Abstract
Objectives.
Concurrent chemoradiation to treat head and neck cancer (HNC) may result in debilitating toxicities. Targeted exercise such as yoga therapy may buffer against treatment-related sequelae; thus, this pilot RCT examined the feasibility and preliminary efficacy of a yoga intervention. Because family caregivers report low caregiving efficacy and elevated levels of distress, we included them in this trial as active study participants.
Methods.
HNC patients and their caregivers were randomized to a 15-session dyadic yoga program or a waitlist control (WLC) group. Prior to randomization, patients completed standard symptom (MDASI-HN) and patients and caregivers completed quality of life (SF-36) assessments. The 15-session program was delivered parallel to patients’ treatment schedules. Participants were re-assessed at patients’ last day of chemoradiation and again 30 days later. Patients’ emergency department visits, unplanned hospital admissions and gastric feeding tube placements were recorded over the treatment course and up to 30 days later.
Results.
With a consent rate of 76%, 37 dyads were randomized. Participants in the yoga group completed a mean of 12.5 sessions and rated the program as “beneficial.” Patients in the yoga group had clinically significantly less symptom interference and HNC symptom severity and better QOL than those in the WLC group. They were also less likely to have a hospital admission (OR = 3.00), emergency department visit (OR = 2.14), and/or a feeding tube placement (OR = 1.78).
Conclusion.
Yoga therapy appears to be a feasible, acceptable, and possibly efficacious behavioral supportive care strategy for HNC patients undergoing chemoradiation. A larger efficacy trial is warranted.
Keywords: Head and neck cancer, Chemoradiation, Yoga, Dyadic intervention, Symptoms, Quality of life, Family caregivers, Healthcare utilization, Gastric feeding tube, Hospital admission
Introduction
Aggressive multi-modal treatments such as concurrent chemoradiation are needed to improve disease outcomes for patients with head and neck cancer (HNC).1–5 Yet, the improved outcomes of chemoradiation may come at the price of debilitating toxicities (e.g., mucositis, dysphagia, and fatigue) which may impair patients’ basic functioning and increase healthcare utilization such as emergency department visits, unplanned hospital admissions, and feeding tube insertions.6–14 In fact, several population-based studies suggest that HNC, particularly when treated with concurrent chemoradiation, is associated with frequent emergency department visits with 28%–55% of HNC patients having at least one visit during treatment.15–20 A considerable portion of these visits and admissions are preventable through improved symptom control.17 For instance, patients with higher depressive symptoms are significantly more likely to visit the emergency department.20 Emergency department visits and hospital admissions are not only financially costly but may interrupt treatment, which has important implications for treatment outcomes as even a 1-day interruption may reduce HNC local control by 1.4%.21 Due to mucositis-related pain, dysphasia, dysgeusia, and xerostomia, eating and drinking become difficult during and after chemoradiation, and some studies have shown as many as 50%-70% of patients require a feeding tube during chemoradiation for nutrition and hydration and to prevent severe weight loss.10,22–24 Weight loss is problematic as it may be associated with sarcopenia, which in turn is associated with decreased survival in HNC patients.25
Considering the high treatment-related burden, HNC patients require extensive and persistent care from their families.26–28 Yet, caregivers of HNC patients may be overwhelmed by the numerous self-management tasks (e.g., oral rinses, swallowing exercises, medication management, calorie counting/food preparation) and thus, resort to negative social control rather than positive social support strategies to encourage adherence.29 Moreover, family caregivers bear the emotional burden of witnessing their loved one struggle with the common HNC symptom burden such as appearance changes, severe weight loss, and high pain levels that require opioid use.29–31 It is not surprising that caregivers are vulnerable to reporting high levels of psychological distress, which may compromise the quality of care and support they are able to provide.32–35 For instance, caregiver distress is associated with the decisions to take the patient to the emergency department and to place a feeding tube.36,37
Consequently, a supportive care approach that includes family caregivers may be advantageous over traditional, patient-only interventions.28,38–41 Considering the multi-faceted needs, a behavioral intervention that integrates physical exercises with relaxation strategies may be a promising approach. Informed by our previous work in thoracic cancers, we developed a dyadic yoga program that includes targeted exercises focusing on facial structures and the neck. While tailored exercise including yoga therapy appears to be an effective rehabilitation strategy for HNC survivor to restore functional losses, the value of prehabilitation to improve peri-operative functional capacity is increasingly recognized.42–46 Considering that definitive chemoradiation is a standard treatment for many patients with locally-advanced disease, we decided to deliver the intervention parallel to standard treatment plans. Because the existing supportive care literature involving HNC patients on chemoradiation is limited, our primary goal was to determine the feasibility of conducting a randomized controlled trial (RCT) assigning patient-caregiver dyads to the dyadic yoga intervention or a waitlist control (WLC) group receiving usual care. Based on our previous dyadic yoga trials, we hypothesized that at least 60% of eligible dyads would consent to participate, those randomized to the dyadic yoga group would attend at least 75% of the intervention sessions, and 80% of the dyads would be retained at the 1-month follow-up assessment.47 Secondarily, we sought to examine preliminary intervention efficacy regarding patients’ cancer-related symptoms, healthcare utilization (i.e., emergency department visits, hospital admissions, and feeding tube insertions) and weight loss, and patients’ and caregivers’ mental and physical health-related quality of life (QOL). We expected that, compared with the WLC group, the DY intervention would yield clinically significant improvements in patient and caregiver outcomes.
Methods
Participants
Patients with stage I-IV HNC starting at least 5 weeks of concurrent chemoradiation with a consenting family caregiver (e.g., spouse/partner, sibling, adult child) were eligible to participate. Both patients and caregivers had to be at least 18 years old, proficient in English, and able to provide informed consent. Patients were excluded if they practiced yoga on a regular basis (self-defined) in the year prior to diagnosis and had a physician-rated Eastern Cooperative Oncology Group (ECOG) performance status of greater than 2.
Design and Endpoints
The current pilot (phase 2) study involves a 2-arm, prospective randomized controlled trial design with parallel group assignment. The primary endpoint is feasibility and secondary outcomes include preliminary efficacy regarding symptoms, quality of life, and healthcare utilization.
