Abstract
CD43 is a transmembrane molecule that contains a 123-aminoacids-long cytoplasmic tail and a highly O-glycosylated extracellular domain of mucin type. Endogenous CD43 expressed in COLO 205, K562 and Jurkat cells revealed a membrane-associated, 20 kDa CD43-specific cytoplasmic tail fragment (CD43-CTF) upon inhibition of γ-secretase. This fragment was formed by an extracellular cleavage, as it was not accumulated after treating cells with 1,10-phenanthroline, a metalloprotease inhibitor. When CD43 was transfected into HEK-293 cells expressing dominant-negative PS1 (presenilin-1), the CD43-CTF was accumulated, but not in cells with wild-type PS1. Owing to its accumulation in the presence of a non-functional PS variant, it may thus be a novel γ-secretase substrate. This CTF is formed by an extracellular cleavage close to the membrane, is a fragment that can be concluded to be a substrate for γ-secretase. However, the intracellular γ-secretase product has not been possible to detect, suggesting a quick processing of this product. During normal growth the CTF was not found without γ-secretase inhibition, but when the cells (COLO 205) were very confluent the fragment could be detected. The intracellular domain of CD43 has previously been shown to contain a functional nuclear localization signal, and has been suggested to be involved in gene activation. From this and the present results, a novel way to explain how mucin-type molecules may transduce intracellular signals can be proposed.
Keywords: leukosialin, MUC1 mucin, presenilin, γ-secretase, sialophorin
Abbreviations: APP, amyloid precursor protein; BCIP/NBT, 5-bromo-4-chloroindol-3-yl phosphate/Nitro Blue Tetrazolium; CD43ct, cytoplasmic tail of CD43; CD43-CTF, CD43-specific cytoplasmic tail fragment; CD43MH, full-length CD43 tagged in the C-terminus with myc/His; CD43ΔE-MH, CD43 lacking the ectodomain and tagged in the C-terminus with myc/His; HRP, horseradish peroxidase; mAb, monoclonal antibody; Ni-NTA, Ni2+-nitrilotriacetate; NLS, nuclear localization signal; PS, presenilin; RIP, regulated intramembrane proteolysis; TAPI-1, tumour necrosis factor-α protease inhibitor 1; WT, wild-type
INTRODUCTION
Type I transmembrane glycoproteins with an extensively O-glycosylated extracellular domain can be classified as members of the mucin family. These proteins are suggested to be involved in cell interactions, but their cytoplasmic C-termini also contain different motifs known to be involved in signalling. As their extracellular domains have variable glycosylation, deficient in typical receptor domains, it has been difficult to understand how signals are transmitted from the outside into the cell. Such signalling has been suggested in many studies, although no mechanism has been provided. An example of such a molecule is the MUC1 mucin, found on epithelia as well as on lymphocytes [1]. When located on normal epithelia, an apical localization is predominant, but in tumour cells it is instead located to the entire cell surface.
CD43 (leukosialin, sialophorin, SPN) is another example of such a mucin-type molecule. It is the major cell-surface molecule in most of the haematopoietic cell lineages. The extracellular domain of CD43 extends 45 nm from the cell surface and contains 93 serine and threonine residues, of which many are O-glycosylated [2]. The cytoplasmic tail of CD43 (CD43ct) has 123 amino acids and includes several serine/threonine phosphorylation sites, together with an SH3 binding domain [3,4]. CD43 is suggested to be involved in the immunological synapse, and is found to both promote and prevent cell–cell interactions in leucocytes [5–7]. It may also be involved in T-cell activation, since antibody ligation results in Fyn and Vav tyrosine phosphorylation by interacting with the SH3 binding domain of CD43ct [4,8]. Cross-linking of CD43 induces protein kinase C activity and Ca2+ mobilization in monocytes [9], and triggers DNA binding of transcription factors such as AP-1 (activator protein 1) [10]. CD43 has also been shown to have a proliferative effect when overexpressed in B-cells of transgenic mice [11,12]. In contrast, cross-linking of CD43 with certain monoclonal antibodies caused apoptosis in myeloid cells [13]. From this, it is evident that CD43 has important regulatory functions in leucocytes, although its precise effects are variable, and dependent on the cell type.
CD43 was first described as an exclusive leucocyte marker, but was later demonstrated to be expressed in different cancer cells [14,15]. A more thorough study revealed that CD43 has a wide distribution in many cell lines, often overlooked due to its variable glycosylation [16]. CD43 has been detected in colon cancer cell lines and in some colon cancer tissue sections, both as protein and mRNA. However, an intriguing observation was a distinct CD43 staining of all colon adenoma where the normal and adenoma cells were clearly delineated [17,18]. This prompted us to study further the CD43 function outside of the haematopoietic cell lineage. Recently, we found that CD43 contains a functional NLS (nuclear localization signal) sequence in the intracellular domain, translocating it to the nucleus. In the nucleus, the cytoplasmic tail of CD43 was found to interact with β-catenin, and was shown to affect genes regulated by β-catenin/TCF-4-responsive promoters [19].
Cleavage of type I transmembrane proteins in the membrane releasing an intracellular fragment capable of transducing nuclear signals has been described for the Notch-1 protein [20], the APP (amyloid precursor protein) [21], ErbB-4 [22], E-cadherin [23] and CD44 [24,25]. Although all mechanistic details have not been revealed for the cleavage of these proteins, a common theme is emerging. The process starts by a proteolytic release of the ectodomain close to the outer cell membrane, e.g. by a membrane-associated metalloprotease. This will then, in one or several steps, activate a PS (presenilin)-dependent γ-secretase activity, cleaving off the cytoplasmic tail a few amino acids into the membrane [26–28]. The released cytoplasmic fragment has in some cases been shown to translocate into the nucleus, where it affects gene activation. This novel signalling pathway has been named RIP (regulated intramembrane proteolysis) [29].
