Abstract
The chimaeric protein Bcr/Abl, the hallmark of chronic myeloid leukaemia, has been connected with several signalling pathways, such as those involving protein kinase B/Akt, JNK (c-Jun N-terminal kinase) or ERKs (extracellular-signal-regulated kinases) 1 and 2. However, no data about the p38 MAPK (mitogen-activated protein kinase) have been reported. Here, we present evidence showing that Bcr/Abl is able to modulate this signalling pathway. Transient transfection experiments indicated that overexpression of Bcr/Abl in 293T cells is able to activate p38 MAPK or induce p73 stabilization, suggesting that c-Abl and Bcr/Abl share some biological substrates. Interestingly, the control exerted by Bcr/Abl on the p38 MAPK pathway was not only mediated by the tyrosine kinase activity of Bcr/Abl, as the use of STI571 demonstrated. In fact, Bcr alone was able to induce p38 MAPK activation specifically through MKK3 (MAP kinase kinase 3). Supporting these observations, chronic myeloid leukaemia-derived K562 cells or BaF 3 cells stably transfected with Bcr/Abl showed higher levels of phosphorylated p38 MAPK compared with Bcr/Abl-negative cells. While Bcr/Abl-negative cells activated p38 MAPK in response to Ara-C (1-β-D-arabinofuranosylcytosine), Bcr/Abl-positive cells were unable to activate p38 MAPK, suggesting that the p38 MAPK pathway is not sensitive to Abl-dependent stimuli in Bcr/Abl-positive cells. Our results demonstrate that the involvement of Bcr/Abl in the p38 MAPK pathway is a key mechanism for explaining resistance to Ara-C, and could provide a clue for new therapeutic approaches based on the use of specific Abl inhibitors.
Keywords: 1-β-D-arabinofuranosylcytosine (Ara-C), Bcr/Abl protein, leukaemia, p38 MAPK (mitogen-activated protein kinase), phosphorylation
Abbreviations: Ara-C, 1-β-D-arabinofuranosylcytosine; BCR, break-point cluster region; CML, chronic myeloid leukaemia; ERK, extracellular-signal-regulated kinase; GFP, green fluorescent protein; HA, haemagglutinin; IL-3, interleukin-3; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MKK, MAP kinase kinase; PI3K, phosphoinositide 3-kinase; SAPK, stress-activated protein kinase
INTRODUCTION
Chronic myeloid leukaemia (CML) is one of the most studied human malignancies. Originating in a haematopoietic stem progenitor cell by a reciprocal translocation between chromosome 9 and 22 t(9:22)(q34:11), it produces the Philadelphia chromosome. This translocation joins the c-Abl tyrosine kinase on chromosome 9, and the BCR (break-point cluster region) on chromosome 22. The consequence of this translocation is the production of a chimaeric Bcr/Abl protein with deregulated activity, which plays an essential role in the pathogenesis of the disease (for a review, see [1]).
Deregulation induced by this chimaeric protein affects the function of several signalling pathways implicated in the malignant phenotype. Consistent with this, activation of the JNK (c-Jun N-terminal kinase), ERKs (extracellular-signal-regulated kinases) 1 and 2, PI3K (phosphoinositide 3-kinase)/Akt (or protein kinase B) and STAT5 pathways by Bcr/Abl has been reported as a mechanism of survival and proliferation independent of growth factors [2–4]. However, no clear relationship has been established with the p38 MAPK (mitogen activated protein kinase) in terms of signal transduction, although recent evidence supports a role for p38 MAPK in interferon response in Bcr/Abl-positive cells [5]. This kinase, one of the SAPKs (stress-activated protein kinases), has been shown to be activated by different types of stress, including genotoxic stress mediated by chemotherapy. Furthermore, this kinase is also implicated in cellular processes, such as cell cycle regulation, differentiation and morphogenesis (for a review, see [6–9]). Moreover, it has recently been demonstrated that this pathway can control the phosphorylation status of the tumour suppressor protein p53 after DNA damage [10], and that blockage of this pathway in the presence of oncogenic Ras can be a mechanism of tumour promotion [11]. Among several molecules upstream of this pathway, c-Abl has been shown to be one of the key regulators of the p38 MAPK pathway in the activation mediated by genotoxic stress induced by DNA-damaging agents such as cisplatin or Ara-C (1-β-D-arabinofuranosylcytosine; for a review, see [12]). It should be mentioned that Ara-C is a classic anti-neoplasic agent widely used in the treatment of CML blast crisis, which provides a typical example of genotoxic stress mediated through p38 MAPK and c-Abl [13–16].
Therefore we decided to investigate the possible connection between Bcr/Abl and the p38 MAPK pathway, and the implications of this in terms of Ara-C-based therapy. Our results demonstrate a connection between Bcr/Abl and the p38 MAPK pathway, which mediates resistance to Ara-C in CML cells.
EXPERIMENTAL
Chemicals and antibodies
Antibodies against the phosphorylated forms of p38 MAPK and MKK (MAP kinase kinase) 3/6 were purchased from Cell Signalling Technology. Antibodies against the non-phosphorylated forms of p38 MAPK, MKK3, Bcr and HA (haemagglutinin) were purchased from Santa Cruz Biotechnology. Antibodies against Abl and E1a were from Oncogene Science, phosphotyrosine (4G10) was from Upstate Biotech, SKF86002 was from Calbiochem and Ara-C was from Pharmacia. STI571 was kindly supplied by Elisabeth Buchdunger (Novartis-Pharma, Basel, Switzerland).