Procedures
Prior to starting the trial (NCT03114501), the MD Anderson Institutional Review Board approved all study procedures. Research staff identified potentially eligible patients in the institution’s electronic medical records, approached patients, and caregivers during routine clinic visits, confirmed their study eligibility, and obtained their written informed consent to participate prior to any data collection. If a caregiver was not present during the clinic visit, the patient’s permission to contact the caregiver was obtained. Prior to randomization, both patients and caregivers completed paper-pen survey measures (baseline/T1). Patients completed standardized self-report measures of cancer-related symptoms at the last day of treatment (T2) and again 1-month later (T3). Both patients and caregivers completed standardized measures of overall QOL at T1 and T3. Surveys were returned either in person during a clinic visit or via prepostage paid return envelopes. We extracted patients’ emergency department visits, hospital admissions, feeding tube insertions and weight from their medical records. Participants were enrolled for a duration of approximately 12 weeks. The trial was conducted between October 2018 and Oct 2019.
Randomization
Dyads were randomized to either the dyadic yoga or WLC group through a form of adaptive randomization called minimization ensuring that the groups were balanced on prognostic factors including patients’ age, sex, and ECOG performance status using a computerized system (FileMaker).48
Masking
Although research coordinators involved in data collection were blind to group assignment, this study was an open-label trial.
DY Group
The manualized yoga program was developed in collaboration with Swami Vivekananda Yoga Anusandhana Samsthana. A yoga therapist (certified by the International Association of Yoga Therapists; C-IAYT) delivered the first 3 sessions in person to individual patient-caregiver dyads. Based on the dyads’ preference, the remaining sessions (4–15) were administered either in person or via videoconferencing. We loaned iPads for the intervention duration to dyads who wanted to attend sessions via videoconference but did not own a device. Dyads had to attend all sessions together over the course of patients’ standard chemoradiation (45-minute sessions, 2–3 times per week for a total of 15 sessions over no more than 6 weeks). This dose was based on our previous yoga efficacy trial in women with breast cancer undergoing radiotherapy.49 While the overall program followed a general Hatha yoga format, we included targeted yoga-based exercises focusing on the face and neck area such as stretching and strengthening of neck (Sukshama Vyama/shakti vikasaka) and facial muscles including tongue, lip, and jaw exercises. The program also included physical postures (asanas) including partner-poses for whole body stretching and strengthening; breathing exercises (pranayama); and relaxation exercises (shavasana) focusing on positive emotions. During sessions 1–4, participants were gradually introduced to the various practices. The remaining sessions (5–15) focused on practicing the components and answering questions pertaining to the techniques and participants’ experiences. Starting with session 1, the interventionist conveyed the notion that each practice is intended to target the needs of both members of the dyad, with a focus on mutual support and togetherness. Dyads received a video and a participant manual of the program and were instructed to practice on their own on the days they did not meet with the instructor. Home practice was assessed (see below).
To ensure treatment fidelity, all sessions were videorecorded (with the participants’ permission obtained during the informed consent process) and reviewed on an ongoing basis using a fidelity checklist. The yoga participants also received routine care as described below for the WLC group.
WLC Group
Dyads in the WLC group received routine care as offered by the multidisciplinary HNC radiation clinic including education pertaining to symptom management (RN-led), prophylactic dental hygiene and possible extraction procedures, speech language pathology evaluation for baseline swallowing assessment and prophylactic treatment, and clinical dietitian assessments for standard-of-care nutrition monitoring during treatment. Patients and caregivers who currently smoked were referred to the Institution’s tobacco cessation program. Psychiatry, social work, gastroenterology (for feeding tube insertions), and supportive care services were consulted as routine clinical care if needed. Dyads were offered the intervention after they completed the T3 assessment. No additional data were collected.
Measures
Demographic Information.
Demographic items (e.g., age, marital status) were included in the baseline questionnaires.
Feasibility Data.
We documented consent rates, class attendance, and completion of assessments. After the last session, participants in the yoga group completed a program evaluation that we had developed for our previously published yoga trials.50,51 Patient and caregivers were asked if they benefited from each the 4 main practice components (e.g., “Did you feel any benefit from practicing the breathing exercises”) using a Likert scale response format (0=”definitely not beneficial”; 1=”not really beneficial”; 2=”not sure if beneficial”; 3=”a little beneficial”; and 4=”definitely beneficial”). The frequency of home yoga practice was assessed weekly with a paper-pencil practice log over the course of treatment. Participants completed perceived exertion during the yoga session on the 0–10 Borg scale on a weekly basis to ascertain safety.52 Instructors monitored for adverse events (falls, practice-related injuries, and psychological distress) during the intervention sessions. For adverse events experienced during the home practice, patients were instructed to contact their radiation oncologist.
Patient Healthcare Utilization and Medical Factors.
Patients’ medical data (e.g., disease site, stage, treatment-related factors) as well as emergency department visits, unplanned hospital admission admissions and feeding tube insertions that occurred from the first fraction of radiation to 30 days after completing radiation were extracted from their electronic medical records. We also extracted patients’ weight measurements that were assessed on a weekly basis during their routine visits with the radiation oncologist.
Cancer-Related Symptoms.
