aHβ=14.9 G) and 0.44 of DMPO carbon-centred radical (aN=15.6 G; aHβ=22.6 G). The six-line spectrum, characteristic of a carbon-centred radical adduct with DMPO, is probably due to a DMPO–CN adduct [31]. Addition of intact mitochondria in either state 4 (Figure 1E) or state 3 (not shown) respiration did not affect EPR signal intensity, thus suggesting that Cu,Zn-superoxide dismutase in mitochondria does not scavenge externally generated O2•−. However, the DMPO–OH signal was abolished (Figure 1F) following the addition of a mitochondrial intermembrane preparation obtained by centrifugation of digitonin-treated mitochondria. Cyanide inhibited the O2•−-scavenging activity elicited by the mitochondrial intermembrane space preparation (Figure 1G).
Figure 1. EPR analysis of O2•−-scavenging activity of intact mitochondria.
The reaction mixtures consisted of: (A) 20 m-units/ml of xanthine oxidase, 7 μM xanthine and 160 mM DMPO; (B) as (A) in the presence of 0.8 μM Cu,Zn-superoxide dismutase; (C) as (B) plus 5 mM KCN; (D) computer simulation of spectrum in (C); (E) as (A) in the presence of intact mitochondria (100 μg of protein/ml); (F) as (A) in the presence of mitochondrial intermembrane space preparation (25 μg of protein/ml); and (G) as (F) plus 5 mM KCN. All reactions were carried out in sucrose/Tris buffer, pH 7.4. The DMPO–OH spin adduct was detected by EPR as described in the Experimental section.
In agreement with these findings, the rates of O2•− formation, monitored spectrophotometrically by following reduction of XTT or acetylated cytochrome c, were not affected by intact mitochondria (Table 2). The mitochondrial intermembrane preparation decreased the rate of reduction of XTT or acetylated cytochrome c. The O2•−-scavenging activity of the mitochondrial intermembrane space content was completely inhibited by cyanide (Table 2).
Table 2. Spectrophotometric analysis of O2•−-scavenging activity in intact mitochondria.
O2•−-scavenging activity of mitochondria was assessed by following the reduction of either XTT (470 nm) or acetylated cytochrome c (550 nm). The reaction mixtures consisted of 20 m-units/ml xanthine oxidase, 50 μM xanthine, and 750 μM XTT or 10 μM acetylated cytochrome c in either sucrose/Tris buffer (in the absence of KCN) or phosphate buffer (50 mM potassium phosphate and 0.1 mM EDTA, pH 7.8) (in the presence of KCN). Where indicated, the following were added to the reaction mixture: bovine Cu,Zn-superoxide dismutase (0.4 μM); intact mitochondria (100 μg of protein/ml); intermembrane space preparation (25 μg of protein/ml); KCN (5 mM). KCN at a concentration of 20 μM was added to the reaction mixture described in the third column in order to inhibit cytochrome c oxidase activity. n.d., not determined.