Cell lines and plasmids
293T cells were maintained in 5% CO2 at 37 °C in Dulbecco's modified Eagle's medium (BioWhittaker) supplemented with 10% (v/v) fetal bovine serum plus antibiotics (BioWhittaker). BaF3, BaF3210 (kindly supplied by Dr Pérez Roger, University of Cardenal Herrera-CEU, Valencia, Spain), U937 and K562 cells were maintained in RPMI medium supplemented with 10% fetal bovine serum plus antibiotics. BaF3 cells were supplemented with 10% conditioned medium from Wehi cells. Plasmids for c-Abl, p38 MAPK, GFP (green fluorescent protein), c-Abl and p73 have been described previously [17]. HA-tagged MKK3 and MKK6 were kindly supplied by Professor J. Woodgett (Experimental Therapeutics, Ontario Cancer Center, Toronto, ON, Canada). The expression vector for Bcr/Abl (p210) was kindly supplied by Dr J. Wang (University of San Francisco, CA, U.S.A.). The plasmid for Bcr was generously given by Dr Ralph B. Arlinghaus (MD Anderson Cancer Center, The University of Texas, TX, U.S.A.).
Western blotting and immunoprecipitation assays
Cells were treated and collected in lysis buffer [25 mM Hepes (pH 7.5)/0.3 M NaCl/1.5 mM MgCl2/0.2 mM EDTA/1% Triton X-100/0.1% SDS/0.5% deoxycholic acid/20 mM β-glycerol phosphate] in the presence of protease and phosphatase inhibitors (0.2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM PMSF and 0.1 mM Na3VO4). Samples (50 μg) were loaded on to SDS/10% polyacrylamide gels, transferred to nitrocellulose filters and blotted against the different proteins using specific antibodies against the phosphorylated form or the total protein. In the immunoprecipitation assay, extracts were pre-cleared and soluble fractions were incubated with anti-HA or anti-p38 MAPK antibody. After at least 2 h, extracts were incubated for 45 min in the presence of Protein G (Gamma-bind Sepharose; Pharmacia Biotech), and then washed three times in the same lysis buffer. Then, immunocomplexes were resuspended in loading buffer and loaded on to SDS/10% polyacrylamide gels. Western blots were quantified using Scion software.
In vitro kinase assays
Kinase assays for p38 MAPK were performed using the p38 MAPK kinase assay kit (non-radioactive) from Cell Signalling Technology. Cells were treated with Ara-c for the indicated times, and processed by following the manufacturer's instructions.
Transfections
293T cells were transiently transfected using Lipofectamine™ (Invitrogen) following the manufacturer's instructions. The total amount of DNA was normalized using an empty vector. Cells were lysed 36 h after transfection and samples were processed for immunoprecipitation or Western blot analysis, as described above.
Flow cytometry assays
Cells were washed with PBS and fixed with 70% (v/v) ethanol for 30 min at −20 °C. Then, cells were washed and finally resuspended in 1 ml of PBS with propidium iodide (10 μg/ml) and RNAse (20 μg/ml). Samples were analysed with Epics XL (Coulter Electronics). Cells with low DNA stainability (sub-G1 peak lower than of G1 cells) were considered as an apoptotic population. The apoptotic population was considered to be the peak prior to the G0/G1 population.
RESULTS
Bcr/Abl activates p38 MAPK and stabilizes p73
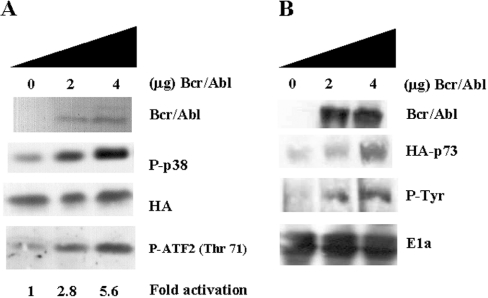
Although the chimaeric Bcr/Abl protein has been connected to other signal transduction pathways, no relationship has been demonstrated with the p38 MAPK pathway. p38 MAPK is extensively related to c-Abl in terms of DNA damage [13], and both molecules have been shown to share biological substrates such as p73 [17]. Therefore we decided to investigate whether Bcr/Abl is able to activate p38 MAPK in transient transfection assays. 293T cells were transiently transfected with HA-tagged p38 MAPKα plus GFP, as a negative control for immunoprecipitation, or Bcr/Abl. Then, 36 h later, the cells were processed to measure p38 MAPK phosphorylation. Clearly, Bcr/Abl was able to activate p38 MAPK, indicating that p38 MAPK is a downstream effector of Bcr/Abl (Figure 1A).
Figure 1. Bcr/Abl activates p38 MAPK and stabilizes p73.
(A) 293T cells were transiently transfected with HA–p38 MAPK (0.5 μg), plus GFP or Bcr/Abl (0, 2 or 4 μg) using LIPOFECTAMINE™. After transfection (36 h), cells were collected in lysis buffer. Anti-HA immunoprecipitates were immunoblotted against phospho (P)-p38 (upper centre panel), and as a loading control membranes were re-probed against HA (lower centre panel). Bcr/Abl expression was evaluated by Western blotting (top panel). In vitro kinase assays were performed under the same conditions. HA–p38 MAPK was immunoprecipitated and incubated for 30 min at 30 °C with 1 μg of GST–ATF2 fusion protein in the presence of cold ATP. Reactions were stopped by adding 50 μl of 5× loading buffer. Samples were blotted against phospho-antibody Thr71 of ATF-2 (bottom panel). (B) 293T cells were transiently transfected with HA-p73 (0.5 μg) and increasing amounts of Bcr/Abl (0, 2 or 4 μg) using LIPOFECTAMINE™. After 36 h, cells were collected in lysis buffer. Samples were immunoblotted against HA and E1A or immunoprecipitated and blotted against phosphotyrosine (P-Tyr). Bcr/Abl was able to stabilize HA–p73 in a dose-dependent fashion, correlating with an increase in tyrosine phosphorylation. As a loading control, lysates were blotted against E1a. The autoradiograms are from a representative experiment that was repeated three times with nearly identical results.