Patients completed the MD Anderson Symptom Inventory-Head and Neck module (MDASI-HN) consisting of 13 core items and 9 HNC-specific items assessing symptom severity as well as 6 items assessing symptom interference with daily life on a scale from 0–10 at baseline (T1), end of radiation treatment (T2), and 1 month later (T3).53 The HNC symptoms were analyzed in a standardized manner based on 2 factors. Factor 1 consists of mouth sores, tasting food, constipation, teeth-gum problems, and skin pain. Factor 2 includes problems with voice-speech, chocking-coughing, chewing-swallowing, and mucus.53
Patient and Caregiver QOL was assessed at baseline and 1 month after completing radiation treatment with the Medical Outcomes Study 36-item short-form survey (SF-36) assessing 8 distinct domains yielding a mental and physical composite summary (MCS and PCS, respectively).54
Statistical Analyses
To examine feasibility, we calculated descriptive statistics of consent, class attendance, assessment completion, and program satisfaction. To examine preliminary efficacy for patients’ MDASI outcomes, we performed multilevel modeling (MLM) with PROC MIXED (SAS, 9.4 version) and controlled for baseline level of the given outcome in addition to randomization factors.55 Because the QOL assessment was only performed twice (T1 and T3), we used the general linear model (GLM), separately for patients and caregivers, controlling for baseline level of the outcome, along with the aforementioned covariates for patients, and age and sex for caregivers (because these variables were associated with caregiver MCS and PCS at P < 0.05). Given the pilot nature of this trial, we evaluated preliminary evidence for efficacy based on clinically meaningful group differences of least square means (LSM) rather than statistically significant group differences. A group difference of 1 point or greater is considered clinically significant for the MDASI symptom severity and interference subscales and a 5-point difference for the MCS and PCS subscales of the SF-36.56,57 We treated emergency department visits and hospital admissions as a binary outcome (yes vs no) regardless of the number of times a patient may have been admitted. For weight loss, we calculated a percentage score deeming a decrease in weight of 5% at the end of chemoradiation versus baseline as clinically significant and created a binary outcome (yes vs no).58 We calculated odds ratios (OR) for having had an emergency department visit, a hospital admission, a feeding tube and clinically significant weight loss.
Considering that the primary goal is to determine feasibility, we based our sample size on typical conventions with 15–20 participants per arm being considered appropriate.59 For the secondary outcomes, we identified QOL (MCS or PCS) as the primary endpoint. A post-attrition sample (assuming 30% attrition rate) of 28 patients (or caregivers; 14 per arm) has 80% power to detect a difference in mean of 6.765 (effect size = 1.10) between the yoga and WLC groups, assuming a standard deviation (SD) of the change of MCS or PCS from T1 to T3 of 6.15 (based on our pilot data in lung cancer patients), and a 0.05 2-sided significance level.
Results
Participant Characteristics
Baseline demographic and medical characteristics by group and role are listed in Table 1. Briefly, patients were male (68%), Non-Hispanic White (84%), educated with at least some college credits (81%), middle aged (mean = 60 years, range = 41–89), and fulltime employed (43%). Patients had mainly oropharyngeal cancer (84%) and high-performance status at study entry (78%). Caregivers were female (81%), educated with at least some college credits (54%), middle aged (mean age = 57 years; range = 37–75), fulltime employed (30%), and married (81%) to the patient. There were no significant group differences regarding baseline participant characteristics.
Table 1.
Baseline Patient and Caregiver Characteristics (N = 37 Dyads)
| Yoga Group (N = 19 Dyads) |
Control Group (N = 18 Dyads) |
|||
|---|---|---|---|---|
| Variable | Patient | Caregiver | Patient | Caregiver |
|
| ||||
| Male sex n (%) | 15 (79) | 2 (11) | 13 (72) | 5 (28) |
| Mean age, years ±SD, (range) | 57.97 ± 8.00 (43–69) | 54.47 ± 9.03 (37–75) | 61.66 ± 10.96 (41–89) | 58.94 ± 7.99 (40–72) |
| Non-Hispanic White, n (%) | 11 (58) | 12 (63) | 13 (72) | 11 (61) |
| Spousal caregiver, n (%) | 15 (79) | 17 (95) | ||
| Highest Level of Education, n (%) | ||||
| Some college or higher | 14 (74) | 15 (79) | 16 (89) | 15 (83) |
| Household Income, n (%) | ||||
| 50,000 or more | 18 (95) | 15 (79) | 16 (89) | 16 (89) |
| Employment Status, n (%) | ||||
| Retried | 4(21) | 5 (26) | 3(17) | 5 (28) |
| Full-time | 10 (53) | 7 (37) | 6 (33) | 4 (22) |
| Medical leave | 4(21) | 2(11) | 3 (17) | 2(11) |
| Part-time/Homemakers | 1 (5) | 5 (26) | 4 (23) | 5 (28) |
| Disease site, n (%) | ||||
| Oropharynx | 16 (84) | 15 (83) | ||
| Nasopharynx | 3 (16) | 1 (6) | ||
| Larynx | 2(11) | |||
| Stage at Diagnosisa, n (%) | ||||
| I | 3 (16) | 2(11) | ||
| II | 6 (32) | 9 (50) | ||
| III | 3 (16) | 2(18) | ||
| IV | 7 (37) | 5 (28) | ||
| Resection, n (%) | ||||
| Yes | 9 (47) | 10 (56) | ||
| HPV Positive, n (%) | ||||
| Yes | 14 (74) | 11 (61) | ||
| ECOG at recruitment, n (%) | ||||
| 0 | 15 (79) | 14 (78) | ||
| 1 | 3(16) | 3(17) | ||
| 2 | 1(5) | 1 (6) | ||
| Time since diagnoses, weeks ±SD, (range) | 9.11 ± 8.73 (3–39) | 6.84 ± 3.55 (3–14) | ||
Abbreviations: SD= Standard deviation; HPV = Human papillomavirus; ECOG = Eastern Cooperative Oncology Group.
Note: Stage is based on the eighth edition of the American Joint Committee on Cancer.
Regarding treatment variables, the most common radiation technique was volumetric modulated arc therapy (VMAT, 49%) followed by intensity-modulated proton therapy (IMPT, 35%) and three dimensional (3D) conformal radiation therapy (16%). Patients received a total centigray of 6815 (SD = 1280) over 32.6 fractions (SD = 1.5). All patients completed the radiation treatment as scheduled. Most patients were treated concurrently with cisplatin (68%), followed by cetuximab (24%) or carboplatin (8%). Regarding toxicities, 46% of patients had grade 2 and 35% had grade 3 dermatitis; 68% had grade 2 and 11% had grade 3 mucositis. There were no significant group differences on treatment regimen, dose reductions, and toxicities.
Feasibility Results
Recruitment and Sample Retention.
