Abstract
Objective:
The purpose of this study is to estimate the prevalence and severity of late oral morbidities in disease-free oropharyngeal cancer (OPC) survivors using patient reported outcomes.
Materials and methods:
Cross-sectional survivorship survey of patients who completed definitive treatment for oropharyngeal carcinoma > 12-months previously without evidence of recurrence, second primary malignancy, or distant metastasis after index cancer. Using MD Anderson Symptom Inventory- Head and Neck Module (MDASI-HN), scores for 4 self-reported oral morbidities: dry mouth, mucus secretions, mouth and throat sores, and teeth and gum issues were evaluated.
Results:
Among 906 survey respondents (57% response rate), (median survival time: 7 years), “dry mouth” and “problems with my mucus” were reported moderate/severe (MDASI-HN item score ≥5) in 39% and 22% of respondents, while 14% reported moderate/severe “problems with teeth and gums”. Smoking at the time of survey was significantly associated with the severity of oral symptoms including “mucus” (p = 0.03), “dry mouth” (p = 0.02), “problems with my teeth and gums” (p = 0.001). All the oral morbidities symptom items significantly, positively correlated with the mean interference scores reflecting adverse impact to quality of life (QOL): “mucus” (r = 0.445, p < 0.001), “problems with teeth” (r = 0.446, p < 0.001), “mouth sores” (r = 0.321, p < 0.001) and “dry mouth” (r = 0.459, p < 0.001).
Conclusion:
This study showed that 45.5% reported at least one late oral morbidity at moderate/severe level which negatively correlated overall function, even years after treatment.
Keywords: Oral morbidities, Oropharyngeal carcinoma, Patient-reported outcomes, Xerostomia, Mouth sores, Dry mouth
Introduction
With the rise in incidence of HPV-positive oropharyngeal cancer (OPC), increased treatment response and decreased recurrence rates, there is a growing population of OPC survivors who now go on to live years (often decades) with long-term effects of therapy [1–3]. Oral morbidities such as mucositis, osteoradionecrosis and xerostomia are common side effects when oral tissues such as mucosa, bone, and salivary glands, are within the volume of tissue irradiated. Furthermore, salivary changes affect the oral flora and overall risk of dental caries further increasing risks of osteoradionecrosis and dental/oral complications [4]. These complications are well described in the literature and can easily be evaluated by clinicians; however, at times, there is a discordance between clinician- and patient-reported measures [5–9]. The best example of this is xerostomia, in which an objective decrease in salivary production does not directly correlate to subjective perception of xerostomia despite the persistent impact of hyposalivation on oral health. This discordance does not negate the need for objective measures; however, clinicians increasing recognize the value of more subjective, patient-reported outcomes assessment to understand toxicities of modern therapy [9].
Patient-reported outcomes (PROs) are quickly becoming a part of the decision-making process for choosing the best and most effective therapy for cancers [10]. The MD Anderson Symptom Inventory- Head and Neck Module (MDASI-HN) is a validated instrument used to assess patient symptoms associated specifically with head and neck cancer and its treatment [11,12]. The MDASI-HN is completed by the patient to assess the severity of 22 common head and neck symptoms such as xerostomia, oral mucositis, challenges with mucus, and difficulty chewing/swallowing. MDASI-HN includes 4 symptom items directly reflecting oral morbidities: “dry mouth,” “mucus,” “problems with teeth/gums,” and “mouth sores.” Patient self-reporting is an opportunity to better understand the impact of treatment which then can inform practitioner decisions and research priorities. Yet, current estimates of late effects of radiation therapy are primarily derived from small or highly selective clinical studies, and often comprise heterogeneous groups of head and neck cancers for whom radiation fields and surgical impact on oral cavity vary greatly [13]. Further, there is lack of data about the association between the oral morbidities and the influence on the quality of life and functional status of survivors, particularly in homogenous groups such as OPC survivors. The purpose of this study, therefore, is to estimate the prevalence and severity of late oral morbidities using a validated PRO questionnaire in a large sample of long-term (> 1-year) disease free OPC survivors at MD Anderson Cancer Center, more specifically to evaluate and characterize the self-reported symptoms that relate to late treatment side effects to salivary glands as well as oral hard and soft tissues.