Here, CD43 is shown to undergo extracellular proteolysis and, upon γ-secretase inhibition, the remaining ‘stub’ of CD43 is accumulated. This fragment includes a small part of the extracellular, transmembrane and the intracellular domains, denoted as the CD43-CTF (CD43-specific cytoplasmic tail fragment). The γ-secretase-dependent accumulation of the CD43-CTF is also shown to be affected by the cell density.
MATERIALS AND METHODS
Cell culture and transfections
All cells were cultivated in Iscove's medium containing 10% (v/v) fetal bovine serum, 1% (v/v) penicillin–streptomycin (Invitrogen, Baltimore, MD, U.S.A.) and supplemented with sodium pyruvate (110 mg/l), L-arginine (116 mg/l), L-glutamine (290 mg/l), L-aspartate (36 mg/l), folic acid (10 mg/l) and 2-mercaptoethanol (3.49 μl/l) (Sigma, St Louis, MO, U.S.A.). HEK-293 cells expressing PS1 WT (wild-type) and a dominant-negative variant (PS1 Asp385→Asn, or PS1 D385N) [30] were grown with the addition of zeocin (0.2 mg/ml). All transfections in these cells were performed using LIPOFECTAMINE™2000 (Invitrogen), and transfected cells were grown for 24 h before analysis. SW480/CD43 and MCF-7/CD43 are stable growing clones that contain the tetracycline-inducible mammalian expression system (Invitrogen). The cells were first transfected with pcDNA4/TR and selected for stable clones using blasticidin (5 μg/ml). These clones were transfected with the pcDNA4/TO-CD43 plasmid, and clones were selected using zeocin (50 μg/ml). The CD43 expression was induced by tetracycline addition (0.75 μg/ml) 24 h before lysis.
Expression plasmids
CD43-inserts were generated by PCR. In the vector pCD43, cDNA encoding the full-length CD43 was inserted into a pcDNA3.1Zeo+ vector (Invitrogen), and the vector pCD43MH was constructed by inserting the CD43 cDNA between BglII and XhoI sites of the pcDNA3.1(+)myc/His-A plasmid (Invitrogen). The CD43 variant lacking the extracellular domain, pCD43ΔE-MH, mimicking an extracellular cleavage, was constructed by inserting the generated PCR fragment between a HindIII and an XhoI site of the vector pSecTagB-myc/His. The constructs were sequenced after construction. The pcDNA4/TO-CD43 plasmid has been described previously [16]. The plasmid expressing full-length MUC1 plasmid (pMUC1-FL) containing 32 tandem repeats was kindly provided by Professor Joyce Taylor-Papadimitriou (Cancer Research UK, Guy's Hospital, London, U.K.).
Antibodies and reagents
The mAb (monoclonal antibody) αCD43-4D2 reacts with the intracellular domain, and has been characterized previously [16]. The αmyc 9E.10 hybridoma (CRL-1729) was obtained from the A.T.C.C. The αMUC1 cytoplasmic tail (CT-2) antibody directed against the C-terminal 17 amino acids was kindly provided by Dr Sandra J. Gendler (Mayo Clinic College of Medicine, Scottsdale, AZ, U.S.A.) [31]. The antibodies were diluted 1:500 for Western blot analysis, except for the β-actin antibody (Sigma), which was diluted 1:5000. The γ-secretase inhibitor L-685,458 (Calbiochem, San Diego, CA, U.S.A.) was added to the cell cultures 18 h before lysis (5 μM), as suggested by Shearman et al. [32]. The metalloprotease inhibitors 1,10-phenanthroline (5 mM; Sigma–Aldrich) and TAPI-1 {tumour necrosis factor-α protease inhibitor 1: N-(R)-[2-(Hydroxyaminocarbonyl)methyl]-4-methylpentanoyl-L-naphthylalanyl-L-alanine,2-aminoethyl amide; 10 μM; Calbiochem} were added to the cell cultures 1 h and 2 h respectively before lysis.
Pull-down experiments and Western blotting
For His6-tagged pull-down experiments, Ni-NTA (Ni2+-nitrilotriacetate) magnetic beads (Qiagen) (50 μl per immunoprecipitation) were used directly in the 1% (v/v) Triton X-100 cell lysate, and eluted by the addition of SDS-loading buffer. All samples were separated on either SDS/PAGE or Tris-Tricine gels (Bio-Rad, Hercules, CA, U.S.A.). The proteins were transferred electrophoretically on to Immobilon PSQ (Millipore, Bedford, MA, U.S.A.), blocked with PBS/0.1% Tween 20 containing 5% milk powder and probed with primary antibodies diluted in block solution. The secondary antibody was either conjugated with alkaline phosphatase or HRP (horseradish peroxidase; Dako, Glostrup, Denmark) diluted 1:1500 and 1:2000 respectively and developed by BCIP/NBT (5-bromo-4-chloroindol-3-yl phosphate/Nitro Blue Tetrazolium) or SuperSignal West Pico Chemiluminescent substrate (Pierce, Rockford, IL, U.S.A.). The preparation of the membranes were made as described by Edbauer et al. [33].