We prescreened and approached 70 consecutive dyads of which 56 were confirmed as study eligible and of those, 43 dyads consented (76%). Ineligibility (n = 14) was due to lack of a caregiver (n = 6), caregiver’s unwillingness/inability to participate (n = 2), ECOG performance status >2 (n = 2), and language barriers (n = 4). Refusal reasons were lack of interest (n = 6), too busy (n = 4) and feeling too distressed to participate (n = 3). Prior to randomization, 5 patients withdrew consent and 1 died so that 37 dyads were randomized (DY = 19; WLC = 18). Of those randomized, 33 completed the end of radiation (89%) and 30 (81%) completed the 1-month follow-up assessments. Based on chi-square analyses, attrition was not a function of group membership (P = 0.74). See Fig. 1 for the Consort Chart.
Fig. 1.
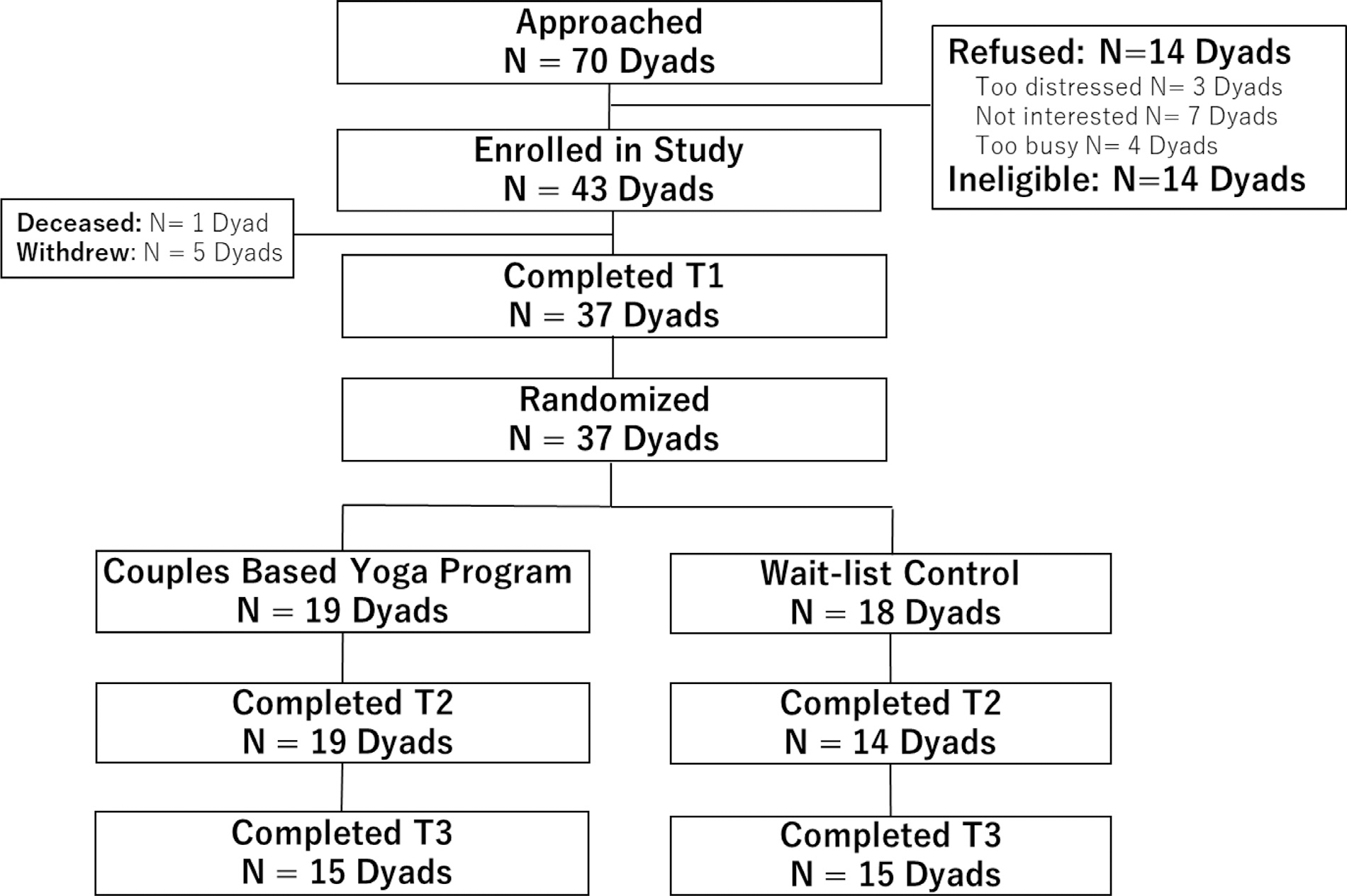
Consort flow diagram.
Adherence and Acceptability.
Dyads randomized to the yoga group attended a mean of 12.5 sessions (SD = 3.3; range: 6–15). All participants in the DY group rated each component of the intervention as either “a little beneficial” or “definitely beneficial” (breathing exercises: μ = 3.43; joint loosening: μ = 3.71; postures: μ = 3.57; relaxation: μ = 3.75). The majority of dyads (87%) in the yoga group chose to attend the yoga sessions in person at the hospital. No related adverse events were observed. Participants rated the sessions as “easy” on the Borg exertion scale (patients: μ = 2.00 σ = 0.57, range = 1–3; caregivers: μ = .83, σ = 0.41, range = 1–2). Weekly home practice varied widely from practicing 5 min to 140 min per week for patients (breathing exercises: μ = 25.7, σ = 50.45; joint loosening: μ = 30.7, σ = 43.4; postures: μ = 26.0, σ = 50.5; relaxation: μ = 27.1, σ = 49.8). Caregivers only practice between 5 min and 30 min per week (breathing exercises: μ = 8, σ = 4.5; joint loosening: μ = 5, σ = 3.5; postures: μ = 6.0, σ = 5.5; relaxation: μ = 13.0, σ = 11.5).
Preliminary Efficacy Results
Cancer related symptoms.