To validate our results with specific phospho-antibodies, an in vitro kinase assay of the transfected HA–p38 MAPK using as a substrate a GST–ATF2 fusion protein was performed, yielding similar results (Figure 1A, bottom panel). Following a similar approach, we decided to see whether Bcr/Abl was able to stabilize p73. This member of the p53 family appears to be stabilized through tyrosine phosphorylation mediated by c-Abl, causing an increase in the half-life of the protein [18–20]. 293T cells were transiently transfected with GFP or Bcr/Abl plus HA-tagged p73α; 36 h later, the cells were lysed to measure p73 levels and tyrosine phosphorylation. Bcr/Abl was able to stabilize p73 in a dose-dependent fashion, which correlates with an increase in tyrosine phosphorylation, as the use of HA and phospho-tyrosine antibodies indicates (Figure 1B). These experiments support the hypothesis that Bcr/Abl and c-Abl are utilizing similar biological substrates.
Bcr/Abl mediates p38 MAPK activation by Abl-dependent and -independent pathways
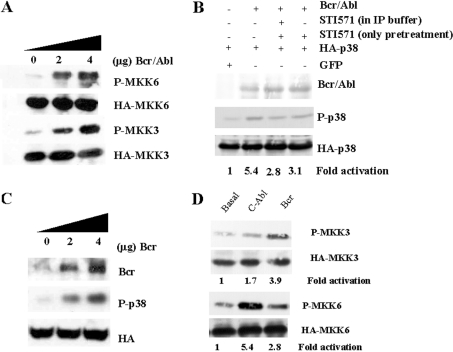
Previous work demonstrated that c-Abl-dependent p38 MAPK activation is mediated through the upstream activator MKK6, with no apparent implication of the other upstream kinase MKK3 [21]. This observation was obtained after treatment with cisplatin, a classic c-Abl stimulus [13]. We decided to study which upstream molecules mediate the p38 MAPK activation in the context of Bcr/Abl expression. Therefore 293T cells were transfected with MKK3 or MKK6 in the presence or absence of Bcr/Abl. Interestingly, both p38 MAPK upstream activators were activated by Bcr/Abl (Figure 2A). To evaluate the participation of c-Abl in the Bcr/Abl-mediated p38 MAPK activation, we decided to use the c-Abl-specific inhibitor STI571 [22,23], an extremely selective drug that only affects Bcr/Abl-positive cells [24]. 293T cells were transfected with HA-p38 MAPK plus GFP or Bcr/Abl. After 24 h, the cells were incubated in the presence/absence of STI571 for 8 h, and then collected in lysis buffer with STI571 in order to keep Bcr/Abl-mediated tyrosine phosphorylation blocked (Figure 2B). As a control for non-specific inhibition, the same approach was performed without STI571 in lysis buffer. Immunoprecipitated samples were blotted against the active form of p38 MAPK. Pre-treatment with STI571, regardless of its presence in the immunoprecipitation buffer, partially blocked the activation of p38 MAPK (by approx. 50%) in the presence of Bcr/Abl (Figure 2B). These data suggest the possibility of activation in a Bcr-dependent manner. Therefore we overexpressed Bcr in 293T cells, and an obvious activation of p38 MAPK was detected (Figure 2C). These results, together with our previous observation with STI517, supports the co-existence of Abl-dependent and Bcr-dependent pathways in p38 MAPK activation mediated through Bcr/Abl. Finally, we studied which p38 MAPK upstream molecules were implicated in the activation of p38 by Bcr (Figure 2D). As expected, Abl was able only to activate MKK6, with almost no effect on MKK3, whereas Bcr preferentially activated MKK3. These data confirmed our observations, and demonstrated the existence of two different mechanisms for the activation of p38 MAPK by Bcr/Abl.
Figure 2. Bcr/Abl activates p38 MAPK through MKK3 and MKK6 pathways in a Abl-dependent and -independent fashion.
(A) 293T cells were transiently co-transfected with HA–MKK6 or HA–MKK3 (0.5 μg) plus increasing amounts of Bcr/Abl (0, 2 or 4 μg) using LIPOFECTAMINE™. After 36 h, cells were collected in lysis buffer and immunoprecipitated against HA. Immunocomplexes were blotted against phospho (P)-MKK6/3, and membranes were re-probed against HA. Bcr/Abl activates both p38 MAPK upstream kinases in a dose-dependent fashion. (B) 293T cells were transiently co-transfected with 0.5 μg of HA-p38 MAPK and GFP, or Bcr/Abl (2 μg) using LIPOFECTAMINE™. After 24 h, cells were incubated in the presence/absence of STI571 (10 μM for 8 h). Samples were collected in lysis buffer with or without STI571, and immunoprecipitated against HA. Immunoprecipitates were blotted against P-p38, and re-probed against HA. Total cell lysates were blotted against P-Bcr/Abl and re-probed against Bcr/Abl. STI571 partially blocks the activation of p38 MAPK. (C) 293T cells were transiently transfected with HA-p38 MAPK (0.5 μg), and Bcr (0, 2 or 4 μg) using LIPOFECTAMINE™. After transfection (36 h), cells were collected in lysis buffer. Anti-HA immunoprecipitates were immunoblotted against P-p38, and as a loading control membranes were re-probed against HA. Bcr expression was evaluated by Western blotting in total cell lysates. (D) 293T cells were transiently transfected using LIPOFECTAMINE™, with 0.5 μg of HA–MKK6 or HA–MKK3 or with 2 μg of GFP, Bcr or c-Abl. After transfection (36 h), cells were collected in lysis buffer and immunoprecipitated against HA. Anti-HA immunoprecipitates were immunoblotted against P-MKK3/MKK6, and as a loading control membranes were re-probed against HA. The autoradiograms are from a representative experiment that was repeated three times with nearly identical results.
Constitutive expression of Bcr/Abl blocks activation of p38 MAPK by Ara-C
In order to evaluate the biological implications of our previous findings, we decided to study whether the p38 MAPK pathway was altered in Bcr/Abl-expressing cells. As a first approach, we evaluated the status of the p38 MAPK pathway in K562 (Bcr/Abl-positive) and U937 (Bcr/Abl-negative) cell lines. Cells expressing endogenous Bcr/Abl showed an increase in levels of phosphorylated p38 MAPK (Figure 3A), supporting our first observation in 293T cells.