| XTT reduction (μM/min) | Acetylated cytochrome c reduction (μM/min) | |||
|---|---|---|---|---|
| Assay conditions | −KCN | +KCN | −KCN | +KCN |
| No additions | 0.57±0.04 | 0.60±0.05 | 1.26±0.10 | 1.30±0.12 |
| Mitochondria | 0.55±0.04 | n.d. | 1.20±0.10 | n.d. |
| Intermembrane space | 0.33±0.03 | 0.56±0.05 | 0.65±0.06 | 1.21±0.10 |
| Bovine Cu,Zn-SOD | 0.005±0.0001 | 0.48±0.04 | 0.01±0.0002 | 1.10±0.10 |
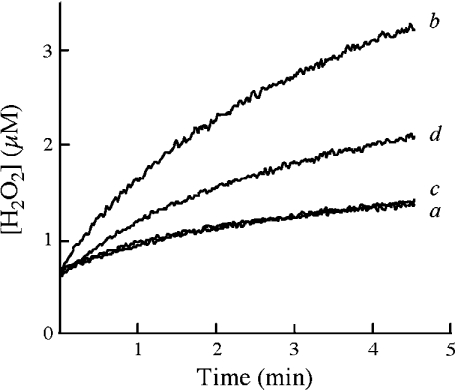
Considering that O2•− can diffuse across the mitochondrial outer membrane [11–13], these findings (Figure 1E and Table 2) suggest that the intermembrane Cu,Zn-superoxide dismutase is inactive in intact mitochondria, but it is activated following selective disruption of the outer membrane by digitonin (Figure 1F and Table 2). This notion was confirmed further by experiments addressing the dismutation of externally generated O2•− by mitochondria. O2•− generated by the xanthine/xanthine oxidase system partially dismutates to H2O2, which was detected by a peroxide-based assay (Figure 2, a). In addition, the xanthine/xanthine oxidase system is known to generate H2O2 directly [30], which contributes to the oxidation of p-hydroxyphenylacetate, the fluorescent probe in the peroxidase-based assay. Bovine Cu,Zn-superoxide dismutase converted all O2•− into H2O2, thereby greatly increasing the rate of p-hydroxyphenylacetate oxidation (Figure 2, b). Intact mitochondria did not alter the rate of H2O2 production (Figure 2, c), whereas the mitochondrial intermembrane preparation significantly increased H2O2 production (Figure 2, d). These findings strengthen the notion that loss of the mitochondrial outer membrane integrity is required for activation of Cu,Zn-superoxide dismutase.
Figure 2. Analysis of O2•− dismutation to H2O2 by intact mitochondria.
H2O2 formation was measured by monitoring horseradish-peroxidase-catalysed H2O2-dependent oxidation of p-hydroxyphenylacetate. Reactions were carried out in sucrose/Tris buffer, pH 7.4, containing 5 m-units/ml xanthine oxidase, 50 μM xanthine, 5 m-units/ml horseradish peroxidase and 1 mM p-hydroxyphenylacetate, with (a) no further additions; (b) 4 μM bovine Cu,Zn-superoxide dismutase; (c) intact mitochondria (100 μg of protein/ml); or (d) mitochondrial intermembrane space content preparation (10 μg of protein/ml).
Thiol groups play a key role in activation of intermembrane Cu,Zn-superoxide dismutase
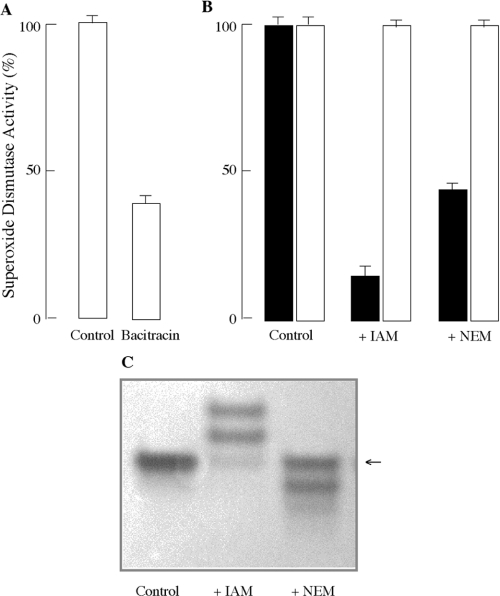
Thiol–disulphide oxidoreductases catalyse thiol–disulphide exchange reactions that result in the formation or reduction of protein disulphide bonds. Protein disulphide-isomerase is a thiol–disulphide oxidoreductase that was recently shown to be located in mitochondrial outer membrane. Bacitracin (10 mM) [32], an inhibitor of protein disulphide-isomerase, considerably decreased intermembrane space Cu,Zn-superoxide dismutase activity (60±5%) (Figure 3A) during digitonin treatment of mitochondria. This suggests that disulphide bond formation, catalysed at least in part by protein disulphide-isomerase, may be indeed the underlying basis for activation of intermembrane Cu,Zn-superoxide dismutase during disruption of the outer membrane by digitonin. Consistent with these findings, incubation of the mitochondrial intermembrane preparation with 4 mM dithiothreitol for 2 h at 37 °C completely inactivated intermembrane space Cu,Zn-superoxide dismutase (results not shown).