The MLM analyses controlling for randomization factors and baseline level of the outcome revealed clinically significant between-group effects for the MDASI symptom interference subscale (LSM: DY = 1.99 vs. WLC = 3.25; d = .30, P = .19) and HN symptom factor 2 (LSM: DY = 3.24 vs. WLC = 4.45; d = .30, P = 0.19) subscale across the follow-up period in favor of the DY group. More specifically, of symptoms comprising Factor 2, clinically significant between-group differences were found in the following 2 symptoms: swallowing (LSM: DY = 6.06 vs. WLC = 7.73; d = .28, P = .19) and mucus (LSM: DY = 3.24 vs. WLC = 4.45; d = .30, P = 0.18). There was no evidence for an effect for the MDASI core symptom severity (LSM: DY = 2.17 vs. WLC = 2.83; d = .21, P = 0.34) or the HN Factor 1 (LSM: DY = 3.44 vs. WLC = 3.96; d = .14, P = 0.54) subscales. See Fig. 2 for patient symptom findings.
Fig. 2.

Least square means for cancer-related symptoms assessed with the MD Anderson Symptom Inventory-Head and Neck Module (MDASI-HN). Higher scores represent greater symptoms. Abbreviations: WLC = waitlist control; HN = Head and Neck. Note: *, denotes a clinically significant group difference (≥1 point). The models controllled for the baseline level of the outcome and randomization factors (patients’ age, sex, and performance status). The effect size Cohen’s d associated with each between-group comparison is interpreted as small (d = .2), medium (d = .5), or large (d = .8).64
QOL.
GLM analyses controlling for randomization factors (sex and age for caregivers) and baseline level of the outcome revealed that group differences for the PCS approached clinical significance (LSM: DY = 44.29 vs. WLC = 39.63; d = .44, P = .22) and reached clinical significance for the MCS (LSM: DY = 53.37 vs. WLC = 47.01; d = .55, P = 0.31) scores so that patients in the yoga group reported better QOL than those in the WLC group at the 1-months follow-up (T3). For caregivers, we found little evidence that the intervention improved their physical QOL (PSC, LSM: DY = 50.97 vs. WLC = 52.97; d = −.27, P = 0.28) or mental QOL (MCS, LSM: DY = 47.72 vs. WLC = 49.96; d = −.23, P = 0.40). Albeit not clinically significant, contrary to our hypothesis, the means were in the opposite direction. See Fig. 3 for patient and caregiver QOL findings.
Fig. 3.
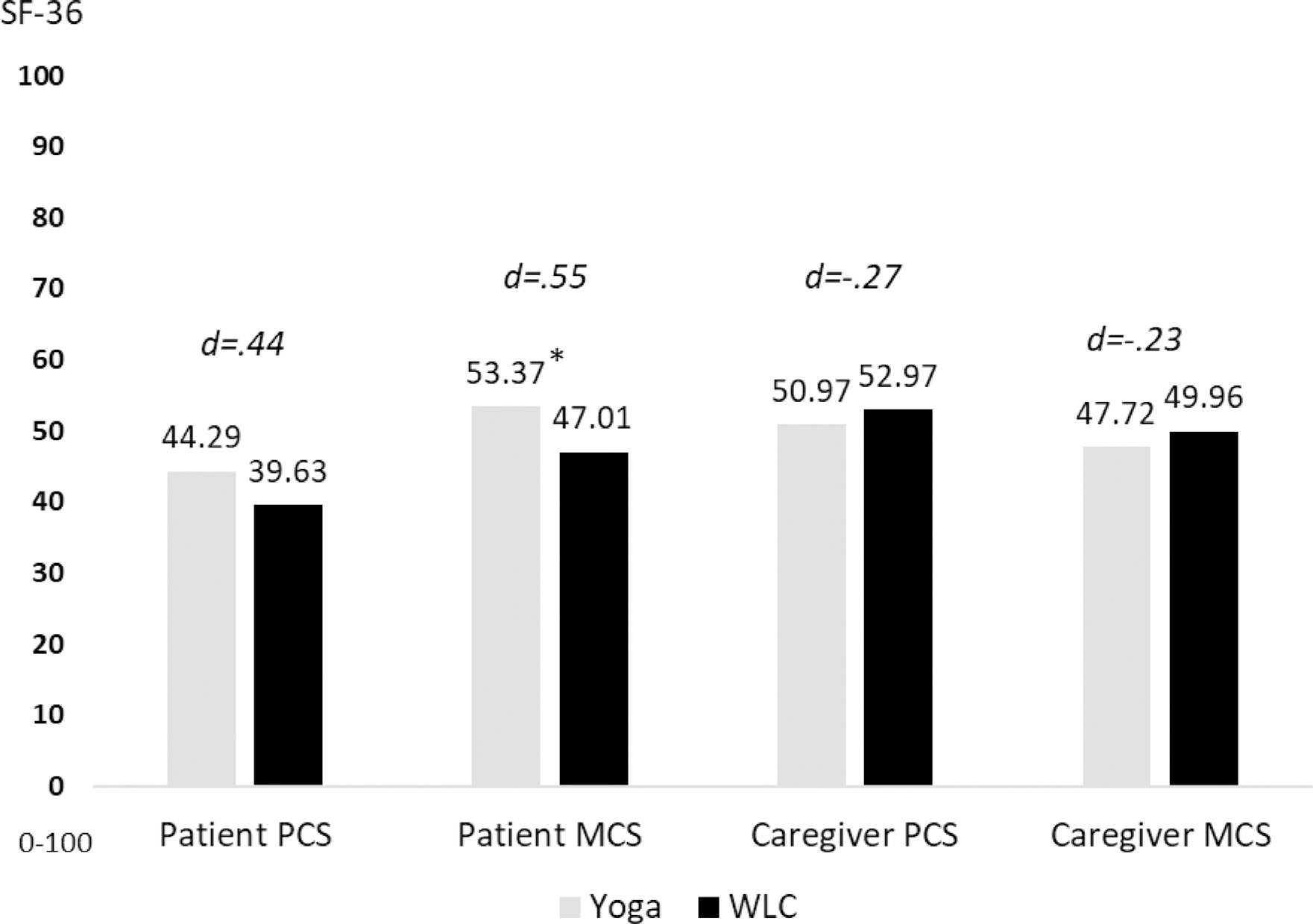
Least square means for physical and mental aspects of quality of life as assessed with the Medical Outcomes Study 36-item Short Form. Higher scores represent better quality of life. Abbreviations: PCS = Physical component summary; MCS = Mental component summary; WLC = waitlist control. Note: *, denotes a clinically significant group difference (≥5 point). The patient models controllled for the baseline level of the outcome and randomization factors (patients’ age, sex, and performance status). The caregiver models controllled for the baseline level of the outcome, caregiver age, and caregiver sex. The effect size Cohen’s d associated with each between-group comparison is interpreted as small (d = .2), medium (d = .5), or large (d = .8).64
Healthcare Utilization and Weight Loss.