RESULTS
γ-Secretase processing of endogenous CD43
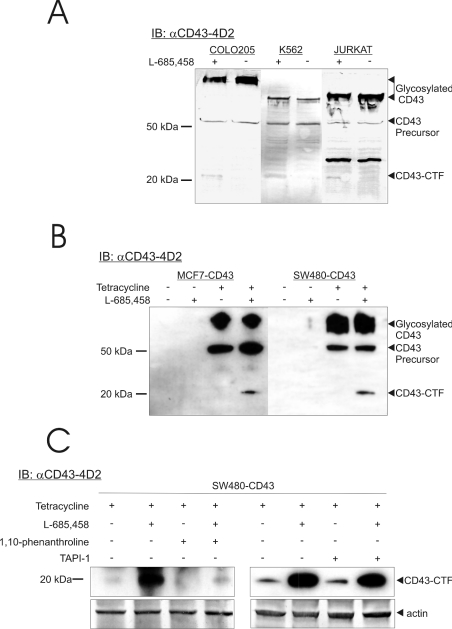
CD43 was previously found to contain a functional NLS in its intracellular C-terminus [19]. This led to the still-unresolved question of how the cytoplasmic tail of CD43 could be released from the plasma membrane. To address this, the possibility of an RIP-like γ-secretase-dependent proteolytic release of the CD43ct was studied. The colon carcinoma cell line COLO 205 and the leucocyte-derived cell lines Jurkat and K562, all expressing high amounts of endogenous CD43 [14,34,35], were studied. The cells were treated with the γ-secretase inhibitor L-685,458 [32] and the cell lysates were analysed by Western blotting using the mAb αCD43-4D2 directed against the intracellular domain of CD43 (Figure 1A). A fragment migrating at an apparent mass of approx. 20 kDa was found in cells treated with the γ-secretase inhibitor. This band, called CD43-CTF, is suggested to be a fragment formed by an extracellular cleavage revealed when this is not further proteolytically processed. That CD43 can be cleaved in its extracellular domain has previously been described [14,36]. In addition to the CD43-CTF, a fragment migrating at approx. 27 kDa was found in the Jurkat cells. This fragment is present regardless of the γ-secretase inhibition, and is probably generated by another extracellular cleavage further away from the membrane and not the substrate for γ-secretase. The presence of several cleavage sites in CD43 has previously been suggested [37]. The non-O-glycosylated CD43 precursor was found as a band at 50 kDa in all cells, and the mature, fully glycosylated CD43 as larger bands migrating differently due to the level of CD43 glycosylation in the different cell lines.
Figure 1. Accumulation of endogenous and expressed CD43.
(A) COLO 205, Jurkat and K562 cells were incubated with the γ-secretase inhibitor (L-685,485) 18 h before lysis. The lysates were separated on a 10–20% Tris-Tricine gel, blotted and probed with αCD43-4D2 mAb followed by an alkaline phosphatase-conjugated secondary antibody and detected with the NBT/BCIP substrate. (B) CD43 (non-tagged) expression in MCF7-CD43 and SW480-CD43 cells was induced by the addition of tetracycline 24 h and incubated with the γ-secretase inhibitor (L-685,485) 18 h before lysis. The samples were analysed by Western blotting, as in (A), but instead developed by enhanced chemiluminescence after probing with an HRP-conjugated secondary antibody. The glycosylated and precursor CD43s (endogenous and induced) are indicated to the right of the gels, together with the CD43-CTF fragment generated after treatment with the γ-secretase inhibitor L-685,458. (C) Tetracycline-induced SW480-CD43 cells were treated with γ-secretase inhibitor for 18 h before lysis. Cells were also subjected to treatment with the metalloprotease inhibitors 1,10-phenanthroline and TAPI-1 for 1 and 2 h respectively. The samples were analysed as in (A) using αCD43-4D2 mAb, followed by secondary HRP-conjugated antibody and enhanced chemiluminescence detection. The membranes were re-probed with β-actin antibody followed by alkaline phosphatase-conjugated secondary antibody and detection using NBT/BCIP substrate.
To prove the CD43 identity of the 20 kDa band, a CD43 tetracycline-inducible system was established in the CD43 low-expressing cell lines SW480 and MCF-7. The addition of tetracycline induced the expression of CD43, as revealed by the 50 kDa precursor and the fully glycosylated bands in the Western blot analysis using the αCD43-4D2 mAb. The 20 kDa CD43-CTF band was found when the two cell lines were treated with the γ-secretase inhibitor L-685,458, but not in the CD43-induced control cells (Figure 1B). This suggests further the CD43-CTF identity of this band, and that CD43 can be a substrate for γ-secretase.
To address whether the CD43-CTF was generated by an extracellular cleavage, the tetracycline-induced SW480-CD43 cells were treated with metalloprotease inhibitors. As shown in the Western blot analysis using the αCD43-4D2 mAb, the more general metalloprotease inhibitor 1,10-phenanthroline blocked the accumulation of CD43-CTF induced by the L-685,458 γ-secretase inhibitor (Figure 1C). The tumor necrosis factor-α-converting enzyme (TACE; also known as ADAM17) metalloprotease inhibitor TAPI-1 did not, however, block the formation of CD43-CTF. No major alteration in the cell-surface CD43 levels was revealed by flow cytometry after the 1,10-phenanthroline or L-685,458 treatments (results not shown). Figure 1(C) also shows, as expected, that small amounts of CD43-CTF were detected without γ-secretase inhibition. This proves the nature of the CD43-CTF, and suggests that CD43 is cleaved extracellularly by a 1,10-phenanthroline-dependent metalloprotease, as shown previously [37].