Materials and methods
This cross-sectional survivorship survey was conducted from September 2015 to July 2016 among OPC patients who received their primary treatment (radiation ± chemotherapy, ± surgery) at The University of Texas, M.D. Anderson Cancer Center (MDACC) between 01/01/2000 and 12/01/2016 were eligible for survey. The study population included adult patients greater than 18 years of age with no evidence of locoregional recurrence, second primary malignancy or distant metastasis at the time of survey. Potential subjects excluded were those having received treatment less than two years prior to the time of the survey. Approval for this study was granted by the Institutional Review Board of MDACC PA11–0936, and patients provided informed consent by survey response.
Survey administration
Participants were asked to complete the survey once. The survey was administered using an adapted version of Dillman method [14], including: (1) a letter of invitation via the US postal service to eligible patients 2–3 weeks prior to the initial contact, (2) the survey questionnaire to all eligible participants via an online server Qualtrics or US postal service; and (3) two reminders were sent to non-responders via US postal service at 2–3 weeks and 4–5 weeks after the initial contact. Participants were contacted by multiple modes of communication, including email (among those with addresses on record) via Qualtrics or myMDAnderson (a secure, personalized patient website), and US postal service via first-class mail with a return envelope.
The MDASI-HN questionnaire
The survey included the MDASI-HN (MD Anderson Symptom Inventory-Head and Neck Module), a psychometrically validated 28-items questionnaire containing 13 “core” items representing important symptoms common to all cancer types, 9 “head and neck cancer specific” items, and 6 questions regarding the extent to which the symptoms interfere with activities of daily life [12]. The validation sample included patients along the continuum of survivorship (pre, during, and post-treatment). MDASI-HN has not, however, been validated in an exclusive sample of long-term cancer survivors. The 6 interference items are rated on numeric scales of 0–10 from” did not interfere” to “interfered completely”; interference mean score is felt to reflect a proxy for health-related quality of life and overall patient function. The MDASI-HN symptoms items are rated on numeric scales of 0–10 from” not present” to “as bad as you can imagine”. MDASI-HN items pertaining to self-reported oral morbidities were the primary endpoints of interest for this analysis. These comprise 4 items including: “dry mouth”, “mucus”, “mouth sores”, and “problems with my teeth/gums”.
Statistical analysis
Mean values for each MDASI- HN oral morbidities items were plotted, and to clarify the clinical interpretation of these data, the percentage of patients with moderate to severe perceived oral symptoms (ratings of ≥5 on the scale of 10) were calculated for each item. Primary covariates considered in subgroup comparisons and correlations of self-reported oral morbidities included sex, age, cancer subsite, tumor classification, therapeutic combination, dental status, follow up time, and smoking at diagnosis and at time of survey, with comparisons Bonferroni corrected (p < 0.006 considered significant for 9 clinical covariates tested). The data were analyzed using one-way ANOVA (categorical covariates), with post hoc students t-test, and pairwise correlations for continuous covariates. Analysis was performed in Stata Data Analysis Software (Version 14.0 College Station, TX).
Selected chart review
To understand the nature of clinical correlates for the symptom item, “problems with my teeth/gums”, chart review was conducted among those respondents who reported moderate to severe values for “problems with teeth/gums” to further clarify the dental challenge. Chart detected dental challenges in this subgroup were coded: as (1) periodontal disease, (2) multiple missing teeth, (3) osteonecrosis, or (4) no chart documented problem.
Results
A total 1728 participants met the inclusion criteria above in order to be eligible for survey; 989 patients responded for an initial response rate of 58%. Eighty-two subjects were excluded after further chart abstraction revealed them ineligible for this analysis yielding a total of 906 subjects included in this study.
Clinical and demographic profiles are detailed in Table 1. The mean age for patients was 65 years (SD 8.4) and 84% of the patients were male. The majority of the tumors were located in the tonsil (46%) and the base of the tongue (49%). The majority of the patients (67%) were treated by combination of radiation and chemotherapy, and 3% of the patients were treated by primary surgery. Thirty-one patients (3%) reported to be current at the time of the survey, whereas at the time of diagnosis, 60 patients (6%) were current smokers and 428 patients (47%) were former smokers. Four hundred and forty-one (49%) tumors tested positive for human papilloma virus (HPV) association (HPV status not tested/unknown in 407 patients, 45%). Median cancer free survival time was 7 years with minimum of 2 years and maximum of 16 years.
Table 1.
