Abstract
Purpose
Age-related hearing loss is the most common form of permanent hearing loss that is associated with various health traits, including Alzheimer’s disease, cognitive decline, and depression. The present study aims to identify genetic comorbidities of age-related hearing loss. Past genome-wide association studies identified multiple genomic loci involved in common adult-onset health traits. Polygenic risk scores (PRS) could summarize the polygenic inheritance and quantify the genetic susceptibility of complex traits independent of trait expression. The present study conducted a PRS-based association analysis of age-related hearing difficulty in the UK Biobank sample (N = 425,240), followed by a replication analysis using hearing thresholds (HTs) and distortion-product otoacoustic emissions (DPOAEs) in 242 young adults with self-reported normal hearing. We hypothesized that young adults with genetic comorbidities associated with age-related hearing difficulty would exhibit subclinical decline in HTs and DPOAEs in both ears.
Methods
A total of 111,243 participants reported age-related hearing difficulty in the UK Biobank sample (> 40 years). The PRS models were derived from the polygenic risk score catalog to obtain 2627 PRS predictors across the health spectrum. HTs (0.25–16 kHz) and DPOAEs (1–16 kHz, L1/L2 = 65/55 dB SPL, F2/F1 = 1.22) were measured on 242 young adults. Saliva-derived DNA samples were subjected to low-pass whole genome sequencing, followed by genome-wide imputation and PRS calculation. The logistic regression analyses were performed to identify PRS predictors of age-related hearing difficulty in the UK Biobank cohort. The linear mixed model analyses were performed to identify PRS predictors of HTs and DPOAEs.
Results
The PRS-based association analysis identified 977 PRS predictors across the health spectrum associated with age-related hearing difficulty. Hearing difficulty and hearing aid use PRS predictors revealed the strongest association with the age-related hearing difficulty phenotype. Youth with a higher genetic predisposition to hearing difficulty revealed a subclinical elevation in HTs and a decline in DPOAEs in both ears. PRS predictors associated with age-related hearing difficulty were enriched for mental health, lifestyle, metabolic, sleep, reproductive, digestive, respiratory, hematopoietic, and immune traits. Fifty PRS predictors belonging to various trait categories were replicated for HTs and DPOAEs in both ears.
Conclusion
The study identified genetic comorbidities associated with age-related hearing loss across the health spectrum. Youth with a high genetic predisposition to age-related hearing difficulty and other related complex traits could exhibit sub-clinical decline in HTs and DPOAEs decades before clinically meaningful age-related hearing loss is observed. We posit that effective communication of genetic risk, promoting a healthy lifestyle, and reducing exposure to environmental risk factors at younger ages could help prevent or delay the onset of age-related hearing difficulty at older ages.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10162-024-00947-0.
Keywords: Polygenic risk scores, Age-related hearing difficulty, Age-related hearing loss, Presbycusis, Hearing loss, Extended-high frequency audiometry, Distortion-product otoacoustic emissions
Introduction
Age-related hearing loss is the most common form of permanent hearing loss. About 15% (37.5 million) of US adults aged > 18 years report hearing difficulty [1]. The prevalence of audiometric hearing loss involving speech frequencies (0.5, 1, 2, and 4 kHz) rises to about 39% in sixth decade of life [2], and about 81% of US adults aged > 79 years have permanent elevation in puretone audiometric hearing thresholds (HTs) [3]. Age-related hearing loss is associated with a wide range of conditions, including social isolation, cognitive decline, depression, dementia, Alzheimer’s disease, and anatomical and physiological changes in the brain [4–6]. Recent research highlighted gaps between individuals reporting hearing difficulty and those receiving timely intervention [7]. There is a need to identify novel biomarkers informative of risk and resilience to age-related hearing difficulty to facilitate the development of individualized prophylactics and therapeutics.
Common adult-onset health conditions, such as age-related hearing difficulty, exhibit polygenic inheritance, where the probability of phenotype expression is determined by complex interactions between genetic, environmental, and lifestyle-related factors [8–10]. The genome-wide association analysis allows unbiased interrogation to identify genetic architecture underlying the polygenic inheritance of a complex trait [11]. In the past decades, genome-wide association studies (GWAS) identified a highly polygenic landscape (i.e., 100 s of variants modifying overall risk by a small effect per variant) of common adult-onset health traits and diseases [for review, 12]. GWAS summary statistics, with effect size estimates and p-values across genomic variants, could be used to construct a risk assessment model for determining a person’s polygenic risk of a complex trait. Polygenic risk scores (PRS) are typically calculated based on the standardized effect size weighted-allele count approach, which counts the standardized effect size of each risk variant associated with a complex trait. The weighted allele count approach allows the quantification of multi-factorial genetic susceptibility of a polygenic condition into a single score [13–15]. Aside from a linear approach to GWAS, PRS models could be obtained with machine learning and artificial intelligence-based methods capable of evaluating linear and non-linear relationships between genetic variants and complex traits [16, 17]. The polygenic score (PGS) catalog provides access to > 2500 PRS models for complex human traits and diseases [18]. These PRS models could be used to calculate PRS in independent cohorts (i.e., those not included in the original GWAS), quantifying the polygenic risk to a complex trait independent of the expression of the trait. Though constructing and verifying PRS models and calculating PRS in independent cohorts impose numerous methodological challenges, recent studies involving large biobank-scale cohorts have documented their predictive utility [19–21].
Age-related hearing difficulty is a genetically complex trait. Recent genetic studies identified highly polygenic architecture underlying age-related hearing difficulty and age-related hearing loss [9, 22–28]. PRS models are designed to ascertain the genetic risk of age-related hearing difficulty and hearing loss [29, 30]. Phenome-wide genetic correlation and latent causal variant analysis identified health-related factors, such as migraine, balance disorder, mania, irritability, prescription drugs (e.g., Zantac, Ranitidine, Pregabalin, anti-epileptic), dusty workplace, and intestine disorders causing hearing loss [23]. The above literature highlights the polygenic inheritance of age-related hearing difficulty.
The present study utilized a PRS-based association approach to identify genetic comorbidities associated with age-related hearing difficulty. We conducted a PRS-based association analysis on the UK Biobank sample, followed by a replication analysis in an independent sample. The replication sample included healthy young adults with self-reported normal hearing. Replication analysis was conducted with two widely used clinical measures for identifying early-stage age-related hearing loss—HTs and distortion product otoacoustic emissions (DPOAEs) across the conventional and extended-high frequency ranges (0.25–16 kHz) [e.g., 31, 32]. Age-related hearing loss is often accompanied by age-related confounders (e.g., systemic diseases), which could adversely influence the PRS-based association analysis of age-related hearing loss in older adults. Therefore, we conducted the replication analysis on healthy young adults with self-reported normal hearing and subclinical variations in HTs and DPOAEs. We reasoned that genetic predisposition to the complex traits associated with age-related hearing loss in the UK Biobank database could be related to a subclinical decline in HTs and DPOAEs at younger ages.
Methods
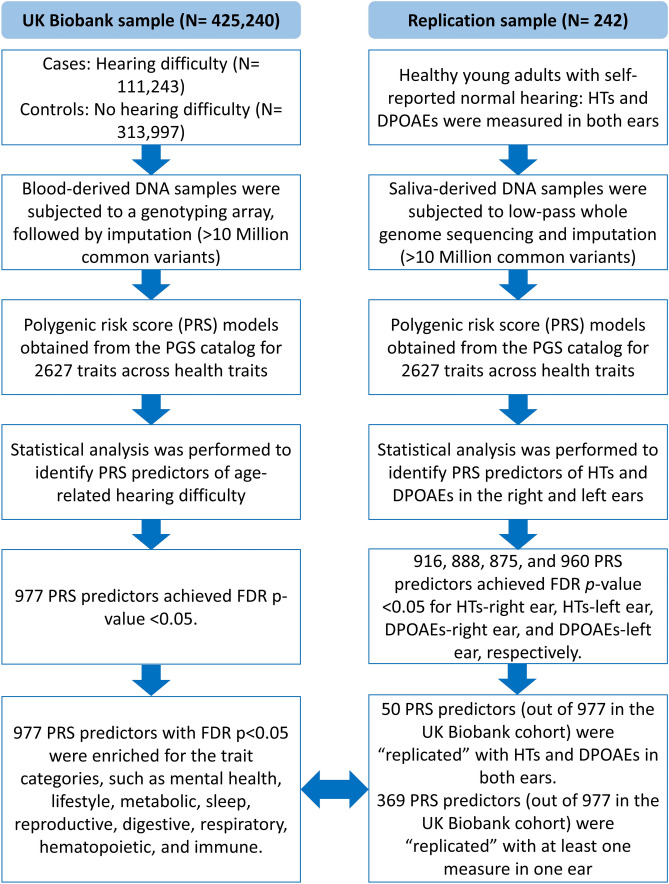
The present study was conducted under the approved project by the UK Biobank (ID#68779). The UK Biobank technical team conducted participant recruitment and data collection. The informed consent form was taken from each participant. We accessed the UK Biobank database containing demographic variables, questionnaires, and interview responses. The University of Iowa institutional review board approved the data handling and analysis procedures (IRB#202103221). The replication analysis was conducted on a sample collected at the University of Iowa campus. An informed consent was obtained from all participants in the replication sample. The University of Iowa Institutional Review Board (IRB#202010165) approved all the procedures used for the replication analysis. Figure 1 presents the overall workflow of the study.
Fig. 1.
Workflow of the study. The study was conducted to identify PRS predictors of age-related hearing difficulty in the UK Biobank cohort. The replication analysis was performed on the replication sample of healthy young adults using HTs and DPOAEs. The results were compared between the two cohorts. PRS predictors were considered “replicated” if they achieved FDR p-value < 0.05 and complementary direction of beta values (for more details, review the “Replication Score for PRS Predictors” section)
The UK Biobank Cohort: Age-Related Hearing Difficulty Phenotype and Questionnaire Responses
The participants responded to a hearing health questionnaire during their first visit to the UK biobank assessment center. The questionnaire investigated hearing difficulty with the question, “Do you have any difficulty with your hearing?”. The response choices included “Yes,” “No,” “I am completely deaf,” “Do not know,” and “Prefer not to answer.” Age-related hearing difficulty phenotype was defined as individuals reporting “Yes” to hearing difficulty. Individuals with “I am completely deaf,” “Do not know,” and “Prefer not to answer” were excluded. We extracted questionnaire responses for demographic factors (e.g., age, sex, ethnicity). Individuals reporting British and Irish ancestral backgrounds were included in the analysis.