Figure 3. Thiol modification and mitochondrial Cu,Zn-superoxide dismutase activity.
(A) Mitochondrial protein disulphide-isomerase activates Cu,Zn-superoxide dismutase following outer membrane disruption. Mitochondria were incubated with digitonin in the presence or absence of 10 mM bacitracin for 1 h at room temperature. Superoxide dismutase activity was determined spectrophotometrically in the mitochondrial intermembrane preparation by following reduction of XTT at 470 nm. (B) Alkylation of thiol groups prevents activation of intermembrane space Cu,Zn-superoxide dismutase during disruption of the mitochondrial outer membrane. Intact mitochondria (■) or intermembrane space preparation (□) were incubated with either IAM or NEM for 1 h at room temperature. The incubation of intact mitochondria with alkylating agents was carried out in the presence of digitonin. Cu,Zn-superoxide dismutase activity was then assessed by monitoring XTT reduction at 470 nm. (C) NBT staining of gels following electrophoresis under non-denaturing conditions. Intact mitochondria were treated as described in (B). The arrow (←) indicates the band for intermembrane space Cu,Zn-superoxide dismutase.
Thiol alkylating agents, such as IAM or NEM, elicited two different effects on mitochondrial Cu,Zn-superoxide dismutase activity depending on the mitochondrial preparation: (i) treatment of intact mitochondria with alkylating agents in the presence of digitonin substantially decreased the activity of intermembrane space Cu,Zn-superoxide dismutase (Figure 3B); this is expected when considering that the enzyme occurs in the reduced (thiol moieties) state in intact mitochondria; (ii) treatment of mitochondrial intermembrane preparation with either NEM or IAM did not affect intermembrane space Cu,Zn-superoxide dismutase activity (Figure 3B), because the enzyme was already in its oxidized (disulphide bonds) state. These findings suggest that thiol groups are critical in the activation process of intermembrane space Cu,Zn-superoxide dismutase during disruption of the mitochondrial outer membrane, and that once intermembrane space Cu,Zn-superoxide dismutase is activated (thiol→disulphide transition), the thiol groups are no longer sensitive to the alkylating agents.
The role of the redox state of cysteine groups in the activation of intermembrane space superoxide dismutase was also assessed by examining its O2•−-scavenging activity in native polyacrylamide gels following electrophoresis by NBT staining. Intact mitochondria revealed a single band in inverted gels with an electrophoretic behaviour similar to that of Cu,Zn-superoxide dismutase (Figure 3C). The O2•−-scavenging activity of this band was inhibited completely by 5 mM KCN, thus indicating the Cu,Zn nature of superoxide dismutase (results not shown). Prior treatment of mitochondria with NEM or IAM substantially decreased the O2•−-scavenging activity of intermembrane space superoxide dismutase, thus supporting the critical role of thiol groups in the enzyme activation (Figure 3C). In IAM-treated mitochondria, however, several bands with residual O2•−-scavenging activity were observed; although the exact identity of these upper bands is unknown, they may be ascribed to intermembrane space Cu,Zn-superoxide dismutase with different extents of alkylation. Under non-denaturing conditions, the movement of proteins through the polyacrylamide gel matrix depends on a combination of their size, shape and charge. Cu,Zn-superoxide dismutase possesses three cysteine residues (6, 57 and 146), of which Cys6 is in the reduced form and is not involved in superoxide dismutase activity. With a pKa of approx. 8.5, the thiol in Cys6 is likely to be ionized, thereby contributing to the overall charge of Cu,Zn-superoxide dismutase. Different levels of alkylation of Cys6 thiol in the superoxide dismutase homodimer may decrease the number of negative charges on the enzyme and thus slow its mobility in native electrophoresis. Therefore the change in the electrophoretic mobility of superoxide dismutase following treatment with IAM could be accounted for, in part, by changes in charge of the enzyme, rather than by its molecular mass or shape. In support of this notion, similar bands with substantially greater intensities were observed when intermembrane space superoxide dismutase was treated with IAM after disruption of mitochondrial outer membrane.