PS1-dependent processing of CD43
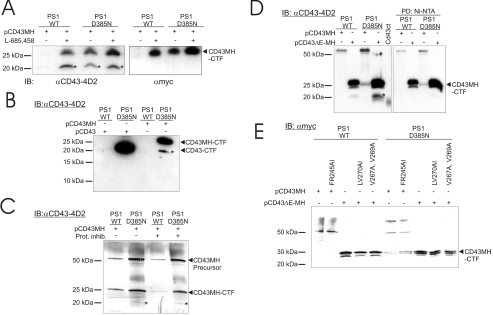
To study the CD43 γ-secretase-dependent processing further, HEK-293 cells stably expressing either PS1 WT or PS1 D385N [30,38] were studied. These cells were transfected with CD43 vectors expressing full-length WT CD43 (pCD43) or full-length CD43 tagged in the C-terminus with myc/His6 (pCD43MH), as well as CD43ΔE-MH (CD43 lacking the ectodomain and tagged in the C-terminus with myc/His6) (Figure 2). When the pCD43MH plasmid was expressed in HEK-293 PS1 D385N cells and analysed by Western blots using mAbs αCD43-4D2 and αmyc respectively, a fragment migrating at approx. 27 kDa was detected (Figure 3A). This fragment, denoted CD43MH-CTF, was not found in cells expressing PS1 WT, but was observed when these cells were subjected to the γ-secretase inhibitor L-685,458 (Figure 3A).
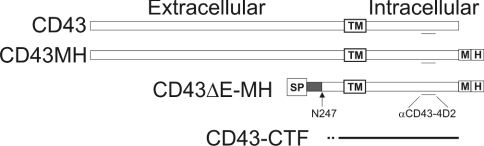
Figure 2. Schematic representation of the CD43 expression plasmid constructs used and the CD43-CTF fragment generated.
The epitope for the αCD43-4D2 antiserum and the location of the myc (M) and His6 (H) tags are shown. TM, the transmembrane domain; SP, signal peptide; N247, the first CD43 amino acid in the CD43ΔE construct.
Figure 3. Generation of the CD43-CTF fragment from CD43 transfected into HEK-293 cells.
(A) PS1 WT and PS1 D385N cells were transfected with pCD43MH and incubated in the presence or absence of the γ-secretase inhibitor L-685,458. Equal protein amounts of the lysates from these cells were separated on a 10–20% Tris-Tricine gel and analysed by Western blotting using αCD43-4D2 or αmyc mAb followed by an HRP-conjugated secondary mAb. (B) The same cells were transfected with pCD43 or pCD43MH 24 h before lysis. Samples were separated on a 10–20% Tris-Tricine gel followed by Western blotting using αCD43-4D2 mAb and HRP-conjugated secondary antibody and detection by enhanced chemiluminescence. The band marked with an asterisk migrating as CD43-CTF shows a degradation with loss of the myc and His6 tags from CD43MH-CTF. (C) Membranes were prepared from PS1 WT and PS1 D385N cells transfected with pCD43MH 24 h before lysis, and incubated for 2 h at 37 °C in the presence or absence of Complete™ protease inhibitors (Roche) and 5 mM EDTA. The samples were separated on a 10–20% Tris-Tricine gel, blotted on to a PVDF membrane and probed with αCD43-4D2 mAb, followed by an alkaline phosphatase-conjugated secondary antibody and detection with NBT/BCIP substrates. (D) HEK-293 cells expressing PS1 WT or PS1 D385N were transfected with either pCD43MH or pCD43ΔE-MH. The left panel shows a Western blot using crude lysates from the cells, and the right panel shows pull-downs using Ni-NTA beads from the same lysates. The samples were separated on SDS/12.5%-PAGE gels and blotted on to a PVDF membrane, probed with the αCD43-4D2 mAb followed by an HRP-conjugated secondary mAb and detected by enhanced chemiluminescence. The precursors of the transfected proteins migrated at approx. 55 and 27 kDa respectively. The ‘CD43ct’ lane shows the migration of recombinant GST (glutathione-S-transferase)–CD43ct after purification and removal of the GST tag. CD43-CTF detected in PS1 D385N cells express full-length CD43 (CD43FL), which migrates similarly to the CD43ΔE-MH precursor. (E) HEK-293 cells expressing PS1 WT or PS1 D385N were transfected with the mutated form of either pCD43MH or pCD43ΔE-MH, as shown. Samples were separated by SDS/PAGE on 10–20% Tris-Tricine gels and blotted on to a PVDF membrane, probed with the αmyc mAb followed by an HRP-conjugated secondary mAb and detected by enhanced chemiluminescence. The CD43-CTF and CD43MH-CTF fragments and the CD43MH precursor are indicated to the right of the gels, and the degradation product lacking the myc/His6 tags is shown by an asterisk.
In Figure 3(A) an unexpected fragment migrating at approx. 20 kDa (marked in the Figure with an asterisk) was detected in the Western blot with the αCD43-4D2 mAb, but not with αmyc mAb. This fragment was only detected when the CD43MH-CTF was found, suggesting it to be a degradation product of CD43MH by a release of the myc/His6 tag. This was illustrated further when HEK-293 cells expressing PS1 WT and PS1 D385N were transfected with either pCD43 or pCD43MH followed by Western blot analysis using αCD43-4D2 mAb (Figure 3B). The CD43MH-CTF (generated from CD43MH) migrated at 27 kDa and the degradation product (marked by an asterisk) occurred at 20 kDa, as before. However, no smaller band corresponding to the CD43-CTF (generated from non-tagged CD43) migrating at 20 kDa was found (Figure 3B). Thus the myc/His6 tag could be cleaved off, a frequent finding for linker sequences generated by cloning.
To verify that CD43-CTF was located in the membrane, preparations of membranes from HEK-293 PS1 WT and PS1 D385N cells transfected with pCD43MH were made. The prepared membranes were incubated in the presence or absence of protease inhibitors at 37 °C for 2 h and analysed by Western blotting using αCD43-4D2 mAb. In this case, the CD43MH-CTF was also found in cells having a functional PS1, but the levels were higher in cells expressing the dominant-negative PS1. The CD43MH-CTF band, as well as the degradation fragment marked with an asterisk, was thus, as expected, located in the membrane (Figure 3C). No alterations in the bands were detected when the protease inhibitortreated membranes and the non-treated membranes were compared, including the fragment lacking the myc/His6 tag.