UK Biobank Cohort: Genotyping
The genotype data were accessed from the UK Biobank (ID#68779). Blood samples were collected from all participants [33]. Blood-derived DNA samples were subjected to genotyping. The genotyping details are found elsewhere: https://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=263. In brief, DNA samples were extracted and subjected to the genotyping array. The genotyping was performed with two platforms: Affymetrix UK BiLEVE Axiom (N ~ 50,000 samples) and Affymetrix UK Biobank Axiom array (N ~ 450,000 samples). The genotypes were imputed using the Haplotype Reference Consortium [34]. The genotype estimates for > 10 million common variants across the genome were obtained. Genetic variants with high heterozygosity and missingness were excluded.
Replication Sample: Audiometric Testing
A sample of 242 healthy young adults (81 males and 161 females) aged 18–40 years (mean age: 21.5 years, SD = 3 years) was recruited by sending a campus-wide invitation email to potential participants. The samples included 174 individuals reporting European ancestral background and 68 with non-European (including Asian, African, Hispanic, Native American, and multi-ethnic backgrounds). Including individuals from multi-ethnic backgrounds in the replication analysis could ensure the generalizability of the findings, as most PRS models are designed based on GWAS conducted on individuals with European ethnicity. Individuals with no significant medical history and those reporting good health were invited for the present study. Individuals reporting significant health conditions, active middle ear conditions, and ear infections were excluded. The otoscopic examination was conducted to rule out outer ear and middle ear conditions. Participants with normal otoscopic findings were tested with immittance audiometry. All audiometric procedures were performed in a double-walled sound-treated booth meeting ANSI standards. Individuals showing normal tympanometric findings (e.g., static compliance: 0.3–1.75 cc, middle ear pressure: 50 to −100 data) were tested further. Audiometry was performed using the modified Hughson-Westlake procedure with MedRx AVANT clinical audiometer (MedRx, Largo, FL). Insert receivers IP30 (RadioEar, MiddelFart, Denmark) were used to test HTs at the conventional frequency range from 0.25 to 8 kHz. HTs at the extended-high frequency range from 9 to 16 kHz were measured using circumaural headphones DD450 (RadioEar, MiddelFart, Denmark). Individuals with HTs ≤ 20 dB HL from 0.25 to 8 kHz were tested further with distortion product otoacoustic emissions (DPOAEs).
DPOAEs were measured using a Mimosa HearID system (Mimosa Acoustics, Champaign, IL) connected to the ER-10C probe assembly (Etymotic Research, Elk Grove Village, IL). HearID and ER-10C were calibrated following the manufacturer’s guidelines. The ER-10C probe was calibrated within the ear canal using the dB SPL calibration procedure before obtaining measurements from each ear. DPOAEs were generated by two primary tones, f1 and f2. DPOAEs were measured at 2f1–f2 for a stimulus frequency ratio of 1.22 and a primary tone level combination of 65/55 dB SPL. DPOAEs were measured for f2 at 1, 2, 3, 4, 6, 8, 9, 10, 11.2, 12.5, 14, and 16 kHz. The recording was stopped until one of the following stopping logics was achieved: a signal-to-noise ratio (SNR) > 12 dB, a noise floor of ≤ 20 dB SPL, or a maximum test duration of > 10 s at each f2. The procedure for DPOAEs data processing and missing data handling were described elsewhere [35].
Replication Sample: Genotyping
Oragene OGR-600 DISCOVER kits (DNA Genotek, Ottawa, Canada) were used to collect saliva samples. The samples were stored at room temperature before DNA extraction. DNA extraction was performed with prepITL2P reagent kits. Saliva-derived DNA samples were subjected to low-pass whole genome sequencing (BGI American Corporation, Cambridge, MA). DNA molecules were fragmented at random locations. Agencourt AMPure XP-Medium kit was used to pick up the randomly fragmented DNA molecules (average size: 200–400 base pairs). The selected DNA fragments were subjected to end-repairing, 3′ adenylation, adapters-ligation, and polymerase chain reaction (PCR) amplifying processes. The amplified PCR products were cleaned up with AxyPrep Mag PCR kit. The double-stranded PCR products were heat denatured and circularized. The final library of the single-strand circle DNAs was subjected to quality control. DNBSEQ-G400 was used to sequence the final library, achieving quality control standards. The resulting sequencing files were subjected to an imputation pipeline generating genotype data for > 10 million common markers [36].
Polygenic Risk Score (PRS) Calculation
PRS models (weights for genetic variants) were obtained from the Polygenic Risk Score Catalog [18]. PRS models were obtained for 2627 common health traits and disease phenotypes, including sensory, metabolic, digestive, cardiovascular, neurological, inflammatory, musculoskeletal, dermatologic, reproductive, mental health, immune, and genitourinary conditions. The PGS catalog is an open-source platform that follows a standardized framework for reporting PRS model design [for review, 37]. The model performance is typically reported for different ethnic groups, following the standardized framework for reporting ancestry [38]. PRS model-specific details on reported traits, mapped traits, development method, genome build, model performance (in multi-ethnic samples when possible), and other study-related details are available on the PGS catalog website (https://www.pgscatalog.org/). A custom PRS calculator was used to calculate PRS for all participants based on the weighted allele count approach, adjusting for differences in genomic ethnicity, as described by the following equation [for review, 13]:
Here, PRS for a given trait for an ith individual is calculated by adding β (i.e., effect size, usually defined as log odds ratio per dosage of each effect allele (0 ≤ ≤ 2), derived from GWAS summary statistics) for genetic variant j. We performed separate calculations on the UK Biobank and replication cohorts to ensure the robustness of the results.
Statistical Analysis
Logistic regression was used to identify PRS predictors for hearing difficulty phenotype while controlling for the effects of age, age2, sex, age * sex, self-reported ethnicity, and genomic ethnicity using the first 10 genomic principal components (PCs). The first 10 genomic PCs were obtained from the UK Biobank and used in the regression analysis to control for potential population stratification influencing the PRS-based association analysis. The association analysis employed the standard statistical approach used for the genome-wide association studies, where a separate logistic regression model was fit for each PRS predictor. The following regression model was fit to the PRS predictors:
Here, i is a vector of PRS predictors. The regression coefficient, 95% confidence limits, and p-value for each PRS predictor were extracted. The Benjamini-Hochberg false discovery rate (FDR) adjusted p-value < 0.05 was used to identify statistically significant PRS predictors.
A linear mixed model (LMM) was used for the replication analysis to evaluate the main effect of PRS predictors on HTs and DPOAEs. Plink2 (version v2.00aLM, https://www.cog-genomics.org/plink2) was used to conduct the principal component analysis on > 10 million genome-wide markers to obtain the first 10 genomic PCs for the replication sample. HTs were nested within frequency. LMM was used to control for the effects of covariates and between-subject factors, such as age, age2, sex, age * sex, self-reported ethnicity, and genomic ethnicity (using the first 10 PCs) while investigating the main effect of PRS predictors on HTs. Frequency was treated as a random effect (log-transformed variable). Similarly, LMM was used to investigate the main effects of PRS predictors on DPOAE amplitudes across the frequency range (f2 ranging from 1 to 16 kHz). The following LMM was fit to the PRS predictors:
Here, i is a vector of PRS predictors. The regression coefficient, 95% confidence limits, and p-value for each PRS predictor were extracted. The FDR p-value < 0.05 was used to identify statistically significant PRS predictors. Two separate analyses were conducted for the right and left ears, building on the opportunity for replication between the two ears to ensure the robustness of the results. The Benjamini-Hochberg FDR adjusted p-value < 0.05 was used to identify statistically significant PRS predictors.
Replication Score for PRS Predictors
The PRS predictors achieving FDR p-value < 0.05 and the direction/sign of beta (effect size estimates) “consistent” with the UK Biobank sample were labeled as replicated. The beta values for age-related hearing difficulty were binary coded (0, control; 1, case) for the UK Biobank sample. Higher HTs and lower DPOAEs represent a poorer physiological status. HTs and DPOAEs are negatively correlated at each test frequency (p < 0.05). Therefore, the PRS predictors showing positive beta values for age-related hearing difficulty in the UK Biobank cohort should exhibit positive beta values for HTs and negative beta values for DPOAEs to be considered replicated (i.e., a “consistent” direction of beta) and vice versa. A replication score of “1” was assigned to a PRS predictor if it achieved the FDR p-value < 0.05 with the direction of beta “consistent” with the UK Biobank cohort for the following analyses: DPOAEs in the left ear, DPOAEs in the right ear, HTs in the right ear, and HTs in the left ear. Therefore, the maximum replication score of “4” indicates that the PRS predictor achieved FDR p-value < 0.05 in the UK Biobank sample and “consistent” beta values for HTs and DPOAEs in both ears for the replication cohort, indicating the most robust evidence of association. In contrast, the replication score of “0” suggests no evidence of replication. Derived measures quantifying the relative agreement between DPOAEs and HTs could provide meaningful insight into pathogenesis [35]. Yet, we limit the replication analysis to individual measures to simplify the interpretation of the results.
Results
Table 1 presents the demographic details of the UK Biobank study sample. The study was conducted on 425,240 adults, with 111,243 reporting hearing difficulty. Individuals reporting hearing difficulty showed significantly higher mean age than those with no hearing difficulty (MD = 2.7 years, p < 0.001). Males showed a significantly higher prevalence of hearing difficulty compared to females. There was no significant difference in the prevalence of hearing difficulty between individuals reporting British and Irish ethnic backgrounds.
Table 1.