Patients in the yoga group had fewer unplanned hospital admissions, emergency department visits and FTs (n = 4, n = 7, n = 5, respectively) than the WLC (n = 8, n = 10, n = 7, respectively) group so that patients in the WLC group were three times more likely to have an unplanned hospital admission (OR 3.00) and two times more likely to have an emergency department visit (OR 2.14) than those in the DY group. Of those who had an emergency department visit during chemoradiation (n = 17; 33%), 10 (59%) visited once, 4 visited twice (24%), and 3 (18%) visited three times. While primary complaints varied widely and were multi-fold, most included nausea and vomiting (76%), oral pain (35%), fever (30%), fatigue (30%), dehydration (24%), altered consciousness (24%), and constipation (18%). Regarding feeding tube and weight loss, patients in the yoga group were 1.78 times less likely to get a feeding tube and 1.45 times less likely to experience clinical weight loss over the course of chemoradiation than those in the WLC group.
Discussion
The goal of this pilot RCT was to demonstrate the feasibility and preliminary efficacy of a yoga-based intervention for HNC patients undergoing concurrent chemoradiation and their caregivers by targeting patient cancer-related symptom burden and healthcare utilization, as well as patient and caregiver QOL. The results revealed that the trial was feasible as it met our a priori feasibility criteria regarding consent, retention, adherence, and acceptability rates. Of note, 80% of the dyads attended at least 10 sessions, and patients practiced at home an additional average of twice per week, which is remarkable considering that the intervention was delivered parallel to multi-model cancer treatment. All patients and caregivers considered the intervention as beneficial, further endorsing the acceptability of this supportive care modality. Because the trial was conducted in the “pre-COVID era,” most sessions (87%) were attended in person rather than via videoconference due to patient preference. As society at large has become more familiar with videoconference technology over the last few years, the desire for remote delivery is expected now to be much higher, and we have demonstrated to be able to successfully deliver remote yoga therapy sessions.60,61
We revealed a signal for preliminary intervention efficacy as patients in the yoga group reported clinically significant improvements in symptom burden particularly oral symptom severity and symptom interference with daily life as well as physical and mental QOL. There is reason to believe that our yoga program may reduce healthcare utilization as measured by unplanned hospital admissions and emergency department visits as patients in the WLC group were three times as likely to have an unplanned hospital admission and twice as likely to have an emergency department visit. The group differences for feeding tube insertions were smaller, but in the expected direction. For caregivers, a benefit based on the current measures was unfortunately not apparent.
Given these encouraging findings regarding feasibility and preliminary efficacy for patients, an efficacy RCT is warranted. Of note, our results revealed important issues to be addressed in future research. A critical question revolves around the utility of the dyadic intervention design considering caregivers did not appear to benefit from the intervention perhaps due to its dyadic delivery. In fact, the intervention and its delivery format may even present an additional burden to caregivers’ schedules and possibly reduce their QOL. Because the program (at least in part) focused on facial muscles including tongue, lip, and jaw exercises, the program may not have been particularly helpful but rather burdensome to the caregivers. Moreover, caregivers were less engaged than patients as seen by their limited home practice. On the other side, participation of the caregiver may improve feasibility and efficacy for the patient. Without the support of a family caregiver, a patient may be less likely to attend the yoga sessions and complete the home practice, which may compromise treatment efficacy. We are currently conducting a 3-arm RCT that compares patient individual versus patient-caregiver dyadic attendance of the yoga intervention to address this central issue. While a dyadic approach to symptom management has gained increasing attention in the supportive care literature, head-to-head comparisons are lacking.
Although pathways by which yoga improves QOL have been identified, future research should examine underlying mechanisms by which yoga improves HNC-related symptom severity and reduces healthcare utilization.62 Our HNC-specific yoga program targeted facial exercises including jaw and neck as well as tongue and lip exercises which may have reduced the development of trismus, lymphedema and fibrosis and thus, improved swallowing processes (i.e., ability for mastication and deglutition) and holistically improved oral symptom severity and interference.63 Future research is encouraged to delineate these mechanisms. Moreover, at this point, we speculatively suggest that, because patients in the yoga group reported less symptom interference with activities of daily life and better mental and physical QOL, they required less healthcare utilization such as unplanned hospital admissions in comparison to the control group. However, it is unclear how the yoga program improves symptom interference and mechanistic work is required.
Study Limitations
In addition to lacking an active control group, our study is limited by the small sample size. Moreover, our sample included patients with high educational attainment and income levels and having the support of a family member. Thus, it is unclear if our findings generalize to patients with more diverse including underprivileged backgrounds. Rather than recruiting solely from an academic hospital, the next steps of this research include pilot testing this intervention in minoritized and underserved populations. The pilot RCT was not powered to examine group differences, and the initial evidence for efficacy presented here must be interpreted with caution. Given the small sample size and overall study goals, we did not evaluate statistically but clinically significant group differences to evaluate a signal for intervention efficacy. Moreover, we reported clinically significant differences between groups, which were based only on point estimates of the least-square means without accounting for the uncertainty of the estimates. As a result, any interpretation of clinical significance should be weighed carefully.