As the CD43-CTF fragment is not accumulated in the WT cells, a functional γ-secretase/PS1 enzyme is expected to cleave quickly within the transmembrane domain to generate a cytoplasmic fragment, CD43ct. Such a fragment was not detected in any of the blots discussed so far. In an attempt to increase the likelihood for detecting such a CD43ct fragment in HEK-293 PS1 WT cells, a vector was constructed expressing an ectodomain-deleted CD43 protein tagged in its C-terminus with myc/His6, i.e. pCD43ΔE-MH (Figure 2). This should mimic the CD43MH-CTF that are processed by the γ-secretase complex. HEK-293 cells expressing PS1 WT and PS1 D385N were transfected with either pCD43MH or pCD43ΔE-MH and lysed after 24 h. Lysates from cells transfected with myc/His-tagged protein were pulled-down with Ni-NTA beads and analysed by Western blotting using αCD43-4D2 mAb. In parallel, a direct Western blot was made using the same lysates and antibody (Figure 3D). The CD43ct is expected to contain approx. 120 amino acids, and when this was recombinantly produced in bacteria and analysed together with cleavage fragments of CD43, it was found to migrate at approx. 17 kDa (Figure 3D), an expected position relative to the CD43-CTF migrating at 20 kDa. No smaller bands that could be a CD43ct fragment were detected in the cells transfected with pCD43ΔE-MH. The precursor protein of CD43ΔE-MH migrated approximately the same distance as the CD43MH-CTF, supporting the hypothesis of the nature of latter fragment and that the normal extracellular CD43 cleavage site must be located close to the transmembrane domain. The CD43ΔE-MH is expected to have 21 amino acids outside of the membrane. In the direct Western blot, but not in the Ni-NTA pull-downs, the degradation products of CD43MH-CTF lacking the myc/His6 tags were again detected (Figure 3D).
CD43 cleavages are not affected by mutations
The extracellular cleavage of CD43 has previously been suggested to occur between amino acids Phe245 and Arg246, located nine amino acids from the membrane [39]. In an attempt to identify the exact location for the observed extracellular cleavage, this potential cleavage site was mutated (Phe245Arg246→Ala/Ile) in the pCD43MH plasmid and transfected into HEK-293 PS1 and PS1 D385N cells. However, no difference in the CTF between WT and mutated CD43 was noticed (Figure 3E).
In an attempt to localize the γ-secretase cleavage site, two sets of mutations were performed in CD43ΔE-MH: Val267 and Val269 were mutated into two alanine residues, and Leu270 and Val271 into alanine and isoleucine, respectively. When these were expressed in HEK-293 cells with PS1 or PS1 D385N, no differences were observed in the appearance of the CTF fragments (Figure 3E).
CD43-CTF levels are dependent on cell density
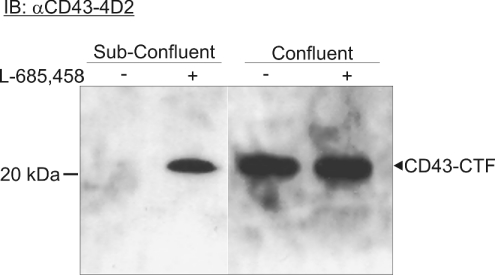
As the CD43-CTF was normally only found upon γ-secretase inhibition, it was important to search for physiological circumstances when this fragment was also generated. When the CD43-high-expressing cell line COLO205 [14] was analysed under confluent and subconfluent growth conditions, the CD43-CTF was observed in the confluent cells also without the addition of the γ-secretase inhibitor L-685,458 (Figure 4). As no major difference of the total level of full-length CD43 was observed, it is suggested that the formation of CD43-CTF could be related to the cell growth and cell density.
Figure 4. Accumulation of CD43-CTF is dependent on cell confluence.
Lysates from subconfluent and confluent COLO 205 cells, treated and not treated with the γ-secretase inhibitor L-685,458, were analysed on a 10–20% Tris-Tricine gel. The gel was blotted and probed with αCD43-4D2 mAb followed by an HRP-conjugated secondary antibody. CD43-CTF was detected regardless of γ-secretase inhibition in the confluent cells.
MUC1 is not cleaved in a γ-secretase-dependent way
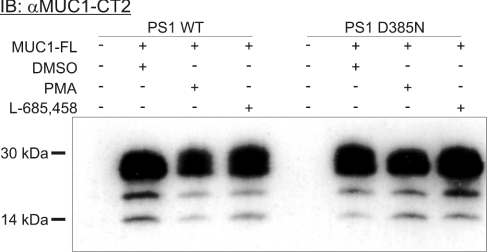
As CD43 was found to be a substrate for γ-secretase, we asked whether this was also a more general property of other mucintype molecules, like MUC1. To study this, full-length MUC1 (MUC1-FL in Figure 5) was transfected into cells expressing PS1 WT or PS1 D385N. In a Western blot using the αMUC1-CT2 mAb reacting with the MUC1ct, no differences, such as additional fragments, were detected between cells having a functional or non-functional PS1 (Figure 5). MUC1 is normally cleaved in its SEA domain (http://www.sanger.ac.uk/Software/Pfam/) just outside of the membrane during biosynthesis, but still held together within this domain. It is when the lysates are first boiled in SDS that these two parts separate and generate the small C-terminal fragments that are reacting with the cytoplasmic tail antibody αMUC1-CT2. Several bands, similar to ones revealed here, are always observed [31]. In addition, no detectable differences were found when the cells were treated with the γ-secretase L-685,458 inhibitor. A PMA-dependent extracellular cleavage of MUC1 has previously been shown in a human uterine epithelial cell line (HES) [40]. In an attempt to induce such an extracellular cleavage and thus promote the formation of a potential MUC1-CTF, PMA was added to the PS1 WT and PS1 D385N cells. No alteration in either the intensity of the bands or signs of any additional cleavages was observed. These results suggest that MUC1, in contrast with CD43, is not a substrate for the γ-secretase proteolytic pathway.