Demographic details of the UK Biobank sample (N = 425,240)
| Characteristic | Hearing difficulty | ||
|---|---|---|---|
| No (N = 313,997) | Yes (111,243) | p-value | |
| Age (mean ± SD, in years) | 56.12 ± 8.09 | 58.89 ± 7.39 | < 0.001 |
| Biological sex (count, %) | |||
| Female | 179,321 (42.2%) | 49,386 (11.6%) | < 0.001 |
| Male | 134,676 (31.7%) | 61,857 (14.5%) | |
| Ethnicity (count, %) | |||
| British | 305,076 (71.7%) | 108,053 (25.4%) | 0.6 |
| Irish | 8921 (2.1%) | 3190 (0.8%) | |
Independent sample t-test was performed for comparing age between the two groups of hearing difficulty. Chi-square was conducted to investigate the influence of sex and ethnicity on hearing difficulty
PRS Predictors of Hearing Difficulty in the UKB Cohort
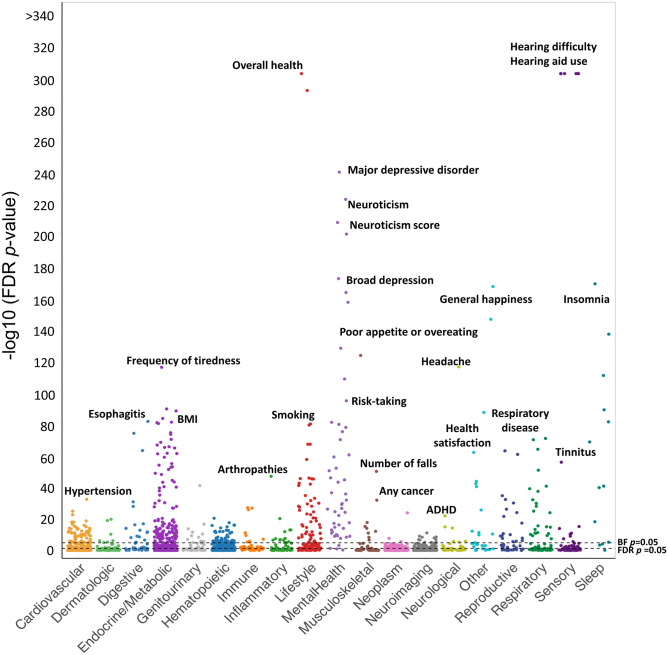
The logistic regression model was fit to 2627 PRS predictors. A total of 977 PRSs achieved FDR p-value < 0.05. Figure 2 presents the results of the regression analysis. Age-related hearing difficulty (PGS000762, PGS001252, PGS001253, PGS001891, PGS002104) and hearing aid use (PGS000763) revealed the lowest p-values (p < 10−300). Among the hearing difficulty-related PRSs, PGS000762 achieved the highest effect size (beta = 1.09, FDR p-value < 10−300). The overall health rating PRS predictors (PGS002000, PGS002218) and general happiness with overall health (PGS002153) revealed significant associations with hearing difficulty. PRS predictors of other sensory traits, such as tinnitus severity, otosclerosis, wearing glasses/contact lenses, myopia, spherical power, disorders of the cornea, and intra-ocular pressure, showed significant association with hearing difficulty. PRSs of mental health traits, such as major depressive disorder, broad depression, anxiety, loneliness, neuroticism, schizophrenia, addiction, and risk-taking tendencies, were associated with hearing difficulty. Among 352 PRS predictors of metabolic traits, 192 PRS predictors revealed significant associations with hearing difficulty. Body mass index (BMI), hip circumference, body fat percentage, obesity, whole body fat, whole body fat-free mass, whole-body water mass, basal metabolic rate, impedance of whole body, type 1 and 2 diabetes, insulin-like growth factor 1, hypothyroidism, disorders of lipid metabolism, vitamin D, folic acid (vitamin B9), and insulin resistance showed significant association with hearing difficulty.
Fig. 2.
A Manhattan-style plot showing the significance levels for the PRS predictors across the trait categories. The significance levels are expressed in FDR p-values (on a −log10 scale). The FDR and BF thresholds for p-value significance are shown with the dotted lines
PRS predictors related to neurological traits, such as stroke, autism spectrum disorder, Parkinson’s disease, attention-deficit hyperactivity disorder, and headache, showed significant association with age-related hearing difficulty. PRS predictors of neuroimaging traits, such as the volume of gray matter and the volumes of gray matter in Heschl’s gyrus (right and left), planum polare (right and left), temporal pole (right and left), thalamus, hippocampus (right and left), parahippocampal gyrus (left), lingual gyrus (right), central opercular cortex (left), frontal pole (left), VIIb cerebellum (right and left), posterior division of temporal fusiform cortex (right and left), caudate (right and left), accumbent (right and left), pallidum (right), brainstem 4th ventricle, later occipital cortex (right superior and inferior divisions), temporal pole (right and left), and insular cortex (right and left), revealed significant association with age-related hearing difficulty.
PRS predictors related to hematopoietic and immune traits, such as high light scatter reticulocyte count, immature reticulocyte fraction, COVID-19 infection, Varicella-Zoster infection, autoimmune diseases, sarcoidosis, connective tissue disorder, allergic diseases, and allergic effect of penicillin, revealed significant associations with age-related hearing difficulty. PRS predictors related to musculoskeletal traits, such as dentures, gonarthrosis, osteoarthritis, falls in the last year, and fractured bones, showed higher odds of reporting hearing difficulty. PRS predictors related to cardiovascular traits, such as systolic blood pressure, HDL cholesterol, apolipoprotein A, atrial fibrillation and flutter, coronary artery disease, triglycerides, chronic ischemic heart disease, congestive heart failure, hypertension, angina, left ventricle stroke volume, and high cholesterol, showed significant association with hearing difficulty.
Lifestyle-related PRS predictors, such as tobacco use, smoking, alcohol use, vitamin B9 intake, tea intake, time spent watching television or computers, salt intake, variation in diet, cannabis use, bread intake, nap during the day, glucosamine intake, and ibuprofen use, revealed significantly higher odds of reporting hearing difficulties. Lifestyle-related PRS predictors, such as high physical activities, chronotype of a morning person, college education, fresh fruit intake, longer sleep duration, use of sun protection, oily fish consumption, cereal consumption, and high-water intake, revealed significantly lower odds of reporting hearing difficulties. Supplementary File S1 provides statistical details about the PRS predictors associated with age-related hearing difficulty. Supplementary File S2 provides trait category-specific volcano plots.
Results of the Replication Analyses Using HTs and DPOAEs
Table 2 presents the demographic details of the replication sample. The replication analysis used HTs and DPOAEs for the right and left ears. Each PRS achieving the FDR p-value < 0.05 in the UK Biobank cohort was assigned a replication score ranging from 0 to 4. Among 977 PRSs achieving the FDR p-value < 0.05, 50, 57, 112, and 150 PRS predictors achieved the replication scores of 4, 3, 2, and 1, respectively (Supplementary File S1). Table 3 presents the PRS predictors achieving the highest replication score.
Table 2.
Demographic details of the replication sample (N = 242)
| Characteristic | Mean (SD)/frequency |
|---|---|
| Age (mean ± SD, in years) | 21.54 ± 0.19 |
| Biological sex (count, %) | |
| Female | 161 (66.5%) |
| Male | 81 (33.5%) |
| Ethnicity (count, %) | |
| European | 174 (71.9%) |
| Non-European | 68 (28.1%) |
| Reoccurring ear infection | |
| Yes | 58 (24.0%) |
| No | 184 (76.0%) |
Table 3.