H2O2 modulates the activity of intermembrane space Cu,Zn-superoxide dismutase
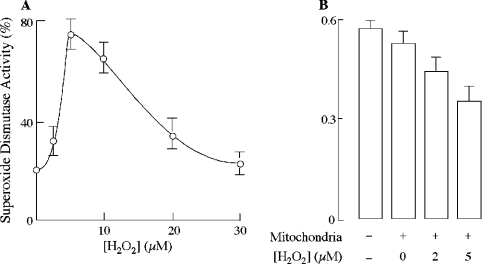
Pre-incubation of intact mitochondria with low micromolar concentrations of H2O2 prevented the inactivation of intermembrane space Cu,Zn-superoxide dismutase following disruption of the outer membrane in the presence of IAM (Figure 4A). Cyanide (5 mM) inhibited this activation, thus suggesting the Cu,Zn nature of the dismutase. A bell-shaped enzyme activation response to H2O2 concentrations was observed, with a maximum effect at 5×10−6 M H2O2 (75±6%) (Figure 4A). It is noteworthy that treatment with H2O2 alone enabled intact mitochondria to scavenge externally generated superoxide (Figure 4B). It may be surmised that an oxidative modification of thiol groups of Cu,Zn-superoxide dismutase is required for its activation in the mitochondrial intermembrane space.
Figure 4. Effect of H2O2 on intermembrane space Cu,Zn-superoxide dismutase activation.
(A) Mitochondria were incubated with the indicated concentrations of H2O2 for 5 min at 37 °C, washed with sucrose/Tris buffer, pH 7.4, and incubated with digitonin in the presence of IAM for 1 h at room temperature. Cu,Zn-superoxide dismutase activity was assayed in the mitochondrial intermembrane space preparation by following the reduction of XTT at 470 nm, and was expressed as percentage of fully activated enzyme prepared by rupturing the outer membrane with digitonin alone. (B) Mitochondria were incubated with indicated concentrations of H2O2 for 5 min at 37 °C. Intermembrane space Cu,Zn-superoxide dismutase activity was then measured spectrophotometrically in intact mitochondria following XTT reduction at 470 nm. Reactions were carried out in the sucrose/Tris buffer consisting of 20 m-units/ml xanthine oxidase, 50 μM xanthine and 750 μM XTT. Where indicated, intact mitochondria were added at a concentration of 100 μg of protein/ml.
DISCUSSION
Recent studies confirmed the localization of Cu,Zn-superoxide dismutase in the mitochondrial intermembrane space of both rat liver [7,8] and yeast [9], and, thereby, ended a 30-year debate following the initial report on the presence of this enzyme in the intermembrane space [33]. The activity of mitochondrial superoxide dismutase is almost equally partitioned between the matrix (Mn-superoxide dismutase) and the intermembrane space (Cu,Zn-superoxide dismutase) [8,12,13] compartments, thus suggesting that the intermembrane space could be one of the major sites of exposure to O2•−. The mitochondrial inner membrane vectorially releases O2•− into the intermembrane space [11] upon autoxidation of the ubisemiquinone at the side O of the cytochrome bc1 complex; intermembrane space O2•− can be released by mitochondria into cytosol via voltage-dependent anion channels [4]. Likewise, cytosolic O2•− can diffuse across the outer membrane [4,12,13]. The NAD(P)H oxidase activity of the apoptosis-inducing factor represents another source of O2•− formation in the intermembrane space [34]. The occurrence of a steady-state level of O2•− in this compartment is expected to be in a functional relationship with the presence of Cu,Zn-superoxide dismutase activity. However, the present study indicates that Cu,Zn-superoxide dismutase is inactive in intact mitochondria and is activated upon oxidation of its thiol groups, thus suggesting a redox-based regulation of the intermembrane space Cu,Zn-superoxide dismutase activity.