Clinical and patient demographic characteristics.
| All patients (%) | |
|---|---|
|
| |
| Sex | |
| Male | 766 (85%) |
| Female | 140 (15%) |
| Age | |
| Median (range) | 66.13 (39–92) |
| T classification | |
| 1 | 335 (37%) |
| 2 | 349 (38%) |
| 3 | 134 (15%) |
| 4 | 88 (10%) |
| N classification | |
| 0 | 84 (9%) |
| 1 | 129 (14%) |
| 2a | 110 (12%) |
| 2b | 404 (45%) |
| 2c | 150 (17%) |
| 3 | 29 (3%) |
| Cancer subsite | |
| Tonsil | 418 (46%) |
| Base of tongue | 445 (49%) |
| Glossopharyngeal sulcus | 11 (1%) |
| Other | 32 (4%) |
| HPV or p16 | |
| No | 58 (6%) |
| Yes | 441 (49%) |
| Unknown | 407 (45%) |
| Therapeutic combination | |
| RT alone | 272 (30%) |
| RT ± systemic* | 610 (67%) |
| Surgery + Adjuvant | 16 (2%) |
| Surgery alone | 8 (1%) |
| Survival time (median) | 7 years |
| Smoking survey | |
| No | 859 (97%) |
| Yes | 31 (3%) |
| Smoking at diagnosis | |
| No | 418 (46%) |
| Former | 428 (47%) |
| Current | 60 (7%) |
| Self-reported teeth at survey | |
| No teeth | 88 (10%) |
| I have all or most of my teeth | 637 (72%) |
| I have many missing teeth | 163 (18%) |
Induction or concurrent systemic therapy
Dental status
Dental status, as self-reported at the time of survey, reflected 72% reported having “most of their teeth” at time of survey, whereas 9.9% reported having no teeth and 18% reported “many missing teeth”.
Symptoms severity assessment
Mean oral morbidity scores suggested expected rank order of self-reported oral morbidities in long-term survivors, with highest burden for “dry mouth” (3.92 ± 2.8) followed by “problems with mucus build up” (2.41 ± 2.79) and “problems with my teeth and gums” (0.56 ± 1.44); “mouth sores” (0.56 ± 1.44) were rarely reported. Salivary symptoms “dry mouth” and “problems with mucus build up” were reported moderate/severe (MDASI-HN item ≥5) in 39% and 22% of respondents, whereas 14% reported moderate/severe “problems with my teeth and gums”. “Mouth sores” were reported as moderate/severe in 4% of population. Complete results are presented in Table 2. Of the subjects reporting moderate to severe “problems with teeth”, chart review indicated that most of these patients suffered from periodontal disease, followed by multiple missing teeth, or osteoradionecrosis. These results are presented in Table 3.
Table 2.
Oral morbidities graded by scale from 0 to 10; 0=‘not present’ to 10=‘as bad as you can imagine’.
| Oral morbidity | MDASI-HN items* | Mean ± SD (symptoms severity R% reporting item score ≥ 5) |
|---|---|---|
|
| ||
| Xerostomia | Your dry mouth at its worst? | 3.92 ± 2.88 (39%) |
| Mucus build up | Your problems with mucus in your mouth and throat at its worst? | 2.41 ± 2.79 (22%) |
| Mouth sores | Your mouth/throat sores at its worst? | 0.56 ± 1.44 (3%) |
| Dental problems | Your problem with teeth or gums at its worst? | 1.59 ± 2.57 (14%) |
Patients were asked to grade symptoms severity at a scale of 0–10.
Table 3.
Dental issues documented in EMR for survivors reporting moderate to severe “problems with teeth”.*
| Teeth problem Item score ≥ 5(%) | ORN | Missing teeth | Periodontal disease | No abnormal findings |
|---|---|---|---|---|
| 124 (13%) | 28 (20%) | 48 (38%) | 54 (43%) | 23 (18%) |
Despite reporting dental challenge, 18% of this cohort had no documented oral/dental problem.
Oral morbidities by clinical and demographic data
Symptom severity assessed in regards to the patient’s characteristics is shown in Figure 1. Smoking at the time of the survey was significantly associated with severity of self-reported “mucus” (p = 0.003), “dry mouth” (p = 0.002), and “problems with my teeth and gums” (p-value 0.001). Patients ≥ 70 years of age at survey reported more mucus symptoms (p < 0.001). Advanced tumor staging was associated with worse mucus, teeth/gum and dry mouth symptoms (p < 0.001). There was a significant correlation between having multiple missing teeth and a greater degree of perceived oral morbidities. Gender and tumor subsite were not significantly associated with oral morbidity symptom severity. Complete results are presented in Figure 1.