Figure 3. Constitutive expression of Bcr/Abl blocks activation of p38 MAPK by Ara-C.
(A) U937 and K562 cells were cultured at 2×105 cells/ml and treated with Ara-C (50 μM) for 3 or 6 h. Cells were collected in lysis buffer, and total cell lysates were blotted against phospho (P)-p38 MAPK and P-MKK3/6. Different basal levels in p38 MAPK and MKK3/6 phosphorylation were observed between U937 and K562 cells. K562 cells showed a lack of activation in response to Ara-C. Membranes were re-blotted against total p38 and MKK3 as loading control. (B) Similar conditions were used for an in vitro kinase assay using as a substrate GST–ATF2. Total p38 MAPK was immunoprecipitated and incubated for 30 min at 30 °C with 1 μg of GST–ATF2 fusion protein in the presence of cold ATP. Reactions were stopped by adding 50 μl of 5× loading buffer. Samples were blotted against phospho-antibody Thr71 of ATF-2. Total p38 MAPK immunoprecipitated was used as a loading control. (C) U937 and K562 cells were cultured at 2×105 cells/ml for 16 h, and then treated with Ara-C (50 μM) for 24 h. Apoptosis was evaluated by flow cytometry. U937 cells underwent apoptosis, whereas no effect was observed in K562 cells. The results presented in the histogram are the average of three independent experiments; the bars represent S.D. (D) U937 and K562 cells were cultured at 2×105 cells/ml for 24 h, after which cells were treated with NaCl (200 mM) for 20 min and collected in lysis buffer. Total cell lysates were blotted against P-p38 MAPK and p38 MAPK. Cells were able to activate p38 MAPK regardless of Bcr/Abl expression.
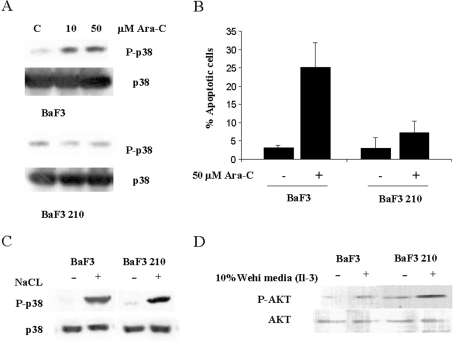
We decided to study the implications of our observations in terms of CML therapy; among several therapeutic stimuli known to activate p38 MAPK, Ara-C has been widely studied. It has been reported that Ara-C induces apoptotic effects through the SAPK pathway mediated by c-Abl, with a very-well-established role for JNK [13–16]. Thus we studied the role of p38 MAPK in the response to Ara-C in the presence/absence of Bcr/Abl. Cells were treated with Ara-C (50 μM), and p38 MAPK activation was evaluated after 3 or 6 h. While a clear activation was observed in U937 cells, K562 showed a lack of activation (Figure 3A). In order to validate our previous observation with phospho-antibodies, we evaluated the kinase activity of p38 MAPK in both cell lines. As expected, U937 showed lower basal levels of endogenous p38 MAPK activity that were increased in the presence of Ara-C (50 μM), whereas K562 had a higher level of activity that was not affected by the presence of Ara-C (Figure 3B). Therefore we decided to correlate p38 MAPK activation with the response to Ara-C in terms of viability/apoptosis. U937 cells, which are human myeloid leukaemia cells but do not express Bcr/Abl, underwent apoptosis, whereas a minimal effect on cell death was observed in K562 (Figure 3C). These data correlate the lack of p38 MAPK activation with resistance to Ara-C. To exclude the possibility of a constitutive blockage of the pathway, we analysed the response to another p38 MAPK stimulus that is not c-Abl-dependent, such as exposure to a high concentration of NaCl. Cells were incubated with NaCl (200 mM) for 20 min, and p38 MAPK activation was measured (Figure 3D). In this case, both cell lines were able to activate p38 MAPK, regardless of Bcr/Abl expression, indicating that the pathway was not completely abolished.
Given that both cell lines have extremely different backgrounds, we decided to use a model with an identical genetic background, in which the only difference was the expression of Bcr/Abl. Therefore we used BaF3 cells with or without Bcr/Abl p210 expression (BaF3 210). We performed experiments similar to those using K562 and U937 cells, which gave nearly identical results (Figures 4A–4C), corroborating our previous observations. To validate the effects of Bcr/Abl on p38 MAPK activation in our experimental model of Baf3 cells, we decided to challenge other signalling pathways also activated by Bcr/Abl, such as PI3K/Akt [4]. In this case, we used conditioned medium from Wehi cells as a source of IL-3 (interleukin-3), which is a potent inducer of Akt activity in this cell type in a c-Abl-independent fashion [25]. From previous observations, Bcr/Abl-expressing cells have been shown to have higher levels of phosphorylated Akt in basal conditions, but after treatment with IL-3 both cell lines activated Akt in a similar fashion (Figure 4D). This result is similar to the one observed for p38 MAPK with NaCl. Therefore our data suggest that interference induced by Bcr/Abl in the p38 MAPK pathway is similar to that observed in the PI3K/Akt pathway, rendering a constitutive activation of the pathway, depending on the stimulus used.
Figure 4. BaF3 and BaF3 p210 cells showed the same features as U937 and K562 cells.