Cu,Zn-superoxide dismutase is inactive in mitochondrial intermembrane space
Folding, metal insertion and thiol oxidation of cytosolic Cu,Zn-superoxide dismutase is a complex process that is only partially understood. Yeast Cu,Zn-superoxide dismutase is imported into yeast mitochondria in an immature form that lacks both Cu2+ and Zn2+ and possesses reduced cysteine residues [10]. While the mechanism for Zn2+ insertion is not known, the copper chaperone for Cu,Zn-superoxide dismutase loads Cu2+ into cytosolic Cu,Zn-superoxide dismutase [35]. Mechanistic studies demonstrated that the copper chaperone for Cu,Zn-superoxide dismutase is localized in yeast mitochondria where it plays a key role for efficient uptake of Cu2+ and its insertion into nascent Cu,Zn-superoxide dismutase [10]. The biochemical pathways for oxidation of critical thiols of the intermembrane space Cu,Zn-superoxide dismutase are not known; however, it appears that thiol oxidation, rather than metal insertion, is the limiting step in the activation of Cu,Zn-superoxide dismutase, for the enzyme is inactive in intact mitochondria and is activated upon rupture of the mitochondrial outer membrane. The oxidation of Cys57 and Cys146, and formation of the intrasubunit disulphide bond is affected by the environmental redox status. These thiol moieties were not oxidized when eukaryotic Cu,Zn-superoxide dismutase was expressed in the prokaryotic cytosol, the latter being a more reducing environment than the former [36]. However, oxidation of critical thiols and activation of eukaryotic Cu,Zn-superoxide dismutase accompanied its export to the prokaryotic periplasmic space, which is a more oxidizing environment than the prokaryotic cytosol, or when it was expressed in the cytosol of prokaryotic cells lacking thioredoxin reductase [36].
Competitive kinetic studies (Figure 1 and Table 2) revealed that intact rat liver mitochondria neither scavenge nor dismutate externally generated O2•−. In agreement with earlier findings [12–15], disruption of the mitochondrial outer membrane led to recovery of the O2•−-scavenging (Figure 1 and Table 2) and -dismutating (Figure 2) activities, which were sensitive to cyanide, thus indicating the Cu,Zn nature of the dismutase. The activation of intermembrane space Cu,Zn-superoxide dismutase upon pre-treatment of intact mitochondria with H2O2 (Figure 4A) and its inactivation following treatment of mitochondria with alkylating agents during disruption of the outer membrane (Figure 3B) suggest that all post-translational modifications necessary for an active state of the enzyme have taken place in mitochondria, except formation of the intrasubunit disulphide bond. The steady-state levels of O2•− and H2O2 in mitochondria and cytosol are similar at 10−9 M and 10−7 M respectively [37]. However, a more reducing mitochondrial environment, compared with the cytosol, might be the underlying reason for the inactive state of Cu,Zn-superoxide dismutase. Liver mitochondria possess both GSH/glutathione reductase and thioredoxin/thioredoxin reductase systems [38,39], which provide reducing equivalents to protect lipid membranes against oxidative damage, permeabilization and, in the case of the inner membrane, loss of the membrane potential. However, the overall redox potential of the mitochondrial inter-membrane space has not been quantitatively compared with those of the mitochondrial matrix and the cytosol.