PRS predictors associated with hearing difficulty in the UK Biobank cohort (N = 425,240)
| PGS ID | Reported trait | Trait category | Beta | FDR p-value |
|---|---|---|---|---|
| PGS000762 | Hearing difficulties | Sensory | 1.09 | <E−300 |
| PGS002161 | Body mass index (BMI) | Metabolic | 0.07 | 9.18E−84 |
| PGS002313 | Body mass index (BMI) | Metabolic | 0.07 | 1.99E−81 |
| PGS001943 | Body mass index (BMI) | Metabolic | 0.07 | 4.26E−81 |
| PGS002679 | Body mass index (BMI) | Metabolic | 0.07 | 1.43E−80 |
| PGS002348 | Smoking status | Lifestyle | 0.07 | 1.33E−79 |
| PGS002096 | Abdominal pain | Digestive | 0.07 | 2.02E−74 |
| PGS000910 | Body mass index (BMI) | Metabolic | 0.07 | 8.78E−74 |
| PGS000921 | Body mass index (BMI) | Metabolic | 0.07 | 9.65E−69 |
| PGS001054 | Snoring | Sleep | 0.05 | 3.27E−41 |
| PGS001995 | Daytime dozing/sleeping (narcolepsy) | Sleep | 0.05 | 2.96E−40 |
| PGS001830 | Tobacco use disorder | Lifestyle | 0.04 | 1.59E−28 |
| PGS001961 | Impedance of whole body | Metabolic | −0.04 | 2.06E−28 |
| PGS002179 | Impedance of whole body | Metabolic | −0.04 | 3.81E−28 |
| PGS000145 | Depression (ICD-10 defined) | Mental health | 0.04 | 4.04E−25 |
| PGS001154 | Impedance (left arm) | Metabolic | −0.03 | 2.84E−20 |
| PGS001161 | Whole body impedance | Metabolic | −0.03 | 4.70E−20 |
| PGS000320 | Body mass index | Metabolic | 0.04 | 2.40E−19 |
| PGS000926 | Daytime dozing/sleeping (narcolepsy) | Sleep | 0.03 | 9.05E−19 |
| PGS002329 | HDL cholesterol | Cardiovascular | −0.03 | 1.35E−18 |
| PGS001156 | Impedance (right arm) | Metabolic | −0.03 | 1.51E−18 |
| PGS002695 | HDL cholesterol | Cardiovascular | −0.03 | 5.00E−17 |
| PGS001954 | HDL cholesterol | Cardiovascular | −0.03 | 1.60E−16 |
| PGS002140 | Wears glasses or contact lenses | Sensory | 0.03 | 9.40E−16 |
| PGS001108 | Basal metabolic rate | Metabolic | 0.03 | 2.03E−14 |
| PGS001959 | High light scatter reticulocyte count | Hematopoietic | 0.03 | 1.74E−13 |
| PGS001976 | Reticulocyte count | Hematopoietic | 0.03 | 2.75E−13 |
| PGS000995 | Dentures | Musculoskeletal | 0.03 | 9.49E−13 |
| PGS001950 | Whole body fat-free mass | Metabolic | 0.03 | 9.49E−13 |
| PGS001234 | Left arm mass (predicted) | Metabolic | 0.03 | 3.80E−12 |
| PGS001165 | Arm fat-free mass (L) | Metabolic | 0.03 | 5.64E−12 |
| PGS002470 | Systolic blood pressure | Cardiovascular | −0.04 | 3.29E−09 |
| PGS001002 | Whole body water mass | Metabolic | 0.02 | 3.40E−09 |
| PGS001146 | Arm fat-free mass (R) | Metabolic | 0.02 | 3.70E−09 |
| PGS001169 | Whole body fat-free mass | Metabolic | 0.02 | 5.14E−09 |
| PGS001235 | Right arm mass (predicted) | Metabolic | 0.02 | 7.36E−09 |
| PGS002101 | Apolipoprotein A | Cardiovascular | −0.02 | 1.20E−07 |
| PGS001846 | Hemorrhoids | Digestive | 0.02 | 6.40E−06 |
| PGS002399 | Forced vital capacity | Cardiovascular | −0.02 | 4.25E−05 |
| PGS002450 | HDL cholesterol | Cardiovascular | −0.03 | 7.58E−05 |
| PGS001168 | Trunk fat-free mass | Metabolic | 0.01 | 2.84E−04 |
| PGS001160 | Trunk mass (predicted) | Metabolic | 0.01 | 5.62E−04 |
| PGS000901 | Systolic blood pressure (male) | Cardiovascular | −0.01 | 1.35E−03 |
| PGS002491 | Diabetes (any type) | Metabolic | 0.35 | 2.07E−03 |
| PGS000356 | Any cancer | Neoplasm | 0.01 | 4.57E−03 |
| PGS002524 | Type 2 diabetes | Metabolic | 0.32 | 5.93E−03 |
| PGS002405 | High light scatter reticulocyte count | Hematopoietic | 0.01 | 6.84E−03 |
| PGS002040 | Retinal detachments and defects | Sensory | −0.01 | 2.81E−02 |
| PGS002401 | HDL cholesterol | Cardiovascular | −0.01 | 3.26E−02 |
| PGS000272 | Pappalysin-1 (PAPPA) serum levels | Hematopoietic | 0.01 | 4.33E−02 |
These PRS predictors achieved the highest replication score in the replication cohort, indicating that the results were replicated for HTs and DPOAEs for both ears
Results of the Enrichment Analysis
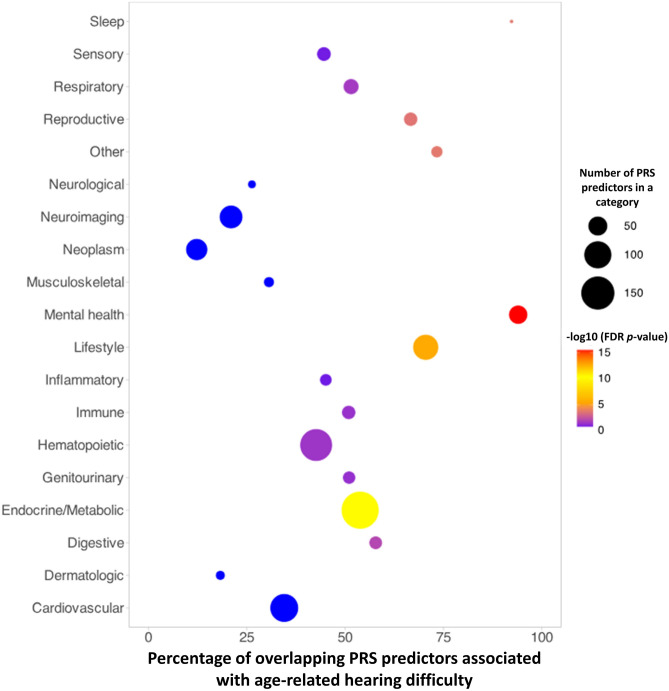
Age-related hearing difficulty was associated with 977 PRS predictors across the health spectrum (Fig. 2). We performed the enrichment analysis to identify the trait categories for which significant PRS predictors were robustly expressed. Fisher’s exact test was conducted on each category, and p-values were obtained. Trait categories with the FDR p-value < 0.05 were identified. Figure 3 presents the results of the enrichment analysis. Mental health, lifestyle, metabolic, sleep, reproductive, digestive, respiratory, hematopoietic, and immune were significantly enriched (Supplementary File S1).
Fig. 3.
A plot presenting the results of the trait category-specific enrichment analysis. The percentage of overlapping PRS predictors associated with age-related hearing difficulty in the UK Biobank (FDR p-value < 0.05) is presented on the X-axis, and trait categories are shown on the Y-axis. The size of the circle represents the number of significant PRS predictors in each category. The color scale presents the significance levels on a log scale. Mental health, lifestyle, metabolic, sleep, reproductive, digestive, respiratory, hematopoietic, and immune categories were significantly enriched for age-related hearing difficulty in the UK Biobank sample
PRS Predictors Associated with HTs and DPOAEs
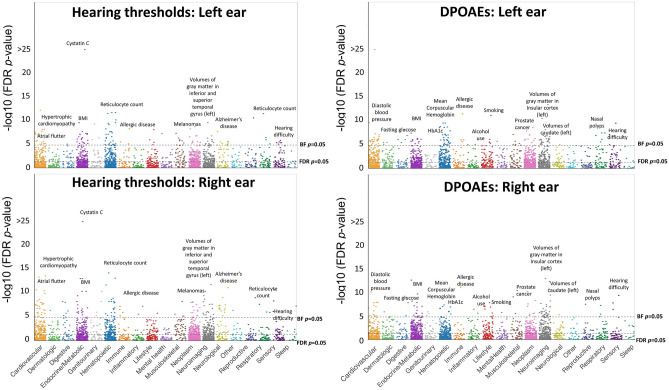
Figure 4 presents the results of the regression analysis for HTs and DPOAEs. The beta values of the PRS predictors revealed high correlation coefficients for HTs (r = 0.83, p < 10−15) and DPOAEs (r = 0.73, p < 10−15) between the ears. The beta values for HTs and DPOAEs revealed inverse relationships for the right (r = −0.65, p < 10−15) and left (r = −0.56, p < 10−15) ears, suggesting that the overall results of the LMM were consistent between HTs and DPOAEs and between both ears. A total of 916, 888, 875, and 960 PRS predictors achieved FDR p-value < 0.05 for HTs-right ear, HTs-left ear, DPOAEs-right ear, and DPOAEs-left ear, respectively (Supplementary File S2). PRS predictor-specific audiograms and DPgrams can be found at 10.6084/m9.figshare.c.7002486.v1.
Fig. 4.
A Manhattan-style plot showing the significance levels for the PRS predictors across the trait categories for HTs and DPOAEs in the right and left ears. The significance levels are expressed in FDR p-values (on a −log10 scale). The FDR and BF thresholds for p-value significance are shown with the dotted lines. The text represents a subset of common PRS predictors replicated in both ears (more details in Supplementary File S1)
Comparison Between PRS Predictors Associated with HTs and DPOAEs in the Replication Cohort
The between-measures replication analysis was conducted to investigate the relative agreement between the results of HTs and DPOAEs. A between-measures replication score of “1” was assigned to a PRS predictor if the direction/sign of the beta value for HTs was “consistent” between both ears and the FDR p-values < 0.05 for both ears. Similarly, a replication score of “1” was assigned to a PRS predictor if the direction of beta value for DPOAEs was consistent between both ears and the FDR p-values for both ears were < 0.05. The replication scores between HTs and DPOAEs were summed to obtain the total between-measures replication score for each PRS predictor. The maximum between-measures replication score of “2” indicates that the PRS predictor achieved the FDR p-value < 0.05 for both ears and measures. A between-measures replication score of “1” indicates that the PRS predictor achieved the FDR p-value of < 0.05 for both ears for only one measure. A total of 179 PRS predictors achieved a replication score of “2,” and 723 achieved a replication score of “1.”
Among the PRS predictors achieving a between-measures replication score of “2,” sensory PRSs, such as hearing difficulty (PGS000762) and wearing glasses or contact lenses, revealed significant relationships with HTs and DPOAEs. Individuals with a higher genetic predisposition to clinical depression showed significantly elevated HTs and reduced DPOAEs. Higher scores of PRS predictors, such as diabetes, hypertrophic cardiomyopathy, Alzheimer’s disease, decreased left ventricular ejection fraction, abdominal pain, varicose veins, melanomas, basal cell carcinomas, and fasting glucose, were associated with significantly elevated HTs and reduced DPOAEs in both ears. Lower scores of PRS predictors, such as cystatin C, monocyte percentage, HDL-cholesterol, chronic airway obstruction, and TNF-related apoptosis-inducing ligand serum levels, were associated with significantly elevated HTs and reduced DPOAEs. PRS predictors related to ossification traits, such as heel broadband ultrasound attenuation, speed of sound through left and right heels, fractured bones, and whole-body impedance, revealed significant associations with HTs and DPOAEs in both ears.
Among 179 PRS predictors with the highest between-measures replication score, 96 PRS predictors revealed a consistent direction of betas with the UK Biobank sample. Among these 96 PRS predictors, 50 revealed FDR p-value < 0.05 and 46 revealed FDR p-value > 0.05 for the UK Biobank sample. Supplementary File S1 presents the complete results of the PRS predictors and their between-measures replication scores.