(A) BaF3 and BaF3 p210 cells were cultured at 2×105 cells/ml. After 24 h, cells were treated with Ara-C (10 or 50 μM). After 6 h, cells were collected in lysis buffer and immunoblotted against phospho (P)-p38 MAPK. As a loading control, lysates were blotted against p38. Cells expressing Bcr/Abl showed a slight increase in the levels of phosphorylated p38 MAPK. (B) BaF3 and BaF3 p210 cells were cultured at 2×105 cells/ml for 16 h and then treated with Ara-C (50 μM) for 24 h to analyse apoptosis by propidium iodide in a FACS apparatus. Bcr/Abl-negative cells underwent apoptosis, while a minimal effect was observed in BaF3 p210 cells. The results shown are the average of three independent experiments; bars represent the S.D. (C) BaF3 and BaF3 p210 cells were cultured at 2×105 cells/ml. After 24 h, cells were treated with NaCl (200 mM) for 20 min, and then collected in lysis buffer and immunoblotted against phospho (P)-p38 MAPK and p38 MAPK. Cells were able to activate p38 MAPK regardless of Bcr/Abl expression. (D) BaF3 and BaF3 p210 cells were cultured at 2×105 cells/ml for 5 h in the absence of serum and conditioned media, and then cells were treated with conditioned medium from Wehi cells for 30 min. Cells were collected in lysis buffer and immunoblotted against P-Akt (Ser473). Cells expressing Bcr/Abl p210 showed an increase in the levels of P-Akt under basal conditions, but after treatment with conditioned medium from Wehi cells, both cell lines activate Akt.
Inhibition of p38 MAPK activation induces resistance to Ara-C
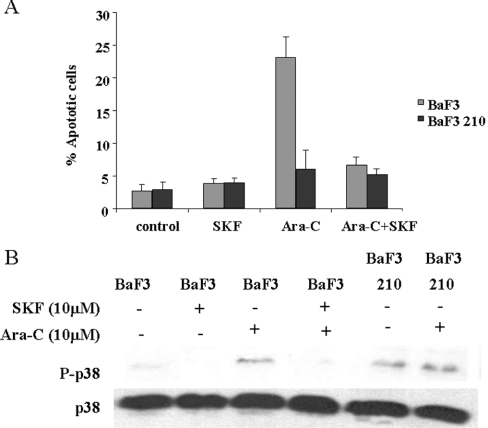
Our previous results prompted us to investigate whether the p38 MAPK pathway is implicated in the cellular resistance to Ara-C. Therefore we decided to block the p38 MAPK pathway using the specific inhibitor SKF86002. This inhibitor is able to block activation of the four members of the p38 MAPK family, due to the blockage of upstream molecules such as MKK6 [26], and is easily evaluated by Western blotting with an antibody against the activated form of p38 MAPK members [27]. Therefore BaF3 210 and BaF3 cells were treated with Ara-C in the presence/absence of SKF86002, and after 6 h samples were collected for biochemical analysis or exposed for 24 h prior to flow cytometry. As expected, no apoptotic effect was observed in p210-expressing cells (Figure 5A). However, SKF86002-mediated inhibition of p38 MAPK induced a marked resistance to Ara-C in BaF3 cells (Figure 5A). To evaluate p38 MAPK status, we performed Western blotting using phospho-p38 MAPK antibody in the presence of Ara-C with or without SKF86002 in BaF3 cells, which corroborated the lack of activation with higher survival rates (Figure 5B). As a control, BaF3 210 cells were exposed to Ara-C and p38 MAPK phosphorylation was evaluated, showing higher basal levels and no activation by Ara-C, as expected. Similar results were obtained for U937 and K562 cells (results not shown), which supported our observations with BaF3 cells and allowed us to consider lack of p38 MAPK activation as a general mechanism for Ara-C resistance, as had been proposed previously [15].
Figure 5. Inhibition of p38 MAPK activation induces resistance to Ara-C.
(A) BaF3 and BaF3 p210 cells were cultured at 2×105 cells/ml. After 24 h, cells were treated with Ara-C (50 μM), SKF86002 (10 μM) or Ara-C plus SKF86002 for 24 h. Apoptosis was measured by propidium iodide in Epics XL. SKF86002 induced a marked resistance to Ara-C in BaF3 cells, whereas no effect was observed in BaF3 p210 cells. The results are the average of three independent experiments. (B) BaF3 cells were cultured at 2×105 cells/ml. After 24 h, cells were treated with Ara-C (50 μM), SKF86002 (10 μM) or Ara-C plus SKF86002. Cells were collected after 6 h in lysis buffer and immunoblotted against phospho (P)-p38 MAPK. As a loading control lysates were blotted against p38 MAPK. There was a correlation between the lack of p38 MAPK activation and Ara-C resistance. As a control, BaF3 p210 cells were treated with Ara-C.
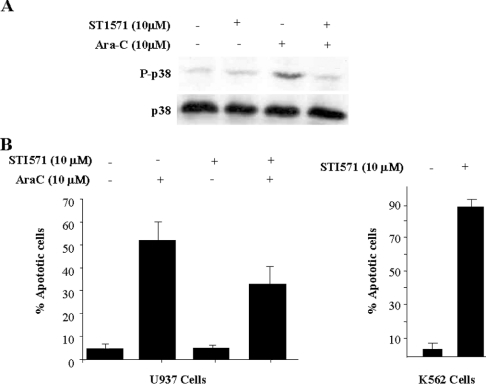
STI571 blocks p38 MAPK activation mediated by Ara-C and induces resistance in U937 cells
According to our previous results with Bcr/Abl-negative cells, inhibition of Abl should block p38 MAPK activity and confer resistance to Ara-C. Therefore we decided to challenge the Bcr/Abl-negative cell line U937 with STI571 in order to increase resistance to Ara-C. Cells were pre-incubated with STI571(10 μM) for 24 h, and then Ara-C (10 μM) was added for 6 h and activation of p38 MAPK was evaluated. p38 MAPK activation was clearly abolished in cells treated with STI571 (Figure 6A). Similar results were obtained after 1 h of incubation (results not shown). These results prompted us to evaluate the effects of STI571 in terms of viability. Again, U937 cells were pre-treated for 24 h with STI571 (10 μM) and then co-incubated with Ara-C (10 μM) up to 72 h. As a positive control for STI571 functionality, K562 cells were incubated with the same amount of STI571 for 24 h, showing an apoptotic fraction close to 90% (Figure 6B, right panel), whereas no effect was observed in U937 cells, as described previously [24]. In the presence of Ara-C alone, U937 cells showed an apoptotic fraction close to 55%, but in the presence of STI571 there was a reduction in the apoptotic fraction to 35% (Figure 6B, left panel), indicating that inhibition of c-Abl can also render resistance to Ara-C. These results, in agreement with those reported on JNK activation mediated by Ara-C [28], support an important role for the SAPK family (JNK and p38 MAPK) in apoptosis mediated by c-Abl in response to Ara-C in Bcr/Abl-negative cells.