Mechanism of intermembrane space Cu,Zn-superoxide dismutase activation
Cellular enzymes known as thiol–disulphide oxidoreductases catalyse thiol–disulphide exchange reactions to promote the formation or reduction of protein disulphide bonds. The prototype of this group of enzymes is protein disulphide-isomerase [40]. Recently, protein disulphide-isomerase was demonstrated to be located in the mitochondrial outer membrane [41,42]. However, whether it resides in the cytosolic or intermembrane space side of the outer membrane is not known. Activation of intermembrane space Cu,Zn-superoxide dismutase upon disruption of outer mitochondrial membrane by digitonin is indicative of gain of access of intermembrane space Cu,Zn-superoxide dismutase to thiol–disulphide oxidoreductases that catalyse disulphide bond formation. Bacitracin [32], a well known inhibitor of protein disulphide-isomerase, partially inhibited intermembrane space Cu,Zn-superoxide dismutase activation following outer membrane disruption (Figure 3A), thus suggesting that protein disulphide-isomerase may constitute a significant part of the thioldisulphide oxidoreductase activities that are possibly located in the cytosolic side of outer mitochondrial membrane. However, it remains to be elucidated whether or not thiol–disulphide oxido-reductases serve to activate intermembrane space Cu,Zn-superoxide dismutase following its release into the cytosol, as may be the case in, for example, the apoptotic disruption of mitochondrial outer membrane. Likewise, it remains to be assessed whether or not cytosolic activation of intermembrane space Cu,Zn-superoxide dismutase is implicated in the regulation of early events of programmed cell death.
The biochemical pathways that are implicated in the activation of Cu,Zn-superoxide dismutase in the intermembrane space are not known. Exposure of intact mitochondria to low concentrations (10−6 M) of H2O2 led to intermembrane space Cu,Zn-superoxide dismutase activation (Figure 4). These low peroxide levels suggest the involvement of an enzyme-mediated thiol oxidation rather than direct oxidation by H2O2. Potential candidates to catalyse such a reaction are thiol oxidases, such as phospholipid hydroperoxide glutathione peroxidase [43], and mitochondrial peroxiredoxins (III and/or V), a family of peroxidases that reduce H2O2 to water with the use of reducing equivalents provided by thiol-containing proteins [44–46]. H2O2 may serve as a substrate for phospholipid hydroperoxide glutathione peroxidase located on the inner mitochondrial membrane, where it may catalyse the oxidation of intermembrane space Cu,Zn-superoxide dismutase. However, it is not known whether the KM of this enzyme for H2O2 is sufficiently low to function at low micromolar concentrations of this substrate. Mitochondrial peroxiredoxins have a very low KM (∼2 μM) for H2O2, and are inactivated by hyperoxidation of their catalytic cysteine residues upon exposure to higher concentrations of H2O2 [47]. Exposure of HeLa or A549 cells to 100 μM H2O2 for 10 min reversibly inactivated mitochondrial peroxiredoxins [48]. This feature of mitochondrial peroxiredoxins may also explain the observed bell-shaped enzyme activation response to H2O2 (Figure 4A), where concentrations higher than 10 μM were less effective in activating intermembrane space Cu,Zn-superoxide dismutase, probably due to a hyperoxidative inactivation of mitochondrial peroxiredoxins. Because the thioredoxin reductase system catalyses the reduction of protein disulphide bonds [49], the activity of intermembrane space Cu,Zn-superoxide dismutase may be regulated through redox changes inherent in the thiol–disulphide conversion as an intermediate in the mitochondrial peroxiredoxin/thioredoxin reductase system. Although peroxiredoxin III and V, and thioredoxin reductase reside in mitochondria, their submitochondrial localization is not known. It is an intriguing possibility that the reversible activation of Cu,Zn-superoxide dismutase in the intermembrane space may function to divert O2•− from its reaction with cytochrome c to form a more diffusible oxidant, H2O2, which can act as a redox signal both in mitochondria and cytosol.
Acknowledgments
This work was supported by grants RO1 ES011342 and RO1 AG16718 from NIH (National Institutes of Health) and a grant from the L. K. Whittier Foundation.