Clinical Implications
Although clinical recommendations regarding the utility of this intervention are premature at this point, the present pilot RCT provides convincing evidence of the feasibility of a yoga intervention for patients undergoing an aggressive form of cancer treatment, namely concurrent chemoradiation. Considering the high safety profile of yoga combined with high patient engagement and the observed clinically significant improvements for patients, yoga therapy has promise in preventing and managing chemoradiation-related toxicities in HNC. Based on these findings, a large, well-controlled efficacy trial of this intervention is warranted.
Acknowledgments
This work was supported by NIH/NCCIH K01 AT007559.
Funding
NIH/NCCIH K01 AT007559, Principal Investigator: Kathrin Milbury
Footnotes
Disclosures
None of the authors competing interests to declare.
Contributor Information
Kathrin Milbury, Department of Behavioral Science, 1155 Pressler St., Houston, Texas 77030, USA.
David I. Rosenthal, Department of Radiation Oncology, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Yisheng Li, Department of Biostatistics, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
An Thuy Ngo-Huang, Department of Palliative, Rehabilitation & Integrative Medicine, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Smitha Mallaiah, Department of Palliative, Rehabilitation & Integrative Medicine, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Sania Yousuf, Department of Behavioral Science, 1155 Pressler St., Houston, Texas 77030, USA.
Clifton D. Fuller, Department of Radiation Oncology, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Carol Lewis, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Eduardo Bruera, Department of Palliative, Rehabilitation & Integrative Medicine, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
Lorenzo Cohen, Department of Palliative, Rehabilitation & Integrative Medicine, 1515 Holcombe Blvd., Houston, Texas 77030, USA.
References
- 1.Howlader N NA, Krapcho M, Garshell J, et al. (eds). SEER cancer statistics review, 2008–2014. National Cancer Institute. Bethesda, MD. 2018. [Google Scholar]
- 2.Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004;22:69–76. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein DJ. Oropharyngeal cancer: the role of chemotherapy. Curr Treat Options Oncol 2003;4:3–13. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–578. [DOI] [PubMed] [Google Scholar]
- 6.Murphy BA. Advances in quality of life and symptom management for head and neck cancer patients. Curr Opin Oncol 2009;21:242–247. [DOI] [PubMed] [Google Scholar]
- 7.Paleri V, Roe JW, Strojan P, et al. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head Neck 2014;36:431–443. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheson KA, Lewin JS. Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep 2012;14:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer 2014;120:1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy BA, Beaumont JL, Isitt J, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage 2009;38:522–532. [DOI] [PubMed] [Google Scholar]
- 11.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society Head and Neck Cancer Survivorship Care guideline. CA Cancer J Clin 2016;66:203–239. [DOI] [PubMed] [Google Scholar]
- 12.Bhayani MK, Hutcheson KA, Barringer DA, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck 2013;35:1634–1640. [DOI] [PubMed] [Google Scholar]
- 13.Head MDA, Neck Cancer Symptom Working GEraj SA, et al. Long-term patient reported outcomes following radiation therapy for oropharyngeal cancer: cross-sectional assessment of a prospective symptom survey in patients >/=65 years old. Radiat Oncol 2017;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiam MK, Mannion K, Dietrich MS, et al. Assessment of musculoskeletal impairment in head and neck cancer patients. Support Care Cancer 2017;25:2085–2092. [DOI] [PubMed] [Google Scholar]
- 15.Moore ZR, Pham NL, Shah JL, et al. Risk of unplanned hospital encounters in patients treated with radiotherapy for head and neck squamous cell carcinoma. J Pain Symptom Manage 2019;57:738–745. e733. [DOI] [PubMed] [Google Scholar]
- 16.Marar M, Gabriel P, Hwang WT, et al. Acute hospital encounters in cancer patients treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2018;101:935–944. [DOI] [PubMed] [Google Scholar]
- 17.Eskander A, Krzyzanowska MK, Fischer HD, et al. Emergency department visits and unplanned hospitalizations in the treatment period for head and neck cancer patients treated with curative intent: a population-based analysis. Oral Oncol 2018;83:107–114. [DOI] [PubMed] [Google Scholar]
- 18.Kligerman MP, Sethi RKV, Kozin ED, Gray ST, Shrime MG. Morbidity and mortality among patients with head and neck cancer in the emergency department: a national perspective. Head Neck 2019;41:1007–1015. [DOI] [PubMed] [Google Scholar]
- 19.Waddle MR, Chen RC, Arastu NH, et al. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol 2015;5:e245–e253. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Gibby CC, Melkonian SC, Hanna EY, et al. Cohort study of oncologic emergencies in patients with head and neck cancer. Head Neck 2017;39:1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 2007;68:654–661. [DOI] [PubMed] [Google Scholar]
- 22.Wopken K, Bijl HP, Langendijk JA. Prognostic factors for tube feeding dependence after curative (chemo-) radiation in head and neck cancer: a systematic review of literature. Radiother Oncol 2018;126:56–67. [DOI] [PubMed] [Google Scholar]
- 23.Ganzer H, Touger-Decker R, Parrott JS, et al. Symptom burden in head and neck cancer: impact upon oral energy and protein intake. Support Care Cancer 2013;21:495–503. [DOI] [PubMed] [Google Scholar]
- 24.Bhayani MK, Hutcheson KA, Barringer DA, et al. Gastrostomy tube placement in patients with hypopharyngeal cancer treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck 2013;35:1641–1646. [DOI] [PubMed] [Google Scholar]
- 25.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol 2016;2:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson JM, Rapley T, Carding PN, Wilson JA, McColl E. Head and neck cancer and dysphagia; caring for carers. Psychooncology 2013;22:1815–1820. [DOI] [PubMed] [Google Scholar]
- 27.Onakoya PA, Nwaorgu OG, Adenipekun AO, Aluko AA, Ibekwe TS. Quality of life in patients with head and neck cancers. J Natl Med Assoc 2006;98:765–770. [PMC free article] [PubMed] [Google Scholar]
- 28.Sterba KR, Zapka J, Cranos C, Laursen A, Day TA. Quality of life in head and neck cancer patient-caregiver dyads: a systematic review. Cancer Nurs 2016;39:238–250. [DOI] [PubMed] [Google Scholar]