Figure 6. STI571 blocks p38 MAPK activation mediated by Ara-C and induces resistance in U937 cells.
(A) U937 cells were cultured at 2×105 cells/ml and pre-treated with STI571 (10 μM) or the same concentration of DMSO for 24 h. Then, Ara-C (10 μM) was added for 6 h. Cells were collected in lysis buffer and immunoblotted against phospho (P)-p38 MAPK. As a loading control, lysates were blotted against p38. The activation of p38 MAPK was abolished by STI571. (B) U937 were cultured at 2×105 cells/ml. Cells were pre-treated with STI571 (10 μM) for 24 h and then treated with Ara-C (10 μM) for 72 h. The combination of Ara-C plus STI571 reduced the apoptotic U937 population. K562 cells were treated with STI571 (10 μM for 24 h) as a control for drug activity. Lethality was close to 80%. The results are the average of three independent experiments; Bars represent S.D.
DISCUSSION
Bcr/Abl has been widely studied in terms of its signal transduction pathways. While a clear relationship has been established with other signal transduction pathways, such as Akt [4], JNK [3] and ERK1/2 [29]. However, no link has been established with the p38 MAPK pathway in terms of signalling; only in the response to interferon has a connection been shown between these molecules [5]. Our results show for the first time that Bcr/Abl is able to interfere with this pathway. From a mechanistic point of view, the control of p38 MAPK by Bcr/Abl could be based on the ability of c-Abl to induce activation of this pathway; therefore it seems logical that Bcr/Abl retains most of the biochemical properties of c-Abl. However, recent evidence suggests that stable overexpression of Bcr is able to activate p38 MAPK, which mediates activation of NF-κβ (nuclear factor κB) [30], indicating that Bcr may be implicated in p38 MAPK activation. Our data, evaluating both p38 MAPK activators, the use of STI571 and the transient transfection experiments in 293T cells overexpressing Bcr alone, suggest that both partners of the chimaeric protein are implicated in the final activation of p38 MAPK. Our results support the hypothesis that c-Abl activates p38 MAPK in a MKK6-dependent manner [21], and that Bcr activates p38 MAPK in a MKK3-dependent manner; therefore the final situation is an increase in p38 MAPK phosphorylation mediated by both MKK6 and MKK3 pathways.
The other c-Abl substrate studied, p73, is also liable to be modified by Bcr/Abl with the same effect as that of c-Abl [18,20,21]. These results, activation of the p38 MAPK pathway and phosphorylation of p73 support the idea that Bcr/Abl retains most of the biochemical properties of c-Abl.
The correlation between Bcr/Abl and other signalling pathways, such as Akt [31], seems to be extremely consistent with a transformed phenotype. Interestingly, p38 MAPK has been widely related to apoptosis in different experimental systems, especially after stress. Therefore activation of this pathway seems to be opposite from that of a transformed phenotype. However, several points should be considered, such as an anti-apoptotic role for p38 MAPK in neutrophils [32]. p38 MAPK is also implicated in the proliferation/differentiation of the immune system [33]. Furthermore, other apoptotic pathways, such as JNK, have been described as downstream effectors for Bcr/Abl, and seem to be critical for the transformed phenotype and maintenance of survival [34]. Our data indicate that this pathway could be modified by the presence of Bcr/Abl, rendering an insensitive p38 MAPK pathway, at least in response to Ara-C. In fact, the lack of a fully functional p38 MAPK pathway, for example by overexpression of the phosphatase PPM1 (protein phosphatase methyltransferase 1) [11], could be implicated in a transformed phenotype, and in our experimental model we demonstrate a lack of function for the p38 MAPK pathway. Furthermore, the same explanation may be used for the p73 protein, again supporting the idea that constitutive activation, through phosphorylation, can render a deregulated function. Taking into account that stabilization seems to be a key event in the biological properties of this type of protein [35–37], constitutive stabilization probably blocks the function of this protein, as has been demonstrated in the case of the p53 protein [38]. Further studies are necessary to clarify the implications of p73 stabilization mediated by Bcr/Abl.