References
- 1.Cadenas E., Davies K. J. A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 2.Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 3.Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. M. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 4.Han D., Antunes F., Canali R., Rettori D., Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 5.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 6.Weisiger R. A., Fridovich I. Superoxide dismutase: organelle specificity. J. Biol. Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 7.Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 8.Iñarrea P. Purification and determination of activity of mitochondrial cyanide-sensitive superoxide dismutase in rat tissue extract. Methods Enzymol. 2002;349:106–114. doi: 10.1016/s0076-6879(02)49326-3. [DOI] [PubMed] [Google Scholar]
- 9.Sturtz L. A., Diekert K., Jensen L. T., Lill R., Culotta V. C. A fraction of yeast Cu/Zn superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria: a physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 10.Field L. S., Furukawa Y., O'Halloran T. V., Culotta V. C. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J. Biol. Chem. 2003;278:28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 11.Han D., Williams E., Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2000;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyler D. D. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem. J. 1975;147:493–504. doi: 10.1042/bj1470493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeters-Joris C., Vandevoorde A.-M., Baudhuin P. Subcellular localization of superoxide dismutase in rat liver. Biochem. J. 1975;150:31–39. doi: 10.1042/bj1500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotilio G., Calabrese L., Finazzi Agro A., Argento-Ceru M. P., Autuori F., Mondovi B. Intracellular localization of superoxide dismutase and its relation to the distribution and mechanism of hydrogen peroxide-producing enzymes. Biochim. Biophys. Acta. 1973;15:98–102. doi: 10.1016/0005-2744(73)90063-6. [DOI] [PubMed] [Google Scholar]
- 15.Lindmark D. G., Muller M. Superoxide dismutase in the anaerobic flagellates, Tritrichomonas foetus and Monocercomonas sp. J. Biol. Chem. 1974;249:4634–4637. [PubMed] [Google Scholar]
- 16.Petit P. X., Goubern M., Diolez P., Susin S. A., Zamzani N., Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 18.Saidha T., Stern A. L., Lee D. H., Schiff J. A. Localization of a sulphate-activating system within Euglena mitochondria. Biochem. J. 1985;232:357–365. doi: 10.1042/bj2320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luchter-Wasylewska E. Continuous assay for acid phosphatase using phenyl phosphate. Anal. Biochem. 1996;241:167–172. doi: 10.1006/abio.1996.0394. [DOI] [PubMed] [Google Scholar]
- 20.Hemsley A., Pegg M., Crane D., Masters C. On the compartmentalization of catalase, fatty acyl-CoA oxidase and urate oxidase in mammalian livers, and the influence of clofibrate treatment on this microlocalization. Mol. Cell Biochem. 1988;83:187–194. doi: 10.1007/BF00226146. [DOI] [PubMed] [Google Scholar]
- 21.Gardner P. R., White C. W. Application of the aconitase method to the assay of superoxide in the mitochondrial matrices of cultured cells: effects of oxygen, redox cycling agent, TNF-α, IL-1, LPS and inhibitors of respiration. In: Davies K. J. A., Ursini F., editors. The Oxygen Paradox. Padova: Cleup University Press; 1995. pp. 33–50. [Google Scholar]
- 22.Garrett R. M., Rajagopalan K. V. Molecular cloning of rat liver sulfite oxidase: expression of a eukaryotic Mo-pterin-containing enzyme in Escherichia coli. J. Biol. Chem. 1994;269:272–276. [PubMed] [Google Scholar]
- 23.McCord J. M., Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 24.Ukeda H., Maeda S., Ishii T., Sawamura M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}–bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine–xanthine oxidase. Anal. Biochem. 1997;251:206–209. doi: 10.1006/abio.1997.2273. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 26.Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal. Biochem. 1984;141:280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- 27.Galvani M., Hamdan M., Herbert B., Righetti P. G. Alkylation kinetics of proteins in preparation for two-dimensional maps: a matrix assisted laser desorption/ionization-mass spectrometry investigation. Electrophoresis. 2001;22:2058–2065. doi: 10.1002/1522-2683(200106)22:10<2058::AID-ELPS2058>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Weaver B. K., Kumar K. P., Reich N. C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders S. P., Harrison S. J., Kuppusamy P., Sylvester J. T., Zweier J. L. A comparative study of EPR spin trapping and cytochrome c reduction techniques for the measurement of superoxide anions. Free Radical Biol. Med. 1994;16:753–761. doi: 10.1016/0891-5849(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 30.Fridovich I. Xanthine oxidase. In: Greenwald R. A., editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 51–53. [Google Scholar]