Fig. 1.
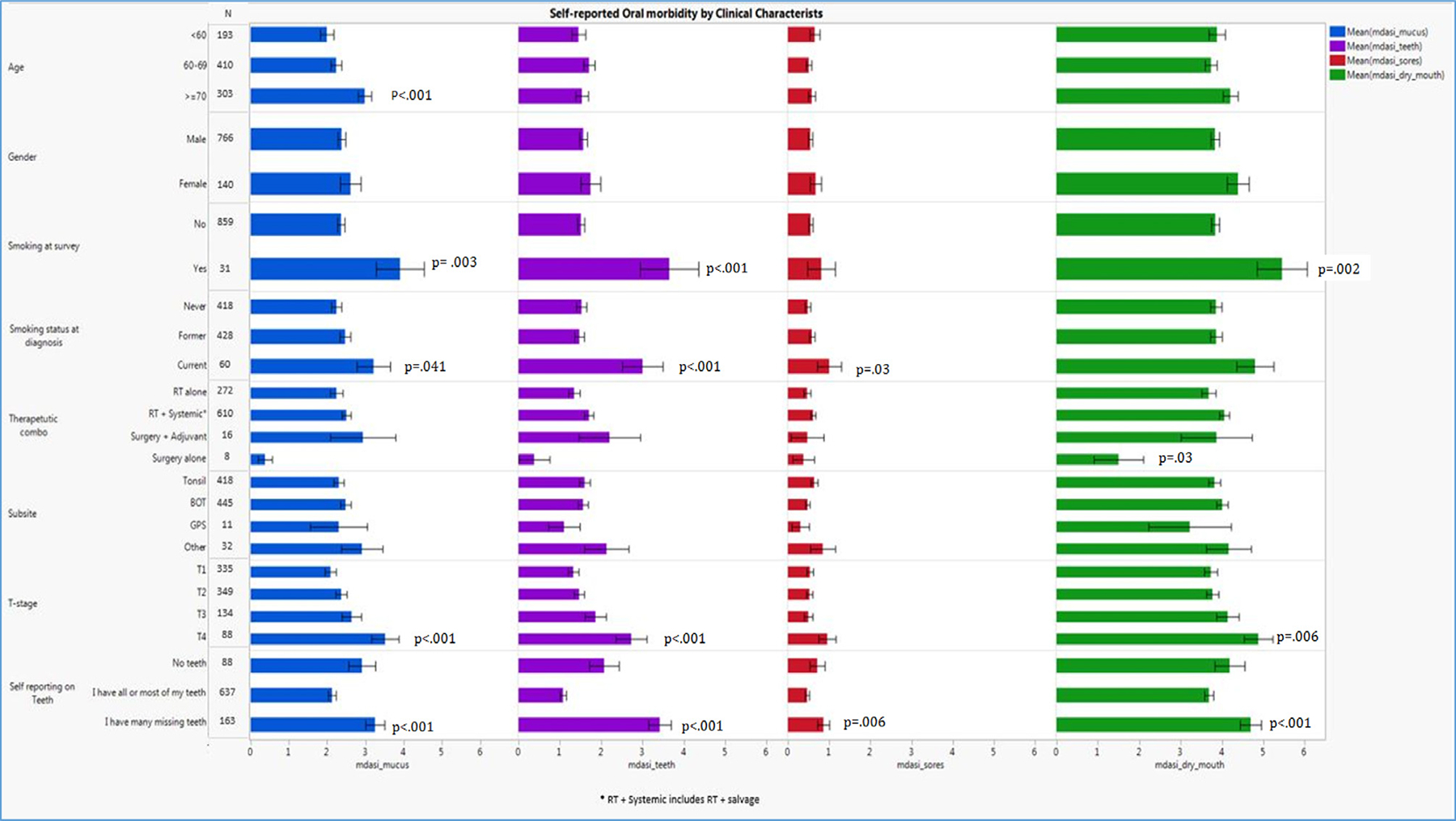
Symptom severity in relation to patient characteristics (Sex, gender, therapeutic combination, and tumor subsite were not associated with oral symptom severity; while, dental status, T-classification, smoking at the time of survey, and age significantly associated with oral morbidity symptom severity p < 0.001. Bonferroni corrected = p < 0.006).