From a clinical point of view, our data support a role for p38 MAPK in Ara-C-based therapy. In this sense, Ara-C is not the most successful therapy for CML, and probably a lack of p38 MAPK activation is involved in this poor response, inducing a resistant phenotype. Ara-C-induced apoptosis is mediated through c-Abl [16] via p38 MAPK activation [13,15]. Accordingly, our data fit in perfectly with these observations, as Bcr/Abl-positive cells have a high level of phosphorylated p38 MAPK that is not increased after Ara-C treatment. It is important to note that higher basal levels of p38 MAPK do not correlate with a more toxic effect in Bcr/Abl-positive cells, probably because other signals related to survival, such as Akt, are also triggered. In fact, other SAPK pathways implicated in the Ara-C-mediated apoptosis, such as JNK, are also activated in Bcr/Abl-positive cells [34]. Therefore we propose a model in which Bcr/Abl induces constitutive activation of the p38 MAPK pathway, rendering it insensitive to further activation in response to Ara-C. Our results using a specific inhibitor of p38 MAPK support a critical role for p38 MAPK in Ara-C-based therapy; this observation is not restricted to Ara-C, as we show here. Recent evidence also supports a role for this signalling pathway in the interferon inhibitory effect [5], suggesting that the p38 MAPK pathway could be a new target in the treatment of CML. A key event in CML therapy is the development of c-Abl inhibitors, such as STI571. As previously described [39], a combination of STI571 with Ara-C leads to an increased death rate of Bcr/Abl-positive cells, with little or no effect on Bcr/Abl-negative cells. Our data demonstrate that this combination is even protective in Bcr/Abl-negative cells. In fact, we also observed the same effect in HL60 cells, although in other experimental systems such as RAMOS, KG-1 and REH, we did not detect any protective effect; however, no potentiation of Ara-C toxicity was observed (results not shown). Considering these data, the combination of the two therapies could be extremely potent against leukaemic cells but less effective, or even protective, in normal cells. However, it should be mentioned that this protective effect is not only due to the inhibition of p38 MAPK, since the JNK pathway is also known to be implicated [28]. The increased sensitivity to Ara-C mediated by STI571 in Bcr/Abl-positive cells is probably p38 MAPK/JNK-independent, at least in K562 cells, where it has been reported that STI571 is able to activate ERK1/2, but is unable to activate p38 MAPK and JNK [40]. Moreover, it has been reported recently that inhibition of the PI3K pathway promotes the toxic effect of STI571, indicating that this signalling pathway is implicated in sensitivity to this drug [41].
Finally, a relation between Bcr/Abl and the p38 MAPK pathway has also been observed recently [42]. Interestingly, these authors showed down-regulation of p38 MAPK expression in embryonic stem cells, using an inducible system for Bcr/Abl expression, and the subsequent lack of p38 MAPK activity. However, several differences should be noted. First, we have used cells either derived from patients (K562) or stably transfected with Bcr/Abl (BaF 3). Secondly, other signalling pathways, such as Akt, seem to be differently regulated by Bcr/Abl in embryonic stem cells than in the model reported by others ([42], and the present study). Thirdly, although IL-3 is able to activate p38 MAPK, it probably is not the best activator of this signalling pathway clearly related to stress. In fact, it has been reported that the withdrawal of IL-3 is able to activate p38 MAPK in the pro-B cell line FL5.12 A [43]. Taken together, our data and those from Wong et al. [42] open up a new and exciting field of research by demonstrating a lack of normal functioning in the p38 MAPK pathway in Bcr/Abl-transformed cells.
In summary, our data show that p38 MAPK is a downstream effector of the chimaeric protein Bcr/Abl. This interference leads to an insensitive p38 MAPK pathway in response to Ara-C, suggesting a role for p38 MAPK in the resistance to Ara-C in CML therapy. Further studies are necessary to evaluate the implication of this pathway in the genesis, prognosis and treatment of CML.
Acknowledgments
This work was supported by grants FIS 03/0763 (to R. S.-P.) and CAM 08.1/0022/2003-1, Bio/2001-0385 from the Ministerio de Ciencia y Tecnología (to L. A.-V). We appreciate the comments of Dr L. Del Peso, Dr C. Muñoz and Dr J. León. V. J. S.-A. is supported by a fellowship from the ‘Fundación Leucemia y Linfoma’. We especially thank S. Jones for editorial assistance.
References
- 1.Deininger M. W., Goldman J. M., Melo J. V. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Danial N. N., Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–2531. doi: 10.1038/sj.onc.1203484. [DOI] [PubMed] [Google Scholar]
- 3.Raitano A. B., Halpern J. R., Hambuch T. M., Sawyers C. L. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skorski T., Bellacosa A., Nieborowska-Skorska M., Majewski M., Martinez R., Choi J. K., Trotta R., Wlodarski P., Perrotti D., Chan T. O., et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer I. A., Verma A., Grumbach I. M., Uddin S., Lekmine F., Ravandi F., Majchrzak B., Fujita S., Fish E. N., Platanias L. C. The p38 MAPK pathway mediates the growth inhibitory effects of interferon-α in BCR-ABL-expressing cells. J. Biol. Chem. 2001;276:28570–28577. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- 6.Nebreda A. R., Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosino C., Nebreda A. R. Cell cycle regulation by p38 MAP kinases. Biol. Cell. 2001;93:47–51. doi: 10.1016/s0248-4900(01)01124-8. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Blanco E. p38 MAPK signalling cascades: ancient roles and new functions. BioEssays. 2000;22:637–645. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Ono K., Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Prieto R., Rojas J. M., Taya Y., Gutkind J. S. A role for the p38 mitogen-activated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60:2464–2472. [PubMed] [Google Scholar]
- 11.Bulavin D. V., Demidov O. N., Saito S., Kauraniemi P., Phillips C., Amundson S. A., Ambrosino C., Sauter G., Nebreda A. R., Anderson C. W., et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 2002;20:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 12.Kharbanda S., Yuan Z. M., Weichselbaum R., Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 13.Pandey P., Raingeaud J., Kaneki M., Weichselbaum R., Davis R. J., Kufe D., Kharbanda S. Activation of p38 mitogen-activated protein kinase by c-Abl-dependent and -independent mechanisms. J. Biol. Chem. 1996;271:23775–23779. doi: 10.1074/jbc.271.39.23775. [DOI] [PubMed] [Google Scholar]