Discussion
The present study conducted a PRS-based association analysis of age-related hearing difficulty using the UK Biobank sample (N = 425,240). The replication analysis was performed using HTs and DPOAEs (0.25–16 kHz) on a sample of healthy young adults with self-reported normal hearing (N = 242). The results of the present study showed the following: (1) Hearing difficulty and hearing aid use PRS predictors revealed the strongest association with age-related hearing difficulty in the UK Biobank sample. Healthy young adults with high PRS scores for hearing difficulty (PGS000762) showed a significant subclinical elevation in HTs and a decline in DPOAEs in both ears. (2) The PRS-based association analysis identified 977 PRS predictors across the health spectrum, showing significant associations with age-related hearing difficulty. The enrichment analysis revealed that the PRS predictors associated with age-related hearing difficulty were enriched for mental health, lifestyle, metabolic, sleep, reproductive, digestive, respiratory, hematopoietic, and immune traits. (3) Fifty out of 977 PRS predictors showing significant association with age-related hearing difficulty in the UK Biobank cohort were replicated for HTs and DPOAEs in both ears. These PRSs belonged to various trait categories, including sensory, metabolic, lifestyle, sleep, mental health, cardiovascular, hematopoietic, musculoskeletal, digestive, and neoplasm. (4) In the replication sample of young adults, 179 PRS predictors achieved the highest between-measures replication score, suggesting they were replicated for DPOAEs and HTs for both ears. The results highlighted a polygenic architecture of age-related hearing difficulty. The study indicates that young adults with a high genetic predisposition to age-related hearing loss and related conditions may have an increased risk of sub-clinical elevation in HTs and a reduction in DPOAEs.
Our study provides novel insight into the genetic comorbidities of age-related hearing difficulty. The study associated several PRS predictors across the health spectrum with age-related hearing difficulty. Our observation is consistent with the polygenic inheritance of age-related hearing difficulty. Under the assumption of polygenic inheritance, it is expected that multiple causal variants distributed across the genome could contribute to the pathogenesis of a complex trait [39]. GWAS applying modern statistical methods for efficiently controlling population stratification and inter-chromosomal linkage disequilibrium showed that complex traits are influenced by 100s of common genetic variants, exerting smaller effects [12]. Recent studies employing novel methods are beginning to elucidate the influence of rare and very rare variants on complex traits [40]. The loci associated with complex traits could influence various molecular pathways and biological processes, collectively orchestrating the phenotypic spectrum [41]; thereby, a high genetic risk to a complex trait could potentially modify risk and resilience to other complex traits.
The PRS-based association analysis could not provide a robust causal inference; instead, it provides insight into genetic comorbidities associated with a complex trait. The genetic comorbidities could be driven by causality (genetic variants → exposure → outcome, this hypothesis can be tested under the Mendelian randomization framework, [42]), genetic correlations/horizontal pleiotropy (i.e., one gene affecting many traits), and common genetic vulnerability (i.e., shared pathways and molecular processes) between complex traits. The results of the PRS analysis must be interpreted considering the general methods employed by the original GWAS, PRS model design, PRS model verification, and PRS-based association analysis [for review, 11, 20].
Association of Hearing Difficulty PRS with Extended-High Frequency Hearing Loss
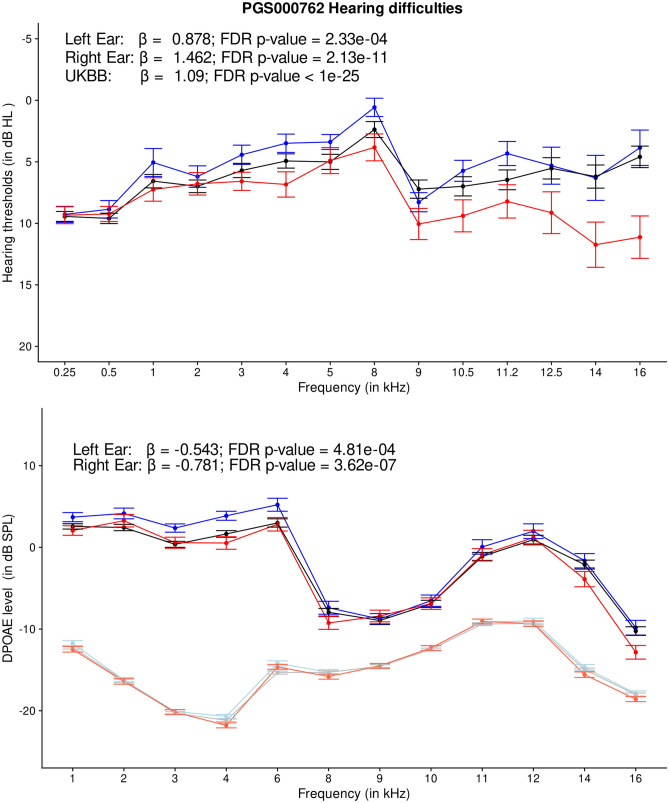
PRS predictors (PGS000762, PGS000763, PGS002104, PGS001252, PGS001891, and PGS002218) related to hearing difficulty traits showed robust evidence of association with age-related hearing difficulty phenotype in the UK Biobank sample (p < 10−300). These were expected findings given that the PRS models for hearing difficulty traits were designed using the GWAS summary statistics or individual-level genotype and phenotype data from the UK Biobank [18]. Among the associated PRS predictors, PGS000762 revealed the highest effect size [30]. Young adults with self-reported normal hearing and high PRS for PGS000762 showed significantly elevated HTs and poorer DPOAEs (Fig. 5). Age-related hearing loss is often characterized by a slopping audiometric configuration, with the early-stage decline in extended-high frequency region [43]. Consistent with the phenotypic characteristics of early-stage age-related hearing loss, young adults with high PRS of age-related hearing difficulty revealed a subclinical elevation in HTs and a decline in DPOAEs at the extended-high frequency region. We hypothesized that (1) youth with a high genetic predisposition to age-related hearing difficulty would experience a significantly accelerated decline in hearing measures with age progression than those with low genetic predisposition to age-related hearing difficulty; (2) individuals with high PRS, and high environmental- and lifestyle-related risk factors would experience the highest decline in their hearing health outcomes with age progression; and (3) individualized precision intervention conveying genetic risk of age-related hearing difficulty, and successfully reducing the risk of environmental and lifestyle factors in younger adults could be efficient at preventing or delaying the onset of age-related hearing decline at older ages. Future research is needed to test these hypotheses.
Fig. 5.
Line charts showing audiogram (top) and DPgram (bottom) for individuals with low (< 20th percentile), mid (20–80th percentile), and high (> 80th percentile) PRS categories for hearing difficulty (PGS000762). The error bar indicates a ± standard error. The light color lines in DPgrams indicate noise floor
Association Between Genetic Predisposition to Metabolic Traits and Age-Related Hearing Difficulty
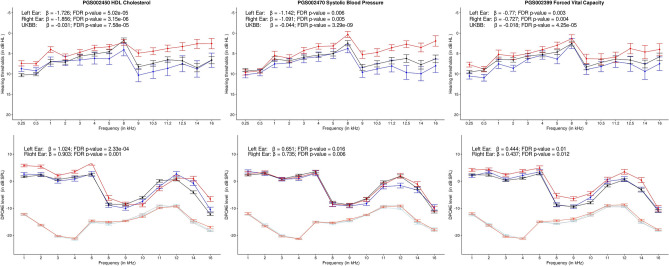
The enrichment analysis revealed that the PRS predictors associated with age-related hearing difficulty were significantly over-represented in a metabolic category (Table 2, Fig. 6). A total of 189 PRS predictors in the metabolic category revealed significant associations with age-related hearing difficulty in the UK Biobank cohort, with various degrees of statistical support for replication (Supplementary File S1). PRS predictors related to metabolic endophenotypes, such as albumin, sex hormone-binding globulin, thyroid, alkaline phosphatase, cystatin C, sodium in the urine, and vitamin D levels, revealed significant associations with age-related hearing difficulty. Large-scale epidemiological studies established the relationship between metabolic syndrome and age-related hearing loss [e.g., 44–47]. Metabolic syndrome is characterized by the cluster of the following complex traits: elevated waist circumference, elevated triglycerides, reduced HDL cholesterol, elevated blood pressure, and elevated fasting plasma glucose level [48]. PRS predictor related to HDL cholesterol (PGS002329, PGS002695, PGS001954, PGS002450, and PGS002401; with replication score = 4), waist circumference (PGS002201, replication score: 2), and triglyceride levels (PGS002523, replication score: 2) revealed significant association with age-related hearing difficulty. However, PRS predictors related to glucose levels (e.g., PGS000305) were not associated. PRS related to systolic blood pressure was negatively correlated with age-related hearing difficulty, indicating that individuals with a genetic predisposition to lower blood pressure are at higher risk of age-related hearing difficulty (PGS002470 and PGS000901; replication score: 4), which is consistent with some observational studies [49, 50]. Taken together, genetic predisposition to HDL cholesterol, waist circumference, and triglyceride levels could contribute to age-related hearing difficulty. In contrast, the association between other components of metabolic syndrome (high blood pressure and high fasting plasma glucose level) and hearing outcomes is likely to be driven by lifestyle and environmental factors. Future research is required to understand the influence of gene-environment interactions on the relationship between metabolic traits and hearing health measures.
Fig. 6.
Line charts showing audiogram (top) and DPgram (bottom) for individuals with low (< 20th percentile), mid (20–80th percentile), and high (> 80th percentile) PRS categories for body mass index, depression, and high light scatter reticulocyte count. The error bar indicates a ± standard error. The light color lines in DPgrams indicate noise floor
PRS Predictors Related to Lifestyle Traits Associated with Age-Related Hearing Difficulty
PRS-related to lifestyle factors, such as smoking (PGS002348, replication score = 4), tobacco use disorder (PGS001830, replication score = 4), problematic alcohol use (PGS000202, replication score = 3), high vitamin B9 intake (PGS001045, replication score = 3), poor physical activity markers (PGS001074, PGS002217, PGS000201, and PGS001999; replication score = 2), chronotype (i.e., morning person being protective, PGS002390, replication score = 2), and college education (PGS002538, replication score = 2), revealed significant association with age-related hearing difficulty. Smoking, alcohol use, and lower physical activity are associated with age-related hearing loss in large-scale epidemiological studies [e.g., 51–53]. PRS related to dietary lifestyle, such as added salt intake, high tea intake, high variation in diet, less oily fish consumption, major dietary changes in the last 5 years, less water intake, high processed meat intake, high cereal consumption, high bread intake, and less fresh fruit intake, increased the risk of age-related hearing difficulty (with replication score ≤ 1). Pro-inflammatory foods with high sugar content are associated with a higher risk. In comparison, greater consumption of fruits and vegetables with antioxidants is associated with a lower risk of age-related hearing loss [e.g., 54, 55]. Of note, the present study might be at risk for underestimating the effects of lifestyle factors as the replication sample was recruited from the university campus, reflecting individuals with a higher-than-national average educational attainment.