Quality of life as measured by symptom interference by oral morbidities symptom severity
All of the MDASI oral items significantly, positively correlated with the mean interference as shown on Fig. 2; “mucus” (r = 0.445, p < 0.001), “problems with teeth” (r = 0.446, p < 0.001), “mouth sores” (r = 0.321, p < 0.001) and “dry mouth” (r = 0.459, p < 0.001).
Fig. 2.
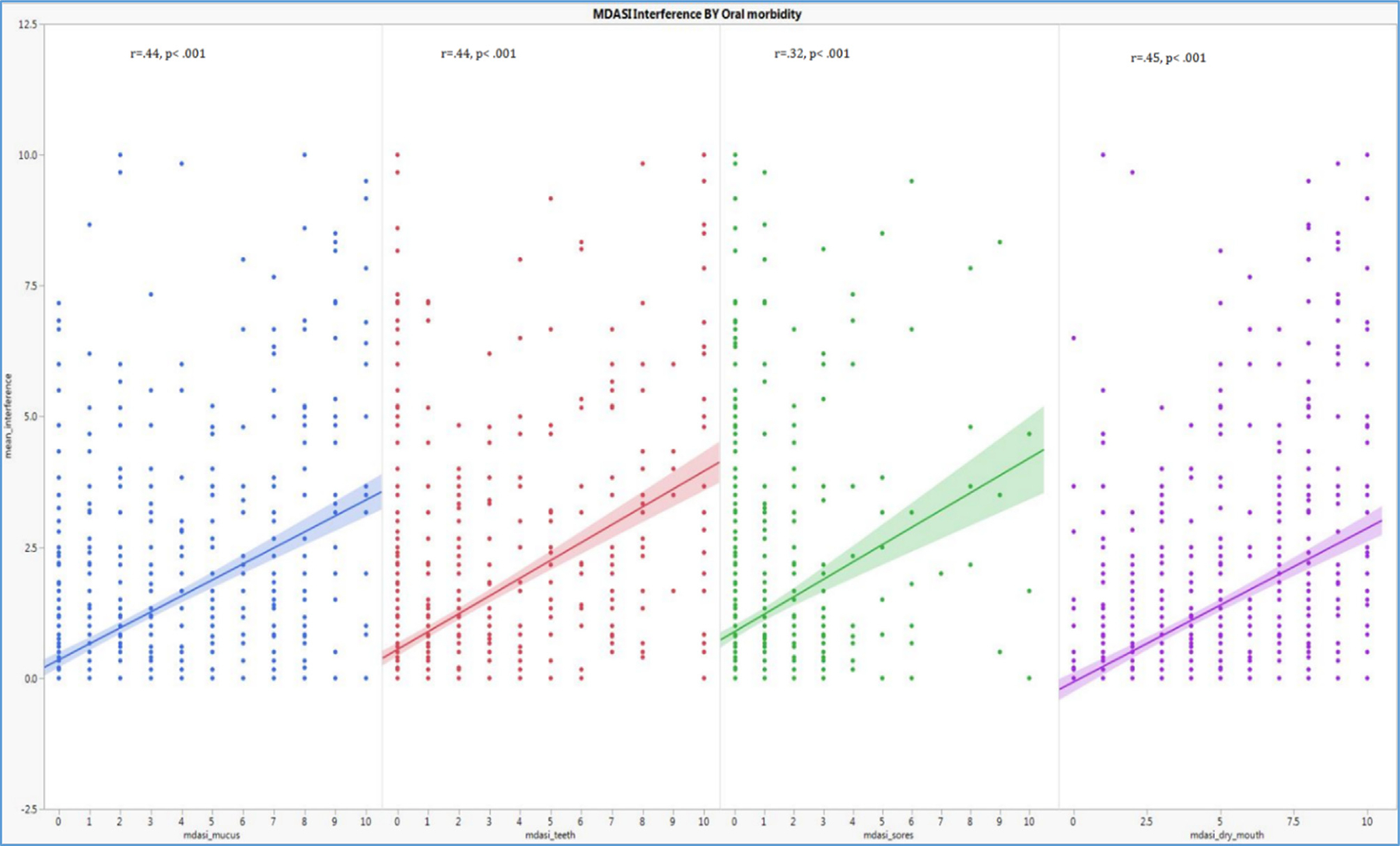
Correlation between symptom severity and symptom interference (All MDASI oral morbidity symptom items significantly, positively correlated with interference which is a surrogate for QOL and patient functioning, p < 0.001).