- 14.Saleem A., Datta R., Yuan Z. M., Kharbanda S., Kufe D. Involvement of stress-activated protein kinase in the cellular response to 1-beta-D-arabinofuranosylcytosine and other DNA-damaging agents. Cell. Growth. Differ. 1995;6:1651–1658. [PubMed] [Google Scholar]
- 15.Stadheim T. A., Saluta G. R., Kucera G. L. Role of c-Jun N-terminal kinase/p38 stress signaling in 1-beta-D-arabinofuranosylcytosine-induced apoptosis. Biochem. Pharmacol. 2000;59:407–418. doi: 10.1016/s0006-2952(99)00330-5. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y., Yuan Z. M., Ishiko T., Nakada S., Utsugisawa T., Kato T., Kharbanda S., Kufe D. W. Pro-apoptotic effect of the c-Abl tyrosine kinase in the cellular response to 1-beta-D-arabinofuranosylcytosine. Oncogene. 1997;15:1947–1952. doi: 10.1038/sj.onc.1201376. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Prieto R., Sanchez-Arevalo V. J., Servitja J. M., Gutkind J. S. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene. 2002;21:974–979. doi: 10.1038/sj.onc.1205134. [DOI] [PubMed] [Google Scholar]
- 18.Agami R., Blandino G., Oren M., Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature (London) 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 19.Gong J. G., Costanzo A., Yang H. Q., Melino G., Kaelin W. G., Jr, Levrero M., Wang J. Y. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature (London) 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Z. M., Shioya H., Ishiko T., Sun X., Gu J., Huang Y. Y., Lu H., Kharbanda S., Weichselbaum R., Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature (London) 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 21.Cong F., Goff S. P. c-Abl-induced apoptosis, but not cell cycle arrest, requires mitogen-activated protein kinase kinase 6 activation. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13819–13824. doi: 10.1073/pnas.96.24.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchdunger E., Zimmermann J., Mett H., Meyer T., Muller M., Druker B. J., Lydon N. B. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. [PubMed] [Google Scholar]
- 23.Druker B. J., Tamura S., Buchdunger E., Ohno S., Segal G. M., Fanning S., Zimmermann J., Lydon N. B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 24.Deininger M. W., Goldman J. M., Lydon N., Melo J. V. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [PubMed] [Google Scholar]
- 25.del Peso L., Gonzalez-Garcia M., Page C., Herrera R., Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. C., Laydon J. T., McDonnell P. C., Gallagher T. F., Kumar S., Green D., McNulty D., Blumenthal M. J., Heys J. R., Landvatter S. W. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 27.Losa J. H., Cobo C. P., Viniegra J. G., Sanchez-Arevalo Lobo V. J., Ramon y Cajal S., Sanchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22:3998–4006. doi: 10.1038/sj.onc.1206608. [DOI] [PubMed] [Google Scholar]
- 28.Raina D., Mishra N., Kumar S., Kharbanda S., Saxena S., Kufe D. Inhibition of c-Abl with STI571 attenuates stress-activated protein kinase activation and apoptosis in the cellular response to 1-beta-D-arabinofuranosylcytosine. Mol. Pharmacol. 2002;61:1489–1495. doi: 10.1124/mol.61.6.1489. [DOI] [PubMed] [Google Scholar]
- 29.Cortez D., Reuther G., Pendergast A. M. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 30.Korus M., Mahon G. M., Cheng L., Whitehead I. P. p38 MAPK-mediated activation of NF-kappaB by the RhoGEF domain of Bcr. Oncogene. 2002;21:4601–4612. doi: 10.1038/sj.onc.1205678. [DOI] [PubMed] [Google Scholar]
- 31.Neshat M. S., Raitano A. B., Wang H. G., Reed J. C., Sawyers C. L. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol. Cell. Biol. 2000;20:1179–1186. doi: 10.1128/mcb.20.4.1179-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado-Kristensson M., Porn-Ares M. I., Grethe S., Smith D., Zheng L., Andersson T. p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase activities have opposite effects on human neutrophil apoptosis. FASEB J. 2002;16:129–131. doi: 10.1096/fj.01-0817fje. [DOI] [PubMed] [Google Scholar]
- 33.Dong C., Davis R. J., Flavell R. A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 34.Hess P., Pihan G., Sawyers C. L., Flavell R. A., Davis R. J. Survival signaling mediated by c-Jun NH2-terminal kinase in transformed B lymphoblasts. Nat. Genet. 2002;32:201–205. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- 35.Ashcroft M., Vousden K. H. Regulation of p53 stability. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 36.Colman M. S., Afshari C. A., Barrett J. C. Regulation of p53 stability and activity in response to genotoxic stress. Mutat. Res. 2000;462:179–188. doi: 10.1016/s1383-5742(00)00035-1. [DOI] [PubMed] [Google Scholar]
- 37.Lohrum M. A., Vousden K. H. Regulation and activation of p53 and its family members. Cell. Death. Differ. 1999;6:1162–1168. doi: 10.1038/sj.cdd.4400625. [DOI] [PubMed] [Google Scholar]
- 38.Blagosklonny M. V. Loss of function and p53 protein stabilization. Oncogene. 1997;15:1889–1893. doi: 10.1038/sj.onc.1201374. [DOI] [PubMed] [Google Scholar]
- 39.Thiesing J. T., Ohno-Jones S., Kolibaba K. S., Druker B. J. Efficacy of STI571, an abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against bcr-abl-positive cells. Blood. 2000;96:3195–3199. [PubMed] [Google Scholar]
- 40.Yu C., Krystal G., Varticovksi L., McKinstry R., Rahmani M., Dent P., Grant S. Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human leukemia cells. Cancer. Res. 2002;62:188–199. [PubMed] [Google Scholar]
- 41.Klejman A., Rushen L., Morrione A., Slupianek A., Skorski T. Phosphatidylinositol-3 kinase inhibitors enhance the anti-leukemia effect of STI571. Oncogene. 2002;21:5868–5876. doi: 10.1038/sj.onc.1205724. [DOI] [PubMed] [Google Scholar]
- 42.Wong S., McLaughlin J., Cheng D., Witte O. N. Cell context-specific effects of the BCR-ABL oncogene monitored in hematopoietic progenitors. Blood. 2003;101:4088–4097. doi: 10.1182/blood-2002-11-3376. [DOI] [PubMed] [Google Scholar]
- 43.Khaled A. R., Moor A. N., Li A., Kim K., Ferris D. K., Muegge K., Fisher R. J., Fliegel L., Durum S. K. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol. Cell. Biol. 2001;21:7545–7557. doi: 10.1128/MCB.21.22.7545-7557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]