Lifestyle-related risk factors of age-related hearing loss could be modifiable. Early identification of individuals with a higher genetic predisposition to lifestyle-related modifiable risk factors could provide a therapeutic window to prevent the expression of undesirable lifestyle traits, thereby reducing the overall risk of age-related hearing loss at older ages. Future research should focus on the genotype-first approach to prevent or delay the expression of age-related hearing difficulty at older ages.
PRS-Related to Mental Health Traits Associated with Age-Related Hearing Difficulty
About 90% of PRS predictors related to mental health showed significant associations with age-related hearing difficulty (Fig. 6). PRS related to clinical depression (PGS000145, replication score = 4) showed a significant association with age-related hearing difficulty. Young adults with high PRS of clinical depression revealed a slopping configuration with elevated HTs and reduced DPOAE amplitudes at high and extended-high frequency ranges in both ears (Fig. 6). Notably, all participants in the replication database reported good overall health. However, subclinical depression in youths, especially in those with high PRS for clinical depression, could not be ruled out. The association between clinical depression PRS and extended-high frequency threshold shift should be investigated further with hypothesis-driven research. Other PRS predictors related to mental health traits, such as neuroticism, worrying, schizophrenia, addiction to substances, risk-taking tendency, loneliness, self-harm or suicidal thoughts, and poor appetite or overeating, revealed significant associations with age-related hearing difficulty. Emerging evidence suggests associations between age-related hearing loss and mental health traits, such as stress, depression, anxiety, and emotional distress [56]. Taken together, our findings highlighted that genetic predisposition to mental health traits strongly correlates with hearing health outcomes in younger and older adults. Clinicians should prioritize mental health while treating individuals with age-related hearing loss. Early preventive interventions addressing mental health could potentially benefit youth with a higher genetic predisposition to age-related hearing loss and mental health traits at older ages.
PRS-Related to Hematopoietic Traits and Age-Related Hearing Difficulty
PRS predictors of hematopoietic traits, such as reticulocyte count (PGS002405, PGS001976, and PGS001959, replication score = 4) and pappalysin-1 serum levels (PGS000272, replication score = 4), revealed significant associations with age-related hearing difficulty. High reticulocyte count and reticulocyte hemoglobin are indicators of iron deficiency anemia [57]. The cochlear tissue is supplied by only the labyrinthine artery, making it vulnerable to ischemic damage [59]. Iron deficiency anemia could impair oxygen-carrying capacity, increase the risk of reactive thrombocytosis, degrade lipid saturase and desaturase, impair glucose metabolism, and myelin production [60–63], potentially contributing to sensorineural hearing loss [58]. Young adults with high PRS of reticulocyte counts, possibly with a subclinical expression of iron deficiency anemia, showed significantly elevated HTs and reduced DPOAE amplitudes in both ears (Fig. 6).
Pregnancy-associated plasma protein A (PAPPA) is a zinc-binding metalloprotein responsible for cleaving inhibitory binding proteins of insulin-like growth factors. It is involved in processes related to inflammation and atherosclerosis [64, 65]. Over-expression of PAPPA could accelerate atherosclerosis and could significantly elevate the risk of cardiovascular diseases and all-cause mortality in patients with chronic kidney disease [66–68]. A high genetic predisposition to PAPPA levels (PGS000272, replication score = 4) was associated with an elevated risk of age-related hearing difficulty in the present study. Consistent with the observation, young adults with high PRS of PAPPA serum levels revealed significantly elevated HTs and DPOAE amplitudes in both ears. Future research is required to investigate the molecular mechanisms underlying the relationship between PAPPA and hearing health.
PRS Predictors Related to Cardiovascular Traits Associated with Age-Related Hearing Difficulty
PRS-related to cardiovascular traits, such as HDL-cholesterol (PGS002329, PGS002695, and PGS001954, replication score = 4) and apolipoprotein A (PGS002101, replication score = 4), revealed significant associations with age-related hearing difficulty. Epidemiological studies identified the association between lower HDL cholesterol levels and HTs [69, 70]. HDL cholesterol and apolipoprotein A are important for preventing oxidative stress-induced cell death [71]. Our past study used a linear model showing that youth with a genetic predisposition to low HDL cholesterol levels exhibit significantly poorer self-reported supra-threshold auditory processing ability without elevation in HTs [72]. The present study employed a linear mixed model to efficiently control for fixed and random factors to detect smaller effect sizes. We identified a significant elevation in HTs and a reduction in DPOAE amplitudes in youth with a genetic predisposition to lower HDL cholesterol (Fig. 7).
Fig. 7.
Line charts showing audiogram (top) and DPgram (bottom) for individuals with low (< 20th percentile), mid (20–80th percentile), and high (> 80th percentile) PRS categories for HDL cholesterol, systolic blood pressure, and forced vital capacity. The error bar indicates a ± standard error. The light color lines in DPgrams indicate noise floor
The present study revealed that the genetic predisposition to lower systolic blood pressure (PGS002470, beta = −0.04, replication score = 4) was associated with a higher risk of age-related hearing difficulty. Youths with high PRS for systolic blood pressure revealed significantly elevated HTs and lower DPOAEs than their counterparts (Fig. 6). These results are consistent with a past epidemiological study showing the association between hypotension and age-related hearing loss [50–52]. Ten PRS predictors of hypertension revealed associations with age-related hearing difficulty; none showed a significant association with HTs and DPOAEs in young adults. Older adults are more likely to express the hypertension phenotype than youth. The phenotypic association between hypertension and age-related hearing loss observed in epidemiological studies [e.g., 73, 74] could be conditional to the expression of hypertension phenotype.
Genetic predisposition to higher forced vital capacity, an indicator of healthy pulmonary and cardiovascular systems [e.g., 75], was associated with a lower risk of age-related hearing loss (PGS002399, replication score = 4). Young adults with high PRS for forced vital capacity revealed better HTs and DPOAEs than their counterparts (Fig. 7). Epidemiological studies observed associations between pulmonary and hearing measures, with better pulmonary health associated with better hearing outcomes [e.g., 76, 77]. Future research is needed to investigate the molecular mechanisms underlying the relationship between genetic predisposition to pulmonary health and hearing outcomes.
Association Between Neuroimaging Traits and Age-Related Hearing Difficulty
Seventy-one PRS predictors revealed significant associations with age-related hearing difficulty in the UK Biobank cohort (Supplementary File S1) (Fig. 8). PRS related to the volumes of gray matter in areas involved in sensory processing, language, and cognition, such as the superior temporal gyrus (posterior division left), Heschl’s gyrus (left and right), planum temporale, central opercular cortex, precentral gyrus, hippocampus, cerebellum, thalamus, lingual gyrus (right), brainstem, insular cortex, precentral gyrus, and cingulate gyrus, revealed significant inverse relationship with age-related hearing difficulty. These observations are generally consistent with emerging literature on the effects of peripheral hearing loss on anatomical and physiological changes in the brain [e.g., 6, 78].
Fig. 8.
A volcano plot presenting the effect sizes as a function of the significance levels for PRS predictors related to neuroimaging traits. The red and blue dots present the PRS categories negatively (i.e., PRS for the volume of gray matter being reduced for individuals with age-related hearing difficulty) and positively associated with hearing difficulty, respectively
HTs and DPOAEs are clinical measures sensitive to cochlear functioning. They are not well suited for the replication analysis of neuroimaging traits as they might not be sensitive to anatomical and physiological changes in the brain. Nevertheless, we observed some PRS predictors of the image-derived phenotypes, such as the volume of gray matter in planum polare (left), the volume of gray matter in the temporal fusiform cortex (anterior division, left), the volume of gray matter in the lateral occipital cortex (inferior division, right), weighted-average L2 in track cingulate gyrus (right and left) (all with replication scores ≥ 2), showing significant association with age-related hearing difficulty. Planum polare, temporal fusiform cortex, occipital cortex, cingulate gyrus, and anterior corona radiata are involved in sensory, language, and cognitive processes [79–82]. Surprisingly, 21 PRS predictors with a between-measures replication score of “2” (i.e., the consistent direction of association between HTs and DPOAEs in both ears) revealed the opposite direction of association with the UK Biobank cohort. Sixteen PRS predictors (out of 21) of MRI-based volumetry, such as volume of gray matter, peripheral cortical gray matter, brainstem, insular cortex, thalamus, caudate, insular cortex, parahippocampal gyrus, and temporal gyrus (left), revealed the opposite direction of association from the UK Biobank cohort, indicating that young adults with high PRS of the volumetric phenotypes exhibit slightly poorer HTs (mean difference ~1 dB) and DPOAEs (mean difference ~0.75 dB) in both ears. Age and peripheral hearing status might affect the direction and strength of associations between PRS predictors and neuroimaging traits. Future research is necessary to understand the effects of genetic predisposition to neuroimaging traits on hearing outcomes across the lifespan.
Summary
The PRS-based association study identified genetic comorbidities associated with age-related hearing difficulty in the UK Biobank cohort (N = 425,240), followed by a replication analysis with HTs and DPOAEs (0.25–16 kHz) using the linear mixed model on a sample of healthy young adults with self-reported normal hearing (N = 242). The study identified 977 PRS predictors across the health spectrum, showing a significant association with age-related hearing difficulty in the UK Biobank cohort. Fifty PRS predictors were replicated for HTs and DPOAEs for both ears in young adults. The PRS predictors associated with age-related hearing difficulty belonged to a wide range of trait categories, including sensory, metabolic, lifestyle, sleep, mental health, cardiovascular, hematopoietic, musculoskeletal, digestive, neurological, neuroimaging, and neoplasm. The results highlighted a polygenic architecture of age-related hearing difficulty. The replication analysis showed that young adults with a high genetic predisposition to age-related hearing difficulty and other related complex traits could increase the risk of sub-clinical decline in HTs and DPOAEs decades before clinically meaningful age-related hearing loss is observed. Effective communication of genetic risk, promoting a healthy lifestyle, and reducing exposure to environmental risk factors at younger ages could help prevent or delay the onset of age-related hearing difficulty at older ages.