Oral morbidities in relation to survival time
The only oral morbidity symptom item that significantly correlated with survival time was “problems with my teeth and gums” with weak, positive linear correlation (r = 0.14, p = 0.002). Severity of the remaining oral morbidity items remained stable over survival time a survey response, as illustrated in Fig. 3.
Fig. 3.

Symptom severity in relation to survival time (Only “problems with teeth” seemed to worsen over time, p = 0.002).
Discussion
The current study estimated that 46% of disease free OPC survivors at MDACC report moderate/severe late oral morbidity symptoms using the validated MDASI-HN instrument. As PROs begin to influence treatment decisions and research priorities; it is critical that the prevalence and nature of oral morbidity symptoms associated with cancer are understood. While there is structured research describing the oral morbidities associated with head and neck cancer; these morbidities are often difficult to assess due to patient/assessor variability and the subjective nature of many toxicities. PROs complement a number of conventional clinical toxicity measures. For instance, patient reported evaluations have been shown to be more reliable than objective, practitioner-assessed measures of salivation [15].
As expected, the most common late oral morbidity associated with cancer treatment in this large OPC survivor survey was “dry mouth” with 39% self-reporting moderate/severe scores. A significant relationship was established between larger T-staging and increased “dry mouth” scores. This is expected because as T-stage increases, so too does the volume of tissue irradiated with potential for target doses and beam path to overlap salivary glands. Most interestingly, however, patients who smoked, at the time of the survey, also reported higher values for “dry mouth”. Similar results were produced by Lee et al. [16], in which smoking seemed to contribute to perception of xerostomia following IMRT; however, this remains mostly under studied in this population. In the general population, data are conflicting. While there is evidence to support reduced salivary flow as a result of smoking [17,18], there is literature to support increased salivary flow as a result of smoking [19]. Further investigation is merited on this topic.
Moderate/severe “problems with my teeth and gums” were reported in 14% of survey respondents. Similarly, it was determined that patients with higher symptoms scores also reported smoking history and many missing teeth. Smoking and alteration of the oral microflora has been established previously in the literature. Wu et al. [20] determined that smokers have increased levels of streptococcus bacteria; the bacteria that is responsible for dental caries. This alteration in oral microflora, coupled with reduced saliva quantity and quality, results in a lower oral pH and subsequently dental caries. This was also one of the only oral morbidities studied that seemed to increase over time. This is true of the population in general, as dental restorations reach their life expectancy, dietary changes that include increased cariogenic foods and a decreased compliance with dental hygiene practices can lead to increased dental needs and “problems with teeth and gums”.
This study showed that the number of missing teeth seemed to increase the degree of perceived oral morbidities. Generally, most head and neck cancer patients are referred to a dentist prior to the initiation of radiation therapy [21,22], while there are guidelines for dental extraction prior to radiation therapy, there is uncertain adherence by treating dentists. Because of this, treatment plans can vary greatly between practitioners ranging from extraction of all teeth in the volume of tissue radiated regardless of dental health to extraction of only non-restorable teeth. While this topic is beyond the scope of this study, our results favor, at least initially, a more conservative approach to dental treatment planning prior radiation therapy.
“Mouth sores” as a symptom were, as expected, the least commonly reported late burden in the oral cavity with only 4% of long-term survivors reporting moderate/severe scores. Oral mucositis as a result of radiation therapy tends to resolve within six to eight weeks [23], so to have mucositis type sores in the oral cavity months years after treatment would be uncommon for the vast majority of survivors. It is possible that those long-term survivors who reported significant “mouth sores” were the result of friable oral tissue that is more prone to injury, both traumatic and/or chemical. The severity of this symptom was compounded by smoking and the number of missing teeth, in that, subgroups of smokers and edentulous patients reported higher scores for “mouth sores”. Additionally, patients who undergo radiation therapy often experience chronic mucosal sensitivity [24], which then can be interpreted as “mouth sores” by patient population. These are usually thin areas of mucosa which remains more sensitive to stimulus (i.e. food or touch). These areas are generally prone to superinfection and thus treatment of these lesions involves prevention and appropriate oral hygiene.