Figure 5. MUC1 is not cleaved by a PS1-dependent mechanism.
Full-length MUC1 was transfected into HEK-293 cells expressing PS1 WT or PS1 D385N. The lysates from these cells were separated on a 10–20% Tris-Tricine gel and analysed on a Western blot using αMUC1-CT2 mAb followed by an HRP-conjugated secondary anti-hamster antibody and developed with enhanced chemiluminescence substrate. No differences in the intensity, migration or number of fragments were detected.
DISCUSSION
Previously, we reported nuclear staining of CD43, and that its intracellular domain contained a functional NLS directing it to the nucleus [19]. It was also suggested to be involved in nuclear signalling events. These observations prompted us to investigate whether CD43 could undergo RIP-like cleavages, i.e. if there was an accumulation of the CD43-CTF when γ-secretase was blocked and a proteolytic release of its intracellular domain. That CD43 could be cleaved in its extracellular domain has been predicted from the presence of a soluble CD43 ectodomain fragment in human plasma [39], secretion of the extracellular part from the colon cancer cell line COLO 205 [14] and the observed cleavage at the cell surface in haematopoietic cells [36,37]. The extracellular cleavage was shown to be inhibited by the more general protease inhibitor 1,10-phenanthroline, but not by inhibitors of the ADAM17/TAPI enzyme. However, the precise localization of these cleavages has not been determined. Here we show that, in the colon carcinoma cell line COLO 205 and in the haematopoietic cell lines Jurkat and K562, endogenous CD43-CTF was found upon γ-secretase inhibition as a fragment migrating at approx. 20 kDa. This accumulation was inhibited by 1,10-phenanthroline, proving further the nature of the CD43-CTF. All three cell lines express high levels of CD43. The 27 kDa CD43 fragment, found as a prominent band in the Jurkat cells, suggested another extracellular cleavage, as has been suggested before [37]. This larger fragment was not a substrate for γ-secretase, as is predicted from our current understanding of how γ-secretase works [26–28]. The proteolytic processing of CD43 was also studied in the CD43-low-expressing cell lines MCF-7 and SW480 after overexpression of CD43 by an inducible system. The expressed CD43 protein in these two cell lines behaved exactly as the endogenous CD43, i.e. accumulation of CD43-CTF upon γ-secretase inhibition.
The extracellular cleavage of the CD43MH-expressed protein gave a CD43MH-CTF fragment of approx. 27 kDa. This fragment is larger than the CD43-CTF due to the 28 extra C-terminal amino acids which constitute the myc/His6 tags. The size of the CD43MH-CTF was slightly smaller than the ectodomain-deleted construct (CD43ΔE-MH), indicating that the extracellular cleavage site for CD43 is located slightly closer to the membrane than the 21 amino acids of this construct. This is compatible with a previously determined extracellular cleavage of CD43 between residues Phe245 and Arg246, located nine amino acids from the membrane [39]. However, mutation of these two amino acids did not block the cleavage. The localization and lack of dependence on the specific amino acid sequence is consistent with the effect of a cell-surface metalloprotease, as suggested by the inhibition by 1,10-phenanthroline. This is in line with previous results and observations for CD43 ectodomain shedding [36,37]. The extracellular α-secretase cleavage site of APP was, in a previous study, mutated with the purpose of blocking the cleavage, but also in this case the APP was efficiently cleaved despite mutations [41]. That CD43-CTF is really derived from ectodomain-cleaved full-length CD43 was supported further by the presence of this fragment in the membrane fraction.
An intramembrane γ-secretase cleavage of CD43, releasing the intracellular domain (CD43ct), was expected in cells having normal PS1, as its inhibition caused an accumulation of CD43-CTF. However, no CD43ct has been observed in any of the cell lines studied, suggesting that CD43-CTF must be rapidly cleaved and the γ-secretase-generated CD43ct fragment quickly metabolized. Difficulties in physically detecting the intracellular domain fragments generated by γ-secretase have also been reported for other proteins known to undergo RIP signalling, e.g. the APP [42,43]. One suggested explanation for the latter observation is that the insulin-degrading enzyme rapidly degrades the APP intracellular domain when released [33]. This is probably not the case for CD43, as no CD43ct was detected in the soluble fractions of membrane preparations incubated with protease inhibitors and EDTA (results not shown). Another possibility is degradation by the proteasome, but the addition of the proteasome inhibitor lactacystin to HEK-293 cells with a functional PS1 showed only an increased amount of the CTF fragment (results not shown). When a plasmid expressing the CD43ct was fused with green fluorescent protein at its N-terminal end, the resulting protein was stabilized and found to be translocated to the nucleus [19]. This was shown to be mediated by a functional NLS located in CD43ct, as suggested by mutagenesis experiments within the NLS, blocking nuclear translocation. An intramembrane γ-secretase-mediated cleavage of CD43 is predicted to generate a fragment containing all the amino acids in the CD43 NLS located close to the membrane. The precise localization of the γ-secretase-mediated cleavage is not known at present. Little is understood of the specificity of the γ-secretase cleavage, but it has been suggested previously that certain valine residues in the membrane domain are important [20,44]. However, two sets of mutations in the membrane domain did not interfere with the cleavage. This lack of inhibitory effect of specific amino acid replacements is similar to what has been observed for γ-secretase cleavage of APP [45].