Supplementary Information
Below is the link to the electronic supplementary material.
Acronyms
- PRS
Polygenic risk score
- GWAS
Genome-wide association study
Funding
National Institute on Deafness and Other Communication Disorders, R21DC016704-01A1. Ishan Bhatt.
Data Availability
The study used the UK Biobank database. The database is publicly available through the UK Biobank website: https://www.ukbiobank.ac.uk/. The data used for the replication analysis will be shared on dbGaP after the completion of the project: R21DC016704-01A1.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NIDCD (2023) Age-related hearing loss (presbycusis). National Institute of Deafness and Other Communication Disorders. https://www.nidcd.nih.gov/health/age-related-hearing-loss
- 2.Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA (2017) Declining prevalence of hearing loss in US adults aged 20 to 69 years. JAMA Otolaryngol Head Neck Surg 143(3):274–285 10.1001/jamaoto.2016.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma RK, Lalwani AK, Golub JS (2020) Prevalence and severity of hearing loss in the older old population. JAMA Otolaryngol Head Neck Surg 146(8):762–763 10.1001/jamaoto.2020.0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abidin FNZ, Wells HR, Altmann A, Dawson SJ (2021) Hearing difficulty is linked to Alzheimer’s disease by common genetic vulnerability, not shared genetic architecture. npj Aging Mech Dis 7(1):17 10.1038/s41514-021-00069-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosemann S, Thiel CM (2020) Neuroanatomical changes associated with age-related hearing loss and listening effort. Brain Struct Funct 225(9):2689–2700 10.1007/s00429-020-02148-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slade K, Plack CJ, Nuttall HE (2020) The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci 43(10):810–821 10.1016/j.tins.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 7.Mahboubi H, Lin HW, Bhattacharyya N (2018) Prevalence, characteristics, and treatment patterns of hearing difficulty in the United States. JAMA Otolaryngol Head Neck Surg 144(1):65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crouch DJ, Bodmer WF (2020) Polygenic inheritance, GWAS, polygenic risk scores, and the search for functional variants. Proc Natl Acad Sci 117(32):18924–18933 10.1073/pnas.2005634117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ (2005) Gene–environment interactions in human diseases. Nat Rev Genet 6(4):287–298 10.1038/nrg1578 [DOI] [PubMed] [Google Scholar]
- 10.Wells HR, Freidin MB, Abidin FNZ, Payton A, Dawes P, Munro KJ, Williams FM (2019) GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK Biobank. Am J Hum Genet 105(4):788–802 10.1016/j.ajhg.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uitterlinden AG (2016) An introduction to genome-wide association studies: GWAS for dummies. In: Seminars in reproductive medicine, vol 34, 4th edn. Thieme Medical Publishers, pp 196–204 [DOI] [PubMed] [Google Scholar]
- 12.Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, Harris LW (2023) The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res 51(D1):D977–D985 10.1093/nar/gkac1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SF, Dias R, Evans D, Salfati EL, Liu S, Wineinger NE, Torkamani A (2020) Genotype imputation and variability in polygenic risk score estimation. Genome Med 12:1–13 10.1186/s13073-020-00801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SW, Mak TSH, O’Reilly PF (2020) Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc 15(9):2759–2772 10.1038/s41596-020-0353-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torkamani A, Wineinger NE, Topol EJ (2018) The personal and clinical utility of polygenic risk scores. Nat Rev Genet 19(9):581–590 10.1038/s41576-018-0018-x [DOI] [PubMed] [Google Scholar]
- 16.Klau JH, Maj C, Klinkhammer H, Krawitz PM, Mayr A, Hillmer AM, Heider D (2023) AI-based multi-PRS models outperform classical single-PRS models. Front Genet 14:1217860 10.3389/fgene.2023.1217860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elgart M, Lyons G, Romero-Brufau S, Kurniansyah N, Brody JA, Guo X, Sofer T (2022) Non-linear machine learning models incorporating SNPs and PRS improve polygenic prediction in diverse human populations. Commun Biol 5(1):856 10.1038/s42003-022-03812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert SA, Gil L, Jupp S, Ritchie SC, Xu Y, Buniello A, Inouye M (2021) The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet 53(4):420–425 10.1038/s41588-021-00783-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marston NA, Pirruccello JP, Melloni GE, Koyama S, Kamanu FK, Weng LC, Ruff CT (2023) Predictive utility of a coronary artery disease polygenic risk score in primary prevention. JAMA Cardiol 8(2):130–137 10.1001/jamacardio.2022.4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanigawa Y, Qian J, Venkataraman G, Justesen JM, Li R, Tibshirani R, Rivas MA (2022) Significant sparse polygenic risk scores across 813 traits in UK Biobank. PLoS Genet 18(3):e1010105 10.1371/journal.pgen.1010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert SA, Abraham G, Inouye M (2019) Towards clinical utility of polygenic risk scores. Hum Mol Genet 28(R2):R133–R142 10.1093/hmg/ddz187 [DOI] [PubMed] [Google Scholar]
- 22.Cornejo-Sanchez DM, Li G, Fabiha T, Wang R, Acharya A, Everard JL, Kadlubowska MK, Huang Y, Schrauwen I, Wang GT, DeWan AT, Leal SM (2023) Rare-variant association analysis reveals known and new age-related hearing loss genes. EJHG 31(6):638–647 10.1038/s41431-023-01302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Angelis F, Zeleznik OA, Wendt FR, Pathak GA, Tylee DS, De Lillo A, Polimanti R (2023) Sex differences in the polygenic architecture of hearing problems in adults. Genome Med 15(1):1–18 10.1186/s13073-023-01186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis MA, Schulte BA, Dubno JR, Steel KP (2022) Investigating the characteristics of genes and variants associated with self-reported hearing difficulty in older adults in the UK Biobank. BMC Biol 20(1):1–20 10.1186/s12915-022-01349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trpchevska N, Freidin MB, Broer L, Oosterloo BC, Yao S, Zhou Y, Nagtegaal AP (2022) Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am J Hum Genet 109(6):1077–1091 10.1016/j.ajhg.2022.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagtegaal AP, Broer L, Zilhao NR, Jakobsdottir J, Bishop CE, Brumat M, Goedegebure A (2019) Genome-wide association meta-analysis identifies five novel loci for age-related hearing impairment. Sci Rep 9(1):15192 10.1038/s41598-019-51630-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, Lustig LR (2016) A large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet 12(10):e1006371 10.1371/journal.pgen.1006371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fransen E, Bonneux S, Corneveaux JJ, Schrauwen I, Di Berardino F, White CH, Friedman RA (2015) Genome-wide association analysis demonstrates the highly polygenic character of age-related hearing impairment. Eur J Hum Genet 23(1):110–115 10.1038/ejhg.2014.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanigawa Y, Kellis M (2023) Power of inclusion: enhancing polygenic prediction with admixed individuals. Am J Hum Genet 110(11):1888–1902 10.1016/j.ajhg.2023.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherny SS, Livshits G, Wells HR, Freidin MB, Malkin I, Dawson SJ, Williams FM (2020) Self-reported hearing loss questions provide a good measure for genetic studies: a polygenic risk score analysis from UK Biobank. Eur J Hum Genet 28(8):1056–1065 10.1038/s41431-020-0603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glavin CC, Siegel J, Dhar S (2021) Distortion product otoacoustic emission (DPOAE) growth in aging ears with clinically normal behavioral thresholds. J Assoc Res Otolaryngol 22:659–680 10.1007/s10162-021-00805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valiente AR, Fidalgo AR, Villarreal IM, Berrocal JRG (2016) Extended high-frequency audiometry (9000–20 000 Hz). Usefulness in audiological diagnosis. Acta Otorrinolaringol (English Edition) 67(1):40–44 10.1016/j.otoeng.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 33.Welsh S, Peakman T, Sheard S, Almond R (2017) Comparison of DNA quantification methodology used in the DNA extraction protocol for the UK Biobank cohort. BMC Genomics 18(1):1–7 10.1186/s12864-016-3391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Marchini J (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726):203–209 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt IS, Lichtenhan J, Tyler R, Goodman S (2023) Influence of tinnitus, lifetime noise exposure, and firearm use on hearing thresholds, distortion product otoacoustic emissions, and their relative metric. J Acoust Soc Am 154(1):418–432 10.1121/10.0019880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinacci S, Ribeiro DM, Hofmeister RJ, Delaneau O (2021) Efficient phasing and imputation of low-coverage sequencing data using large reference panels. Nat Genet 53(1):120–126 10.1038/s41588-020-00756-0 [DOI] [PubMed] [Google Scholar]
- 37.Wand H, Lambert SA, Tamburro C, Iacocca MA, O’Sullivan JW, Sillari C, Wojcik GL (2021) Improving reporting standards for polygenic scores in risk prediction studies. Nature 591(7849):211–219 10.1038/s41586-021-03243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales J, Welter D, Bowler EH, Cerezo M, Harris LW, McMahon AC, MacArthur JA (2018) A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS catalog. Genome Biol 19(1):1–10 10.1186/s13059-018-1396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell KJ (2012) What is complex about complex disorders? Genome Biol 13(1):1–11 10.1186/gb-2012-13-1-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Momozawa Y, Mizukami K (2021) Unique roles of rare variants in the genetics of complex diseases in humans. J Hum Genet 66(1):11–23 10.1038/s10038-020-00845-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lappalainen T, MacArthur DG (2021) From variant to function in human disease genetics. Science 373(6562):1464–1468 10.1126/science.abi8207 [DOI] [PubMed] [Google Scholar]