- 29.Badr H, Yeung C, Lewis MA, Milbury K, Redd WH. An observational study of social control, mood, and self-efficacy in couples during treatment for head and neck cancer. Psychol Health 2015;30:783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langius JA, van Dijk AM, Doornaert P, et al. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr Cancer 2013;65:76–83. [DOI] [PubMed] [Google Scholar]
- 31.Schaller A, Liedberg GM, Larsson B. How relatives of patients with head and neck cancer experience pain, disease progression and treatment: a qualitative interview study. Eur J Oncol Nurs 2014;18:405–410. [DOI] [PubMed] [Google Scholar]
- 32.Northouse LL, Katapodi MC, Song LX, Zhang LL, Mood DW. Interventions with family caregivers of cancer patients meta-analysis of randomized trials. Ca-Cancer J Clin 2010;60:317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swore Fletcher BA, Dodd MJ, Schumacher KL, Miaskowski C. Symptom experience of family caregivers of patients with cancer. Oncol Nurs Forum 2008;35:E23–E44. [DOI] [PubMed] [Google Scholar]
- 34.Manne SL, Ostroff J, Winkel G, Grana G, Fox K. Partner unsupportive responses, avoidant coping, and distress among women with early stage breast cancer: patient and partner perspectives. Health Psychol 2005;24:635–641. [DOI] [PubMed] [Google Scholar]
- 35.Litzelman K, Kent EE, Mollica M, Rowland JH. How does caregiver well-being relate to perceived quality of care in patients with cancer? Exploring associations and pathways. J Clin Oncol 2016;34:3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott CL, Ramsey SD, Engelberg R, et al. Care coordination and emergency department (ED) use at the end of life for patients with cancer: findings from structured interviews with primary caregivers. J Clin Oncol 2018;36(30_suppl):161. [Google Scholar]
- 37.Verdonck-de Leeuw IM, Eerenstein SE, Van der Linden MH, et al. Distress in spouses and patients after treatment for head and neck cancer. Laryngoscope 2007;117:238–241. [DOI] [PubMed] [Google Scholar]
- 38.Nightingale CL, Sterba KR, Tooze JA, et al. Vulnerable characteristics and interest in wellness programs among head and neck cancer caregivers. Support Care Cancer 2016;24:3437–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paek MS, Nightingale CL, Tooze JA, et al. Contextual and stress process factors associated with head and neck cancer caregivers’ physical and psychological well-being. Eur J Cancer Care (Engl) 2018;27:e12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longacre ML, Ridge JA, Burtness BA, Galloway TJ, Fang CY. Psychological functioning of caregivers for head and neck cancer patients. Oral Oncol 2012;48:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellanos EH, Dietrich MS, Bond SM, et al. Impact of patient symptoms and caregiving tasks on psychological distress in caregivers for head and neck cancer (HNC). Psychooncology 2019;28:511–517. [DOI] [PubMed] [Google Scholar]
- 42.Adair M, Murphy B, Yarlagadda S, et al. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther 2018;17:774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch PT HS, Lee R, Sumer BD, et al. Effectiveness of physical activity interventions in improving objective and patient-reported outcomes in head and neck cancer survivors: a systematic review. Oral Oncol 2021;117:105253. [DOI] [PubMed] [Google Scholar]
- 44.Samuel SR VS, Maiya AG, Fernandes DJ, McNeely ML. Exercise-based interventions for cancer survivors in India: a systematic review. Springerplus 2015;4:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch PT, Horani S, Lee R, et al. Effectiveness of physical activity interventions in improving objective and patient-reported outcomes in head and neck cancer survivors: a systematic review. Oral Oncol 2021;117:105253. [DOI] [PubMed] [Google Scholar]
- 46.Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol 2015;33:3314–3321. [DOI] [PubMed] [Google Scholar]
- 47.Badr H, Herbert K, Chhabria K, et al. Self-management intervention for head and neck cancer couples: results of a randomized pilot trial. Cancer 2019;125:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pocock S. PocockSJ: clinical trials: a practical approach. New York: John Wiley & Sons; 1983. [Google Scholar]
- 49.Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol 2014;32:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol 2010;8:43–55. [PubMed] [Google Scholar]
- 51.Milbury K, Mallaiah S, Lopez G, et al. Vivekananda Yoga Program for patients with advanced lung cancer and their family caregivers. Integr Cancer Ther 2015;14:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 53.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck 2007;29:923–931. [DOI] [PubMed] [Google Scholar]
- 54.Ware JE, Johnston SA, Davies-Avery A, al e. Conceptualization and measurement of health for adults in the health insurance study (Mental health R-1987/3-HEW: 3). In: Santa Monica, CA: RAND Corporation; 1994. [Google Scholar]
- 55.Kahan BC MT. Improper analysis of trials randomized using stratified blocks or minimization. Stat Med 2012;31:328–340. [DOI] [PubMed] [Google Scholar]
- 56.Cleeland CS. The m. D. Anderson symptom inventory user guide. In: Center MAC, ed2009. [Google Scholar]
- 57.Ware JE, Kosinski M, Keller SK. Physical and mental health summary scales. A user’s manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 58.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007;25:3205–3209. [DOI] [PubMed] [Google Scholar]
- 59.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016;25:1057–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snyder S, Silva RF, Whisenant MS, Milbury K. Video conferenced yoga interventions for cancer patients and their caregivers during the COVID-19 pandemic: a report from a clinician’s perspective. Integr Cancer Ther 2021;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milbury K, Whisenant M, Weathers SP, et al. Dyadic versus individual delivery of a yoga program for family caregivers of glioma patients undergoing radiotherapy: results of a 3-arm randomized controlled trial. Cancer Med 2023;12:7567–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley KE, Park CL. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychol Rev 2015;9:379–396. [DOI] [PubMed] [Google Scholar]
- 63.Deng J, Murphy BA, Dietrich MS, et al. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck 2013;35:1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen J. Statistical power analysis for the behavioral sciences 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]