Membrane-bound proteins with a highly glycosylated extracellular domain, such as the mucins, have been suggested to act as receptors. However, it has been difficult to understand how these types of protein could bind a ligand, and even more how this could trigger an outside-in signal. In this study, we suggest that CD43 signals via a RIP-like pathway. This also suggests a mechanism of how mucin-type proteins might cause outside-in signalling. Several mucin-type molecules of a similar type to CD43 are known to be proteolytically cleaved in the extracellular domain (e.g. MUC1, MUC3, MUC4 and MUC13). Whether any of these proteins can act via RIP-type signalling is unknown at present. Interestingly, MUC1, with a molecular topology similar to that of CD43 and recently suggested to appear in the nucleus [46,47], did not appear to act as a γ-secretase substrate.
The observation that CD43 is a substrate for γ-secretase, and that the level of the CD43-CTF can vary with cell density, suggests that CD43 can act as a signalling molecule, perhaps by nuclear translocation via its functional NLS in the CD43ct [19]. We propose that CD43 can undergo an RIP-like signalling process.
Acknowledgments
This work was supported by The Swedish Cancer Foundation, The Swedish Research Council, The Swedish Institute, and IngaBritt and Arne Lundberg's Foundation. We thank Dr Sandra Gendler for the CT2 mAb and Professor Joyce Taylor-Papadimitriou for the full-length MUC1-expressing plasmid.
References
- 1.Gendler S. J. MUC1, the renaissance molecule. J. Mammary Gland Biol. Neoplasia. 2001;6:339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 2.Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piller V., Piller F., Fukuda M. Phosphorylation of the major leukocyte surface sialoglycoprotein, leukosialin, is increased by phorbol 12-myristate 13-acetate. J. Biol. Chem. 1989;264:18824–18831. [PubMed] [Google Scholar]
- 4.Pedraza-Alva G., Merida L. B., Burakoff S. J., Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J. Biol. Chem. 1996;271:27564–27568. doi: 10.1074/jbc.271.44.27564. [DOI] [PubMed] [Google Scholar]
- 5.Allenspach E. J., Cullinan P., Tong J., Tang Q., Tesciuba A. G., Cannon J. L., Takahashi S. M., Morgan R., Burkhardt J. K., Sperling A. I. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 6.Delon J., Kaibuchi K., Germain R. N. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 7.Ostberg J. R., Barth R. K., Frelinger J. G. The Roman god Janus: a paradigm for the function of CD43. Immunol. Today. 1998;19:546–550. doi: 10.1016/s0167-5699(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 8.Pedraza-Alva G., Merida L. B., Burakoff S. J., Rosenstein Y. T cell activation through the CD43 molecule leads to Vav tyrosine phosphorylation and mitogen-activated protein kinase pathway activation. J. Biol. Chem. 1998;273:14218–14224. doi: 10.1074/jbc.273.23.14218. [DOI] [PubMed] [Google Scholar]
- 9.Wong R. C., Remold-O'Donnell E., Vercelli D., Sancho J., Terhorst C., Rosen F., Geha R., Chatila T. Signal transduction via leukocyte antigen CD43 (sialophorin). Feedback regulation by protein kinase C. J. Immunol. 1990;144:1455–1460. [PubMed] [Google Scholar]
- 10.Santana M. A., Pedraza-Alva G., Olivares-Zavaleta N., Madrid-Marina V., Horejsi V., Burakoff S. J., Rosenstein Y. CD43-mediated signals induce DNA-binding activity of AP-1, NF-AT and NFkB transcription factors in human T lymphocytes. J. Biol. Chem. 2000;275:31460–31468. doi: 10.1074/jbc.M005231200. [DOI] [PubMed] [Google Scholar]
- 11.Dragone L. L., Barth R. K., Sitar K. L., Disbrow G. L., Frelinger J. G. Dysregulation of leukosialin (CD43, Ly48, sialophorin) expression in the B-cell lineage of transgenic mice increases splenic B-cell number and survival. Proc. Natl. Acad. Sci. U.S.A. 1995;92:626–630. doi: 10.1073/pnas.92.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misawa Y., Nagaoka H., Kimoto H., Ishii Y., Kitamura K., Tsunetsugu-Yokota Y., Shibuya M., Takemori T. CD43 expression in a B cell lymphoma, WEHI 231, reduces susceptibility to G1 arrest and extends survival in culture upon serum depletion. Eur. J. Immunol. 1996;26:2573–2581. doi: 10.1002/eji.1830261106. [DOI] [PubMed] [Google Scholar]
- 13.Cermak L., Simova S., Pintzas A., Horejsi V., Andera L. Molecular mechanisms involved in cd43-mediated apoptosis of TF-1 cells. J. Biol. Chem. 2002;277:7955–7961. doi: 10.1074/jbc.M108048200. [DOI] [PubMed] [Google Scholar]
- 14.Baeckstrom D., Zhang K., Asker N., Ruetschi U., Ek M., Hansson G. C. Expression of the leukocyte-associated sialoglycoprotein CD43 by a colon carcinoma cell line. J. Biol. Chem. 1995;270:13688–13692. doi: 10.1074/jbc.270.23.13688. [DOI] [PubMed] [Google Scholar]
- 15.Santamaria M., Lopez-Beltran A., Toro M., Pena J., Molina I. J. Specific monoclonal antibodies against leukocyte-restricted cell surface molecule CD43 react with nonhematopoietic tumor cells. Cancer Res. 1996;56:3526–3529. [PubMed] [Google Scholar]
- 16.Fernandez-Rodriguez J., Andersson C. X., Laos S., Baeckstrom D., Sikut A., Sikut R., Hansson G. C. The leukocyte antigen CD43 is expressed in different cell lines of nonhematopoietic origin. Tumour Biol. 2002;23:193–201. doi: 10.1159/000067252. [DOI] [PubMed] [Google Scholar]
- 17.Sikut R., Nilsson O., Baeckstrom D., Hansson G. C. Colon adenoma and cancer cells aberrantly express the leukocyte-associated sialoglycoprotein CD43. Biochem. Biophys. Res. Commun. 1997;238:612–616. doi: 10.1006/bbrc.1997.7334. [DOI] [PubMed] [Google Scholar]