- 42.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, Davey Smith G (2022) Mendelian randomization. Nat Rev Methods Primers 2(1):6 10.1038/s43586-021-00092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra SK, Saxena U, Rodrigo H (2022) Extended high-frequency hearing impairment despite a normal audiogram: relation to early aging, speech-in-noise perception, cochlear function, and routine earphone use. Ear Hear 43(3):822–835 10.1097/AUD.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 44.Vaden KI Jr, Eckert MA, Matthews LJ, Schmiedt RA, Dubno JR (2022) Metabolic and sensory components of age-related hearing loss. J Assoc Res Otolaryngol 23(2):253–272 10.1007/s10162-021-00826-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rim HS, Kim MG, Park DC, Kim SS, Kang DW, Kim SH, Yeo SG (2021) Association of metabolic syndrome with sensorineural hearing loss. J Clin Med 10(21):4866 10.3390/jcm10214866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung DJ, Han KD, Cho YS, Rhee CS, Lee KY (2019) Association of metabolic syndrome with the incidence of hearing loss: a national population-based study. PLoS ONE 14(7):e0220370 10.1371/journal.pone.0220370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aghazadeh-Attari J, Mansorian B, Mirza-Aghazadeh-Attari M, Ahmadzadeh J, Mohebbi I (2017) Association between metabolic syndrome and sensorineural hearing loss: a cross-sectional study of 11,114 participants. Diabetes Metab Syndr Obes 10:459–465 10.2147/DMSO.S150893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson PM, Tuomilehto J, Rydén L (2019) The metabolic syndrome–what is it and how should it be managed? Eur J Prev Cardiol 26(2_suppl):33–46 10.1177/2047487319886404 [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Hagiwara M, Roehm PC (2012) Spontaneous intracranial hypotension presenting with severe sensorineural hearing loss and headache. Otol Neurotol 33(8):e65 10.1097/MAO.0b013e318254ed98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirodda A, Ferri GG, Modugno GC, Borghi C (2001) Systemic hypotension and the development of acute sensorineural hearing loss in young healthy subjects. Arch Otolaryngol Head Neck Surg 127(9):1049–1052 10.1001/archotol.127.9.1049 [DOI] [PubMed] [Google Scholar]
- 51.Tang D, Tran Y, Dawes P, Gopinath B (2023) A narrative review of lifestyle risk factors and the role of oxidative stress in age-related hearing loss. Antioxidants 12(4):878 10.3390/antiox12040878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian P, Zhao Z, Liu S, Xin J, Liu Y, Hao Y, Yang L (2023) Alcohol as a risk factor for hearing loss: a systematic review and meta-analysis. PLoS ONE 18(1):e0280641 10.1371/journal.pone.0280641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo PL, Di J, Ferrucci L, Lin FR (2021) Analysis of hearing loss and physical activity among US adults aged 60–69 years. JAMA Netw Open 4(4):e215484–e215484 10.1001/jamanetworkopen.2021.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigo L, Campos-Asensio C, Rodríguez MÁ, Crespo I, Olmedillas H (2021) Role of nutrition in the development and prevention of age-related hearing loss: A scoping review. J Formos Med Assoc 120(1):107–120 10.1016/j.jfma.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Sardone R, Lampignano L, Guerra V, Zupo R, Donghia R, Castellana F, Quaranta N (2020) Relationship between inflammatory food consumption and age-related hearing loss in a prospective observational cohort: results from the Salus in Apulia Study. Nutrients 12(2):426 10.3390/nu12020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bigelow RT, Reed NS, Brewster KK, Huang A, Rebok G, Rutherford BR, Lin FR (2020) Association of hearing loss with psychological distress and utilization of mental health services among adults in the United States. JAMA Netw Open 3(7):e2010986–e2010986 10.1001/jamanetworkopen.2020.10986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoenemann C, Ostendorf N, Zarbock A, Doll D, Hagemann O, Zimmermann M, Luedi M (2021) Reticulocyte and erythrocyte hemoglobin parameters for iron deficiency and anemia diagnostics in patient blood management. A narrative review. J Clin Med 10(18):4250 10.3390/jcm10184250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schieffer KM, Chuang CH, Connor J, Pawelczyk JA, Sekhar DL (2017) Association of iron deficiency anemia with hearing loss in US adults. JAMA Otolaryngol Head Neck Surg 143(4):350–354 10.1001/jamaoto.2016.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Nuttall AL (2003) Disorders of cochlear blood flow. Brain Res Rev 43(1):17–28 10.1016/S0165-0173(03)00189-9 [DOI] [PubMed] [Google Scholar]
- 60.Hilton C, Sabaratnam R, Drakesmith H, Karpe F (2023) Iron, glucose and fat metabolism and obesity: an intertwined relationship. Int J Obes 47(7):554–563 10.1038/s41366-023-01299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuku I, Kaya E, Yologlu S, Gokdeniz R, Baydin A (2009) Platelet counts in adults with iron deficiency anemia. Platelets 20(6):401–405 10.1080/09537100903137306 [DOI] [PubMed] [Google Scholar]
- 62.Franchini M, Targher G, Montagnana M, Lippi G (2008) Iron and thrombosis. Ann Hematol 87:167–173 10.1007/s00277-007-0416-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478 10.1002/glia.20784 [DOI] [PubMed] [Google Scholar]
- 64.Boldt HB, Conover CA (2007) Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Hormon IGF Res 17(1):10–18 10.1016/j.ghir.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 65.Harrington SC, Simari RD, Conover CA (2007) Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E–deficient mice challenged with a high-fat diet. Circ Res 100(12):1696–1702 10.1161/CIRCRESAHA.106.146183 [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Meng X, Zhou C, Zhou X (2020) Pregnancy-associated plasma protein A as a predictor of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a meta-analysis of prospective studies. Arch Med Sci 16(1):8–15 10.5114/aoms.2020.91283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lund J, Wittfooth S, Qin QP, Ilva T, Porela P, Pulkki K, Voipio-Pulkki LM (2010) Free vs total pregnancy-associated plasma protein A (PAPP-A) as a predictor of 1-year outcome in patients presenting with non–ST-elevation acute coronary syndrome. Clin Chem 56(7):1158–1165 10.1373/clinchem.2009.136960 [DOI] [PubMed] [Google Scholar]
- 68.Iversen KK, Teisner AS, Teisner B, Kliem A, Thanning P, Nielsen H, Grande P (2009) Pregnancy associated plasma protein A, a potential marker for vulnerable plaque in patients with non-ST-segment elevation acute coronary syndrome. Clin Biochem 42(9):828–834 10.1016/j.clinbiochem.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 69.Sun YS, Fang WH, Kao TW, Yang HF, Peng TC, Wu LW, Chen WL (2015) Components of metabolic syndrome as risk factors for hearing threshold shifts. PLoS ONE 10(8):e0134388 10.1371/journal.pone.0134388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki K, Kaneko M, Murai K (2000) Influence of serum lipids on auditory function. Laryngoscope 110(10):1736–1738 10.1097/00005537-200010000-00033 [DOI] [PubMed] [Google Scholar]
- 71.Lüscher TF, Landmesser U, von Eckardstein A, Fogelman AM (2014) High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 114(1):171–182 10.1161/CIRCRESAHA.114.300935 [DOI] [PubMed] [Google Scholar]
- 72.Bhatt IS, Ramadugu SK, Goodman S, Bhagavan SG, Ingalls V, Dias R, Torkamani A (2023) Polygenic risk score-based association analysis of speech-in-noise and hearing threshold measures in healthy young adults with self-reported normal hearing. J Assoc Res Otolaryngol 24(5):513–525 10.1007/s10162-023-00911-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S, Park JM, Han JS, Seo JH, Han KD, Joo YH, Park KH (2020) Age-related hearing loss in the Korea national health and nutrition examination survey. PLoS ONE 15(12):e0243001 10.1371/journal.pone.0243001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reed NS, Huddle MG, Betz J, Power MC, Pankow JS, Gottesman R, Deal JA (2019) Association of midlife hypertension with late-life hearing loss. JAMA Otolaryngol Head Neck Surg 161(6):996–1003 10.1177/0194599819868145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang HK, Park HY, Jeong BH, Koh WJ, Lim SY (2015) Relationship between forced vital capacity and Framingham cardiovascular risk score beyond the presence of metabolic syndrome: the fourth Korea National Health and nutrition examination survey. Medicine 94(47):e2089 10.1097/MD.0000000000002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dem𝗂rel OB, Ersözlü T, Den𝗂z M (2021) Evaluation of the hearing system in chronic obstructive pulmonary disease patients. Namık Kemal Tıp Dergisi 9(2):174–178 10.4274/nkmj.galenos.2021.26818 [DOI] [Google Scholar]
- 77.Żebrowska A, Zwierzchowska A, Manowska B, Przybyła K, Krużyńska A, Jastrzębski D (2016) Respiratory function and language abilities of profoundly deaf adolescents with and without cochlear implants. Adv Exp Med Biol 912:73–81 10.1007/5584_2016_233 [DOI] [PubMed] [Google Scholar]
- 78.He J, Cabrera-Mendoza B, De Angelis F, Pathak GA, Koller D, Curhan SG, Curhan GC, Mecca AP, van Dyck CH, Polimanti R (2024) Sex differences in the pleiotropy of hearing difficulty with imaging-derived phenotypes: a brain-wide investigation. Brain awae077 [DOI] [PMC free article] [PubMed]
- 79.Turker S, Kuhnke P, Eickhoff SB, Caspers S, Hartwigsen G (2023) Cortical, subcortical, and cerebellar contributions to language processing: a meta-analytic review of 403 neuroimaging experiments. Psychol Bull [DOI] [PubMed]
- 80.Neuschwander P, Schmitt R, Jagoda L, Kurthen I, Giroud N, Meyer M (2023) Different neuroanatomical correlates for temporal and spectral supra-threshold auditory tasks and speech in noise recognition in older adults with hearing impairment. Eur J Neurosci 57(6):981–1002 10.1111/ejn.15922 [DOI] [PubMed] [Google Scholar]
- 81.Taddeo S, Schulz M, Andermann M, Rupp A (2022) Neuromagnetic representation of melodic contour processing in human auditory cortex. Front Hum Neurosci 16:909159 10.3389/fnhum.2022.909159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giampiccolo D, Duffau H (2022) Controversy over the temporal cortical terminations of the left arcuate fasciculus: a reappraisal. Brain 145(4):1242–1256 10.1093/brain/awac057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study used the UK Biobank database. The database is publicly available through the UK Biobank website: https://www.ukbiobank.ac.uk/. The data used for the replication analysis will be shared on dbGaP after the completion of the project: R21DC016704-01A1.