- 31.Moreno S. N., Stolze K., Janzen E. G., Mason R. P. Oxidation of cyanide to the cyanyl radical by peroxidase/H2O2 systems as determined by spin trapping. Arch. Biochem. Biophys. 1988;265:267–271. doi: 10.1016/0003-9861(88)90127-0. [DOI] [PubMed] [Google Scholar]
- 32.Roth R. A. Bacitracin: an inhibitor of the insulin degrading activity of glutathione-insulin transhydrogenase. Biochem. Biophys. Res. Commun. 1981;98:431–438. doi: 10.1016/0006-291x(81)90858-5. [DOI] [PubMed] [Google Scholar]
- 33.Weisiger R. A., Fridovich I. Mitochondrial superoxide dismutase: site of synthesis and intramitochondrial localization. J. Biol. Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 34.Miramar M. D., Constantini P., Ravagnan L., Saraiva L. M., Haouzi D., Brothers G., Penninger J. M., Peleato M. L., Kroemer G., Susin S. A. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 2001;276:16391–16398. doi: 10.1074/jbc.M010498200. [DOI] [PubMed] [Google Scholar]
- 35.Culotta V. C., Klomp L. W., Strain J. J., Casareno R. L., Krems B., Gitlin J. D. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 36.Battistoni A., Mazzetti A. P., Rotilio G. In vivo formation of Cu,Zn superoxide dismutase disulfide bond in Escherichia coli. FEBS Lett. 1999;443:313–316. doi: 10.1016/s0014-5793(98)01725-6. [DOI] [PubMed] [Google Scholar]
- 37.Boveris A., Cadenas E. Cellular sources and steady-state levels of reactive oxygen species. In: Clerch L. B., Massaro D. J., editors. Oxygen, Gene Expression, and Cellular Function. New York: Marcel Dekker; 1997. pp. 1–25. [Google Scholar]
- 38.Wudarczyk J., Debska G., Lenartowicz E. Relation between the activities reducing disulfides and the protection against membrane permeability transition in rat liver mitochondria. Arch. Biochem. Biophys. 1996;327:215–221. doi: 10.1006/abbi.1996.0112. [DOI] [PubMed] [Google Scholar]
- 39.Rigobello M. P., Callegaro M. T., Barzon E., Benetti M., Bindoli A. Purification of mitochondrial thioredoxin reductase and its involvement in the redox regulation of membrane permeability. Free Radical Biol. Med. 1998;24:370–376. doi: 10.1016/s0891-5849(97)00216-5. [DOI] [PubMed] [Google Scholar]
- 40.Sevier C. S., Kaiser C. A. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari D. M., Soling H. D. The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J. 1999;339:1–10. [PMC free article] [PubMed] [Google Scholar]
- 42.Rigobello M. P., Donella-Deana A., Cesaro L., Bindoli A. Distribution of protein disulphide isomerase in rat liver mitochondria. Biochem. J. 2001;356:567–570. doi: 10.1042/0264-6021:3560567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takebe G., Yarimizu J., Saito Y., Hayashi T., Nakamura H., Yodoi J., Nagasawa S., Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J. Biol. Chem. 2002;277:41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 44.Seo M. S., Kang S. W., Kim K., Baines I. C., Lee T. H., Rhee S. G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- 45.Kang S. W., Chae H. Z., Seo M. S., Kim K., Baines I. C., Rhee S. G. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J. Biol. Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 46.Watabe S., Hiroi T., Yamamoto Y., Fujioka Y., Hasegawa H., Yago N., Takahashi S. Y. SP-22 is a thioredoxin-dependent peroxide reductase in mitochondria. Eur. J. Biochem. 1997;249:52–60. doi: 10.1111/j.1432-1033.1997.t01-1-00052.x. [DOI] [PubMed] [Google Scholar]
- 47.Wood Z. A., Poole L. B., Karplus P. A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 48.Woo H. A., Kang S. W., Kim H. K., Yang K. S., Chae H. Z., Rhee S. G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid: immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 49.Wood Z. A., Schroder E., Robin Harris J., Poole L. B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]