- 18.Pimenidou A., Madden L. A., Topping K. P., Smith K. A., Monson J. R., Greenman J. Novel CD43 specific phage antibodies react with early stage colorectal tumours. Oncol. Rep. 2004;11:327–331. [PubMed] [Google Scholar]
- 19.Andersson C. X., Fernandez-Rodriguez J., Laos S., Sikut R., Sikut A., Baeckstrom D., Hansson G. C. CD43 has a functional NLS, interacts with beta-catenin, and affects gene expression. Biochem. Biophys. Res. Commun. 2004;316:12–17. doi: 10.1016/j.bbrc.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Schroeter E. H., Kisslinger J. A., Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 21.De Strooper B. Aph-1, Pen-2, and Nicastrin with presenilin generate an active gamma-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 22.Ni C. Y., Yuan H., Carpenter G. Role of the ErbB-4 carboxyl terminus in gamma-secretase cleavage. J. Biol. Chem. 2003;278:4561–4565. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]
- 23.Marambaud P., Shioi J., Serban G., Georgakopoulos A., Sarner S., Nagy V., Baki L., Wen P., Efthimiopoulos S., Shao Z., et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammich S., Okochi M., Takeda M., Kaether C., Capell A., Zimmer A. K., Edbauer D., Walter J., Steiner H., Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J. Biol. Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 25.Murakami D., Okamoto I., Nagano O., Kawano Y., Tomita T., Iwatsubo T., De Strooper B., Yumoto E., Saya H. Presenilin-dependent gamma-secretase activity mediates the intramembranous cleavage of CD44. Oncogene. 2003;22:1511–1516. doi: 10.1038/sj.onc.1206298. [DOI] [PubMed] [Google Scholar]
- 26.Haass C., De Strooper B. The presenilins in Alzheimer's disease – proteolysis holds the key. Science. 1999;286:916–919. doi: 10.1126/science.286.5441.916. [DOI] [PubMed] [Google Scholar]
- 27.Ebinu J. O., Yankner B. A. A RIP tide in neuronal signal transduction. Neuron. 2002;34:499–502. doi: 10.1016/s0896-6273(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 28.Aguzzi A., Haass C. Games played by rogue proteins in prion disorders and Alzheimer's disease. Science. 2003;302:814–818. doi: 10.1126/science.1087348. [DOI] [PubMed] [Google Scholar]
- 29.Brown M. S., Ye J., Rawson R. B., Goldstein J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature (London) 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder J. A., Thompson M. C., Gardner M. M., Gendler S. J. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 2001;276:13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 32.Shearman M. S., Beher D., Clarke E. E., Lewis H. D., Harrison T., Hunt P., Nadin A., Smith A. L., Stevenson G., Castro J. L. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–9704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- 33.Edbauer D., Willem M., Lammich S., Steiner H., Haass C. Insulin-degrading enzyme rapidly removes the beta-amyloid precursor protein intracellular domain (AICD) J. Biol. Chem. 2002;277:13389–13393. doi: 10.1074/jbc.M111571200. [DOI] [PubMed] [Google Scholar]
- 34.Brown T. J., Shuford W. W., Wang W. C., Nadler S. G., Bailey T. S., Marquardt H., Mittler R. S. Characterization of a CD43/leukosialin-mediated pathway for inducing apoptosis in human T-lymphoblastoid cells. J. Biol. Chem. 1996;271:27686–27695. doi: 10.1074/jbc.271.44.27686. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J. Biol. Chem. 1986;261:12787–12795. [PubMed] [Google Scholar]
- 36.Bazil V., Strominger J. L. Metalloprotease and serine protease are involved in cleavage of CD43, CD44, and CD16 from stimulated human granulocytes. Induction of cleavage of L-selectin via CD16. J. Immunol. 1994;152:1314–1322. [PubMed] [Google Scholar]
- 37.Remold-O'Donnell E., Parent D. Two proteolytic pathways for down-regulation of the barrier molecule CD43 of human neutrophils. J. Immunol. 1994;152:3595–3605. [PubMed] [Google Scholar]
- 38.Steiner H., Haass C. Intramembrane proteolysis by presenilins. Nat. Rev. Mol. Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- 39.Schmid K., Hediger M. A., Brossmer R., Collins J. H., Haupt H., Marti T., Offner G. D., Schaller J., Takagaki K., Walsh M. T., et al. Amino acid sequence of human plasma galactoglycoprotein: identity with the extracellular region of CD43 (sialophorin) Proc. Natl. Acad. Sci. U.S.A. 1992;89:663–667. doi: 10.1073/pnas.89.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thathiah A., Blobel C. P., Carson D. D. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 2003;278:3386–3394. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 41.Sisodia S. S. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita A., Whelan C. M., Smith C. J., Berezovska O., Hyman B. T. Direct visualization of the gamma secretase-generated carboxyl-terminal domain of the amyloid precursor protein: association with Fe65 and translocation to the nucleus. J. Neurochem. 2002;82:839–847. doi: 10.1046/j.1471-4159.2002.01016.x. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y., Pimplikar S. W. The gamma-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14979–14984. doi: 10.1073/pnas.261463298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Strooper B., Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 45.Sastre M., Steiner H., Fuchs K., Capell A., Multhaup G., Condron M. M., Teplow D. B., Haass C. Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y., Liu D., Chen D., Kharbanda S., Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen Y., Caffrey T. C., Wheelock M. J., Johnson K. R., Hollingsworth M. A. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J. Biol. Chem. 2003;278:38029–38039. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]