Thick secretions and mucus build up is considered one of the most reported acute side effects of OPC treatment and can last up to 6 months, but the evolution of this symptom into long-term survivorship is not well characterized [9,25]. It is, therefore, noteworthy that roughly one-quarter of long-term survivors reported moderate to severe mucus symptoms which did not appear to worsen over time. This result conflicts with previous research completed by Nordgren et al. in 2006 in that who reports that problems with mucous worsened over time [26]. These were patients that were treated in the early to mid 1990s with conventional radiation techniques leading to higher doses to parotid glands and smaller minor salivary glands. Thus, divergent results may reflect differing radiation techniques or differing assessment styles. Our results, however, do show a significant relationship between age and mucous production. In fact, survivors ≥70 years old reported the higher mucus symptoms when compared to younger patients. Oral morbidities, such as those studied, are multifactorial in nature. Considering “dry mouth”, for example, multiple factors contribute the perception of xerostomia such as age and medications [27]. Therefore, these findings maybe correlate with ageing salivary glands but also could reflect the fact that an ageing population is more likely to be taking more medications and consequently, some symptoms may be less a direct result of the treatment itself. This leads to a limitation of this study, because of the multifactorial nature of some of these morbidities, it is difficult to isolate treatment-attributable prevalence of symptoms such as “dry mouth” that can result from radiation, medication, age, or a combination thereof.
Other limitations include lack of specificity as to the type of “problems with my teeth and gums”. That is, the most ambiguous oral morbidity symptom item we evaluated was “problems with my teeth and gums”. What respondents considered a dental problem can vary widely, ranging from staining or tooth color changes on otherwise healthy teeth to loose/ill-fitting denture prostheses, or even issues with more significant health implications like dental caries causing periapical infection, cellulitis, and pain. This was confirmed by the chart review completed in spite of reporting a moderate to severe score regarding “problems with teeth and gums”, 18% of participants did not appear to have a recorded dental/gingival problem. Further complicating this matter is the fact that early dental disease is often asymptomatic. Dental caries become symptomatic when the lesion approximates or invades the dental nerve; at this point, the tooth will develop sensitivity and eventually pain. The dental status of these patients is not different than that of the general population with a wide variety of oral/dental issues and varying range of dental knowledge. The performance of these oral morbidity PRO items in the general population is not benchmarked, and this cross-sectional survey cannot adjust for severity of these symptoms items at baseline before cancer therapy. The inability to compare oral symptoms or more objective dental pre-treatment findings to post-treatment survey responses is a limitation of this study as prior perceptions of oral health may certainly influence post-treatment PRO responses.
Dental decay is a multifactorial process and while there are obvious implications from radiation therapy such as increased bacteria levels and xerostomia; there are other factors which are not related specifically to cancer care, i.e. lack of access to dental care (due to finances or distance), poor oral hygiene in general, or patient non-compliance.
An avenue for future research is to explore other oral morbidities such as dysgeusia, increased mucosal sensitivity and other oral morbidities which this study did not address. Similarly, patients’ adaptation or acclimation to chronic oral morbidities may attenuate the degree of symptoms reported on PROs such as MDASI-HN over time. While this is beyond the scope of this research, future research should address this topic through longitudinal analysis.
Conclusion
In conclusion, this survivorship survey found that 45.5% long-term, disease-free OPC survivors report moderate/severe late oral morbidity symptoms at a median survival time of 7 years. Oral symptom severity significantly correlated with QOL.
Acknowledgement (Funding)
This work was completed with support of the MD Anderson Oropharynx Program Patient-Reported Outcomes/Function Core. Dr. Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (R03 CA188162). Drs. Lai, Fuller, and Hutcheson receive funding support from the National Institute for Dental and Craniofacial Research (R01 DE025248 and 1R56DE025248-01). Dr. Fuller received/receives grant and/or salary support from: the National Institutes of Health/National Cancer Institute’s Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. This work was supported in part infrastructure support by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
Footnotes
Conflict of interest disclosures
The authors made no disclosures.
References
- [1].Pezzuto F, Buonaguro L, Caponigro F, et al. Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology 2015;89(3):125–36. [DOI] [PubMed] [Google Scholar]
- [2].Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol 2014;50(5):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol 2014;50(5):387–403. [DOI] [PubMed] [Google Scholar]
- [4].Epstein JB, Chin EA, Jacobson JJ, Rishiraj B, Le N. The relationships among fluoride, cariogenic oral flora, and salivary flow rate during radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86(3):286–92. [DOI] [PubMed] [Google Scholar]
- [5].Hutcheson KA, Barringer DA, Rosenthal DI, et al. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg 2008;134(2):178–83. [DOI] [PubMed] [Google Scholar]
- [6].Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg 2010;18(3):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oh HK, Chambers MS, Martin JW, Lim HJ, Park HJ. Osteoradionecrosis of the mandible: treatment outcomes and factors influencing the progress of osteoradionecrosis. J Oral Maxillofac Surg 2009;67(7):1378–86. [DOI] [PubMed] [Google Scholar]
- [8].Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 2006;24(17):2636–43. [DOI] [PubMed] [Google Scholar]
- [9].Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 2012;62(6):400–22. [DOI] [PubMed] [Google Scholar]
- [10].Mott FE. Patient Reported Outcomes (PROs) as part of value-based care can shape therapy guidelines: impact on emerging targeted agents and immunotherapy protocols in resource-limited regions. Oncol Ther 2017;5(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 2014;120(13):1975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck 2007;29(10):923–31. [DOI] [PubMed] [Google Scholar]
- [13].Rutten H, Pop LA, Janssens GO, et al. Long-term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head-and-neck cancer: results of a multidisciplinary late morbidity clinic. Int J Radiat Oncol Biol Phys 2011;81(4):923–9. [DOI] [PubMed] [Google Scholar]
- [14].Dillman DA, Smyth JD, Christian LM. Internet, phone, mail, and mixed-mode surveys: the tailored design method. 4th ed. Hoboken: Wiley; 2014. [Google Scholar]
- [15].Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 2012;62. [DOI] [PubMed] [Google Scholar]
- [16].Lee TF, Chao PJ, Ting HM, et al. Using multivariate regression model with least absolute shrinkage and selection operator (LASSO) to predict the incidence of xerostomia after intensity-modulated radiotherapy for head and neck cancer. PLoS One 2014;9(2):e89700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rad M, Kakoie S, Niliye Brojeni F, Pourdamghan N. Effect of Long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospects 2010;4(4):110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].da Silva L, Kupek E, Peres KG. General health influences episodes of xerostomia: a prospective population-based study. Commun Dent Oral Epidemiol 2017;45(2):153–9. [DOI] [PubMed] [Google Scholar]
- [19].Bouquot D, Schroeder K. Oral effect of tobacco abuse. J Am Dent Inst Cont Educ 1992;43:3–17. [Google Scholar]
- [20].Wu J, Peters BA, Dominianni C, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 2016;10(10):2435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brennan MT, Woo SB, Lockhart PB. Dental treatment planning and management for the patient who has cancer. Dent Clin North Am 2008;52(1):19–37. [DOI] [PubMed] [Google Scholar]
- [22].Hong CH, Hu S, Haverman T, Stokman M, Napenas JJ, Braber JB, Gerber E, Geuke M, Vardas E, Waltimo T, Jenson SB, Saunder SP. A systemic review of dental disease management in cancer patients. Support Care Cancer 2018;26(10):155–74. [DOI] [PubMed] [Google Scholar]
- [23].Rubin P Cancer of the head and neck. Oral cavity: neck nodes. JAMA 1971;217(4):451–2. [PubMed] [Google Scholar]
- [24].Samim F, Epstein JB, Zumsteg ZS, Ho AS, Barasch A. Oral and dental health in head and neck cancer survivors. Cancers Head Neck 2016;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cooperstein E, Gilbert J, Epstein JB, et al. Vanderbilt Head and Neck Symptom Survey version 2.0: report of the development and initial testing of a subscale for assessment of oral health. Head Neck 2012;34(6):797–804. [DOI] [PubMed] [Google Scholar]
- [26].Nordgren M, Jannert M, Boysen M, et al. Health-related quality of life in patients with pharyngeal carcinoma: a five-year follow-up. Head Neck 2006;28(4):339–49. [DOI] [PubMed] [Google Scholar]
- [27].Quock RL. Xerostomia: current streams of investigation. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122(1):53–60. [DOI] [PubMed] [Google Scholar]


